Abstract
Nearly three out of four survivors experience Cancer-Related Cognitive Impairment (CRCI) for months or years following treatment. Both clinical and animal studies point to the hippocampus as a likely brain region affected in CRCI, however no previous study has investigated the functional connectivity of the hippocampus in CRCI. We compared hippocampal connectivity in cancer survivors and healthy controls and tested the relationship between functional connectivity differences and measures of objective and subjective cognition. Exploratory analysis of inflammatory markers was conducted in a small subset of participants as well. FMRI data were acquired during a memory task from 16 breast cancer survivors and 17 controls. The NIH Toolbox was used to assess cognitive performance and Neuro-QoL was used to measure self-reported cognitive concerns. Whole-brain group-level comparisons identified clusters with different connectivity to the hippocampus in survivors versus controls during task. Average connectivity was extracted from clusters of significant difference between the groups and correlated with cognitive performance and subjective report. Survivors performed worse on a test of episodic memory and reported greater cognitive concern than controls. Exploratory analysis found higher IL6 in cancer survivors compared to controls. Cancer survivors demonstrated higher connectivity of hippocampus with left cuneus, left lingual, left precuneus, and right middle prefrontal gyrus compared with controls. In survivors, higher task-related hippocampal-cortical connectivity was related to worse subjective measures of cognitive concern. Of the four significant clusters, higher connectivity of the precuneus with hippocampus was significantly associated with worse cognitive concern in survivors. The observed greater hippocampal-cortical connectivity in survivors compared to controls is the first reported fMRI biomarker of subjective concern, and may represent a compensatory response to cancer and its treatments. This compensation could explain, in part, the subjective feelings of cognitive impairment that were reported by survivors.
Highlights
-
•
Cancer survivors performed worse on a test of episodic memory and reported greater cognitive concern than controls
-
•
Cancer survivors demonstrated significantly higher hippocampal-cortical connectivity
-
•
Higher functional connectivity was associated with worse self-reported cognitive functioning in cancer survivors
1. Introduction
Up to 75% of survivors experience Cancer-related Cognitive Impairment (CRCI) for months or years following treatment (Ahles et al., 2012; Janelsins et al., 2014). CRCI can have significant negative impacts on survivors, including problems with treatment adherence and decreased quality of life (Janelsins et al., 2014). Developing methods to detect and mitigate CRCI is essential to improving cancer survivors' quality of life.
Both objective and subjective cognitive impairment have been reported in survivors following cancer treatment (Biglia et al., 2012; Hurria et al., 2006; Hutchinson et al., 2012; Jenkins et al., 2006; O'Farrell et al., 2013; Scherling and Smith, 2013; Shilling and Jenkins, 2007); the most frequently impaired cognitive domains include working and long-term memory, executive functioning, processing speed and attention (Ahles et al., 2010; Bender et al., 2006; Debess et al., 2010; Hermelink et al., 2007; Janelsins et al., 2011; Wefel et al., 2010). However, most studies have found that objective cognitive deficits measured through laboratory tests did not represent and could not explain the subjective cognitive complaints reported by cancer survivors (Hutchinson et al., 2012; Jansen et al., 2011; O'Farrell et al., 2013). It would be important to understand the neural mechanisms related to CRCI, identify the neurophysiological correlates of CRCI, and develop neuroimaging biomarkers of both objective and subjective deficits in CRCI.
Chemotherapy, hormone therapy and other cancer treatments are thought to impair cognitive functioning by altering specific brain structures and/or impairing connectivity between brain regions (Meyers, 2008). Collectively, neuroimaging studies suggest that adjuvant cancer therapies induce dysregulations to the brain's network hubs, including the hippocampus, prefrontal cortex, and the default mode network (Bruno et al., 2012; Chen et al., 2017; de Ruiter et al., 2011; Dumas et al., 2013; Ferguson et al., 2007b; Kesler et al., 2009; Kesler et al., 2013b; Meyers, 2008). The hippocampus, which is of critical importance to memory, has been shown to be vulnerable to the effects of cancer treatments (Inagaki et al., 2007; Kesler et al., 2013a). Both human and animal studies have shown associations between chemotherapeutic treatments with common chemotherapeutics and a variety of abnormal changes to the hippocampus, including loss of gray and white matter, decreased neurogenesis, increased cell death, and blood vessel damage (Dietrich et al., 2006; Inagaki et al., 2007; Nobakht et al., 2009; Seigers et al., 2010).
Recent work by our group revealed a localized loss of hippocampal volume in breast cancer survivors undergoing adjuvant therapy as compared with healthy controls (Apple et al., 2017). Moreover, the hippocampal structural loss co-localized to a region of decreased activity in the same survivors during a covert spatial memory task using functional magnetic resonance imaging (fMRI) (Ryals et al., 2015a). Most interestingly, survivors and controls did not differ in cognitive task performance, and that none of the measures of structural loss or reduced activity were correlated with objective tests or subjective Patient Reported Outcomes (PRO) of cognition in the survivors. To gain deeper insight into a potential brain-based mechanism in the context of CRCI, we sought to explore ways in which the brain may compensate for structural and functional deficits while maintaining cognitive task performance.
In fMRI studies reported in the literature (Bruno et al., 2012; de Ruiter et al., 2011; Kesler, 2014), no increases in task-related activity has explained a compensatory response in CRCI, such as ones reported by Dickerson and colleagues in individuals with mild cognitive impairment (Dickerson et al., 2005). Functional connectivity, on the other hand, could be investigated for its potential role in compensation. Research has shown that improved cognitive performance can be attributable to increased resting-state network functional connectivity. Specifically, research on healthy adults has found a relationship between higher performance on perceptual tasks and increased functional connectivity between visual and prefrontal regions (Baldassarre et al., 2012). A study in healthy adults using noninvasive high-frequency repetitive transcranial magnetic stimulation showed improved memory was accompanied by strengthened hippocampal-cortical functional connectivity (Wang et al., 2014). In a study by Seeley and colleagues, stronger functional connectivity within the executive-control network was related to higher executive task performance in younger healthy adults (Seeley et al., 2007). Studies in aging pollutions have found increased connectivity in the default mode network in healthy older adults compared with MCI subjects (Dong et al., 2012). In the current study, we compared hippocampal functional connectivity during the covert spatial memory task (Ryals et al., 2015a) between survivors and healthy controls, and hypothesized that compensatory differences in task-based functional connectivity would be observed in survivors and they would be related to measures of objective and subject tests of cognition. Additionally, research has found an association between cytokine concentration and cognitive performance in breast cancer patients (Cheung et al., 2015). For example, increased sTNFRI and sTNFRII concentrations have been associated with poorer visual memory performance (Williams et al., 2018). To explore relationships of connectivity imaging markers with systemic inflammatory markers as a protentional mechanism for CRCI, several pro-inflammatory cytokine markers including interleukin-1 (IL-1), IL-6, and IL-10 as well as c-reactive protein (CRP) and tumor necrosis factor (TFNα) were collected and analyzed in the survivors. Relationships between elevated cytokines and measures of imaging, cognition and self-report were also explored.
