Abstract
Background
Reasoning biases such as the jumping-to-conclusions bias (JTC) are thought to contribute to delusions. Interventions targeting these biases such as metacognitive training (MCT) may improve delusions. So far, it is not clear whether JTC depends on dopaminergic reward areas that constitute the main action locus of antipsychotic drugs, or on additional cortical areas. The present study aimed to investigate fMRI activation and functional connectivity patterns underlying JTC, and their changes following MCT, in patients with delusions.
Methods
Participants were 25 healthy individuals and 26 patients with current delusions who were either medication-free or on stable medication without sufficient response. We assessed (1) BOLD activity in the task-positive (TPN), task-negative (TNN), and subcortical reward network (RN); (2) Psychophysiological interactions (PPI) of peak activation areas.
Results
Presence of JTC (irrespective of group) was associated with lower RN activity during conclusion events, and with increased effective connectivity between TPN and TNN during draw events. Following MCT, changes were observed in TPN activity and in effective connectivity of inferior parietal cortex (part of the TPN) with all three target networks.
Conclusion
JTC is associated not only with reward system areas that constitute the main target of antipsychotic drugs, but also with cortical areas, particularly of the TPN.
Keywords: Evidence gathering, Prediction error, Schizophrenia, Psychosis, Striatum
Highlights
-
•
Faulty evidence gathering (jumping to conclusions, JTC) is associated with delusions.
-
•
We assessed data gathering with fMRI in patients with delusions vs healthy controls.
-
•
JTC was associated with abnormal activity and connectivity patterns.
-
•
Changes in the task-positive network were observed following metacognitive training.
1. Introduction
Delusions are defined as erroneous beliefs based on misinterpretation of events or perception (American Psychiatric Association, 2000). Their most prominent features are their incomprehensibility and incorrigibility; delusional ideas are held with a conviction unwarranted by evidence, and resist revision (Langdon et al., 2010).
The introduction of chlorpromazine in 1950 and the investigation of its effects on psychotic states led to the dopamine hypothesis which, in its initial and simplest formulation, states that psychotic symptoms such as delusions result from elevated striatal presynaptic dopamine activity. Today still, all currently licensed antipsychotic drugs have as a common denominator their ability to block dopamine D2 receptors (Howes and Kapur, 2009). However, it is still not fully understood how changes in the dopaminergic system translate to clinical symptoms such as delusions. A very influential account supported by a wealth of neuroimaging studies (Heinz and Schlagenhauf, 2010) posits that dysregulated striatal dopamine activity leads to delusions by affecting prediction error signaling, a process that uses the mismatch between a predicted outcome (based on previous experience) and an actual outcome to update associations and drive new learning; aberrant prediction error signaling is thought to result in a tendency to detect spurious associations between unrelated events (Corlett et al., 2010; Deserno et al., 2016; Fletcher and Frith, 2009; Howes et al., 2012).
Although the prediction error account provides a good explanation for the generation of implausible associations, it does not, in and of itself, explain how delusional ideas are adopted and maintained in spite of contrary evidence (Broyd et al., 2017; Langdon et al., 2010). One approach of dealing with this theory gap (for another, see Corlett et al., 2016) is to assume a second step in delusion formation (Broyd et al., 2017; Garety and Freeman, 2013; Moritz et al., 2017b) – specifically, distruptions in belief evaluation processes, subsumed under the term ‘reasoning biases’ (Garety and Freeman, 2013). The best investigated among these disruptions is the jumping-to-conclusion (JTC) bias, a tendency to make inferences based on limited evidence (Garety and Freeman, 1999). JTC is typically demonstrated using probabilistic reasoning tasks such as the beads task (Huq et al., 1988), in which patients with delusions consistently require fewer evidence than healthy controls to arrive to a conclusion. Importantly, this response pattern (JTC) cannot be accounted for by motivational deficits (Moutoussis et al., 2011), impulsivity (Speechley et al., 2010), risk propensity or intelligence (van der Leer et al., 2015), and has been proposed to result from a ‘liberal acceptance’ reasoning style, i.e. a lowered subjective probability threshold for the acceptance of hypotheses (Moritz et al., 2017b).
JTC is of particular interest with respect to the treatment of delusions: It does not appear to be influenced by dopamine antagonists (i.e., antipsychotic drugs) (Andreou et al., 2015b, 2014; Ermakova et al., 2014; Menon et al., 2008). In contrast, it is amenable to psychological interventions targeting reasoning biases (Moritz et al., 2012; Moritz et al., 2013a,b; Waller et al., 2011), which makes it a compelling treatment target for patients with delusions. Indeed, the above interventions have been shown to improve delusions when used as adjunctive treatment to antipsychotic medication (Andreou et al., 2017b; Eichner and Berna, 2016; Garety et al., 2015; Moritz et al., 2014a,b), an effect that may be mediated by improvement of JTC in patients (Andreou et al., 2015a). Given that the efficacy of antipsychotic drugs is unsatisfactory for many patients (Jaaskelainen et al., 2013) and often limited by high non-compliance rates (Lieberman et al., 2005), research into the neurophysiological substrate of JTC is of great relevance both for understanding delusion emergence and for the development of new treatment options.
A major difficulty in disentangling the neural substrates of JTC is to pinpoint the areas involved in its defining feature, i.e. insufficient evidence gathering before arriving to a conclusion. The reason is that probabilistic reasoning engages a large number of widely distributed brain areas involved in a variety of functions such as attention, information updating, salience processing, or representation of uncertainty (Demanuele et al., 2015; Esslinger et al., 2013; Furl and Averbeck, 2011; Krug et al., 2014b; Whitman et al., 2013). It has been suggested that JTC may result from dysregulated dopaminergic activity in the reward system through aberrant salience processing (Broyd et al., 2017; Speechley et al., 2010); however, Evans et al. (2015) have alternatively suggested that JTC results from a failure of a central comparator mechanism in the inferior parietal cortex, which continuously weighs the costs and benefits of seeking more information against those of terminating evidence gathering.
So far, there is no clear support for either of the above hypotheses. Two previous studies investigating probabilistic reasoning in schizophrenia yielded discordant findings; one study reported decreased activity in subcortical reward areas (Rausch et al., 2014), while another found decreased activity in parietal and prefrontal areas (Krug et al., 2014a), in patients compared to healthy controls. Findings of these two studies are difficult to interpret, though, given that patient populations were mixed with respect to current presence of delusions, and that the presence of JTC was not taken into account. Moreover, impaired BOLD activation patterns in patients were associated with executive function deficits rather than JTC or delusions (Krug et al., 2014a; Rausch et al., 2014).
