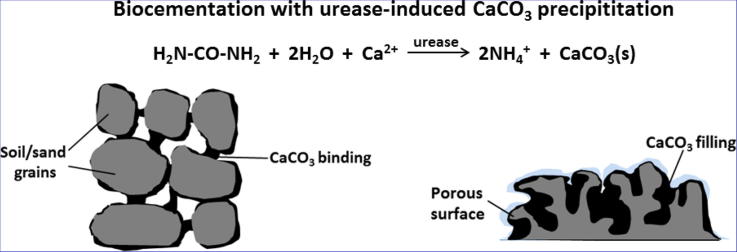
Graphical abstract
Keywords: Urease, CaCO3, Biomineralization, Soil and sand, Construction materials, Groundwater
Abstract
Inducing calcium carbonate precipitation is another important function of urease in nature. The process takes advantage of the supply of carbonate ions derived from urea hydrolysis and of an increase in pH generated by the reaction, effects that in the presence of Ca2+ ions lead to the precipitation of CaCO3. Further to its importance in nature, if performed in a biomimetic manner, the urease-aided CaCO3 mineralization offers enormous potential in innovative engineering applications as an eco-friendly technique operative under mild conditions, to be used for remediation and cementation/deposition in field applications in situ. These include among others, the strengthening and consolidation of soil/sand, the protection and restoration of stone and concrete structures, conservation of stone cultural heritage materials, cleaning waste- and groundwater of toxic metals and radionuclides, and plugging geological formations for the enhancement of oil recovery and geologic CO2 sequestration. In view of the potential of this newly emerging interdisciplinary branch of engineering, this article presents the principles of urease-aided calcium carbonate mineralization apposed to other biomineralization processes, and reviews the advantages and limitations of the technique compared to the conventional techniques presently in use. Further, it presents areas of its existing and potential applications, notably in geotechnical, construction and environmental engineering, and its future perspectives.
Introduction
Discovered in the 19th century and identified to be responsible for the hydrolysis of urea to carbonic acid and ammonia (ureolysis) [1], the first enzyme ever crystallized [2], and the first enzyme ascertained to contain Ni(II) ions essential for the activity [3], urease has been a subject of extensive research [4], [5], [6], [7]. This included structure and functions of the enzyme, kinetics and the catalytic mechanism, and more recently, amino acid sequencing, crystal structures, and genetic organization. In addition to gaining theoretical understanding of urease biochemistry, this knowledge is of key importance for controlling the processes occurring with the participation of the enzyme. These include both natural processes and those man-devised, in which the activity of the enzyme is exploited. The latter ones have emerged, as parallel to the theoretical research, the practical potential of ureases has been increasingly studied for various laboratory, biotechnological and engineering applications.
One example of novel engineering applications of urease is in biomineralization of calcium carbonate [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]. Relying on the activity of urease, the process is termed urease-aided CaCO3 mineralization or exchangeably, ureolysis-driven CaCO3 mineralization. By mimicking CaCO3 bio-formation in nature, in contrast to typical synthetic techniques, the process can be performed in an environmentally benign manner under mild conditions and importantly, in field applications in situ. Accordingly, the process offers exciting innovative potential in multiple engineering fields, notably geotechnical, construction and environmental, where it is used as a means of biocementation and bioremediation [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], for instance for the strengthening and consolidation of soil and sand, the protection and restoration of stone and concrete structures, conservation of stone cultural heritage materials, cleaning waste- and groundwater of toxic metals, radionuclides and excess soluble Ca2+, plugging oil reservoir bedrocks for the enhancement of oil recovery, and as a sealant in geologic CO2 sequestration.
In this context this article looks at the principles of urease-aided calcium carbonate mineralization and reviews its advantages and limitations compared to the conventional techniques presently in use. Further it presents areas of its applications and future perspectives.
Biomineralization
Biomineralization is the formation of minerals by living organisms (typically to stiffen or harden the tissues). It is a widespread natural phenomenon that occurs in almost all groups of organisms from prokaryotes to humans. In the synthesis of minerals the organisms utilize different mechanisms, generally classified as active (type 1) and passive (type 2) [8], [10], [11], [14], [16], [19]:
-
(1)
biologically-controlled mineralization, where mineral formation, including their growth, composition, morphology and location, is entirely controlled by the cellular processes specific for a given organism,
-
(2)
biologically-induced mineralization, where the metabolic activity of an organism (e.g. bacteria) modifies the local environment, thus creating conditions favourable for mineral precipitation (typically with cell walls serving as crystal nucleation sites).
