Abstract
Sarcoidosis is a multisystemic inflammatory disease of unknown origin characterized by the formation of noncaseating granulomas and accumulation of inflammatory cells. Sarcoidosis most commonly affects the lungs and lymphoid system. However, the liver can also be involved in 50%-65% of cases. On magnetic resonance imaging, sarcoidosis lesions usually present as hypointense lesions on all sequences. However, we present a rare case of nodular liver sarcoidosis presenting with T2 hyperintense lesions. In addition, while most cases of hepatic nodular sarcoidosis present with multiple small hepatic nodules, liver masses of our case are larger than usual. Moreover, this case suggested that when intact vascular structures penetrating liver nodular lesions are observed as in the current case, liver sarcoidosis can be included in a list of differential diagnosis.
Keywords: Hepatic sarcoidosis, Atypical manifestations, CT, MRI
Introduction
Sarcoidosis is a multisystemic granulomatous disease of unknown origin which typically affects the lungs and mediastinal lymph nodes [1]. However the central nervous systems, bones, eyes, skin, spleen, and liver can also be involved, in addition, 50%-65% of the patients' biopsies show a histological involvement of the liver [2]. Homogeneous hepatomegaly is often detected on computed tomography (CT). Focal nodular lesions observed on both CT and magnetic resonance imaging (MRI) represent additional radiological manifestations of Sarcoidosis [3]. Here, we report atypical manifestations of liver sarcoidosis on both CT and MRI.
Case
A 68-year-old woman presented to our hospital following a suspicion of liver tumors detected on noncontrast CT during a medical check-up. She had no significant clinical history and was not on any medication. Initial laboratory results were unremarkable. Physical examination results and blood pressure were also normal.
Portal phase of dynamic CT revealed several hypodense lesions with homogeneous enhancement in the liver (Fig. 1). These lesions measured approximately 1-4 cm. Intrahepatic vascular structures penetrating the lesions seemed to be intact. CT showed no organomegaly or enlargement of abdominal lymph nodes.
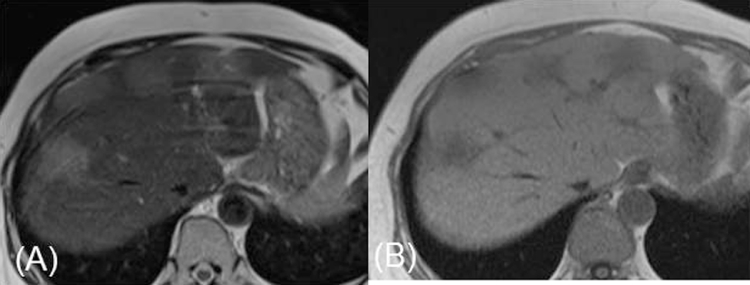
Fig. 1.
(A) Arterial phase of enhanced computed tomography scan revealed hypodense tumors in the liver. (B) Portal phase of enhanced computed tomography scan further evidenced tumor hypodensity and the intact vascular architecture in the tumor region (arrow).
Furthermore, a contrast-enhanced MRI using gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid also revealed several mass lesions in the liver. The lesions were slightly hyperintense on T2-weighted MR images and hypointense on T1-weighted MR images as compared with the surrounding hepatic parenchyma (Fig. 2). On hepatobiliary-phase these lesions were identified as hypointensity areas (Fig. 3). Diffusion-weighted image revealed tumors with high signal intensity with a restriction of water diffusivity on the apparent diffusion coefficient maps (Fig. 4).
Fig. 2.
(A) T2-weighted magnetic resonance imaging images showed slightly hyperintense tumors. (B) T1-weighted magnetic resonance imaging images showed hypointense tumors.
Fig. 3.
Hepatobiliary-phase of contrast MR images revealed a hypointensity.
Fig. 4.
(A) Diffusion-weighted images revealed high intensity tumors. (B) The tumors were restricted on apparent diffusion coefficient maps.