Another issue not yet addressed in existing patient imaging studies are the complex interactions between brain areas involved in various stages of evidence gathering – a particularly important point, in light of the current trend of neuroimaging literature to focus on the functional integration of brain areas into networks (Bressler and Menon, 2010; Sporns, 2014) and the conceptualization of schizophrenia as a ‘dys-connectivity’ disorder (Andreasen et al., 1998; Friston, 1999). Our own group (Andreou et al., 2017a) has, for example, recently provided evidence suggesting that probabilistic reasoning in healthy individuals is dependent on the connectivity between a task-positive (TPN) and task-negative (TNN) network, of which the former consists of areas typically activated during cognitively demanding tasks, while the latter (also referred to as the default mode network, DMN) includes regions that are commonly deactivated during cognitive processing and show greater activity during rest (Fox et al., 2005; see also the discussion on an intrinsic and an extrinsic mode network in Hugdahl et al., 2015). More specifically, we observed that connectivity between the TPN and TNN decreased during evidence gathering, but increased at the moment of arriving to a conclusion (Andreou et al., 2017a). Our finding highlights connectivity between these two networks as a further potential factor that may contribute to JTC, which however needs to be confirmed in patients with delusions.
In the present study, we aimed to provide further insights into the pathophysiology of delusions and their treatment by identifying brain networks and mechanisms associated with an aspect (i.e., JTC) currently thought to be non-responsive to available antipsychotic drugs. We investigated fMRI activation and functional connectivity patterns underlying JTC, and their changes following Metacognitive Training (MCT), a psychological intervention targeting reasoning biases, in patients with delusions. Based on the above findings, we particularly focused on subcortical reward areas with a high expression of dopamine D2 receptors, the TPN and the TNN. We expected that JTC would be associated with abnormal activity and/or connectivity patterns in cortical (most particularly the TPN, which includes the inferior parietal cortex) rather than subcortical areas during probabilistic reasoning; further, we assumed that more cautious reasoning following a course of MCT would be associated with normalization of these patterns.
2. Material and methods
2.1. Participants and measures
Participants were 30 patients with schizophrenia spectrum disorders and current delusions, and 27 healthy individuals. To minimize the effects of antipsychotic medication changes over the study period and thus relatively isolate the effects of MCT from those of medication, we only included patients who were either medication-free or on stable medication without sufficient response (for a detailed description of recruitment, inclusion and exclusion criteria, see Supplementary Methods).
The study was approved by the Ethics Committee of the German Psychological Association, and was performed in accordance with the most recent version of the Declaration of Helsinki ethical standards. All participants provided written informed consent, and were reimbursed with 40 EUR for their participation.
Diagnosis of psychotic disorders in patients, and absence of psychiatric morbidity in healthy controls, were confirmed with the Mini International Neuropsychiatric Interview (Sheehan et al., 1998). Presence of subthreshold delusional ideas in healthy controls was assessed with the Schizotypal Personality Questionnaire (Klein et al., 1997; Raine, 1991). Psychopathology in patients was assessed with the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987). Scores were derived for positive, negative, disorganization, excitement and distress factors of the scale (van der Gaag et al., 2006). PANSS item P1 was used as an index of delusion severity.
Probabilistic reasoning was assessed with an fMRI-adapted version of the Box Task (Andreou et al., 2015b; Balzan et al., 2017), the validity and reliability of which have been confirmed in previous studies (Andreou et al., 2015b; Moritz et al., 2017a). Because the Box Task does not provide a cut-off for JTC, presence or absence of the latter was assessed with the Fish Task (Moritz et al., 2010), where JTC was defined as decisions made after 1 or 2 draws. For a detailed description of the Box Task and correlations between Box Task and Fish Task performance, see Supplementary Methods.
After baseline assessments, patients underwent a course of Metacognitive Training (MCT), a manualized group intervention that consists of 8 twice-weekly sessions lasting 45–60 min and aims to foster awareness for delusion-related reasoning biases such as JTC by using entertaining, predominantly delusion-neutral, exercises (Moritz et al., 2013a,b; see Supplementary Methods for details).
2.2. fMRI data acquisition & preprocessing
fMRI data was acquired once in healthy controls and twice in patients (at baseline and after completing at least 6 MCT sessions) using a 3 Tesla MRI scanner (Siemens Magnetom Trio, Siemens, Erlangen, Germany) equipped with a 32-channel head coil. Blood oxygenation level-dependent (BOLD) signals were measured with an echo planar imaging sequence (33 transversal slices, 2.0 mm thickness, 1.0 mm gap, voxel size 2 × 2 × 2 mm3, TE = 25 ms, TR = 1980 ms, flip angle = 75°, 108 × 108 matrix, field of view 216 × 216 mm2, GRAPPA with PAT-factor 2). For structural imaging, a high-resolution 3D T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) data set was acquired (240 slices, FOV 256 × 256 mm2, voxel size 1 × 1 × 1 mm3) in the same session as the T2 sequence.
Data were processed using standard procedures implemented in the Statistical Parameter Mapping software (SPM12, www.fil.ion.ucl.ac.uk/spm). The first 5 volumes of each block were discarded to allow for MRI saturation effects. Preprocessing steps included slice timing, realignment, registration to standard space (Montreal Neurological Institute, MNI) and spatial smoothing with an 8 mm Gaussian kernel. Outliers in global mean image time series (>1.3% variation in global intensity) and movement (>1 mm/scan) were detected using an artifact detection and repair toolbox (ArtRepair; Mazaika et al., 2009) and entered as regressors of no interest in the first-level models.
Two healthy controls and four patients were excluded from fMRI analyses because of intracranial pathology (1 control); rate of fMRI image outlier data as defined above >20% (1 control, 1 patient); failure to complete all 3 paradigm runs (2 patients); and consistent opening of only one box per trial resulting in null regressors in the design matrix (1 patient). Thus, the final sample included in fMRI analyses consisted of 25 healthy controls and 26 patients with delusions (13 medication-free, 13 medicated); follow-up fMRI analyses included 22 patients.
2.3. BOLD analyses
2.3.1. Whole-brain analyses
BOLD responses to stimuli were examined using the general linear model (GLM) approach. Regressors corresponding to all trial events were convolved with the standard hemodynamic function (see Supplementary Methods); six motion correction parameters and outlier indices were added to the model as predictors of no interest.
First-level contrasts were calculated for each of the following events of interest compared to baseline: (1) box opening in the 80:20 condition leading to a conclusion; (2) box opening in the 60:40 condition leading to a conclusion; (3) box opening in the 80:20 condition leading to a choice for a further draw; (4) box opening in the 60:40 condition leading to a choice for a further draw. Please note that the events of interest corresponded to a stimulus (opening of a box) leading to a behavioral choice (another draw, or conclusion); the actual time at which the choice was made is unknown.
First-level contrasts were entered into a second-level 2 (Group: patients vs. controls) × 2 (JTC vs. non-JTC) × 2 (Decision Event: conclusion vs. draw) × 2 (Ratio: 80:20 vs. 60:40) mixed factorial model. The Multilevel and Repeated Measures (MRM) toolbox (McFarquhar et al., 2016) was used for these analyses, because the second-level inference procedures included in SPM do not allow for partitioned error modelling in the case of mixed designs. MRM makes use of permutation testing to improve p-value approximation and provides a familywise error (FWE) analogue to standard Gaussian random theory approach (McFarquhar et al., 2016). For the present analysis, effects observed at p < 0.001 (uncorrected) and surviving a cluster level FWE correction at p < 0.05 are reported as significant.