Frequently combinations of the above mechanisms are operative in biomineralization.
Notably, the processes involved in biomineralization provide stringent control from the nano- to macro-level over the composition, structure, size and morphology of biominerals, giving rise to materials of remarkable complexity, with strictly defined structures and with distinct properties that greatly contrast with those of geological minerals and often surpass those of synthetic analogues. Of importance, in contrast to biomineralization that typically occurs at ambient temperatures and under mild conditions, the chemical syntheses of minerals are frequently energy inefficient, requiring elevated temperatures and strong chemical solutions, and often producing noxious by-products. In this context, there is a growing interest in exploiting biomineralization in practical applications as an eco-friendly, energy-saving technique with a potential to be carried out in situ.
Biomineralization of calcium carbonate
Of all the minerals produced by biomineralization in nature, calcium carbonate (CaCO3) is a major product [10], [16], [19]. Comprising more than 4% of the earth’s crust, it is a common substance found in rocks, such as chalk, marble, travertine, tufa, and others. It is also the main component in shells as well as in pearls and corals. Having been utilized for ages as a building material, now also in different industries, agriculture and medicine, in addition to the newly emerging engineering field of biomineralization, calcium carbonate has always been one of the most useful and versatile materials known to man.
Calcium carbonate forms different polymorphs seen in nature as minerals. These include three anhydrous polymorphs: calcite, aragonite and vaterite, of which calcite is most stable, and two hydrated polymorphs: monohydrocalcite (CaCO3·H2O) and ikaite (CaCO3·6H2O), in addition to various amorphous phases (ACC).
The equilibrium of calcium carbonate precipitation follows the equation:
| (1) |
where Ksp is the solubility product constant, most commonly reported for calcite to be 4.8·10−9 [10], [12], [15]. It follows from Eq. (1) that CaCO3 precipitation occurs when the ion activity product [Ca2+] [CO32−] exceeds the solubility product Ksp.
Apart from the concentrations of (i) calcium ions and (ii) carbonate ions (Eq. (1)), the precipitation of calcium carbonate relies on two more factors: (iii) the pH and (iv) the availability of crystal nucleation sites [20]. The process requires alkaline pH as an indispensable condition to shift the bicarbonate equilibrium to form carbonate ions [20]:
| (2) |
while nucleation sites facilitate the accumulation of calcium ions for the precipitation.
Microorganisms can secrete calcium carbonate in environments rich in Ca2+ by changing any of the above precipitation factors [10], [12], [20], separately or in combinations, their primary role being in increasing pH. Even though the actual roles of bacteria and their activities in carbonate precipitation have not been precisely resolved, various biological pathways have been proposed for this precipitation. The pathways include [20], [21]:
-
(1)
photosynthesis associated with the activity of algae and cyanobacteria,
-
(2)
sulphate cycle, with the activity of sulphate reducing bacteria in dissimilatory sulphate reduction,
-
(3)
nitrogen cycle, including ammonification of amino acids, nitrate reduction and the hydrolysis of urea. These pathways have in common the production of CO2 and NH3.
Of all the above pathways, the method that is most widely utilized for the precipitation of CaCO3 for technical applications, is arguably the one based on the hydrolysis of urea. The reaction is catalyzed by the enzyme urease.
Urease
Urease (urea amidohydrolase, EC 3.5.1.5) is an enzyme widespread in nature [4], [5], [6], [7]. It is synthesized by numerous organisms, including plants, bacteria, algae, fungi and invertebrates, and also occurs in soils as a soil enzyme. The enzyme is counted among the most proficient enzymes known to date. All ureases are high molecular weight, multisubunit enzymes. Bacterial ureases differ from plant and fungal ones, typically homohexameric, in that they are composed of heteromeric subunits. Most importantly, irrespective of the source and structural composition, the salient feature of ureases is the active site that contains a bi-nickel centre with a characteristic structure found conserved among the enzymes. As a result, all ureases exert one catalytic function that is the hydrolysis of urea (Eq. (3)) and follow the same catalytic mechanism. The products of the reaction and the resulting increase in pH of the reaction environment that can reach pH up to 9.2, are consequential characteristics of the action of ureases. These can be deleterious (medicine and agriculture) or beneficial (biomineralization of CaCO3).
