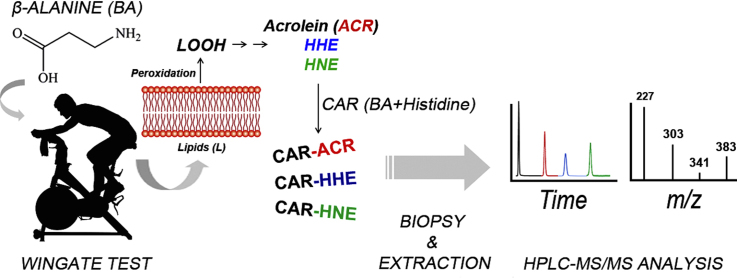
Abstract
Previous studies have demonstrated that exercise results in reactive aldehyde production and that β-alanine supplementation increases carnosine content in skeletal muscle. However, little is known about the influence exercise and β-alanine supplementation have on the formation of carnosine-aldehydes. The goal of the present study was to monitor the formation of carnosine-aldehyde adducts, following high-intensity intermittent exercise, before and after β-alanine supplementation. Vastus lateralis biopsy samples were taken from 14 cyclists, before and after a 28 day β-alanine supplementation, following 4 bouts of a 30 s all-out cycling test, and carnosine and CAR-aldehyde adducts [carnosine-acrolein, CAR-ACR (m/z 303), carnosine-4-hydroxy-2-hexenal, CAR-HHE (m/z 341) and carnosine-4-hydroxy-2-nonenal, CAR-HNE (m/z 383)] were quantified by HPLC-MS/MS. β-alanine supplementation increased muscle carnosine content by ~50% (p = 0.0001 vs. Pre-Supplementation). Interestingly, there was a significant increase in post-exercise CAR-ACR content following β-alanine supplementation (p < 0.001 vs. post-exercise before supplementation), whereas neither exercise alone nor supplementation alone increased CAR-ACR formation. These results suggest that carnosine functions as an acrolein-scavenger in skeletal muscle. Such a role would be relevant to the detoxification of this aldehyde formed during exercise, and appears to be enhanced by β-alanine supplementation. These novel findings not only have the potential of directly benefiting athletes who engage in intensive training regimens, but will also allow researchers to explore the role of muscle carnosine in detoxifying reactive aldehydes in diseases characterized by abnormal oxidative stress.
Keywords: Carnosine, β-alanine, Acrolein, Carnosine-aldehyde adducts, Skeletal muscle
Graphical abstract
Highlights
-
•
Lipid peroxidation generates electrophilic reactive aldehydes.
-
•
β-Alanine supplementation increases muscle carnosine content in skeletal muscle.
-
•
The carnosine-acrolein levels were higher in muscle following β-alanine supplementation in post-exercise.
-
•
This acrolein-scavenger role for muscle carnosine may contribute to its ergogenic and therapeutic effects.
1. Introduction
Lipid peroxidation occurs during normal physiological and pathological processes, and generates a complex mixture of phospholipid products, including hydroperoxides, which then decompose to form electrophilic reactive aldehydes [1]. These secondary lipid oxidation products are capable of reacting with DNA and proteins, potentially compromising the structure and function of these biomolecules. Therefore, aldehyde detoxification is essential for normal cellular function. Some of the most well studied lipid oxidation products include: malondialdehyde, 4-hydroxy-2-nonenal (HNE), 4-hydroxy-2-hexenal (HHE), 4-oxo-(2E)-nonenal, 2,4-decadienal, 4,5-epoxy-(2E)-decenal, hexenal, acrolein, and crotonaldehyde, which have been detected in human tissues under normal conditions, as well as in clinical conditions associated with redox stress disorders [2], [3], [4], [5].
Aldehyde detoxification can occur via reactions catalyzed by alcohol dehydrogenase, aldo-keto reductase, aldehyde dehydrogenase and through conjugation with glutathione [6]. Additionally, histidine-containing dipeptides, such as carnosine (β-alanyl-L-histidine, CAR), homocarnosine (gamma-amino-butyryl-histidine) and anserine (β-alanyl-L-1-methylhistidine), have been shown to detoxify aldehydes, in vivo [7], [8]. The reaction between carnosine and α, β-unsaturated aldehydes (i.e., HNE and acrolein) is well described [9], [10] and carnosine-aldehyde products have been detected during normal metabolism and in the urine of mice and adult human non-smokers [6], [10].
Carnosine is abundantly expressed in the skeletal and cardiac muscles, as well as in other excitable cells [9]. In skeletal muscle, β-alanine availability is a limiting factor for carnosine synthesis [11], and β-alanine supplementation has been shown to consistently increase carnosine content in skeletal muscle [11], [12], [13]. Previous studies have demonstrated that carnosine functions as an intracellular buffer in skeletal muscle [14]. Other properties of carnosine include metal quenching [15], anti-glycation [16] and aldehyde detoxification [6], [10]. For these reasons, carnosine has long been considered to have a number of relevant physiological roles, such as healthy ageing and disease prevention [17]. However, little is known about which tissues carnosine targets, or to what conditions carnosine is physiologically relevant. Moreover, β-alanine supplementation leads to a substantial increase in muscle carnosine content, which may potentiate the putative reactive aldehyde detoxifying effect of carnosine. Thus it is plausible that if carnosine plays a role in aldehyde detoxification, then β-alanine supplementation should enhance this process.