2.3.2. Region-of-interest analyses
Additionally, we implemented region-of-interest (ROI) analyses to specifically assess the effects of Group and JTC on three networks of interest: TPN (3 ROIs: bilateral inferior parietal lobule and dorsal anterior cingulate cortex), TNN (2 ROIs: ventromedial prefrontal cortex and posterior cingulate cortex) and subcortical reward network (3 ROIs: ventral tegmental area, ventral and dorsal striatum); for a detailed description of ROI selection and construction, see Supplementary Methods.
For each ROI, mean activity in each condition was extracted using MarsBar (marsbar.sourceforge.net), and included in a linear mixed model with Group and JTC as between-subject factors, Decision Event, Ratio and Network as within-subject factors, as well as all resulting two- and three-way interactions (fixed effects); participant ID was included as a random effect. For longitudinal analyses, the model included Time (pre- vs. post-MCT), Decision Event, Ratio, Network and all resulting interactions as within-subjects fixed effects, and participant ID as a random effect. Linear mixed model analyses were conducted in SPSS 24.0.
2.4. Effective connectivity analyses
Psychophysiological interaction (PPI) analysis, a data-driven approach that maps task-dependent functional connectivity between a seed region of interest (ROI) and the rest of the brain (Friston et al., 1997), was employed. PPI represents a measure of stimulus-dependent connectivity, describing responses in one ROI in terms of the interaction between responses in another ROI and a psychological process.
Seeds for the PPI analysis were derived from the results of the above GLM analysis of BOLD activity. The strongest group-level peaks of activation were identified for the contrast conclusion > draw (bilateral BA40, MNI coordinates: 52 -34 44 / -42 -44 48) and draw > conclusion (PCC, MNI coordinates -6 -52 26); for a detailed description of ROI construction and time-series extraction, see Supplementary Methods. PPI coefficients for seed ROIs were entered into a second-level model in MRM similar as above. Because PPI is associated with low statistical power, especially in event-related designs (O'Reilly et al., 2012), we used a more lenient multiple comparison correction for these analyses and defined the significance threshold as FWE-corrected p < 0.05 at the cluster level with a voxel-level threshold of p < 0.005 (uncorrected).
ROI analyses were also conducted for PPI coefficients extracted as described above, using the same ROIs and linear mixed model set-up as for BOLD analyses; separate linear mixed models were implemented for the BA40 (conclusion > draw) and PCC (draw > conclusion) sources.
2.5. Specificity of longitudinal effects following MCT
In this first fMRI investigation of longitudinal effects following MCT, the study design was observational, i.e. there was no control intervention; hence, any improvements in delusions could be unspecific and not necessarily reflect an improvement in reasoning. This was taken into account by dividing the patient group into responders and non-responders for longitudinal analyses. Response was defined as statistically significant increase in draws to decision in the Box Task (i.e., more cautious evidence gathering, the postulated mediator of symptom improvement after MCT) over time (see Supplementary methods).
However, due to the small sample size of the responder group (see Results, below), subgroup analyses on fMRI data were not deemed appropriate. In order to assess whether observed effects were related to changes in probabilistic reasoning over time, Box Task performance (i.e., mean number of draws to decision) was included as a condition- and time-dependent covariate in longitudinal ROI analyses. In MRM analyses, which do not allow for inclusion of time-dependent covariates, change in Box Task performance (difference score averaged over the two ratio conditions) was used as covariate.
3. Results
Baseline characteristics and between-group comparisons are reported in Table 1. SPQ scores in healthy controls are reported in Table S1 (Supplementary Results).
Table 1.
Baseline characteristics of participant groups and between-group differences.
| Healthy controls (n = 25) |
Patients with delusions (n = 26) |
Patients vs. controls |
Medication free vs. medicated patients |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antipsychotic medication-free (n = 13) |
Medicated (n = 13) |
||||||||||||
| n | mean | SD | n | mean | SD | n | mean | SD | χ2/t | p | χ2/t | p | |
| Gender (male/female) | 15/10 | 9/4 | 7/3 | 0.013 | 0.910 | 0.650 | 0.420 | ||||||
| Age | 34.76 | 10.1 | 37.00 | 12.2 | 36.69 | 13.0 | 0.659 | 0.513 | 0.062 | 0.951 | |||
| Premorbid IQ | 107.32 | 8.8 | 108.77 | 11.7 | 103.33 | 10.4 | 0.408 | 0.685 | 1.228 | 0.232 | |||
| Jumping-to-conclusions (yes/no) | 6/19 | 1/12 | 5/8 | 0.006 | 0.938 | 3.467 | 0.063 | ||||||
| Box Task - mean number of draws | |||||||||||||
| 60:40 | 9.49 | 5.1 | 10.59 | 5.5 | 10.86 | 5.7 | 0.831 | 0.410 | 0.123 | 0.903 | |||
| 80:20 | 5.80 | 2.7 | 6.16 | 2.4 | 6.74 | 4.1 | 0.776 | 0.441 | 0.441 | 0.663 | |||
| Fish Task - draws to decision | 3.92 | 1.9 | 5.00 | 2.8 | 4.69 | 3.1 | 1.338 | 0.187 | 0.266 | 0.793 | |||
| PANSS | |||||||||||||
| Item P1 (delusions) | 4.85 | 1.0 | 4.62 | 1.0 | 0.579 | 0.568 | |||||||
| Positive | 21.46 | 6.1 | 21.08 | 5.2 | 0.174 | 0.863 | |||||||
| Negative | 11.69 | 3.8 | 16.92 | 6.4 | 2.544 | 0.018 | |||||||
| Disorganization | 16.23 | 3.7 | 16.38 | 4.0 | 0.102 | 0.920 | |||||||
| Excitement | 11.15 | 3.1 | 11.46 | 2.6 | 0.272 | 0.788 | |||||||
| Distress | 16.69 | 3.9 | 18.15 | 3.6 | 0.981 | 0.337 | |||||||
| Medication⁎ | |||||||||||||
| Chlorpromazine equivalents | 828.15 | 806.6 | |||||||||||
| Aripiprazol | 7 | ||||||||||||
| Clozapine | 3 | ||||||||||||
| Olanzapine | 1 | ||||||||||||
| Quetiapine | 3 | ||||||||||||
| Paliperidone | 1 | ||||||||||||
| Risperidone | 1 | ||||||||||||
PANSS: Positive and Negative Syndrome Scale.
Four patients received treatment with two antipsychotics.
There was a significant positive correlation between number of draws to decision in the Box Task and the Fish Task at a medium effect size (Pearson's r = 0.338, p = 0.01). Participants displaying a JTC response style in the Fish Task had a lower number of draws in the Box Task (6.34 ± 3.85 vs. 8.76 ± 3.77 in participants who did not display JTC); this difference was marginally significant [t(51) = 1.975, p = 0.05]. Quite unexpectedly, patients and healthy controls did not significantly differ in any measures of evidence gathering at baseline.