2. Participants and methods
2.1. Participants
The Institutional Review Board at Northwestern University approved this study in accordance with the Declaration of Helsinki. As described in our previous paper (Apple et al., 2017), 16 pre-menopausal breast cancer survivors and 18 healthy controls gave written informed consent and were enrolled into the study. Breast cancer survivors had invasive ductal carcinoma, metastatic lobular carcinoma or inflammatory breast cancer without brain metastases, confirmed with histology. All survivors had completed systemic chemotherapy interventions within 18 months of the study, and were undergoing estrogen blockade therapy (Tamoxifen) at the time of the study. Only breast cancer survivors who scored a 0 or 1 on the physician-rated Eastern Cooperative Oncology Group (ECOG) were included in the study (0 – good functional status, 1 – symptomatic and restricted in physically strenuous activity but otherwise ambulatory, 2 – capable of all self-care but requiring rest up to half of the waking day, 3 – requiring rest more than half of the waking day, 4 – bedridden) (Oken et al., 1982). As an exploratory analysis, inflammatory markers were collected in a subset of the participants. Serum was harvested and assayed in duplicate by custom multiplex immunoassay (MesoScale Disovery V-Plex) on a SECTOR Imager 2400A (MesoScale Discovery) and IL-10 and IL-10 M450 from 11 cancer survivors and 12 controls, and IL1β, IL1β M450, IL6, IL6 M450, TNFα, TNFα M450, CRP and CRP M450 were collected from 12 participants per group.
Participants were right handed 18–45 years old, had normal or corrected vision, reported no history of current or past neurological or psychiatric disorders or psychoactive drugs at the time of the study. Of the 18 controls, one was unable to complete fMRI, and one did not complete the cognitive testing. Of the 16 survivors, one did not complete self-report questionnaires and cognitive testing. Objective cognitive performance data included 15 survivors and 17 controls, self-report data included 15 survivors and 18 controls, and fMRI data included 16 survivors and 17 controls.
2.2. Cognitive assessment
The NIH Toolbox Cognition Battery (www.nihtoolbox.org) (Weintraub et al., 2013) was administered to participants on site, consisting of seven subtests including picture Sequence Memory Test (measure of episodic memory thought to be related to hippocampal functioning (Bauer et al., 2013)), List Sorting Working Memory Test (for working memory), Flanker Inhibitory Control and Attention Test (for executive function, attention and inhibitory control), Pattern Comparison Processing Speed Test (for processing speed), and Dimensional Change Card Sort Test (for executive function and set shifting), Picture Vocabulary Test, and Oral Reading Recognition Test (for language). Raw scores on each subtest were standardized to a standardized T-scores with a normative mean of 50 and a standard Deviation of 10.
2.3. Self-report measures
Participants completed two computerized adaptive tests to assess their subjective daily function, Neuro-QoL and PROMIS pain interference. Neuro-QoL (www.neuroqol.org) reports cognitive, emotional, and functional concerns in the past week. PROMIS pain interference (www.nihpromis.org) assesses the extent to which pain effects their functioning (Cella et al., 2012). In Neuro-QoL, the Applied cognition-General Concerns subtest assesses cognitive functioning including perceived difficulties in memory, attention and decision making (e.g. “I had to read something several times to understand it,” “I had difficulty doing more than one thing at a time,” “I had trouble thinking clearly,” “My thinking was slow,” “I had trouble remembering new information, like phone numbers or simple instructions,” “I had to work really hard to pay attention or I would make a mistake,” and “I had trouble concentrating”) on a scale from one to five. The Neuro-QoL Applied cognition-Executive Function subtest assesses applications of mental function related to planning, organizing, calculating, working with memory and learning (e.g. “How much difficultly currently having checking the accuracy of financial documents? counting the correct amount of money when making purchases? reading and following complex instructions? planning for and keeping appointments that are not part of your weekly routine? managing your time to do most of your daily activities? taking care of complicated tasks like managing a checking account or getting appliances fixed? keeping important personal papers such as bills, insurance documents and tax forms organized? learning new tasks or instructions?”). Neuro-QoL also contains self-reported anxiety, depression, fatigue, and sleep subtests (Cella et al., 2012). PROMIS pain interference scale (www.nihpromis.org) was to assess the extent to which pain affects functioning on a 5-point Likert scale (e.g. “How difficult was it for you to take in new information because of pain?” “How much did pain interfere with your enjoyment of life?”) (Cella et al., 2012). Neuro-QoL and PROMIS pain interference yielded T-scores for each participant (standardized with a mean = 50, sd = 10).
2.4. MRI data acquisition and processing
Participants were scanned on a 3 T TIM Trio scanner (Siemens Medical Systems) with a 32-channel head coil. Anatomical MRI was acquired using a high-resolution 3D T1-weighted MPRAGE sequence (TR = 2400 ms, TE = 3.16 ms, voxel size = 1 mm3, FOV = 25.6 cm, flip angle = 8°, 176 sagittal slices, slice thickness = 1 mm, matrix = 256 × 256, sagittal, time = 8:09 min). Three fMRI runs were acquired during a configural covert spatial memory task (Ryals et al., 2015a). Each of the three runs consisted of a study and test block. During the study block, participants viewed 12 scenes for 8 s each. During the test phase, participants viewed 24 novel scenes in which half of the scenes were similar in configuration to the 12 study scenes and the other half were configurally different or “new.” After each scene, participants rated how familiar the scene was on a 4-point scale. See Supplemental Fig. 1 (Ryals et al., 2013, 2015b) for more details. All images were acquired axially, parallel to the anterior-posterior commissure plane using a T2* echo-planar sequence (TR = 2000 ms, TE = 20 ms, voxel size = 1.7 × 1.7 × 3 mm3, FOV = 220 × 220 mm, flip angle = 80°, time~ = 16 min each run). The first 3 images of each run were excluded to account for nuclear magnetic resonance and eddy-current equilibrium leaving a total of 1464 images for analysis.
Functional data preprocessing and analyses were conducted using Analysis of Functional NeuroImages (AFNI) (Cox, 1996). Preprocessing included slice-timing and motion correction, registration, spatial smoothing to 8-mm FWHM, and alignment to Talairach-Tournoux template. Images were censored if their translational or rotational change was >0.7 mm or radians. The median percentage of images that were censored for the survivors and controls were 0.51% and 1.51% respectively (survivors mean = 1.69%, interquartile range = 1.57%; control mean = 2.22%, interquartile range = 2.08%). No participants had >15% of their images censored, therefore all subjects were included in subsequent analysis.