Physical exercise is a potent physiological stimulus that can lead to acute intracellular modifications, such as the formation of lipid peroxidation by-products, which results in an increased formation of hydroperoxides and malondialdehyde [18]. Thus, to explore the roles of exercise and β-alanine supplementation on the formation of carnosine-aldehyde adducts, CAR-ACR, CAR-HHE and CAR-HNE were quantified in human skeletal muscle samples, following a bout of acute exercise, before and after chronic β-alanine supplementation. We hypothesized that β-alanine supplementation would increase CAR-aldehyde adduct formation, therefore providing further evidence for the physiological role of carnosine in the detoxification of lipid peroxidation by-products formed during exercise.
2. Materials and methods
2.1. Participants and study design
This study is part of a broader project aimed at assessing the effects of β-alanine combined or not with sodium bicarbonate on high-intensity intermittent exercise performance and underpinning physiological mechanisms. Participants were randomly allocated to one of the following groups: β-alanine+placebo (BA+PL); sodium bicarbonate+placebo (SB+PL), β-alanine+sodium bicarbonate (BA+SB) or placebo+placebo (PL+PL) using a 2:2:2:1 ratio. The study followed a double-blind randomized design. The placebo for β-alanine and sodium bicarbonate were dextrose and calcium carbonate, respectively. β-alanine or dextrose (6.4 g day−1 split in 4 single doses of 1.6 g) was taken for 28 days whereas sodium bicarbonate or calcium carbonate (0.5 g kg−1 day−1 split in 4 single doses of 1.25 g kg−1 day−1) was taken for 5 days, commencing on the 5th day before the last day of β-alanine/dextrose supplementation.
For this specific study, muscle samples from 31 of these participants (BA+PL: n = 9; SB+PL: n = 11; BA+SB: n = 8; PL+PL: n = 3) were available for the determination of carnosine content and CAR-aldehyde adducts. In order to increase the number of observations in each group and time-point, thereby increasing the statistical power of our analyses, these samples were grouped as follows: individuals taking β-alanine (i.e., those ingesting β-alanine+placebo or β-alanine+sodium bicarbonate) were grouped as “β-alanine group” (BA: n = 17), while those not taking β-alanine (i.e., sodium bicarbonate+placebo or placebo+placebo) were grouped as “placebo group” (PL: n = 14). Since none of the other substances used (i.e., dextrose, calcium carbonate or sodium bicarbonate) have any predictable effect on muscle carnosine content or CAR-aldehyde adducts, the simplified BA or PL grouping strategy emerged as an appealing way to examine the effects of β-alanine and exercise on carnosine-aldehyde adducts in these individuals.
Muscle carnosine and CAR-aldehyde adducts could not be quantified in samples from 3 individuals of the PL group due to insufficient amount of sample available. Therefore, all analyses were carried out in muscle samples from 28 individuals (BA: n = 14; PL: n = 14). All participants included in the analyses (BA: age 36 ± 6 years; body mass 77.9 ± 12.6 kg; height 1.77 ± 0.1 m; fat 17.5 ± 6.9%; PL: age 36 ± 6 years; body mass 73.0 ± 7.6 kg; height 1.76 ± 0.04 m; fat 16.3 ± 4.6%) had been actively training for the past 2 years or longer (total training volume: BA: 13 ± 5 h·week−1, totaling 259 ± 148 km week−1; PL: 10 ± 7 h week−1, totaling 232 ± 182 km week−1). No significant differences between groups were shown for any of the baseline participant's characteristics (two-tailed t-test for independent samples: all p > 0.05). Exclusion criteria were: previous or current use of anabolic steroids or any other performance-enhancing drug, use of supplements containing creatine in the 3 months before the study, or β-alanine in the 6 months before the study. Participants were required to visit the exercise physiology laboratory on three different occasions. On the first visit, they were assessed for eligibility and familiarized with the acute exercise protocol. The second visit took place 2–7 days later, and the participants undertook the pre-supplementation trial (PRE-Supplementation). The same procedures were repeated on the third visit, which took place following a 28-day supplementation period (POST-Supplementation). During both the PRE- and POST-Supplementation trials, all participants had a muscle sample biopsy taken from the vastus lateralis at rest and immediately after the acute exercise protocol for the quantification of muscle carnosine, CAR-HNE, CAR-HHE and CAR-ACR adducts in skeletal muscle. When using a S/N = 7 for the limit of quantification (LOQ), it was possible to quantify carnosine in all 28 individuals, whereas CAR-ACR was quantified in 26, CAR-HNE in 8 and CAR-HHE was quantified in none individuals (see HPLC-ESI+-MS/MS method validation).
All participants were fully informed about the risks and benefits associated with participation before signing the informed consent form. All procedures were in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee for Human Research.
2.2. β-Alanine supplementation
Participants were given 6.4 g of β-alanine (Compound Solutions, California, USA) per day in gelatin capsules with carboxymethylcellulose, which was added to slow β-alanine release to the blood and prevent paraesthesia, as previously described [19]. The total daily dose was split into four individual 1.6 g doses to be taken along with main meals (breakfast, lunch, afternoon snack and dinner). They were also asked to maintain the same diet throughout the experimental period, which was verbally confirmed during the POST-Supplementation trial.
2.3. Exercise protocol
A high-intensity intermittent cycling exercise protocol was used as a model to study the formation of lipid peroxidation by-products due to evidence suggesting that exercise intensity and muscle acidosis play a role in the formation of lipid peroxidation by-products [20], [21]. The test was conducted on a mechanically-braked cycle-ergometer, with the resistance set at 5% of the individual's body mass. The participants completed four 30-s bouts of all-out cycling exercise (Wingate Test), and were given a 3-min recovery period between bouts.