Delusions and all PANSS symptom dimensions improved significantly post-MCT, with the exception of negative symptoms (trend-wise improvement) (Table 2). A trend towards increase in draws to decision post-MCT was observed in the Box Task, though not in the Fish Task. Seven MCT responders were identified based on Box Task performance; these patients also showed trend-wise greater increase in draws to decision in the Fish Task than non-responders. Delusions showed somewhat larger improvement in MCT responders than non-responders, but this was a small effect that did not reach significance. There was a positive correlation at a medium effect size between delusion improvement (percent decrease from baseline) and change in data gathering (percent increase from baseline), which also missed significance (Spearman's rho = 0.332, p = 0.13).
Table 2.
Probabilistic reasoning and symptoms in patients with delusions before and after Metacognitive Training (MCT). Statistics are provided for a linear mixed model with time (pre- vs. post-MCT) as within-subjects fixed effect, JTC response as between-subjects fixed effect, and participant ID as a random effect.
| JTC response |
Statistics |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-responders (n = 15) |
Responders (n = 7) |
|||||||||||
| Baseline |
Follow-up |
Baseline |
Follow-up |
Time |
Time × response |
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | p | F | p | |
| Fish Task - draws to decision | 4.53 | 2.7 | 4.07 | 1.8 | 5.29 | 3.9 | 6.29 | 3.2 | 0.428 | 0.520 | 3.239 | 0.086 |
| Box Task – mean number of draws⁎ | ||||||||||||
| 80:20 | 6.36 | 3.7 | 5.38 | 2.2 | 6.06 | 2.3 | 8.73 | 1.3 | 3.225 | 0.086 | 15.024 | <0.001 |
| 60:40 | 10.33 | 6.0 | 8.29 | 5.1 | 10.77 | 4.2 | 15.50 | 2.6 | 3.934 | 0.060 | 25.060 | <0.001 |
| PANSS | ||||||||||||
| Item P1 (delusions) | 4.73 | 1.0 | 3.53 | 1.6 | 5.14 | 0.9 | 3.29 | 1.6 | 29.508 | <0.001 | 1.363 | 0.255 |
| Positive | 22.00 | 5.7 | 16.47 | 7.2 | 21.57 | 4.4 | 15.57 | 4.9 | 46.289 | <0.001 | 0.076 | 0.786 |
| Negative | 13.87 | 4.8 | 11.6 | 3.6 | 12.57 | 4.8 | 10.71 | 2.9 | 4.135 | 0.054 | 0.041 | 0.842 |
| Disorganization | 16.87 | 4.1 | 13.87 | 3.7 | 15.57 | 3.9 | 13.14 | 2.7 | 18.020 | <0.001 | 0.200 | 0.659 |
| Excitement | 11.27 | 2.7 | 9.8 | 1.4 | 11.57 | 2.8 | 9.43 | 0.8 | 11.938 | 0.002 | 0.419 | 0.524 |
| Distress | 16.53 | 3.3 | 13.13 | 3.5 | 19.57 | 3.7 | 16.00 | 4.8 | 9.685 | 0.005 | 0.006 | 0.939 |
PANSS: Positive and Negative Syndrome Scale.
Please note that time × response statistics are redundant in this case, since response was defined based on Box Task performance (i.e., significant increase in draws to decision in the Box Task).
3.1. BOLD analyses
In whole-brain analyses over both groups, Decision Event was associated with extensive bilateral brain activations including the insula, dorsal and ventral medial frontal areas, dorsolateral prefrontal cortex, posterior cingulate cortex, medial occipital areas, inferior parietal cortex, the thalamus and lentiform nucleus (F-contrast conclusion vs. draw, cluster-level FWE p < 0.05). ROI analyses indicated a significant interaction between Decision Event and Network [F(2, 833.71) = 46.94, p < 0.001], with the TPN and subcortical reward network showing higher BOLD activity in conclusion compared to draw events, whereas the opposite was the case in the TNN.
3.1.1. Baseline group comparisons
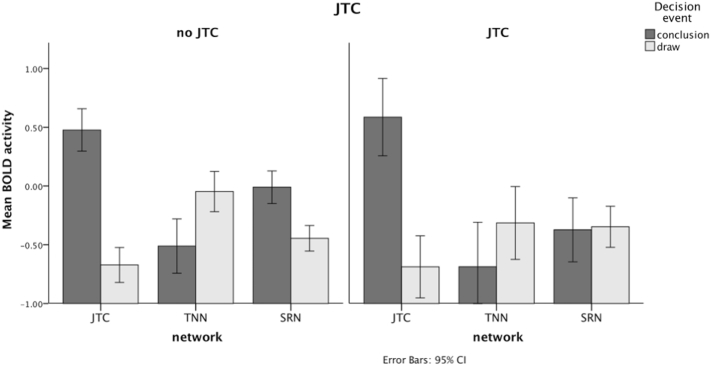
Whole-brain analyses did not yield any significant effects or interactions involving Group or JTC in baseline analyses. In ROI analyses, we observed a significant JTC × Network × Decision Event interaction [F(2, 834.71) = 4.37, p = 0.01]. Follow-up analyses conducted for each network separately showed that this was due to the fact that conclusion events in JTC subjects were not associated with the expected activity increase in the subcortical reward network (Fig. 1).
Fig. 1.
BOLD activity in three networks of interest (TPN: task-positive network; TNN: task-negative network; SRN: subcortical reward network) during conclusion and draw events in the Box Task in participants with and without a jumping-to-conclusions response pattern [Decision Event × JTC interaction: RSN: F(1,348.36) = 10.47, p = 0.00; TPN: p = 0.46; TNN: p = 0.48].
Analyses were repeated including mean number of draws to decision in the Box Task at baseline as a covariate, in place of the dichotomous factor JTC; results of these additional analyses are reported in Supplementary results.
3.1.2. Longitudinal effects (pre- vs post-MCT)
There were no significant findings with respect to Time or Box Task performance change in longitudinal whole-brain analyses.
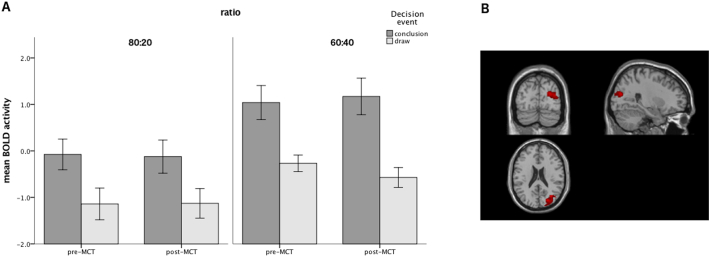
In longitudinal ROI analyses, there was a significant 4-way interaction between Time (pre- vs. post-MCT), Decision event, Ratio and Network [F(2,649.23) = 3.439, p = 0.03]. Separate linear mixed model analyses for each network separately yielded no significant main effects or interactions involving Time in the TNN and the subcortical reward network. In the TPN however, a significant 3-way interaction between Time, Decision event and Ratio [F(1,312.30) = 12.117, p = 0.001], resulting from greater difference in TPN activity between conclusion and draw events post-MCT in the 60:40 condition (β = 1.062, SE = 0.175 vs. β = 1.632, SE = 0.191 for pre- and post-MCT respectively) compared to the 80:20 condition (β = 1.074, SE = 0.157 vs. β = 0.332, SE = 0.153 for pre- and post-MCT respectively; see Fig. 2A).