Multiple linear regression was used to model motion parameters with a hemodynamic response function and its temporal derivative to yield least squares estimates of the linear regression coefficients as well as the residual time series used in all subsequent analyses. Average signals from the whole brain, cerebrospinal fluid, and white matter were regressed from the residual time series followed by temporal filtering (0.009–0.08 Hz). For task-based functional connectivity, bilateral hippocampal segmentation extracted from the Talairach atlas was used to extract the time series for each participant. The mean time series across the seed was cross-correlated to every other gray matter voxel and then z-transformed. These task-based hippocampal functional connectivity maps were used in subsequent statistical analyses.
2.5. Statistical analysis
Group differences on demographic, cognitive, self-report and inflammatory variables were tested with t-tests, chi-square tests and F tests where appropriate. Group differences on hippocampal functional connectivity were tested with analysis of variance (ANOVA) using AFNI's 3dttest++. Pearson correlations were used in the exploratory analysis examining the relationship between inflammatory markers and imaging, cognitive and self-report measures in the survivor group. AFNI's 3dClustSim (Forman et al., 1995) was used to determine a minimum cluster size of ≥131 contiguous voxels at uncorrected voxel threshold of p = .005 for a corrected p = .05. Clusters showing significant difference between the groups were reported and used to further examine relationships with objective or subjective measures that differed between groups using multiple linear regression models. In addition, the average functional connectivity measures across all significant clusters was related to objective and subjective measures using linear regression models. Since age differed between groups (see results), we controlled for the effect of age in the following two ways: 1) Within the NIH Toolbox Cognition Battery (e.g. Picture Sequence Memory test), age was controlled for by using Standard Scores, typically used to reflect performance independent of age. 2) For measures that did not have standardized scores available, i.e. self-report measures and fMRI measures, age was included as a statistical covariate.
3. Results
3.1. Demographic, cognitive, self-report and inflammatory measures
Demographic, cognitive and self-report data can be found in Table 1. Survivors were 10 years older than controls [t(31) = 6.69, p = .001], therefore, all subsequent analyses were performed while accounting for age. Survivors reported greater cognitive concern than controls [F(1,30) = 4.71, p = .038], but not in perceived executive function, anxiety, depression, fatigue, sleep disturbance or pain. Episodic memory performance (i.e., the Picture Sequence Memory Test from the NIH Toolbox Cognition Battery) was significantly poorer in survivors [t(30) = 2.13, p = .041]. No group differences were observed for the rest of the NIH Toolbox Cognition Battery. Exploratory analysis looking at pro-inflammatory markers found higher levels of IL6 concentration in the survivors (t = 2.54, p = .019). No other group differences in inflammatory markers were observed.
Table 1.
Patient demographics, self-report and cognition.
| Oncology group (n = 16%) | Control group (n = 18) | t-test (df) | p value | Cohen's D | |
|---|---|---|---|---|---|
| Demographics mean (SD) [Range] | |||||
| Age | 38.31 (5.25) | 27.42 (4.06) | 6.69 (32) | 0.001b | 2.321 |
| Years of education | 16.73 (1.62) | 16.22 (1.86) | 0.831 (31) | 0.413 | 0.292 |
| Handedness (R/L) | 100% R | 100% R | – | – | |
| Self-report mean T-score (SD) | (n = 15) | (n = 18) | F test (df) | p value | Cohen's D |
| Neuro-QoL | |||||
| Applied cognition - general concernsa | 36.96 (5.42) | 42.08 (4.18) | 4.71 (1,30) | 0.038b | 1.058 |
| Applied cognition - executive functiona | 40.55 (5.96) | 43.56 (5.58) | 0.76 (1,30) | 0.389 | 0.521 |
| Anxiety | 53.95 (4.78) | 51.37 (4.66) | 2.58 (1,30) | 0.119 | 0.547 |
| Depression | 48.24 (6.08) | 44.77 (4.51) | 0.38 (1,30) | 0.543 | 0.648 |
| Fatigue | 47.86 (7.76) | 46.30 (6.01) | 0.72 (1,30) | 0.402 | 0.225 |
| Sleep disturbance | 50.37 (9.72) | 46.50 (6.10) | 0.002 (1,30) | 0.965 | 0.477 |
| PROMIS | |||||
| Pain interference | 47.91 (10.22) | 42.71 (5.81) | 1.43 (1,30) | 0.241 | 0.626 |
| NIH toolbox mean standard score (SD) | (n = 15) | (n = 17) | t-test (df) | p value | Cohen's D |
| Picture Sequence Memory Test (EM) | 96.96 (13.73) | 107.05 (13.01) | 2.13 (30) | 0.041b | 0.754 |
| Flanker Inhibitory Control and Attention Test (Att., EF) | 95.61 (7.68) | 95.29 (12.02) | 0.09 (30) | 0.930 | 0.032 |
| Pattern Comparison Processing Speed Test (PS) | 88.51 (12.21) | 82.65 (10.03) | 1.49 (30) | 0.147 | 0.524 |
| Dimensional Change Card Sort (EF) | 95.92 (8.57) | 98.72 (11.84) | 0.76 (30) | 0.455 | 0.271 |
| List Sorting Working Memory Test (WM) | 101.84 (13.29) | 107.03 (13.43) | 1.10 (30) | 0.282 | 0.388 |
| Picture Vocabulary Test (lang.) | 134.54 (20.24) | 136.03 (17.49) | 0.22 (30) | 0.824 | 0.079 |
| Oral Reading Recognition Test (lang.) | 111.61 (10.93) | 118.77 (15.11) | 1.52 (30) | 0.140 | 0.543 |
% = years of education were only recorded for 15 survivors; EM = episodic memory, EF = executive function, Att. = attention, WM = working memory, PS = processing speed, lang. = language.
Lower scores signify worse perceived functioning, in all other self-report, lower scores signify fewer symptoms (i.e. less anxiety); NIH toolbox measures are adjusted for age, ethnicity, gender and level of education.
Statistically differs between groups.