2.4. Muscle biopsy
Muscle samples (approximately 70–150 mg) were taken from the mid-portion of the vastus lateralis using the percutaneous needle biopsy technique [22] with suction [23]. In order to make the muscle accessible, a 1-cm wide incision was made in the skin and fascia under local anesthesia (3 ml, 2% xylocaine). Samples obtained after exercise were taken from a position ~1-cm away and at a similar depth to the biopsy location taken at rest. Immediately after sample collection, blood was removed, and visible fat and connective tissue were dissected away. The samples were then frozen in liquid nitrogen and stored at − 80 °C until analysis.
2.5. Synthesis of the unlabeled and isotopically labeled carnosine-aldehyde standards
CAR-ACR, CAR-HNE, CAR-HHE and CAR-HNEd11 and CAR-HHEd5 were prepared, purified and analyzed as previously described [10].
2.6. Carnosine-aldehyde adducts extraction from muscle samples
Carnosine-aldehyde adduct extraction was performed according to Baba et al. [6], with some modifications. Wet vastus lateralis biopsies (20–50 mg) were added to 150 µL of a solution containing 150 mM KH2PO4, 1 mM EDTA, 1 mM DTT, a diluted (1:1000) protease inhibitor cocktail (Sigma Chemical Co., St. Louis, MO) and isotopically labeled internal standards (7.5 nM of CAR-HNEd11, 7.5 nM of CAR-HHEd5 and 0.2 µM of CARd4), and homogenized using a PowerGen 1000 homogenizer (Thermo Fisher Scientific, Waltham, MA). Samples were centrifuged at 13,000 × g for 20 min at 9 °C, the pellet was discarded and the supernatant was retained for further processing. Total protein content of the supernatant was assessed using the Pierce™ BCA Protein Assay kit (Thermo Fisher Scientific, Waltham, MA) following the instructions of the manufacturer. Supernatants were then submitted to a protein precipitation protocol using 15 µL of 70% HClO4 (v/v) for each 100 µL of sample. Samples were centrifuged at 13,000 × g for 15 min at 9 °C. The protein precipitate was discarded and the pH of the supernatant was adjusted to 7.0 using concentrated NaOH prior to injection of 10 µL onto the HPLC-ESI+-MS/MS system. Each sample was injected in duplicate.
Calibration curves for CAR-HNE (from 5 to 45 nM), CAR-HHE (from 10 to 70 nM), CAR-ACR (from 0.1 to 30 μM) and CAR (from 0.5 to 5 µM) were prepared using the extraction solution described above and injected in duplicate. Results were expressed as pmol of adduct/mg of protein for CAR-HNE, nmol of adduct/mg of protein for CAR-ACR, µmol of CAR/mg of protein and mmol of CAR/kg of wet muscle.
2.7. Analysis of carnosine and carnosine-aldehyde adducts using HPLC-ESI+-MS/MS
On-line HPLC-ESI+-MS/MS analyses were carried out in the positive mode and detection was conducted on a triple quadrupole mass spectrometer API 6500 (Sciex, Washington D.C, WA), using selected reaction monitoring (SRM). An Agilent HPLC system (Agilent Technologies, Santa Clara, CA) equipped with an autosampler (1200 High performance), a column oven set at 45°C (1200 G1216B), an automated high pressure flow switching valve, a 1200 Binary Pump SL and a Shimadzu 10-AVp Isocratic Pump (Shimadzu, Tokyo, Japan) were used for sample injection and cleanup on a Kinetex C18 column, with an i.d. of 100 × 4.6 mm and particle diameter of 2.6 µm (Phenomenex, Torrance, CA) followed by a second Kinetex C18 column, with an i.d. of 100 × 2.1 mm and particle diameter 2.6 µm (Phenomenex, Torrance, CA). The mobile phase consisted of 5 mM ammonium acetate pH 5.5 (A) and acetonitrile (B). Prior to use, both solutions were filtered through a 0.22 µm PVDF membrane (Millipore, Bedford, MA). The adducts were eluted from the columns according to the following method: from 0 to 6 min, 10% acetonitrile and 150 µL/min; from 6 to 10 min, 10–90% acetonitrile and 150–300 µL/min; from 10 to 15 min, 90% acetonitrile and 300 µL/min; from 15 to 20 min, 90–10% acetonitrile and 300–150 µL/min allowing the first column to re-equilibrate until 30 min.
A high-pressure flow switching valve composed of 2-positions and 6-ports was inserted between the two columns. The valve discarded the eluent from the first column until 3 min of run while kept the second column supplied with a solution of water: acetonitrile (9:1, v/v) at a constant flow of 100 µL/min using a Shimadzu 10-AVp Isocratic Pump. After 3 min of run, the valve switched position allowing the eluent from the first column to enter the second column. Upon elution from the second column, the samples were injected into the mass spectrometer. After 14 min of run, the valve switched back to the initial position, allowing both columns to re-equilibrate.
The aldehyde-adducts and carnosine were analyzed by electrospray ionization (ESI) in the positive mode, and detection was made using selected reaction monitoring (SRM) on a triple quadrupole mass spectrometer API 6500. The Turbo Ionspray Voltage was kept at 5500 V, the curtain gas at 15 psi and the nebulizer and auxiliary gas at 50 psi. The temperature was set to 500 °C, and the pressure of nitrogen in the collision cell was adjusted to high. The signal to noise ratio (S/N) of ≥ 3 was used as the detection criteria for the adducts and a S/N ≥ 7 was used as the quantification criteria. Transitions for CAR-HNE, CAR-HNEd11, CAR and CARd4 were monitored using a dwell time of 150 ms and transitions for CAR-ACR, CAR-HHE and CAR-HHEd5 were monitored using a dwell time of 250 ms. All precursor ions monitored corresponded to the single protonated adduct [M+H]+. Table 1 shows all transitions monitored for carnosine and each carnosine-aldehyde adduct.