Fig. 2.
Pre- vs. post-MCT comparisons regarding (A) BOLD activity in the task-positive network (TPN), separately for conclusion and draw events and for the two ratio conditions (80:20 and 60:40); (B) psychophysiological interactions of the bilateral posterior inferior parietal cortex [seed regions: left and right BA40 (MNI coordinates: 52–34 44 / -42 -44 48)] for the contrast conclusion > draw. F image thresholded at FWE-corrected cluster p < 0.05 with a voxel-level threshold of p < 0.005 (uncorrected).
Inclusion of Box Task performance as a time-varying covariate revealed a significant interaction with Network [F(2,404.05) = 9.125, p < 0.001], qualified by a 3-way interaction of Box Task performance, Network and Decision event [F(2, 403.86) = 6.591, p = 0.002]. Follow-up analyses for each network separately showed positive correlations between number of draws in the Box Task and MRI activity mainly in the TPN [F(1,93.99) = 33.298, p < 0.001; β = 0.036, SE = 0.014] and less so in the TNN [F(1,51.085) = 4.038, p = 0.05; β = 0.019, SE = 0.017] and subcortical reward network [F(1,114.12) = 3.566, p = 0.06; β = 0.024, SE = 0.013]; only in the TPN, a significant interaction with Decision event [F(1,185.89) = 19.879, p < 0.001] indicated that the effect of Box Task performance on MRI activity was more prominent for Conclusion compared to Draw events.
3.2. Effective connectivity analyses
3.2.1. Baseline group comparisons
There were no significant effects of Group or JTC in baseline whole-brain PPI analyses. In ROI analyses, a significant interaction between JTC and network was observed [F(2, 580.17) = 4.884, p = 0.008] for the PCC source ROI; this interaction resulted from greater PCC connectivity with the TPN [F(1, 41.4) = 12.104, p = 0.001], but not TNN [F(1, 45.68) = 0.530, p = 0.470] or subcortical reward network [F(1, 44.60) = 0.783, p = 0.381], for the contrast draw > conclusion in subjects displaying JTC.
Analyses were repeated including mean number of draws to decision in the Box Task at baseline as a covariate, in place of the dichotomous factor JTC; results of these additional analyses are reported in Supplementary results.
3.2.2. Effect of metacognitive training
In longitudinal whole-brain PPI analyses, there was significantly increased connectivity between BA40 and right medial occipital areas (BA18, BA19 and BA17; Fig. 2B) following MCT; there was no significant effect of change in Box Task performance as a covariate. No significant Time effects were observed in PCC.
A significant main effect of Time in longitudinal ROI analyses indicated significantly higher connectivity between BA40 and all three target networks (TPN, TNN and subcortical reward network) following MCT [F(1,1142.24) = 17.059, p < 0.001] for the conclusion > draw contrast, but no significant effect or interaction of Time for PCC connectivity and the opposite contrast. There were no significant effects or interactions of Box Task performance as a time-varying covariate.
4. Discussion
The present study used fMRI to (a) investigate probabilistic reasoning in patients with delusions, focusing both on activity and effective connectivity, and to (b) assess changes in activity and connectivity patterns in patients following Metacognitive Training, an intervention that specifically targets reasoning biases such as JTC. Presence of a JTC bias was associated with lower activity in subcortical reward areas during conclusion events and with greater connectivity between PCC and the TPN during draw events in ROI analyses. Following MCT, there was greater difference in TPN activity between draw and conclusion events in the 60:40 condition; moreover, there was increased connectivity for the conclusion > draw contrast between bilateral BA40 and medial occipital areas in whole-brain analyses, as well as with all three studied networks (TPN, TNN and subcortical reward network) in ROI analyses. Increase in draws to decision in the Box Task following MCT was associated with higher activity overall in the TPN during conclusion events.
Our findings constitute new evidence regarding the role of the dopaminergic reward system in the emergence of JTC. It has been previously argued that JTC may result directly from increased mesolimbic dopaminergic signaling and aberrant salience through ‘overvaluing’ acquired evidence (Broyd et al., 2017; Speechley et al., 2010). Contradicting this view, a previous study indicated reduced, rather than increased, striatal activity during probabilistic reasoning in patients with schizophrenia (Rausch et al., 2014), a finding that was confirmed in patients at high risk for psychosis (Rausch et al., 2015). Moreover, drug-challenge studies failed to find evidence of JTC modulation by dopaminergic agents (Andreou et al., 2014; Andreou et al., 2015b; Ermakova et al., 2014). Our present findings too are difficult to explain in terms of mesolimbic hyperactivity, as JTC subjects failed to show the expected activity increase in subcortical reward areas during conclusion events. This discrepancy may be solved by considering an updated dopamine hypothesis (Maia and Frank, 2016), according to which schizophrenia is characterized by a combination of high tonic dopaminergic activity (and/or increased spontaneous dopamine transients) and decreased adaptive dopamine activity in response to relevant stimuli. Our finding of blunted reward system responses to conclusion events in JTC subjects might thus represent such a case of reduced adaptive dopaminergic signaling.
TPN and TNN activity was not associated with presence of JTC at baseline. However, we observed greater connectivity between PCC (part of the TNN) and the TPN for the draw > conclusion contrast in subjects displaying JTC. We have argued before, based on a previous analysis of healthy participant data from this study (Andreou et al., 2017a), that a functional separation (i.e., decreased connectivity) between TPN and TNN is required during evidence gathering to enable the execution of several parallel mental operations. Therefore, increased connectivity between the two systems during evidence gathering in JTC individuals may trigger premature conclusions.
Furthermore, activity in the TPN was longitudinally associated with changes in evidence gathering following MCT, possibly suggesting more effective processing of the stimulus sequence. More importantly, the gain in effective connectivity during conclusion events between the posterior inferior parietal cortex (BA40) and the rest of the TPN as well as with the TNN and subcortical reward network increased following MCT. These findings support the view of the posterior inferior parietal cortex as a central coordinator of evidence gathering (Evans et al., 2015; Furl and Averbeck, 2011). They also raise the compelling possibility that this region may be able to compensate disrupted reward functioning and thereby contribute to JTC improvement following metacognitive intervention, which, in turn, may mediate delusion improvement, as suggested by previous studies (Andreou et al., 2015a; Garety et al., 2015): Although JTC responders did not show significantly greater delusion improvement in the present study (possibly because of the small sample size), increase in evidence gathering correlated positively with delusion improvement. Although this correlation missed significance and thus needs replication in a larger sample, it reached a medium effect size, similar to that observed for the MCT arm in a previous controlled clinical trial by our group in a non-overlapping patient sample (Andreou et al., 2015a).