Ultrasound-guided liver biopsy using a 16-gauge true-cut needle was performed. Histological specimen showed several multinucleated giant cells as well as multiplications of epithelioid cells were observed in the portal and periportal spaces (Fig. 5). The lobular structure of the liver was intact. Hepatic sarcoidosis was diagnosed based on such pathological findings. After starting treatment with prednisolone (30 mg), the patient showed good response with near regression of the liver lesions on CT 12 months later.
Fig. 5.
(A) Hematoxylin-eosin stain (original magnification × 100) revealed the granulomatous nodule of multiplications of epithelioid cells (arrowhead) in the portal and periportal spaces. (B) Hematoxylin-eosin (original magnification × 400) revealed several multinucleated giant cells (arrow) in the portal area.
Discussion
Sarcoidosis is a multisystemic idiopathic disease characterized by formation of noncaseating granulomas due to the accumulation of inflammatory cells. Such granulomas can involve any organ typically affecting the lungs and mediastinal lymph nodes in about 90% of the patients [4]. Gezer et al. reported that sarcoidosis could be located in the extrapulmonary region in approximately 30% of the cases [5]. The common sites of extrapulmonary sarcoidosis included the central nervous system, bones, skin, eyes, liver, and spleen. Although the liver was histologically involved in 50%-65% of the patients, hepatic sarcoidosis is usually clinically silent [2].
In 2%-60% of patients with hepatic sarcoidosis, laboratory tests show abnormality indicating liver dysfunction [6]. Only about 15% of the patients with hepatic sarcoidosis presented an elevated serum alkaline phosphatase level, which is a common indicator of liver dysfunction. Although rare, portal hypertension, cirrhosis and chronic cholestatic disease may also manifest in such patients [7], [8].
On CT, hepatomegaly with homogeneous appearance of parenchyma is the most commonly seen liver abnormality [9]. This may be associated with splenomegaly and an enlargement of abdominal lymph nodes close to the liver hilus or in celiac regions [10].
Only 5%-15% of patients with hepatic sarcoidosis show nodular lesions in the liver on CT and/or MRI ranging from 1 mm to several centimeters [9]. Moreover, they are typically located in the portal and periportal spaces of the hepatic sinuses [11]. The relatively large hepatic lesions (6 cm long) as seen in our case were reported by Jung et.al. [12]. These large lesions could be formed by the histological confluence of small granulomatous nodules (<2 mm) [13].
Such nodular lesions are hypodense on CT and generally low-signal intensity on both T2-weighted and gadolinium-enhanced T1-weighted images of MRI [11]. In our case, signal intensity of these lesions was relatively higher on T2-weighted MR images than usual. Signal intensity of the lesions on T2-weighted MR images is related to the disease activity and hyperintensity possibly is due to inflammation-related edema and high vascular permeability [14]. Hence, liver lesions in our case are supposed to have relatively high activity.
Seemingly, intrahepatic vascular structures penetrating liver nodular lesions were intact in our case suggesting the presence of a benign tumor-like lesion or infiltrative tumor growth. Malignant lymphoma and cholangiocellular carcinoma exhibit infiltrative growth. Thus, when cases with intact vascular structures penetrating liver nodules are encountered, a list of differential diagnosis includes these [15], [16]. In addition, inflammatory pseudotumor could be another possibility because active inflammation might increase the surrounding parenchymal blood supply and display early parenchymal enhancement. In case of hepatic sarcoidosis, this could be attributed to the location of the granulomatous nodule in the portal and periportal spaces of the hepatic sinuses and agglutination of small granulomatous nodules.
In our case, sarcoidosis formed nodular lesions in liver. These nodular lesions were larger than usual and their signal intensity on T2-weighted MR images was relatively high presumably due to active inflammation. These findings were atypical for liver sarcoidosis.