3.2. Hippocampal connectivity: group comparison
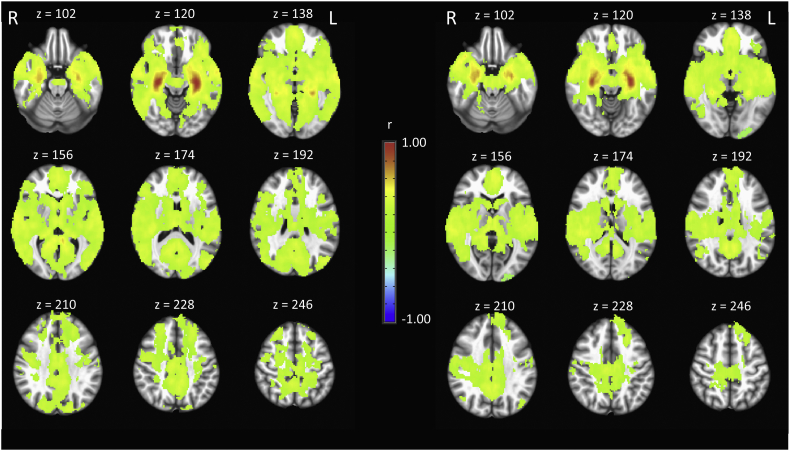
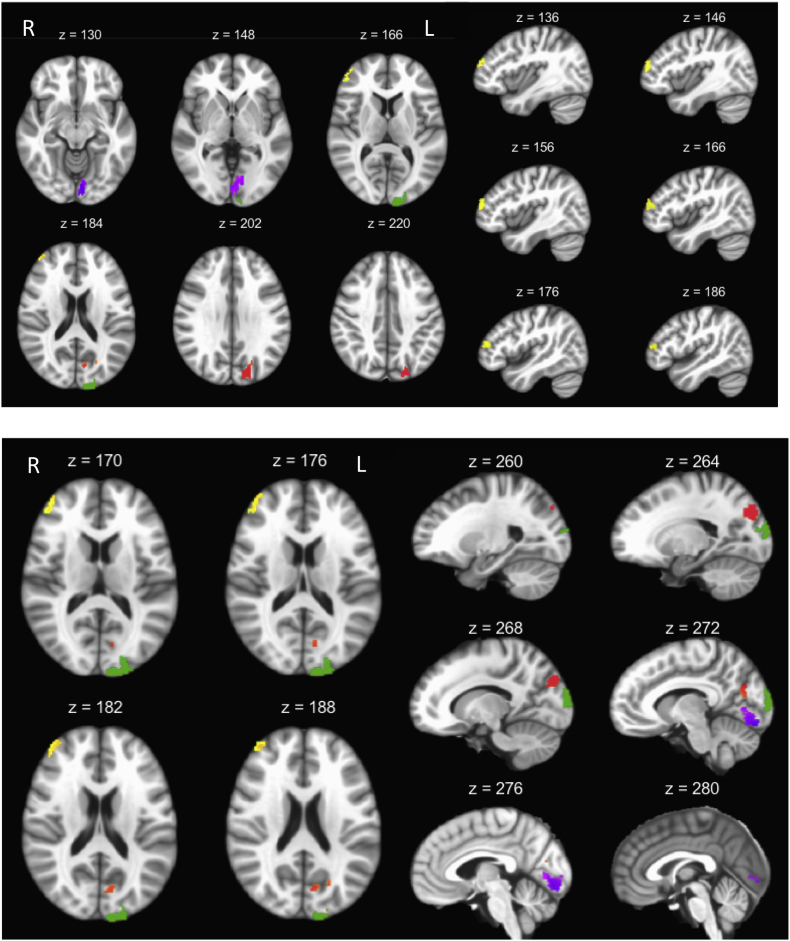
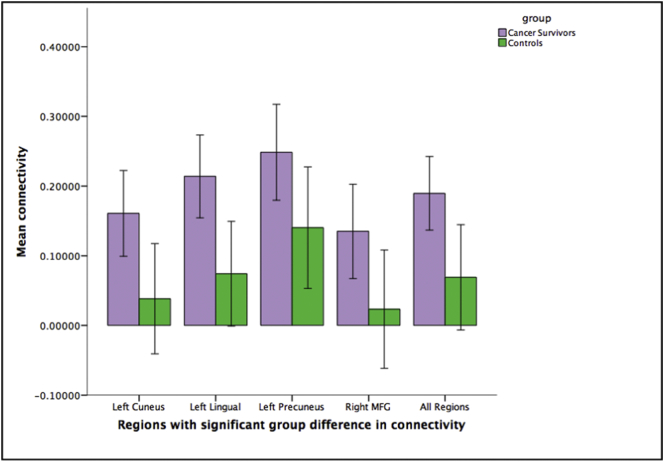
Hippocampal functional connectivity maps for survivors and controls (Fig. 1) revealed expected patterns in both groups based on previous literature (i.e. strong intra-hippocampal connectivity) (Ranganath et al., 2005; Stein et al., 2000). Whole-brain group-level comparisons identified four clusters with significantly different hippocampal connectivity (Table 2). After controlling for differences in age, cancer survivors showed higher hippocampal connectivity in the left cuneus, left lingual, left precuneus, and right middle frontal gyrus compared to controls (Fig. 2, Fig. 3).
Fig. 1.
Hippocampal functional connectivity map within groups of cancer survivors (left panel) and controls (right panel). All voxels represent positive hippocampal connectivity with warmer colors indicating higher correlations. The statistical threshold was set at p = .005 cluster size >131. View is radiological.
Table 2.
Clusters showing group differences in hippocampal-cortical functional connectivity between cancer survivors and controls.
| Region | BA | Cluster Size | Coordinates (Talairach RAI) |
|---|---|---|---|
| Left Cuneus/Middle Occipital Gyrus | 18, 19 | 360 | +10 +100 +16 |
| Left Lingual Gyrus | 17, 18 | 334 | +6 +86 -14 |
| Left Precuneus/Cuneus | 7, 19, 18, 31 | 332 | +20 +78 +34 |
| Right middle frontal/Superior frontal | 10, 46 | 153 | -40 -52 +22 |
Voxels size = 2 mm3; BA = Brodmann's area.
Fig. 2.
The difference in task related hippocampal-whole brain connectivity between patients and controls. Clusters within the left cuneus (green), left lingual (purple), and left precuneus (red) in the top panel, and clusters in the right middle frontal gyrus (yellow) represent higher connectivity in the survivors when compared to the controls, after covarying for age. View is radiological.
Fig. 3.
Mean connectivity differences across the four clusters of significant group difference (p < .005 cluster size >131). Error bars: 95% CI.
3.3. Hippocampal connectivity: correlations
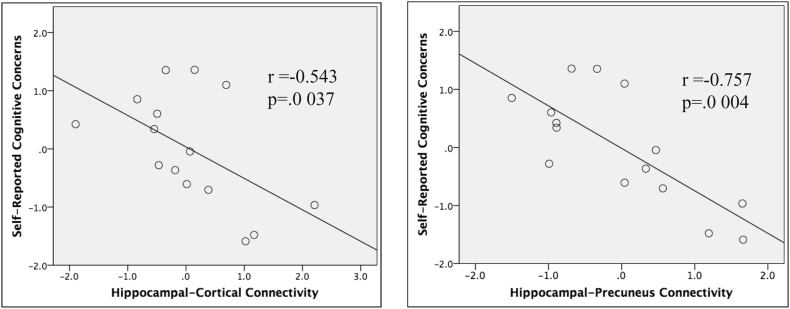
To analyze relationships between hippocampal connectivity and the significant objective and subjective group differences (NeuroQoL general cognition measure and NIH Toolbox episodic memory subtest), age-corrected residuals for both the connectivity in the significant clusters as well as the general cognition self-report measures were used in linear regression models. Linear regression predicting self-reported cognitive concerns found a significant group by connectivity interaction [F(1,31) = 4.58, p = .041]. However, linear regression predicting performance on the episodic memory subtest of the NIH toolbox was not significant for the interaction between group and connectivity. To explore this relationship further, linear regression models were run in each group separately. Two analyses per group were run between average connectivity of the four clusters and the general cognitive concerns (Fig. 4). Analyses revealed that higher connectivity averaged across all of the above four clusters (left cuneus, left lingual, left precuneus, and right middle frontal gyrus) was significantly associated with lower general cognitive concerns score for the survivors [F(1,13) = 5.43, p = .037, R2 = 0.29, standardized beta = −0.54]. Multiple linear regression revealed that only the hippocampal-precuneus connectivity was significantly associated with subjective cognitive concern scores (p = .004) out of the four clusters [overall model F(4,10) = 4.66, p = .022, R2 = 0.65]. Analyses did not reveal a significant relationship between hippocampal connectivity and the Picture Sequence Memory cognitive test. No significant relationships were observed in the control group.