4.1. Limitations
Some limitations of the current study need to be taken into account when considering its findings. A major limitation results from the surprising lack of baseline differences in JTC between the two participant groups. Although JTC rates in our healthy control sample were comparable to those reported in previous studies (Balzan et al., 2017; Dudley et al., 2016; Freeman, 2007; Lincoln et al., 2010), only 23% of patients exhibited a JTC response style, which is quite low compared to the rates reported in patients with psychotic disorders in a recent meta-analysis (Dudley et al., 2016). Looking at Table 1, it becomes clear that these low rates were mainly driven by the medication-free patient group, in which only 8% displayed JTC. This finding cannot be attributed to the lack of antipsychotic medication, since increased JTC is also observed in clinical or psychometric high-risk subjects, who are generally not treated with antipsychotics (for a summary of related studies, see Rausch et al., 2015). Moreover, it cannot be explained by demographic variables, IQ or positive symptoms, as all of these variables were similar to the medicated patient group. It is therefore likely that the low JTC rates observed in this patient group represent a chance finding, especially given the small sample size. Whatever their cause might be, low JTC rates in our patient sample may have been responsible for the lack of an overall significant JTC improvement following MCT, in contrast to previous trials (Garety et al., 2015; Moritz et al., 2014a,b, 2013a,b); they also complicate interpretation of results, especially given our choice of paradigm and the observational design of the study.
We intentionally removed additional rewarding stimuli by explicit feedback from our probabilistic reasoning paradigm, in order to better assess evidence gathering in isolation. However, this may have led to the somewhat counter-intuitive finding of a complete lack of baseline differences (e.g. in striatal areas) between patients with delusions and healthy controls, especially given the lack of JTC differences between the two groups, as discussed above.
A final limitation of the study results from the selection of an observational design without a control intervention. We only included stable patients who were either medication-free or resistant to their current medication, in order to minimize the influence of natural illness course and antipsychotic medication during the study period. We also focused on evidence gathering rather than symptoms as an outcome variable because the latter are known to be confounded by unspecific study effects in open studies; in contrast, evidence gathering exhibits no consistent longitudinal changes in the absence of a metacognitive intervention (Andreou et al., 2018, 2015b; Menon et al., 2008; Peters and Garety, 2006; So et al., 2012; Woodward et al., 2009), and practice effects have been often reported to lead to a decrease in draws to decision (rather than the expected increase following MCT) (Andreou et al., 2015b; Peters and Garety, 2006; Woodward et al., 2009). Still, the lack of a control intervention limits causal inferences regarding MCT effects. Moreover, only correlational analyses were possible, since the small size of the JTC-responder group precluded subgroup analyses. Thus, although the longitudinal association of TPN activity with Box Task performance is a promising finding, larger controlled trials are warranted to confirm the relevance of changes observed following MCT for probabilistic reasoning and delusions.
4.2. Conclusions
The present study investigated fMRI activity and effective connectivity patterns associated with jumping-to-conclusions bias in patients with delusions, and their changes following MCT, an intervention designed to target reasoning biases. JTC was associated with disrupted activity in subcortical regions associated with the dopaminergic reward system, but also with aberrant effective connectivity between PCC and the TPN. Moreover, changes were observed following MCT on TPN activity and on effective connectivity between posterior inferior parietal cortex and other cortical and subcortical areas. Given the surprising lack of increased JTC in the present patient sample, findings should be considered preliminary. Pending replication in larger studies, the pattern of results indicates additional pathophysiological mechanisms, supplementary to dopaminergic antagonism, which may help improve JTC and delusions.
Acknowledgments
Acknowledgments
This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) through a research grant awarded to CM and CA (AN 970/4-1; MU 2705/2-1).
Declarations of interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.07.004.
Appendix A. Supplementary data
Supplementary Methods
Supplementary Results
References
- American Psychiatric Association . 4th ed. American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders. Text revision. [Google Scholar]
- Andreasen N.C., Paradiso S., O'Leary D.S. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr. Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Andreou C., Moritz S., Veith K., Veckenstedt R., Naber D. Dopaminergic modulation of probabilistic reasoning and overconfidence in errors: a double-blind study. Schizophr. Bull. 2014;40:558–565. doi: 10.1093/schbul/sbt064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou C., Schneider B.C., Balzan R., Luedecke D., Roesch-Ely D., Moritz S. Neurocognitive deficits are relevant for the jumping-to-conclusions bias, but not for delusions: a longitudinal study. Schizophr. Res. Cogn. 2015;2:8–11. doi: 10.1016/j.scog.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou C., Schneider B.C., Braun V., Kolbeck K., Gallinat J., Moritz S. Dopamine effects on evidence gathering and integration. J. Psychiatry Neurosci. 2015;40:422–428. doi: 10.1503/jpn.140306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou C., Steinmann S., Kolbeck K., Leicht G., Moritz S., Mulert C. The role of effective connectivity between the task-positive and task-negative network for evidence gathering. NeuroImage. 2017 doi: 10.1016/j.neuroimage.2018.02.039. [DOI] [PubMed] [Google Scholar]
- Andreou C., Wittekind C.C.E., Fieker M., Heitz U., Veckenstedt R., Bohn F., Moritz S. Individualized metacognitive therapy for delusions: a randomized controlled rater-blind study. J. Behav. Ther. Exp. Psychiatry. 2017;56:144–151. doi: 10.1016/j.jbtep.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Andreou C., Veckenstedt R., Lüdtke T., Bozikas V.P., Moritz S. Differential relationship of jumping-to-conclusions and incorrigibility with delusion severity. Psychiatry Res. 2018;264:297–301. doi: 10.1016/j.psychres.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Balzan R.P.R.P., Ephraums R., Delfabbro P., Andreou C. Beads task vs. box task: the specificity of the jumping to conclusions bias. J. Behav. Ther. Exp. Psychiatry. 2017;56:42–50. doi: 10.1016/j.jbtep.2016.07.017. [DOI] [PubMed] [Google Scholar]
- Bressler S.L., Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn. Sci. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Broyd A., Balzan R.P., Woodward T.S., Allen P. Dopamine, cognitive biases and assessment of certainty: a neurocognitive model of delusions. Clin. Psychol. Rev. 2017 doi: 10.1016/j.cpr.2017.04.006. [DOI] [PubMed] [Google Scholar]