Table 1.
Monitored transitions for carnosine and carnosine-aldehydes adducts in Selected Reaction Monitoring (SRM) in the HPLC-ESI+-MS/MS analyses.
| Quantification Transition (m/z) | Confirmation Transition 1 (m/z) | Confirmation Transition 2 (m/z) | |
|---|---|---|---|
| CAR | 227→110 | 227→210 | – |
| CARd4 | 231→110 | 231→214 | – |
| CAR-ACR | 303→166 | 303→110 | 303→210 |
| CAR-HNE | 383→366 | 383→110 | 383→266 |
| CAR-HNEd11 | 394→377 | 394→110 | 394→277 |
| CAR-HHE | 341→324 | 341→110 | 341→224 |
| CAR-HHEd5 | 346→329 | 346→110 | 346→229 |
2.8. HPLC-ESI+-MS/MS method validation
The validation methodology employed is suggested by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Tripartite Guideline of Validation of Analytical Procedures: Text and Methodology [24]. The limit of detection (LOD), limit of quantification (LOQ) and inter- and intra-day repeatability were evaluated for the developed HPLC-ESI+-MS/MS method. The LOD and LOQ of each carnosine-aldehyde adduct was evaluated on a signal-to-noise (S/N) ratio basis, being the concentration that provided S/N = 3 the LOD and S/N = 7 the LOQ. For repeatability inter- and intra-day evaluation, three samples were spiked with the same concentration of each adduct (7 pmol of CAR, 4 pmol of CAR-ACR, 150 fmol of CAR-HNE and 100 fmol of CAR-HHE) and injected in triplicate on two consecutive days. Blanks were injected between samples to ensure that no carryover of adducts from the previous analyses.
2.9. Statistical analysis
Data are presented as mean ± standard deviation (SD). Statistical analysis was carried out with SAS UNIVERSITY EDITION (2.7 9.4 M5 - SAS Institute Inc., Cary, NC, USA). Mixed models were used to examine changes in carnosine and CAR-ACR, with ‘treatment’ and ‘time’ as fixed factors and participants as random factors. Four covariance structures were tested and the Bayesian-information criterion (lowest BIC) was used to choose the structure that best fit to each data set. Hypothesis-driven single-degree-of-freedom contrast analyses were carried out to identify specific within- and between-group effects whenever a significant main effect of time of group-by-time interaction was observed. Statistical significance was accepted when the two-tailed p-value was < 0.05.
3. Results
A sensitive methodology based on HPLC-ESI+-MS/MS was employed for the quantification of carnosine and CAR-aldehyde adducts (CAR-ACR, CAR-HHE and CAR-HNE) in vastus lateralis samples from cyclists, before and after chronic β-alanine supplementation. The LODs for the standards (S/N = 3) were 100 fmol for CAR, 150 fmol for CAR-ACR, 750 amol for CAR-HNE and 7.5 fmol for CAR-HHE, and the LOQs were 300 fmol, 450 fmol, 2.2 fmol and 20 fmol, respectively. Table 2 summarizes the adduct quantification data and the coefficients of variation of the analysis within the same day and on a subsequent day. The method described herein provides a highly specific and precise way to quantify carnosine and carnosine-aldehyde adducts in tissues, and could be effectively utilized in future studies focused on assessing the concentrations of these compounds in muscle samples exposed to exacerbated lipid peroxidation processes.
Table 2.
Repeatability intra- and inter-day assay of the developed HPLC-ESI+-MS/MS method for carnosine-aldehyde adducts, detection and quantification. Mean ± SD are shown for two subsequent days of injection of three different standards containing 7 pmol of CAR, 4 pmol of CAR-ACR, 150 fmol of CAR-HNE and 100 fmol of CAR-HHE.
| Day 1 | Day 2 | Intra-day CV (%) |
Inter-day CV (%) | ||
|---|---|---|---|---|---|
| Day 1 | Day 2 | ||||
| CAR (pmol) | 6.91 ± 0.08 | 6.92 ± 0.10 | 1.18 | 1.47 | 1.48 |
| CAR-ACR (pmol) | 4.09 ± 0.13 | 4.12 ± 0.01 | 3.29 | 0.30 | 3.27 |
| CAR-HNE (fmol) | 166 ± 4 | 170 ± 4 | 2.33 | 2.37 | 2.27 |
| CAR-HHE (fmol) | 97 ± 5 | 94 ± 3 | 1.54 | 1.48 | 1.50 |
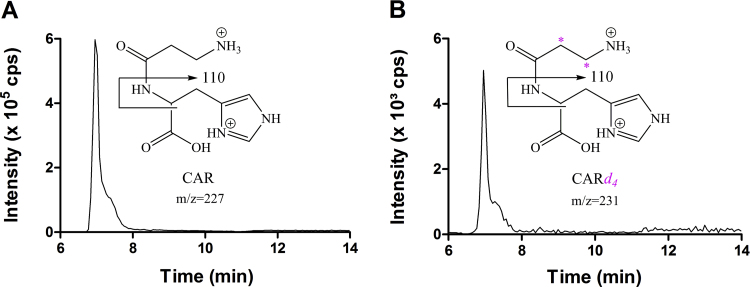
A representative chromatogram of the HPLC-ESI+-MS/MS analyses of carnosine from a muscle sample is presented in Fig. 1A. The use of the stable isotopically labeled internal standard CARd4, which is four mass units larger than the unlabeled endogenous carnosine, ensured accurate quantification (Fig. 1B).