Fig. 4.
Significant correlations between objective and subjective cognition in breast cancer survivors. Subjective cognitive concern was negatively correlated with hippocampus-precuneus/cortical connectivity in cancer survivors.
Although exploratory analysis found higher levels of IL6 concentration in the survivors (t = 2.54, p = .019), linear regression predicting IL6 concentration was not significant for the interaction between group and self-reported cognitive concern or episodic memory subtest of the NIH toolbox.
4. Discussion
In breast cancer survivors receiving adjuvant therapy, we previously reported localized hippocampal volume loss and reduced hippocampal activity in the absence of significant worsening performance in a covert spatial memory task, as compared with healthy controls (Apple et al., 2017; Ryals et al., 2015a). In the current study, we examined whether differences in hippocampal functional connectivity may be a marker of a compensatory mechanism for the structural and functional deficits in order to maintain task performance in the same group of breast cancer survivors. We observed higher functional connectivity between the hippocampus and left cuneus, left precuneus, left lingual gyrus, and right middle frontal cortex in cancer survivors compared to the controls. This higher connectivity was related to higher levels of self-reported general cognitive concerns in survivors, but not in controls. The survivors demonstrated elevated IL6 concentration compared with controls, which corroborates previous studies (Cheung et al., 2015; Dethlefsen et al., 2013; Williams et al., 2016). However, no show relationships between IL6 and imaging, cognition and self-report measures were found. To our knowledge, this is the first study to suggest that higher task-based hippocampal-cortical functional connectivity may reflect the brain's compensatory response to cancer or cancer-treatment related loss of hippocampal structure and function to maintain cognitive task performance.
In our study, the survivors reported significantly greater subjective concerns of general cognitive impairment as compared with healthy controls, and the degree of concern was significantly associated with the increases in task-based hippocampal-cortical functional connectivity. I.e., survivors who were more similar to controls in terms of strength of functional connection between the hippocampus and the cortex reported less cognitive concerns. It is possible that this was due to the fact that they were not exerting as much “effort” or calling upon other regions of the brain to complete cognitive tasks. On the other hand, survivors with more general concerns with their own cognition may have compensated more (i.e., “worked harder”) during the task. Previous studies have observed aberrant activity and resting-state functional connectivity in breast cancer survivors (Bruno et al., 2012; Janelsins et al., 2014), and some suggest that increased activity and connectivity during task may help preserve cancer survivor's behaviors and bring them closer to their premorbid abilities that are comparable to that of controls (Ferguson et al., 2007a; Hosseini and Kesler, 2014; Janelsins et al., 2014). That breast cancer survivors may be working harder to restore to “normal” performance level may explain, in part, the difficulty of detecting these cognitive changes or deficits in cancer populations through subjective testing (Reuter-Lorenz and Cimprich, 2013). While cognitive neuroscience approaches are being leveraged to improve assessment of CRCI (see National Cancer Institute 2016 FOA PAR-16-212), our hippocampal-cortical functional connectivity measure reported here may serve as an excellent neuroimaging biomarker for CRCI as it relates to the survivors self-reported cognitive concerns, therefore captures the subtle cognitive deficits at an earlier stage. Utilizing tools and techniques other than standard cognitive assessments to study complex disorders is important given the often subtle ways in which CRCI presents.
Research has shown that higher functional connectivity in both task negative and task-positive networks is associated with improved cognitive performance in young and older adults (Baldassarre et al., 2012; Dong et al., 2012; Meier et al., 2012; Seeley et al., 2007). Older adults who perform similarly behaviorally to younger adults on cognitive tasks recruit more brain regions comparatively (Berlingeri et al., 2013; Cabeza et al., 2002). In breast cancer survivors, a study among twins found more activation in the twin cancer with in bilateral frontal and parietal regions during a working memory task compared to their non-affected twin (Ferguson et al., 2007b). Additionally, an EEG study of breast cancer survivors found higher EEG amplitude following motor and processing speed tasks and that the increases correlated with elevated self-reported physical and mental fatigue. This was thought to reflect increased effort to maintain performance during these physical and cognitive tasks (Moore et al., 2014). Perceived effort and/or a sense of mental fatigue may be greater when compensatory processes are needed compared to when they are not needed (Reuter-Lorenz and Cimprich, 2013). Although none of the survivors in the current study reported higher fatigue compared to controls, they reported worse perceived cognitive functioning; it is possible this higher effort is contributing to this notion of worse cognitive ability.
Compensation by means of increased connectivity has been observed in other populations. In alcohol-dependent adults whose default mode network was disrupted (Dupuy and Chanraud, 2016), functional connectivity between the left posterior cingulate and left cerebellar regions was found to be increased in during a spatial working memory task, and alcohol-dependent adults performed as well as the control (Chanraud et al., 2011). Increased recruitment of cortical areas has been observed in survivors with hippocampal damage (due to medial temporal lobe resection for sclerosis or tumor). The survivors demonstrated intact memory performance but showed increased recruitment of cortical areas including the dorsolateral prefrontal cortex and the posterior parietal cortex (Finke et al., 2013). Additionally, survivors with mild Alzheimer's disease show higher functional connectivity in prefrontal areas during memory tasks compared to controls (Grady et al., 2003).
In the current study, the higher functional connectivity observed in breast cancer survivors may be suggestive of a compensatory recruitment of cortical regions to aid in successful cognitive performance (Cabeza and Dennis, 2013). Furthermore, the brain regions that demonstrated significantly higher functional connectivity during task with the hippocampus (i.e., precuneus/cuneus, middle frontal gyrus) are thought to play key roles in episodic memory (Euston et al., 2012; Fletcher et al., 1995). However, this difference in connectivity did not account for the observed degradation in the objective measures of episodic memory conducted outside of the scanner, suggesting the successful compensatory effort, marked by the higher functional connectivity, may be insufficient to meet the demands of the episodic memory tasks. Future research can examine if the overall hippocampal-cortical network can be further strengthened by noninvasive means such as repetitive transcranial magnetic stimulation to overcome cancer and treatment-related loss of hippocampal structure and function, thus improving memory performance (Wang et al., 2014).