Notwithstanding that urease has been extensively studied over the years, its catalytic mechanism still remains debatable [7], [22], [23], [24]. Its elucidation is crucial for the efficacious use of the enzyme and for the control of its activity. One way to achieve this control is by using inhibitors [5], [25], [26], [27], [28], the primary among them being: amides and esters of phosphoric acid [22], [29], [30], thiols [30], [31], [32], hydroxamic acids [29], [30], [33], [34], phosphinic and thiophosphinic acids [35], boric acid [29], [32], [36], phosphate [30], [32], [37], [38], heavy metal ions [39], [40], bismuth compounds [41], quinones [42], [43], [44], and fluoride [29], [45].
Another way of controlling the action of urease is by immobilizing the enzyme [46]. The immobilization consists of converting enzymes into insoluble form, most frequently by fixing them to or within solid supports, as a result of which enzyme systems are obtained, where structures of enzymes, hence their activities, are stabilized. Despite the fact that upon immobilization the kinetic properties of enzymes may be worsened, their stabilities, operational lifetimes and sensitivities to inhibitions [34], [46], [47] are improved, thus providing robust and reliable enzyme preparations.
Urease-aided calcium carbonate mineralization
The process of urease-aided calcium carbonate mineralization is triggered by the catalytic action of urease in the hydrolysis of urea [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [48], [49]. The products of the reaction are carbonic acid and ammonia [5]:
| (3) |
The products equilibrate in water to give bicarbonate, ammonium and hydroxide ions, respectively:
| (4) |
| (5) |
The production of hydroxide ions from reaction (5) brings about an increase in pH, which in turn leads to the formation of carbonate ions (Eq. (2)):
| (6) |
In the presence of dissolved Ca2+, the ions combine and calcium carbonate precipitates:
| (7) |
The overall process can thus be presented:
| (8) |
As shown the precipitation process takes advantage of the supply of carbonate ions derived from urea hydrolysis and of an increase in pH generated by the reaction. If the process is carried out with use of ureolytic bacteria, in addition to the bulk phase, most commonly the crystallization takes place on bacterial cell walls, which serve as crystal nucleation sites. This is because the cell walls possess negatively charged functional groups and attract and bind Ca2+ ions, resulting in their deposition and accumulation. Consequently, carbonate crystals grow on the external surfaces of cells by successive stratification with the cells finally encased within [10], [49]. In such a case Eq. (7) should be expressed as:
| (9) |
Urease-aided calcium carbonate mineralization has proved to be a rapid process capable of producing large quantities of CaCO3 in short periods of time [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [48], [49]. In terms of biological mechanisms on the other hand, with the reaction scheme presented above it classifies as representing biologically-induced mineralization (type 2).
Procedures
To perform the precipitation of CaCO3 in real sites, the aqueous mixture of the three mineralization components, i.e. the ureolytic agent (ureolytic bacteria or free urease), urea and Ca2+, is introduced to the setting where the intended precipitation is to take place, be it (i) granular materials, such as soil or sand to be consolidated (by binding the grains together), (ii) stone and concrete structures to be repaired (by filling pores, cracks and fissures), (iii) their surfaces to be covered with a protective layer, or (iv) rock formations to be plugged up (by filling pores). The three components of the mineralization mixture may be introduced to the material in different order to achieve the desired effects. For instance, the application of the bacteria, urea and Ca2+ mixed together prior to the injection to the material, most commonly gives rise to immediate flocculation of bacteria and crystal precipitation. This results in clogging the surface pores, making deeper layers of the material inaccessible to the mineralization mixture. While this technique is effective in the formation of surface layers and in the treatment of very coarse grained materials and mixed-in-place applications [50], it is not adequate for homogeneous distribution of CaCO3 over larger distances in the material. To accomplish such homogenous precipitation, a two-phase injection procedure was developed [51], [52]. In the procedure a mixture of bacteria and nutrients is firstly injected into the material, immediately followed by a fixation solution (i.e. a solution with high salt concentration). The fixation solution is applied to control the distribution of cells on grains to assure their in-depth penetration and uniform attachment. Finally, this stage is followed by the injection of urea-Ca2+ solution to effectuate the precipitation. When sufficient calcium carbonate is precipitated, durable stabilization of the material is achieved.