Fig. 1.
Representative chromatogram of vastus lateralis sample of a β − alanine supplemented individual at pre-Wingate test showing the quantification transitions (A) m/z 227→110 for CAR, where the fragment m/z 110 is produced by the simultaneous loss of the β-alanine residue and the carboxyl group of the L-histidine moiety [CAR+H-C4H7NO3]+ and (B) m/z 231→110 for the isotopic internal standard CARd4.
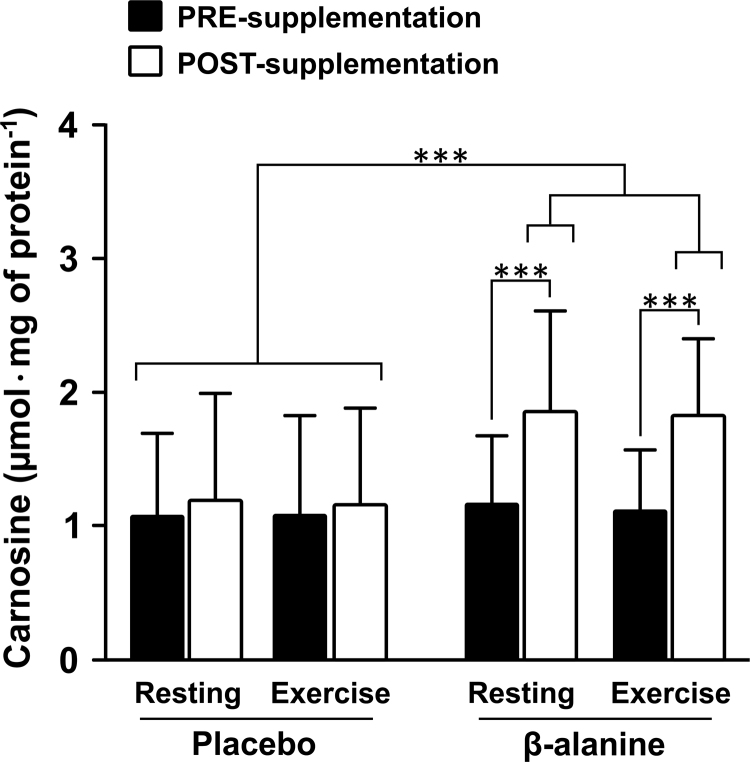
Consistent with previous studies [11], [13], muscle carnosine content measured at rest increased by ~50% after 28 days of β-alanine supplementation (group by time interaction: F=13.45, p < 0.0001; PRE vs. POST in PL group: t = −0.36, p = 0.72; PRE vs. POST in BA group: t = 6.84, p < 0.0001) (Fig. 2). Interestingly, there were no acute changes in carnosine content in muscle immediately after exercise, irrespective of group, either before (single effect of time: t = −1.06, p = 0.29) or after the supplementation period (single effect of time: t = 0.59, p = 0.56). This indicated that (i) acute exercise (at least in this type of exercise) does not induce any measurable loss of muscle carnosine; and/or (ii) carnosine-mediated aldehyde scavenging does not result in a substantial depletion of muscle carnosine content.
Fig. 2.
Carnosine content measured in vastus lateralis at rest and after high-intensity intermittent exercise PRE- and POST-supplementation. POST-supplementation carnosine values were significantly higher than all other conditions. ***p < 0.001.
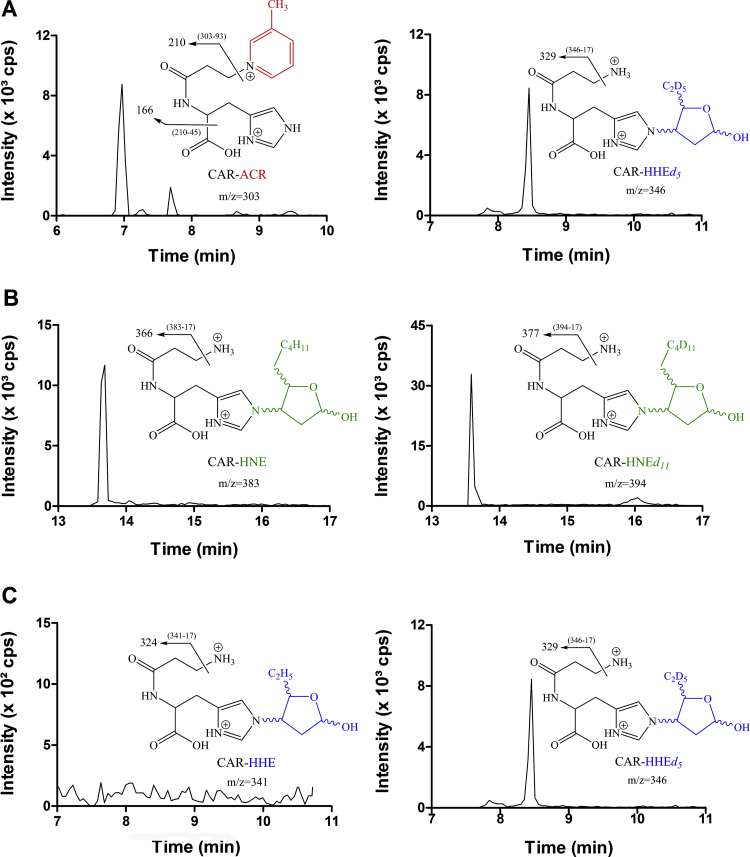
Representative chromatograms depicting the detection of the CAR-ACR and CAR-HNE adducts and the isotopically labeled internal standards are shown in Figs. 3A and 3B, respectively. Interestingly, CAR-HHE was not detected under our experimental conditions (Fig. 3C). Since CAR-HHE has been previously detected in the urine of young healthy individuals [10], the origin of this reactive aldehyde is likely a tissue other than skeletal muscle. This result also suggests that carnosine aldehyde detoxification is not specific to skeletal muscle.