In conclusion, we have reported a rare case of hepatic sarcoidosis. Radiologically, there are several atypical points for liver sarcoidosis such as multiple and large (about 4 cm) nodular formation and hyperintensity on T2-weighted MR images. When we see such cases with intact vascular structures penetrating liver nodules, a list of differential diagnosis includes hepatic sarcoidosis as well as malignant lymphoma, cholangiocellular carcinoma, and inflammatory pseudotumor.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997;336(17):1224–1234. doi: 10.1056/NEJM199704243361706. [DOI] [PubMed] [Google Scholar]
- 2.MacArthur KL, Forouhar F, Wu GY. Intra-abdominal complications of sarcoidosis. J Formosan Med Assoc. 2010;109(7):484–492. doi: 10.1016/S0929-6646(10)60082-4. [DOI] [PubMed] [Google Scholar]
- 3.Warshauer DM, Semelka RC, Ascher SM. Nodular sarcoidosis of the liver and spleen: appearance on MR images. J Magn Reson Imaging. 1994;4(4):553–557. doi: 10.1002/jmri.1880040407. [DOI] [PubMed] [Google Scholar]
- 4.(1999) Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med, 160(2):736–755. [DOI] [PubMed]
- 5.Gezer NS, Basara I, Altay C, Harman M, Rocher L, Karabulut N. Abdominal sarcoidosis: cross-sectional imaging findings. Diagn Interventional Radiol. 2015;21(2):111–117. doi: 10.5152/dir.2014.14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warshauer DM, Lee JK. Imaging manifestations of abdominal sarcoidosis. Am J Roentgenol. 2004;182(1):15–28. doi: 10.2214/ajr.182.1.1820015. [DOI] [PubMed] [Google Scholar]
- 7.Judson MA. Hepatic, splenic, and gastrointestinal involvement with sarcoidosis. Seminars Respir Crit Med. 2002;23(6):529–541. doi: 10.1055/s-2002-36517. [DOI] [PubMed] [Google Scholar]
- 8.Ayyala US, Padilla ML. Diagnosis and treatment of hepatic sarcoidosis. Curr Treat Options Gastroenterol. 2006;9(6):475–483. doi: 10.1007/s11938-006-0004-9. [DOI] [PubMed] [Google Scholar]
- 9.Koyama T, Ueda H, Togashi K, Umeoka S, Kataoka M, Nagai S. Radiologic manifestations of sarcoidosis in various organs. Radiographics. 2004;24(1):87–104. doi: 10.1148/rg.241035076. [DOI] [PubMed] [Google Scholar]
- 10.Karagiannidis A, Karavalaki M, Koulaouzidis A. Hepatic sarcoidosis. Ann Hepatol. 2006;5(4):251–256. [PubMed] [Google Scholar]
- 11.Palmucci S, Torrisi SE, Caltabiano DC, Puglisi S, Lentini V, Grassedonio E. Clinical and radiological features of extra-pulmonary sarcoidosis: a pictorial essay. Insights Imaging. 2016;7(4):571–587. doi: 10.1007/s13244-016-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung G, Brill N, Poll LW, Koch JA, Wettstein M. MRI of hepatic sarcoidosis: large confluent lesions mimicking malignancy. Am J Roentgenol. 2004;183(1):171–173. doi: 10.2214/ajr.183.1.1830171. [DOI] [PubMed] [Google Scholar]
- 13.Warshauer DM, Dumbleton SA, Molina PL, Yankaskas BC, Parker LA, Woosley JT. Abdominal CT findings in sarcoidosis: radiologic and clinical correlation. Radiology. 1994;192(7):93–98. doi: 10.1148/radiology.192.1.8208972. [DOI] [PubMed] [Google Scholar]
- 14.Sekine T, Amano Y, Hidaka F, Takagi R, Machida T, Naito Z. Hepatosplenic and muscular sarcoidosis: characterization with MR imaging. Magn Reson Med Sci. 2012;11(2):83–89. doi: 10.2463/mrms.11.83. [DOI] [PubMed] [Google Scholar]
- 15.Asayama Y, Tajima T, Okamoto D, Nishie A, Ishigami K, Ushijima Y. Imaging of cholangiolocellular carcinoma of the liver. Eur J Radiol. 2010;75(1):120–125. doi: 10.1016/j.ejrad.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko K, Honda H, Kajiyama K, Yokomizo Y, Hashiguchi N, Fukuya T. Radiologically identifiable intratumoral portal vein in intrahepatic cholangiomas: a diagnostic pitfall. Abdominal Imaging. 1996;21(5):445–447. doi: 10.1007/s002619900100. [DOI] [PubMed] [Google Scholar]