Ureolytic bacteria vs. free urease
As an ureolytic agent, the process uses either urease-producing bacteria [8], [9], [10], [11], [12], [13], [14], [15], [16], [17] or purified enzyme [18]. The most common bacterium utilized is arguably the soil bacterium Sporosarcina pasteurii (formerly Bacillus pasteurii) [49], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], while the purified enzyme is that extracted from plant sources, mainly from jack bean (Canavalia ensiformis) [62], [63], [64], [65], [66], [67], [68], [69].
Although the microbial precipitation has received the most attention and is most advanced in terms of development for field applications, the use of the enzyme offers a number of advantages. Unlike microbial, the enzymatic process is more straightforward in that the growth and the storage of bacteria are avoided. Normally, the production of highly active ureolytic bacteria on larger scale is expensive, representing a major cost factor in the precipitation process [70], whereas the enzyme is obtainable from plants in a relatively simple process, also crude plant extracts can be used [68], in addition to the fact that, though costly, the enzyme is available commercially.
Furthermore, in the efficiency of the process decisive is the size of the molecules. The size of plant urease molecule is ca. 12 nm [71], whereas that of most bacteria is higher than 0.3 μm, with the majority in the range from 0.5 to 5 μm [18], [72]. The small size of the molecule compared to the bacteria permits a considerably easier and more efficient penetration of urease into the small pores in the material and besides, allows urease, urea and Ca2+ to be used together in the injection solution [64]. By contrast, being unable to enter pore throats smaller than ca. 0.4 μm, the bacteria commonly remain on the surface of the material giving rise to the formation of a superficial film consisting of biological remains (surface bioplugging), resulting in an adverse effect that prevents the access of the mineralizing mixture deeper in the material [18], [48], [72].
The choice between free urease and bacteria is also important in respect of the activity. On this point it was observed that the enzymatic mineralization resulted in higher quantities of the precipitated CaCO3 compared to the corresponding bacteria, an effect that was ascribed to the inhibition of the bacterial activity by high concentrations of urea and/or calcium chloride [63] and to the encasement of bacterial cells in the precipitate resulting in the limited nutrient transport to the cells and/or cell membrane disruption [8], [49]. In practical terms, the activity of the free enzyme during the process can be affected and/or controlled by inhibitors [5], [56], while its longevity may be extended and the resistance to inhibitions enhanced when an immobilized form of the enzyme is used [56].
Another aspect of consequential importance is that unlike bacteria, the free enzyme is short-lived. This is beneficial in that the enzyme naturally degrades, thereby eliminating long term effects on the ecosystem, while ureolytic bacteria may remain active after the process thereby posing a risk of uncontrolled bacterial growth and biofilm formation [73], [74]. In relation to the above, it was also observed that in the case of bacterially formed CaCO3, the precipitated mass inside the material consisted not only of CaCO3 but also of biomass, this by being degradable impaired the durability of the formed material [62], [63].
Process parameters
The parameters that have influence on the course of the precipitation process and its efficacy, primarily include the concentration of bacteria/urease and the concentrations of urea and Ca2+, in addition to environmental parameters, such as temperature and salinity [48], [55], [62], [63], [69]. It was found that an increase in urease concentration enhanced the rate of CaCO3 precipitation [62], [63], while increases in urea and Ca2+ concentrations increased the quantity of precipitated CaCO3, however, only up to a certain level [48], [55], [62], [63], [69]. In this regard, it appeared that for a given concentration of cells/urease there exist the optimal urea and Ca2+ concentrations, above which the beneficial effects for the precipitation are counterbalanced by the detrimental effects, i.e. accumulation of salts and urea in the material [48], [62]. By contrast, temperature was found to increase the rate of CaCO3 precipitation in the enzymatic process, and practically to have no influence on the bacterial process [62], [63].