Fig. 3.
Representative chromatogram of vastus lateralis sample of a β-alanine supplemented individual at pre-Wingate test showing the quantification transitions (A) m/z 303→166 for CAR-ACR and m/z 346→329 for its internal standard CAR-HHEd5 (B) m/z 383→366 for CAR-HNE and m/z 394→377 for its isotopic internal standard CAR-HNEd11 and (C) m/z 341→324 for CAR-HHE and m/z 346→329 for its isotopic internal standard CAR- HHEd5.
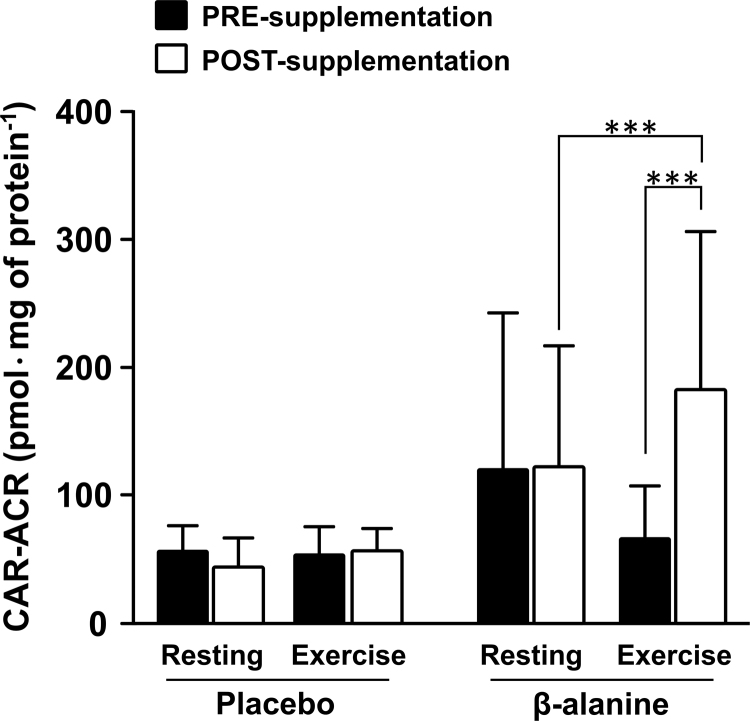
Neither acute exercise alone (single effect of time: t = −1.58, p = 0.13) nor β-alanine supplementation alone (single effect of time: t = −0.65, p = 0.52) elicited any significant change in CAR-ACR levels in skeletal muscle. However, a significant increase in muscle CAR-ACR levels was observed in Post-Supplementation samples following exercise (group by time interaction: F=4.72, p = 0.01) in the BA (t = 4.6, p = 0.0001, within-group effect) but not in the PL group (t = −0.06, p = 0.95, within-group effect; BA vs. PL at POST: t = 3.46, p = 0.002, between-group effect) (Fig. 4). This indicates that carnosine can effectively scavenge acrolein, which appears to be particularly relevant for exercise-induced aldehyde formation when muscle carnosine stores are loaded via β-alanine supplementation. Additionally, the contribution of carnosine to acrolein detoxification appears to be dependent on carnosine availability (i.e., carnosine loading via supplementation), and increases during conditions of exacerbated lipid peroxidation (i.e., exercise). In contrast, carnosine-HHE adduct was not detected, and there were no observable changes in CAR-HNE levels in response to supplementation or exercise (CAR-HNE levels: PRE-supplementation, resting 0.58 ± 0.20 and exercise 1.79 ± 3.06 pmol/mg of protein; POST-supplementation, resting = 0.80 ± 0.66 and exercise = 0.90 ± 0.58 pmol/mg of protein).
Fig. 4.
Carnosine-acrolein (CAR-ACR) content measured in vastus lateralis at rest and after high-intensity intermittent exercise PRE- and POST-supplementation. POST-supplementation carnosine values measured after exercise were significantly higher than those measured at rest and those PRE-supplementation measured after exercise. ***p < 0.001.
4. Discussion
Exercise has been shown to increase the activity of the antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase (GPX), which is indicative of increased redox stress [25]. Augmented lipid peroxidation, evidenced by the quantification of malondialdehyde levels, has also been reported following exercise [25]. Furthermore, increased HNE-modified albumin levels were detected following an acute bout of variable intensity exercise [26]. Therefore, exercise emerges as an ideal model for studying the role of carnosine as a scavenger of lipid peroxidation by-products.
In the present study, CAR-ACR (m/z 303), CAR-HHE (m/z 341) and CAR-HNE (m/z 383) were quantified following high-intensity intermittent exercise, before and after chronic of β-alanine supplementation. There was an observed increase (ca. 50%) in muscle carnosine content after β-alanine supplementation. Interestingly, there was also a significant increase in post-exercise CAR-ACR levels following β-alanine supplementation, whereas neither exercise or supplementation alone increased CAR-ACR formation. These results indicate that carnosine plays an acrolein-scavenger role in skeletal muscle, which may be important to the detoxification of this aldehyde generated during exercise. It is important to note that the bimolecular rate constant for carnosine with acrolein is one order of magnitude higher than those for carnosine with HNE and HHE [27]. This can explain, at least partially, the undetectable levels of CAR-HHE as well as the lower levels of CAR-HNE found in the muscle samples.