Our study has limitations, including a cross-sectional design, differences in age between groups, and small sample size which may have contributed to the lack of relationships between inflammatory markers and other measures. Although we controlled for the difference in statistical analysis, future studies should recruit aged-matched controls and collect pre−/post- treatment data to better track longitudinal change in network functioning and how it relates to subjective or objective measures. Additionally, inclusion of resting-state fMRI data can be beneficial for better understanding of the neural mechanisms underlying CRCI. Our study did not collect resting-state fMRI, nor did it contain enough fixation volumes to extract pseudo-resting state BOLD data (Fair et al., 2007). A caveat of this study is that the differences in connectivity between cancer survivors and controls are not necessarily selective, and survivors could demonstrate other aberrant connectivity patterns which may be related to other cognitive domains if tested with different paradigms. Finally, the observed differences in hippocampal-cortical connectivity may be due to the survivors' chemotherapy regimen, the effects of ongoing Tamoxifen treatment, or a combination of the two. Future studies should consider differences in stage (e.g. stages I-IV), type of treatment (e.g. different types of chemotherapy drugs including anthracyclines, taxanes, 5-fluorouracil, cyclophosphamide, carboplatin or a combination of the above), and therapy regimen (e.g. 3-month course vs a 6-month course, daily vs weekly etc.) of cancer to ascertain their effects on hippocampal-cortical connectivity.
5. Conclusions
The current study observed greater task related hippocampal functional connectivity in breast cancer survivors as compared with healthy controls. The higher connectivity was correlated with greater subjective feelings of cognitive concern in the survivors. These findings suggest that hippocampal-cortical task-based functional connectivity may be a biomarker for a compensatory mechanism in CRCI. As the field evolves, it may be important to utilize research to inform clinical practice as it is critical to develop strategies to palliate symptoms and improve patient quality of life. Along this same vein, self-reported cognition may be increasingly useful for identifying differences in brain behavior at an earlier time in order to implement a more effective intervention.
Conflicts of interest
Alexandra C. Apple, Anthony J. Ryals, Matthew P. Schroeder, Lynne I. Wagner, Pei-An Shih, David Cella, Frank J. Penedo, James Reilly, Joel L. Voss, and Lei Wang declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Acknowledgments and funding
This work was supported by award numbers 1F31CA210719-01 from the National Cancer Institute, T32-NS047987 from the National Institute of Neurological Disorders and Stroke, R01-NR014182 from the National Institute of Nursing Research, P30 CA060553 (L Platanias) from the Robert H. Lurie Comprehensive Cancer Center, and by a grant from the Lynn Sage Cancer Research Foundation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.07.010.
Appendix A. Supplementary data
Covert scene configuration memory test and stimuli example. (A) Example study block (top) and test block trials (bottom). During the test portion of this task, participants viewed a series of 12 scenes for 8 seconds and were asked to rate how well they thought they learned that scene; ISIs were randomized between scenes. During the test portion, participants saw 24 novel scenes, 12 of which were new (configurally unfamiliar) and 12 of which were “old” (non-identical configurally similar to previously studied scenes). Participants indicated whether they remembered studying the scene in a yes/no paradigm. (B) Examples of study (left) and test (right) scene stimuli which have similar configurations. ISI = Interstimulus interval.
References
- Ahles T.A., Saykin A.J., McDonald B.C., Li Y., Furstenberg C.T., Hanscom B.S., Mulrooney T.J., Schwartz G.N., Kaufman P.A. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J. Clin. Oncol. 2010;28(29):4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles T.A., Root J.C., Ryan E.L. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J. Clin. Oncol. 2012;30(30):3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apple A.C., Ryals A.J., Alpert K.I., Wagner L.I., Shih P.A., Dokucu M., Cella D., Penedo F.J., Voss J.L., Wang L. Subtle hippocampal deformities in breast cancer survivors with reduced episodic memory and self-reported cognitive concerns. Neuroimage Clin. 2017;14:685–691. doi: 10.1016/j.nicl.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassarre A., Lewis C.M., Committeri G., Snyder A.Z., Romani G.L., Corbetta M. Individual variability in functional connectivity predicts performance of a perceptual task. Proc. Natl. Acad. Sci. U. S. A. 2012;109(9):3516–3521. doi: 10.1073/pnas.1113148109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer P.J., Dikmen S.S., Heaton R.K., Mungas D., Slotkin J., Beaumont J.L. III. NIH toolbox cognition battery (CB): measuring episodic memory. Monogr. Soc. Res. Child Dev. 2013;78(4):34–48. doi: 10.1111/mono.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender C.M., Sereika S.M., Berga S.L., Vogel V.G., Brufsky A.M., Paraska K.K., Ryan C.M. Cognitive impairment associated with adjuvant therapy in breast cancer. Psychooncology. 2006;15(5):422–430. doi: 10.1002/pon.964. [DOI] [PubMed] [Google Scholar]
- Berlingeri M., Danelli L., Bottini G., Sberna M., Paulesu E. Reassessing the HAROLD model: is the hemispheric asymmetry reduction in older adults a special case of compensatory-related utilisation of neural circuits? Exp. Brain Res. 2013;224(3):393–410. doi: 10.1007/s00221-012-3319-x. [DOI] [PubMed] [Google Scholar]
- Biglia N., Bounous V.E., Malabaila A., Palmisano D., Torta D.M., D'Alonzo M., Sismondi P., Torta R. Objective and self-reported cognitive dysfunction in breast cancer women treated with chemotherapy: a prospective study. Eur. J. Cancer Care (Engl.) 2012;21(4):485–492. doi: 10.1111/j.1365-2354.2011.01320.x. [DOI] [PubMed] [Google Scholar]
- Bruno J., Hosseini S.M., Kesler S. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol. Dis. 2012;48(3):329–338. doi: 10.1016/j.nbd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R., Dennis N.A. Frontal lobes and aging: deterioration and compensation. In: Stuss D.T.K., editor. Principles of Frontal Lobe Function. Oxford University Press; New York: 2013. 2nd Edition. [Google Scholar]