By-products
Independent of whether the precipitation is performed with use of bacteria or the free enzyme, the process produces the same by-products. One is the ammonium ion NH4+ resulting from the hydrolysis of urea (Eqs. (3), (5)). The ion poses ecological risk that includes NH3 toxicity to humans and acidification arising from NH3 nitrification, in addition to stone discoloration [12], [14], [15], [48], [53]. These effects, however, can be minimized by using balanced low urea concentrations in the process or by applying additional decontamination after the process [9], [12]. The other by-product is an anion remaining from the calcium salt applied. CaCl2 is the salt of choice both in laboratory mineralization experiments and in real sites [48], [49], [51], [52], [53], [57], [58], [62], [63], [64], [65], [66], [67], [68], [72]. Indeed, CaCl2 was shown to promote the precipitation of CaCO3 in markedly higher quantities than Ca(NO3)2, Ca(OH)2 and (CH3COO)2Ca [69], [75]. However, the choice of salt for a given applications should be careful in that some anions may be detrimental for the treated material, e.g. Cl− ions for the steel reinforcement in concrete, a case for which calcium acetate was suggested [12].
CaCO3 polymorphs
Interestingly, the formation of different polymorphs of calcium carbonate was observed in the process depending on the type of urease and reaction conditions. For instance, it was reported that urease from jack bean provoked the formation of calcite, while that from Sporosarcina pasteurii, vaterite [67]. Also, when the solution was unstirred jack bean urease produced an amorphous precipitate that crystallized to vaterite and later to calcite [66]. By contrast, in the processes driven bacterially by Sporosarcina pasteurii, it was calcite that was typically obtained [49], [55], [57], [58]. Despite extensive research, the origin of polymorph selection during CaCO3 biomineralization has not as yet been resolved [10], [19], [76].
Given the above presented characteristics, for the implementation of the urease-aided CaCO3 mineralization in a commercial application, all beneficial and detrimental effects of whether a bacterium or the free enzyme is chosen, whether the mineralization components are used together or separately in a sequence, whether in lower or higher concentrations, have to be weighed taking into account all relevant aspects, health and environmental included, in addition to obvious economic viability. To date, urease-aided mineralization procedures are mostly under research and optimization, but some have proved economic and have been implemented. A selection of existing and potential applications of urease-aided calcium carbonate mineralization is presented in a subsequent section.
Applications of urease aided-calcium carbonate mineralization
Improvements of soil and sand (strengthening and consolidation)
Mechanical properties of soils are a resultant of natural conditions, erosion and human interventions. Frequently the properties are insufficient for the desired use of land, especially to meet today’s ever-increasing demands in the area of civil engineering infrastructure [10], [13], [14], [52]. This requires improvement of soils, mainly their strength and stiffness as well as volume stability, permeability and durability, done through strengthening and consolidation. Various techniques are presently available, including drainage, densification and vibration techniques, sand or stone columns, and chemical grouting, all of them known to be energy-consuming [9], [64]. Among these techniques chemical grouting is most common. In the technique, the soil is injected with a hardening fluid grout, which once in the soil, solidifies filling spaces between soil grains simultaneously acting as a grain binder. Examples of fluid grouts include cement, bentonite, lignosulfonates, silicate, epoxy, acrylamide and polyurethane. Effective as it is, chemical grouting raises serious economic and environmental concerns. Among other techniques, it is reckoned as one of most expensive. By contrast, the environmental concerns it causes, involve the adverse impacts, such as excessive reduction of soil permeability, as well as the disturbance of the ecosystem by both the toxic/hazardous character of the chemical grouts (except silicates) and by a considerable increase in pH of groundwater [9], [13], [64]. Additionally, cement, the chief grout, is a problematic substance in that its production worldwide accounts for as much as ca. 5% of global anthropogenic CO2 emissions and to ca. 5% of the total industrial energy consumption [77].