A previous study reported that overweight individuals treated with carnosine (2 g/day) displayed a significant increase in the urinary excretion of carnosine-acrolein adducts [28]. Moreover, our group elucidated the structure of a 3-methylpyridinium carnosine (m/z 303) from the reaction of carnosine and acrolein, and simultaneously quantified carnosine-aldehyde adducts in human urine [10]. Although the presence of carnosine-aldehyde adducts in the urine supports the role of carnosine in cellular detoxification, it does not provide any information about where it was formed. In this regard, our findings extend the current knowledge by showing that the human skeletal muscle is a source of carnosine-aldehyde adducts, CAR-ACR in particular. Acrolein can be formed endogenously by the oxidation of fatty acids from cell membranes and by the myeloperoxidase-catalyzed oxidation of the amino acid threonine; this aldehyde can be found extensively, including in food, water, and environmental pollutants [29].
In different models of exacerbated oxidative stress, it has been shown that reactive aldehydes may damage cells [5]; hence, enhanced detoxification of reactive aldehydes may counteract some of these deleterious effects [7]. For instance, a previous study showed that exercise-induced increases in ALDH2 activity, a mitochondrial enzyme responsible for detoxification, and ameliorated oxidative stress in hypertensive rats [30]. Thus, it is tempting to speculate that increased carnosine levels could confer a similar protection against oxidative stress, by acting as an aldehyde scavenger in human skeletal muscle. It would be interesting to determine if this process exists in other conditions in which oxidative stress is potentially elevated, such as intensive training routines, a plethora of cardiovascular, metabolic and neurodegenerative diseases, and cancers.
In conclusion, these results showed for the first time that CAR exerts an aldehyde-detoxification role, in the skeletal muscle, through the formation of CAR-ACR adducts. Through this apparent scavenging function, CAR prevents the reactive aldehydes from modifying other biomolecules, such as proteins and DNA. These results reveal a physiological role for muscle carnosine, especially during intense exercise, and may partially explain its ergogenic and therapeutic effects.
Acknowledgements
Supported by CEPID-Redoxoma (São Paulo Research Foundation FAPESP: Proc. 2013/07937-8), NAP-Redoxoma (PRPUSP: Proc. 2011.1.9352.1.8), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior). Ana Helena Sales de Oliveira was supported by a postdoctoral fellowship grant FAPESP#2016/24761-9. Guilherme Artioli and Bruno Gualano are supported by FAPESP (2014/11948-8 and 2013/14746-4). The authors wish to thank the volunteers who participated in this study. We also thank Manoel Neves, Luiz Augusto Riani and Vitor Painelli for their help with data collection.
The authors have no conflicts of interest to disclose.
Contributor Information
Guilherme G. Artioli, Email: artioli.gg@gmail.com.
Marisa H.G. Medeiros, Email: mhgdmede@iq.usp.br.
References
- 1.West J.D., Marnett L.J. Endogenous reactive intermediates as modulators of cell signaling and cell death. Chem. Res. Toxicol. 2006;19(2):173–194. doi: 10.1021/tx050321u. [DOI] [PubMed] [Google Scholar]
- 2.Nair U., Bartsch H., Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Radic. Biol. Med. 2007;43(8):1109–1120. doi: 10.1016/j.freeradbiomed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Blair I.A. DNA adducts with lipid peroxidation products. J. Biol. Chem. 2008;283(23):15545–15549. doi: 10.1074/jbc.R700051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medeiros M.H.G. Exocyclic DNA adducts as biomarkers of lipid oxidation and predictors of disease. Challenges in developing sensitive and specific methods for clinical studies. Chem. Res. Toxicol. 2009;22(3):419–425. doi: 10.1021/tx800367d. [DOI] [PubMed] [Google Scholar]
- 5.Marnett L.J., Riggins J.N., West J.D. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J. Clin. Investig. 2003;111(5):583–593. doi: 10.1172/JCI18022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baba S.P., Hoetker J.D., Merchant M., Klein J.B., Cai J., Barski O.A., Conklin D.J., Bhatnagar A. Role of aldose reductase in the metabolism and detoxification of carnosine-acrolein conjugates. J. Biol. Chem. 2013;288(39):28163–28179. doi: 10.1074/jbc.M113.504753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carini M., Aldini G., Beretta G., Arlandini E., Facino R.M.J. Acrolein-sequestering ability of endogenous dipeptides: characterization of carnosine and homocarnosine/acrolein adducts by electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2003;38(9):996–1006. doi: 10.1002/jms.517. [DOI] [PubMed] [Google Scholar]
- 8.Yeum K.J., Orioli M., Regazzoni L., Carini M., Rasmussen H., Russell R.M., Aldini G. Profiling histidine dipeptides in plasma and urine after ingesting beef, chicken or chicken broth in humans. Amino Acids. 2010;38(3):847–858. doi: 10.1007/s00726-009-0291-2. [DOI] [PubMed] [Google Scholar]
- 9.Boldyrev A.A., Aldini G., Derave W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013;93(4):1803–1845. doi: 10.1152/physrev.00039.2012. [DOI] [PubMed] [Google Scholar]