- Corlett P.R., Taylor J.R., Wang X.-J., Fletcher P.C., Krystal J.H. Toward a neurobiology of delusions. Prog. Neurobiol. 2010;92:345–369. doi: 10.1016/j.pneurobio.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett P.R., Honey G.D., Fletcher P.C. Prediction error, ketamine and psychosis: an updated model. J. Psychopharmacol. 2016;30:1145–1155. doi: 10.1177/0269881116650087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demanuele C., Kirsch P., Esslinger C., Zink M., Meyer-Lindenberg A., Durstewitz D. Area-specific information processing in prefrontal cortex during a probabilistic inference task: a multivariate fMRI BOLD time series analysis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0135424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deserno L., Schlagenhauf F., Heinz A. Striatal dopamine, reward, and decision making in schizophrenia. Dialogues Clin. Neurosci. 2016;18:77–89. doi: 10.31887/DCNS.2016.18.1/ldeserno. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley R., Taylor P., Wickham S., Hutton P. Psychosis, delusions and the “jumping to conclusions” reasoning bias: a systematic review and meta-analysis. Schizophr. Bull. 2016;42:652–665. doi: 10.1093/schbul/sbv150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner C., Berna F. Acceptance and efficacy of metacognitive training (mct) on positive symptoms and delusions in patients with schizophrenia: a meta-analysis taking into account important moderators. Schizophr. Bull. 2016;42:952–962. doi: 10.1093/schbul/sbv225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermakova A.O., Ramachandra P., Corlett P.R., Fletcher P.C., Murray G.K. Effects of methamphetamine administration on information gathering during probabilistic reasoning in healthy humans. PLoS One. 2014;9 doi: 10.1371/journal.pone.0102683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esslinger C., Braun U., Schirmbeck F., Santos A., Meyer-Lindenberg A., Zink M., Kirsch P. Activation of midbrain and ventral striatal regions implicates salience processing during a modified beads task. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S.L., Averbeck B.B., Furl N. Jumping to conclusions in schizophrenia. Neuropsychiatr. Dis. Treat. 2015;11:1615–1624. doi: 10.2147/NDT.S56870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P.C., Frith C.D. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat. Rev. Neurosci. 2009;10:48–58. doi: 10.1038/nrn2536. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D. Suspicious minds: the psychology of persecutory delusions. Clin. Psychol. Rev. 2007;27:425–457. doi: 10.1016/j.cpr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Friston K.J. Schizophrenia and the disconnection hypothesis. Acta Psychiatr. Scand. Suppl. 1999;395:68–79. doi: 10.1111/j.1600-0447.1999.tb05985.x. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Furl N., Averbeck B.B. Parietal cortex and insula relate to evidence seeking relevant to reward-related decisions. J. Neurosci. 2011;31:17572–17582. doi: 10.1523/JNEUROSCI.4236-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garety P.A., Freeman D. Cognitive approaches to delusions: a critical review of theories and evidence. Br. J. Clin. Psychol. 1999;38:113–154. doi: 10.1348/014466599162700. Pt 2. [DOI] [PubMed] [Google Scholar]
- Garety P.A., Freeman D. The past and future of delusions research: from the inexplicable to the treatable. Br. J. Psychiatry. 2013;203:327–333. doi: 10.1192/bjp.bp.113.126953. [DOI] [PubMed] [Google Scholar]
- Garety P., Waller H., Emsley R., Jolley S., Kuipers E., Bebbington P., Dunn G., Fowler D., Hardy A., Freeman D. Cognitive mechanisms of change in delusions: an experimental investigation targeting reasoning to effect change in paranoia. Schizophr. Bull. 2015;41:400–410. doi: 10.1093/schbul/sbu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A., Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr. Bull. 2010;36:472–485. doi: 10.1093/schbul/sbq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes O.D., Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr. Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes O.D., Kambeitz J., Kim E., Stahl D., Slifstein M., Abi-Dargham A., Kapur S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch. Gen. Psychiatry. 2012;69:776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K., Raichle M.E., Mitra A., Specht K. On the existence of a generalized non-specific task-dependent network. Front. Hum. Neurosci. 2015;9 doi: 10.3389/fnhum.2015.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq S.F., Garety P.A., Hemsley D.R. Probabilistic judgements in deluded and non-deluded subjects. Q. J. Exp. Psychol. 1988;40A:801–812. doi: 10.1080/14640748808402300. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen E., Juola P., Hirvonen N., McGrath J.J., Saha S., Isohanni M., Veijola J., Miettunen J. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr. Bull. 2013;39:1296–1306. doi: 10.1093/schbul/sbs130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Klein C., Andresen B., Jahn T. Psychometric assessment of the schizotypal personality according to DSM-III-R criteria. Psychometric properties of an authorized German translation of Raine's “Schizotypal Personality Questionnaire” (SPQ) Diagnostica. 1997;43:347–369. [Google Scholar]
- Krug A., Cabanis M., Pyka M., Pauly K., Kellermann T., Walter H., Wagner M., Landsberg M., Shah N.J., Winterer G., Wölwer W., Brinkmeyer J., Müller B.W., Kärgel C., Wiedemann G., Herrlich J., Vogeley K., Schilbach L., Rapp A., Klingberg S., Kircher T. Attenuated prefrontal activation during decision-making under uncertainty in schizophrenia: a multi-center fMRI study. Schizophr. Res. 2014;152:176–183. doi: 10.1016/j.schres.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Krug A., Cabanis M., Pyka M., Pauly K., Walter H., Landsberg M., Shah N.J., Winterer G., Wölwer W., Musso F., Müller B.W., Wiedemann G., Herrlich J., Schnell K., Vogeley K., Schilbach L., Langohr K., Rapp A., Klingberg S., Kircher T. Investigation of decision-making under uncertainty in healthy subjects: a multi-centric fMRI study. Behav. Brain Res. 2014;261:89–96. doi: 10.1016/j.bbr.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Langdon R., Ward P.B., Coltheart M. Reasoning anomalies associated with delusions in schizophrenia. Schizophr. Bull. 2010;36:321–330. doi: 10.1093/schbul/sbn069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J.A., Stroup T.S., McEvoy J.P., Swartz M.S., Rosenheck R.A., Perkins D.O., Keefe R.S., Davis S.M., Davis C.E., Lebowitz B.D., Severe J., Hsiao J.K., Clinical Antipsychotic Trials of Intervention Effectiveness, I Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Lincoln T.M., Lange J., Burau J., Exner C., Moritz S. The effect of state anxiety on paranoid ideation and jumping to conclusions. An experimental investigation. Schizophr. Bull. 2010;36:1140–1148. doi: 10.1093/schbul/sbp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia T.V., Frank M.J. An integrative perspective on the role of dopamine in schizophrenia. Biol. Psychiatry. 2016;81:52–66. doi: 10.1016/j.biopsych.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaika P., Hoeft F., Glover G.H., Reiss A.L. Methods and software for fMRI analysis for clinical subjects. NeuroImage. 2009;47:S58. [Google Scholar]