- Cabeza R., Anderson N.D., Locantore J.K., McIntosh A.R. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 2002;17(3):1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cella D., Lai J.S., Nowinski C.J., Victorson D., Peterman A., Miller D., Bethoux F., Heinemann A., Rubin S., Cavazos J.E., Reder A.T., Sufit R., Simuni T., Holmes G.L., Siderowf A., Wojna V., Bode R., McKinney N., Podrabsky T., Wortman K., Choi S., Gershon R., Rothrock N., Moy C. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012;78(23):1860–1867. doi: 10.1212/WNL.0b013e318258f744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S., Pitel A.L., Pfefferbaum A., Sullivan E.V. Disruption of functional connectivity of the default-mode network in alcoholism. Cereb. Cortex. 2011;21(10):2272–2281. doi: 10.1093/cercor/bhq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., He X., Tao L., Li J., Wu J., Zhu C., Yu F., Zhang L., Zhang J., Qiu B., Yu Y., Wang K. The working memory and dorsolateral prefrontal-hippocampal functional connectivity changes in long-term survival breast cancer patients treated with tamoxifen. Int. J. Neuropsychopharmacol. 2017;20(5):374–382. doi: 10.1093/ijnp/pyx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung Y.T., Ng T., Shwe M., Ho H.K., Foo K.M., Cham M.T., Lee J.A., Fan G., Tan Y.P., Yong W.S., Madhukumar P., Loo S.K., Ang S.F., Wong M., Chay W.Y., Ooi W.S., Dent R.A., Yap Y.S., Ng R., Chan A. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann. Oncol. 2015;26(7):1446–1451. doi: 10.1093/annonc/mdv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Debess J., Riis J.O., Engebjerg M.C., Ewertz M. Cognitive function after adjuvant treatment for early breast cancer: a population-based longitudinal study. Breast Cancer Res. Treat. 2010;121(1):91–100. doi: 10.1007/s10549-010-0756-8. [DOI] [PubMed] [Google Scholar]
- Dethlefsen C., Hojfeldt G., Hojman P. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res. Treat. 2013;138(3):657–664. doi: 10.1007/s10549-013-2488-z. [DOI] [PubMed] [Google Scholar]
- Dickerson B.C., Salat D.H., Greve D.N., Chua E.F., Rand-Giovannetti E., Rentz D.M., Bertram L., Mullin K., Tanzi R.E., Blacker D., Albert M.S., Sperling R.A. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65(3):404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J., Han R., Yang Y., Mayer-Proschel M., Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J. Biol. 2006;5(7):22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Shen Y., Lei X., Luo C., Li Q.W., Wu W.Y., Yao D.Z., Li C.B. The heterogeneity of aging brain: altered functional connectivity in default mode network in older adults during verbal fluency tests. Chin. Med. J. 2012;125(4):604–610. [PubMed] [Google Scholar]
- Dumas J.A., Makarewicz J., Schaubhut G.J., Devins R., Albert K., Dittus K., Newhouse P.A. Chemotherapy altered brain functional connectivity in women with breast cancer: a pilot study. Brain Imaging Behav. 2013;7(4):524–532. doi: 10.1007/s11682-013-9244-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy M., Chanraud S. Imaging the addicted brain: alcohol. Int. Rev. Neurobiol. 2016;129:1–31. doi: 10.1016/bs.irn.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Euston D.R., Gruber A.J., McNaughton B.L. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76(6):1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Schlaggar B.L., Cohen A.L., Miezin F.M., Dosenbach N.U., Wenger K.K., Fox M.D., Snyder A.Z., Raichle M.E., Petersen S.E. A method for using blocked and event-related fMRI data to study "resting state" functional connectivity. NeuroImage. 2007;35(1):396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson R.J., Ahles T.A., Saykin A.J., McDonald B.C., Furstenberg C.T., Cole B.F., Mott L.A. Cognitive-behavioral management of chemotherapy-related cognitive change. Psychooncology. 2007;16(8):772–777. doi: 10.1002/pon.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson R.J., McDonald B.C., Saykin A.J., Ahles T.A. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J. Clin. Oncol. 2007;25(25):3866–3870. doi: 10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke C., Bruehl H., Duzel E., Heekeren H.R., Ploner C.J. Neural correlates of short-term memory reorganization in humans with hippocampal damage. J. Neurosci. 2013;33(27):11061–11069. doi: 10.1523/JNEUROSCI.0744-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P.C., Frith C.D., Baker S.C., Shallice T., Frackowiak R.S., Dolan R.J. The mind's eye--precuneus activation in memory-related imagery. NeuroImage. 1995;2(3):195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Grady C.L., McIntosh A.R., Beig S., Keightley M.L., Burian H., Black S.E. Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer's disease. J. Neurosci. 2003;23(3):986–993. doi: 10.1523/JNEUROSCI.23-03-00986.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermelink K., Untch M., Lux M.P., Kreienberg R., Beck T., Bauerfeind I., Munzel K. Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer. 2007;109(9):1905–1913. doi: 10.1002/cncr.22610. [DOI] [PubMed] [Google Scholar]
- Hosseini S.M., Kesler S.R. Multivariate pattern analysis of FMRI in breast cancer survivors and healthy women. J. Int. Neuropsychol. Soc. 2014;20(4):391–401. doi: 10.1017/S1355617713001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurria A., Rosen C., Hudis C., Zuckerman E., Panageas K.S., Lachs M.S., Witmer M., van Gorp W.G., Fornier M., D'Andrea G., Moasser M., Dang C., Van Poznak C., Hurria A., Holland J. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: a pilot prospective longitudinal study. J. Am. Geriatr. Soc. 2006;54(6):925–931. doi: 10.1111/j.1532-5415.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- Hutchinson A.D., Hosking J.R., Kichenadasse G., Mattiske J.K., Wilson C. Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat. Rev. 2012;38(7):926–934. doi: 10.1016/j.ctrv.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Inagaki M., Yoshikawa E., Matsuoka Y., Sugawara Y., Nakano T., Akechi T., Wada N., Imoto S., Murakami K., Uchitomi Y. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109(1):146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- Janelsins M.C., Kohli S., Mohile S.G., Usuki K., Ahles T.A., Morrow G.R. An update on cancer- and chemotherapy-related cognitive dysfunction: current status. Semin. Oncol. 2011;38(3):431–438. doi: 10.1053/j.seminoncol.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins M.C., Kesler S.R., Ahles T.A., Morrow G.R. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int. Rev. Psychiatry. 2014;26(1):102–113. doi: 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen C.E., Cooper B.A., Dodd M.J., Miaskowski C.A. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19(10):1647–1656. doi: 10.1007/s00520-010-0997-4. [DOI] [PubMed] [Google Scholar]