- 10.Bispo V.S., de Arruda Campos I.P., Di Mascio P., Medeiros M.H.G. Structural elucidation of a carnosine-acrolein adduct and its quantification in human urine samples. Sci. Rep. 2016;6:19348. doi: 10.1038/srep19348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris R.C., Tallon M.J., Dunnett M., Boobis L., Coakley J., Kim H.J., Fallowfield J.L., Hill C.A., Sale C., Wise J.A. The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids. 2006;30(3):279–289. doi: 10.1007/s00726-006-0299-9. [DOI] [PubMed] [Google Scholar]
- 12.Hill C.A., Harris R.C., Kim H.J., Harris B.D., Sale C., Boobis L.H., Kim C.K., Wise J.A. Influence of beta-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids. 2007;32(2):225–233. doi: 10.1007/s00726-006-0364-4. [DOI] [PubMed] [Google Scholar]
- 13.Saunders B., De Salles Painelli V., DE Oliveira L.F., DA Eira Silva V., DA Silva R.P., Riani L., Franchi M., Gonçalves L.S., Harris R.C., Roschel H., Artioli G.G., Sale C., Gualano B. Twenty-four weeks of β-alanine supplementation on carnosine content, related genes, and exercise. Med. Sci. Sports Exerc. 2017;49(5):896–906. doi: 10.1249/MSS.0000000000001173. [DOI] [PubMed] [Google Scholar]
- 14.Sale C., Saunders B., Harris R.C. Effect of beta-alanine supplementation on muscle carnosine concentrations and exercise performance. Amino Acids. 2010;39(2):321–333. doi: 10.1007/s00726-009-0443-4. [DOI] [PubMed] [Google Scholar]
- 15.Matsukura and Tanaka Applicability of zinc complex of L-carnosine for medical use. Biochemistry. 2000;65(7):817–823. [PubMed] [Google Scholar]
- 16.Pepper E.D., Farrell M.J., Nord G., Finkel S.E. Antiglycation effects of carnosine and other compounds on the long-term survival of Escherichia coli. Appl. Environ. Microbiol. 2010;76(24):7925–7930. doi: 10.1128/AEM.01369-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Artioli G.G., Sale C., Jones R.J. Carnosine in health and disease. Eur. J. Sport Sci. 2018;4:1–10. doi: 10.1080/17461391.2018.1444096. [DOI] [PubMed] [Google Scholar]
- 18.Bailey D.M., Davies B., Young I.S. Intermittent hypoxic training: implications for lipid peroxidation induced by acute normoxic exercise in active men. Clin. Sci. 2001;101(5):465–475. [PubMed] [Google Scholar]
- 19.Tobias G., Benatti F.B., Painelli V.D., Roschel H., Gualano B., Sale C., Harris R.C., Lancha A.H., Artioli G.G. Additive effects of beta-alanine and sodium bicarbonate on upper-body intermittent performance. Amino Acids. 2013;45(2):309–317. doi: 10.1007/s00726-013-1495-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman J.R., Im J., Kang J., Maresh C.M., Kraemer W.J., French D., Nioka S., Kime R., Rundell K.W., Ratamess N.A., Faigenbaum A.D., Chance B. Comparison of low- and high-intensity resistance exercise on lipid peroxidation: role of muscle oxygenation. J. Strength Cond. Res. 2007;21(1):118–122. doi: 10.1519/00124278-200702000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Seifi-Skishahr F., Siahkohian M., Nakhostin-Roohi B. Influence of aerobic exercise at high and moderate intensities on lipid peroxidation in untrained men. J. Sports Med. Phys. Fit. 2008;48(4):515–521. [PubMed] [Google Scholar]
- 22.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand. J. Clin. Lab. Investig. 1962;35(7):609–616. [PubMed] [Google Scholar]
- 23.Neves M., Jr, Barreto G., Boobis L., Harris R., Roschel H., Tricoli V., Ugrinowitsch C., Negrao C., Gualano B. Incidence of adverse events associated with percutaneous muscular biopsy among healthy and diseased subjects. Scand. J. Med Sci. Sports. 2012;22(2):175–178. doi: 10.1111/j.1600-0838.2010.01264.x. [DOI] [PubMed] [Google Scholar]
- 24.ICH harmonised tripartite guideline validation of analytical procedures: text and methodology Q2 (R1) (1994)International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. European Union, Japan and USA.
- 25.Bouzid M.A., Filaire E., Matran R., Robin S., Fabre C. Lifelong voluntary exercise modulates age-related changes in oxidative stress. Int. J. Sports Med. 2018;39(1):21–28. doi: 10.1055/s-0043-119882. [DOI] [PubMed] [Google Scholar]
- 26.Shi M., Wang X., Yamanaka T., Ogita F., Nakatani K., Takeuchi T. Effects of anaerobic exercise and aerobic exercise on biomarkers of oxidative stress. Environ. Health Prev. Med. 2007;12:202–208. doi: 10.1265/ehpm.12.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barski O.A., Xie Z., Baba S.P., Sithu S.D., Agarwal A., Cai J., Bhatnagar A., Srivastava S. Dietary carnosine prevents early atherosclerotic lesion formation in Apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 2013;33:1162–1170. doi: 10.1161/ATVBAHA.112.300572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regazzoni L., de Courten B., Garzon D. A carnosine intervention study in overweight human volunteers: bioavailability and reactive carbonyl species sequestering effect. Sci. Rep. 2016;6:27224. doi: 10.1038/srep27224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burcham P.C. Acrolein and human disease: untangling the knotty exposure scenarios accompanying several diverse disorders. Chem. Res. Toxicol. 2017;30:145–161. doi: 10.1021/acs.chemrestox.6b00310. [DOI] [PubMed] [Google Scholar]
- 30.Campos J.C., Fernandes T., Bechara L.R. Increased clearance of reactive aldehydes and damaged proteins in hypertension-induced compensated cardiac hypertrophy: impact of exercise training. Oxid. Med. Cell Long. 2015;464195:11. doi: 10.1155/2015/464195. [DOI] [PMC free article] [PubMed] [Google Scholar]