- McFarquhar M., McKie S., Emsley R., Suckling J., Elliott R., Williams S. Multivariate and repeated measures (MRM): a new toolbox for dependent and multimodal group-level neuroimaging data. NeuroImage. 2016;132:373–389. doi: 10.1016/j.neuroimage.2016.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon M., Mizrahi R., Kapur S. “Jumping to conclusions” and delusions in psychosis: relationship and response to treatment. Schizophr. Res. 2008;98:225–231. doi: 10.1016/j.schres.2007.08.021. S0920-9964(07)00380-5 [pii] [DOI] [PubMed] [Google Scholar]
- Moritz S., Veckenstedt R., Hottenrott B., Woodward T.S., Randjbar S., Lincoln T.M. Different sides of the same coin? Intercorrelations of cognitive biases in schizophrenia. Cogn Neuropsychiatry. 2010;15:406–421. doi: 10.1080/13546800903399993. doi:919171028 [pii]10.1080/13546800903399993. [DOI] [PubMed] [Google Scholar]
- Moritz S., Veckenstedt R., Randjbar S., Vitzthum F. Springer; Heidelberg, Germany: 2012. Individualisierte Metakognitive Therapie für Menschen mit Psychose (MKT+) [Google Scholar]
- Moritz S., Veckenstedt R., Bohn F., Hottenrott B., Scheu F., Randjbar S., Aghotor J., Köther U., Woodward T.S.T.S., Treszl A., Andreou C., Pfueller U., Roesch-Ely D. Complementary group Metacognitive Training (MCT) reduces delusional ideation in schizophrenia. Schizophr. Res. 2013;151:61–69. doi: 10.1016/j.schres.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Moritz S., Veckenstedt R., Bohn F., Köther U., Woodward T.S. Metacognitive training in schizophrenia. Theoretical rationale and administration. In: Roberts D.L., Penn D.L., editors. Social Cognition in Schizophrenia. From Evidence to Treatment. Oxford University Press; New York, NY: 2013. pp. 358–383. [Google Scholar]
- Moritz S., Andreou C., Schneider B.C., Wittekind C.E., Menon M., Balzan R.P., Woodward T.S. Sowing the seeds of doubt: a narrative review on metacognitive training in schizophrenia. Clin. Psychol. Rev. 2014;34:358–366. doi: 10.1016/j.cpr.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Moritz S., Veckenstedt R., Andreou C., Bohn F., Hottenrott B., Leighton L., Köther U., Woodward T.S., Treszl A., Menon M., Schneider B.C., Pfueller U., Roesch-Ely D. Sustained and “sleeper” effects of group metacognitive training for schizophrenia. JAMA Psychiatry. 2014;71:1103–1111. doi: 10.1001/jamapsychiatry.2014.1038. [DOI] [PubMed] [Google Scholar]
- Moritz S., Göritz A.S., Balzan R.P., Gawȩda L., Kulagin S.C., Andreou C. A new paradigm to measure probabilistic reasoning and a possible answer to the question why psychosis-prone individuals jump to conclusions. J. Abnorm. Psychol. 2017;126:406–415. doi: 10.1037/abn0000262. [DOI] [PubMed] [Google Scholar]
- Moritz S., Pfuhl G., Lüdtke T., Menon M., Balzan R.P.R.P., Andreou C. A two-stage cognitive theory of the positive symptoms of psychosis. Highlighting the role of lowered decision thresholds. J. Behav. Ther. Exp. Psychiatry. 2017;56:12–20. doi: 10.1016/j.jbtep.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Moutoussis M., Bentall R.P., El-Deredy W., Dayan P. Bayesian modelling of jumping-to-conclusions bias in delusional patients. Cogn. Neuropsychiatry. 2011;16:422–447. doi: 10.1080/13546805.2010.548678. [DOI] [PubMed] [Google Scholar]
- O'Reilly J.X., Woolrich M.W., Behrens T.E.J., Smith S.M., Johansen-Berg H. Tools of the trade: psychophysiological interactions and functional connectivity. Soc. Cogn. Affect. Neurosci. 2012;7:604–609. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters E., Garety P. Cognitive functioning in delusions: a longitudinal analysis. Behav. Res. Ther. 2006;44:481–514. doi: 10.1016/j.brat.2005.03.008. S0005-7967(05)00068-9 [pii] [DOI] [PubMed] [Google Scholar]
- Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr. Bull. 1991;17:555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- Rausch F., Mier D., Eifler S., Esslinger C., Schilling C., Schirmbeck F., Englisch S., Meyer-Lindenberg A., Kirsch P., Zink M. Reduced activation in ventral striatum and ventral tegmental area during probabilistic decision-making in schizophrenia. Schizophr. Res. 2014;156:143–149. doi: 10.1016/j.schres.2014.04.020. [DOI] [PubMed] [Google Scholar]
- Rausch F., Mier D., Eifler S., Fenske S., Schirmbeck F., Englisch S., Schilling C., Meyer-Lindenberg A., Kirsch P., Zink M. Reduced activation in the ventral striatum during probabilistic decision-making in patients in an at-risk mental state. J. Psychiatry Neurosci. 2015;40:163–173. doi: 10.1503/jpn.140191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan, D. V, Lecrubier, Y., Sheehan, K.H., Amorim, P., Janavs, J., Weiller, E., Hergueta, T., Baker, R., Dunbar, G.C., 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59 Suppl. 2, 22–33–57. [PubMed]
- So S.H., Freeman D., Dunn G., Kapur S., Kuipers E., Bebbington P., Fowler D., Garety P.A. Jumping to conclusions, a lack of belief flexibility and delusional conviction in psychosis: a longitudinal investigation of the structure, frequency, and relatedness of reasoning biases. J. Abnorm. Psychol. 2012;121:129–139. doi: 10.1037/a0025297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speechley W.J., Whitman J.C., Woodward T.S. The contribution of hypersalience to the “jumping to conclusions” bias associated with delusions in schizophrenia. J. Psychiatry Neurosci. 2010;35:7–17. doi: 10.1503/jpn.090025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. Contributions and challenges for network models in cognitive neuroscience. Nat. Neurosci. 2014;17:652–660. doi: 10.1038/nn.3690. [DOI] [PubMed] [Google Scholar]
- van der Gaag M., Hoffman T., Remijsen M., Hijman R., de Haan L., van Meijel B., van Harten P.N., Valmaggia L., de Hert M., Cuijpers A., Wiersma D. The five-factor model of the positive and negative syndrome scale II: a ten-fold cross-validation of a revised model. Schizophr. Res. 2006;85:280–287. doi: 10.1016/j.schres.2006.03.021. [DOI] [PubMed] [Google Scholar]
- van der Leer L., Hartig B., Goldmanis M., McKay R. Delusion proneness and “jumping to conclusions”: relative and absolute effects. Psychol. Med. 2015;45:1253–1262. doi: 10.1017/S0033291714002359. [DOI] [PubMed] [Google Scholar]
- Waller H., Freeman D., Jolley S., Dunn G., Garety P. Targeting reasoning biases in delusions: a pilot study of the Maudsley review training Programme for individuals with persistent, high conviction delusions. J. Behav. Ther. Exp. Psychiatry. 2011;42:414–421. doi: 10.1016/j.jbtep.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman J.C., Metzak P.D., Lavigne K.M., Woodward T.S. Functional connectivity in a frontoparietal network involving the dorsal anterior cingulate cortex underlies decisions to accept a hypothesis. Neuropsychologia. 2013;51:1132–1141. doi: 10.1016/j.neuropsychologia.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Woodward T.S., Munz M., Leclerc C., Lecomte T. Change in delusions is associated with change in “jumping to conclusions”. Psychiatry Res. 2009;170:124–127. doi: 10.1016/j.psychres.2008.10.020. S0165-1781(08)00388-0 [pii] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods
Supplementary Results