- Jenkins V., Shilling V., Deutsch G., Bloomfield D., Morris R., Allan S., Bishop H., Hodson N., Mitra S., Sadler G., Shah E., Stein R., Whitehead S., Winstanley J. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br. J. Cancer. 2006;94(6):828–834. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S.R. Default mode network as a potential biomarker of chemotherapy-related brain injury. Neurobiol. Aging. 2014;35(Suppl 2):S11–S19. doi: 10.1016/j.neurobiolaging.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S.R., Bennett F.C., Mahaffey M.L., Spiegel D. Regional brain activation during verbal declarative memory in metastatic breast cancer. Clin. Cancer Res. 2009;15(21):6665–6673. doi: 10.1158/1078-0432.CCR-09-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S., Janelsins M., Koovakkattu D., Palesh O., Mustian K., Morrow G., Dhabhar F.S. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav. Immun. 2013;30(Suppl):S109–S116. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S.R., Wefel J.S., Hosseini S.M., Cheung M., Watson C.L., Hoeft F. Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proc. Natl. Acad. Sci. U. S. A. 2013;110(28):11600–11605. doi: 10.1073/pnas.1214551110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier T.B., Wildenberg J.C., Liu J., Chen J., Calhoun V.D., Biswal B.B., Meyerand M.E., Birn R.M., Prabhakaran V. Parallel ICA identifies sub-components of resting state networks that covary with behavioral indices. Front. Hum. Neurosci. 2012;6:281. doi: 10.3389/fnhum.2012.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers C.A. How chemotherapy damages the central nervous system. J. Biol. 2008;7(4):11. doi: 10.1186/jbiol73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H.C., Parsons M.W., Yue G.H., Rybicki L.A., Siemionow W. Electroencephalogram power changes as a correlate of chemotherapy-associated fatigue and cognitive dysfunction. Support Care Cancer. 2014;22(8):2127–2131. doi: 10.1007/s00520-014-2197-0. [DOI] [PubMed] [Google Scholar]
- Nobakht M., Najafzadeh N., Kordestani Shargh B. Effects of tamoxifen on morphological and ultrastructural aspects of developing hippocampus of rat. Iran. Biomed. J. 2009;13(4):237–243. [PubMed] [Google Scholar]
- O'Farrell E., MacKenzie J., Collins B. Clearing the air: a review of our current understanding of "chemo fog". Curr. Oncol. Rep. 2013;15(3):260–269. doi: 10.1007/s11912-013-0307-7. [DOI] [PubMed] [Google Scholar]
- Oken M.M., Creech R.H., Tormey D.C., Horton J., Davis T.E., McFadden E.T., Carbone P.P. Toxicity and response criteria of the eastern cooperative oncology group. Am. J. Clin. Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- Ranganath C., Heller A., Cohen M.X., Brozinsky C.J., Rissman J. Functional connectivity with the hippocampus during successful memory formation. Hippocampus. 2005;15(8):997–1005. doi: 10.1002/hipo.20141. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P.A., Cimprich B. Cognitive function and breast cancer: promise and potential insights from functional brain imaging. Breast Cancer Res. Treat. 2013;137(1):33–43. doi: 10.1007/s10549-012-2266-3. [DOI] [PubMed] [Google Scholar]
- de Ruiter M.B., Reneman L., Boogerd W., Veltman D.J., van Dam F.S., Nederveen A.J., Boven E., Schagen S.B. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum. Brain Mapp. 2011;32(8):1206–1219. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals A.J., Cleary A.M., Seger C.A. Recall versus familiarity when recall fails for words and scenes: the differential roles of the hippocampus, perirhinal cortex, and category-specific cortical regions. Brain Res. 2013;1492:72–91. doi: 10.1016/j.brainres.2012.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals A.J., Apple A.C., Wang J.X., Cella D., Penedo F.J., Wang L., Voss J.L. Paper presented at the ASCO Annual Meeting, Chicago, IL. 2015. Hippocampal memory impairment in breast cancer survivors after chemotherapy measurement using covert testing. [Google Scholar]
- Ryals A.J., Wang J.X., Polnaszek K.L., Voss J.L. Hippocampal contribution to implicit configuration memory expressed via eye movements during scene exploration. Hippocampus. 2015;25(9):1028–1041. doi: 10.1002/hipo.22425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherling C.S., Smith A. Opening up the window into "chemobrain": a neuroimaging review. Sensors (Basel) 2013;13(3):3169–3203. doi: 10.3390/s130303169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigers R., Timmermans J., van der Horn H.J., de Vries E.F., Dierckx R.A., Visser L., Schagen S.B., van Dam F.S., Koolhaas J.M., Buwalda B. Methotrexate reduces hippocampal blood vessel density and activates microglia in rats but does not elevate central cytokine release. Behav. Brain Res. 2010;207(2):265–272. doi: 10.1016/j.bbr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Shilling V., Jenkins V. Self-reported cognitive problems in women receiving adjuvant therapy for breast cancer. Eur. J. Oncol. Nurs. 2007;11(1):6–15. doi: 10.1016/j.ejon.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Stein T., Moritz C., Quigley M., Cordes D., Haughton V., Meyerand E. Functional connectivity in the thalamus and hippocampus studied with functional MR imaging. AJNR Am. J. Neuroradiol. 2000;21(8):1397–1401. [PMC free article] [PubMed] [Google Scholar]
- Wang J.X., Rogers L.M., Gross E.Z., Ryals A.J., Dokucu M.E., Brandstatt K.L., Hermiller M.S., Voss J.L. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science. 2014;345(6200):1054–1057. doi: 10.1126/science.1252900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefel J.S., Saleeba A.K., Buzdar A.U., Meyers C.A. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116(14):3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- Weintraub S., Dikmen S.S., Heaton R.K., Tulsky D.S., Zelazo P.D., Bauer P.J., Carlozzi N.E., Slotkin J., Blitz D., Wallner-Allen K., Fox N.A., Beaumont J.L., Mungas D., Nowinski C.J., Richler J., Deocampo J.A., Anderson J.E., Manly J.J., Borosh B., Havlik R., Conway K., Edwards E., Freund L., King J.W., Moy C., Witt E., Gershon R.C. Cognition assessment using the NIH toolbox. Neurology. 2013;80(11 Suppl 3):S54–S64. doi: 10.1212/WNL.0b013e3182872ded. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A.M., Zent C.S., Janelsins M.C. What is known and unknown about chemotherapy-related cognitive impairment in patients with haematological malignancies and areas of needed research. Br. J. Haematol. 2016;174(6):835–846. doi: 10.1111/bjh.14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A.M., Shah R., Shayne M., Huston A.J., Krebs M., Murray N., Thompson B.D., Doyle K., Korotkin J., van Wijngaarden E., Hyland S., Moynihan J.A., Cory-Slechta D.A., Janelsins M.C. Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. J. Neuroimmunol. 2018;314:17–23. doi: 10.1016/j.jneuroim.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Covert scene configuration memory test and stimuli example. (A) Example study block (top) and test block trials (bottom). During the test portion of this task, participants viewed a series of 12 scenes for 8 seconds and were asked to rate how well they thought they learned that scene; ISIs were randomized between scenes. During the test portion, participants saw 24 novel scenes, 12 of which were new (configurally unfamiliar) and 12 of which were “old” (non-identical configurally similar to previously studied scenes). Participants indicated whether they remembered studying the scene in a yes/no paradigm. (B) Examples of study (left) and test (right) scene stimuli which have similar configurations. ISI = Interstimulus interval.