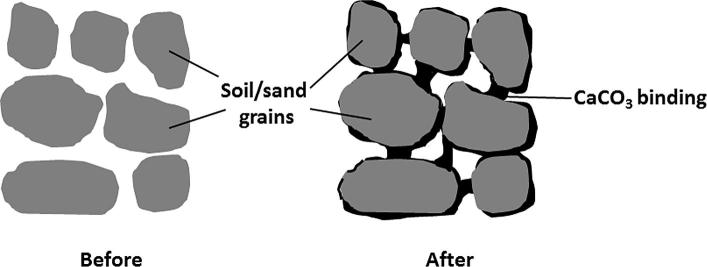
In this context, to address the sustainability considerations, the established techniques of the strengthening and consolidation of soil and sand need to be either replaced or supplemented by novel materials and eco-friendly practices. The ureolysis-driven carbonate precipitation is an obvious alternative [8], [9], [10], [11], [13], [14], [17], where CaCO3 precipitated in situ in soil/sand takes a role of a binding medium (Fig. 1), rightly termed biocement or biogrout. Numerous laboratory experiments and attempts to upscale the process [51], [52], [53], [63], [64], [69], [72] demonstrated that a significant improvement of soil/sand strength can be achieved on demand, crucially, with a control of soil permeability. The research suggests that the process can address a wide variety of geotechnical problems, including erosion and scour, slope stability, the bearing capacity of soil for foundations and constructions, tunnelling, under-seepage of levees, and liquefiable soils in areas prone to earthquakes [8], [9], [10], [13], [17]. Other promising applications involve constructing subsurface barriers to prevent the intrusion of salt water to freshwater aquifers, sealing ponds/reservoirs with a crust to control seepage into underlying soil, and finally treatments to suppress dust [8], [13], [17]. Noteworthily, all soil grouting treatments are hindered by the limited in-depth access of the grout. In general, chemical grouting is effective up to 1–2 m from the injection point [13], whereas for the biogrouting it was shown in a laboratory test that it was possible to precipitate CaCO3 at a distance of 5 m from the injection point [51].
Fig. 1.
Schematic representation of soil/sand biogrouting with CaCO3.
Interestingly, chemical grouting and biogrouting differ significantly in terms of costs. The costs of the raw materials for the chemical grouting were evaluated to be in the range 2–72 USD/m3 of soil, whereas those for the biogrouting, in cases where cheap waste materials were used as bacterial nutrients, in the range 0.5–9 USD/m3 of soil [17], the injection costs being approximately the same for the two techniques. Thus, in addition to sustainability issues, cost effectiveness, if cheap nutrients are used, may be another factor that argues in favour of the implementation of CaCO3 biogrouting in place of chemical grouting [17].
Protection and restoration of construction materials (deposition and cementation)
Construction (building) materials undergo progressive deterioration due to physical, chemical and biological weathering [12], [15], [50], [73], [78]. Disadvantageously, the process has today been tremendously accelerated by atmospheric pollution, products of the burning of fossil fuels (sulphur and nitrogen oxides) in particular. The weathering factors induce an ongoing dissolution of the mineral components of the material. This increases the porosity of the material, which in turn results in the weakening of the stability and durability of the construction, be it a building or a statue, made of calcareous materials (limestone, marble), bricks or concrete. Several chemical treatments are in use to protect and restore the materials [12], [73]. While water repellents are employed to protect the surfaces of the materials from the intrusion of water and other weathering agents carried therewith, pollutants included, consolidants (cementing or hardening agents) are aimed to strengthen their inner structures. Yet neither of the treatments has been recognized as fully satisfactory. Organic treatments, performed e.g. with acrylic or epoxy resins, when applied for the formation of surface layers frequently develop incompatible and harmful surface films, and when applied for cementation yield pore fillings with mechanical and thermal expansion properties different from those of the material. Additionally, the use of large amounts of organic solvents contributes to pollution. By contrast, inorganic treatments, such as the lime-water technique applied to produce CaCO3 cementation with use of Ca(OH)2 solution, end in the formation of fragile and ineffectual crusts. The treatments also suffer from irreversibility and limited durability [12], [73].
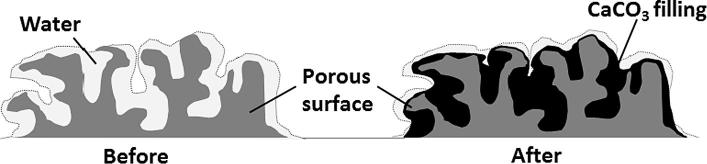
In response to these circumstances, ureolysis-driven CaCO3 formation has been widely studied mainly for the deposition (Fig. 2), also for the cementation, and has been implemented in real sites, especially in the sector of the restoration of cultural heritage for the conservation of historic stoneworks.
Fig. 2.
Schematic representation of biodeposition of CaCO3 layer on the porous surface of a construction material.
A construction material that is especially befitted for this treatment is limestone [12], [50], [73], [74], [79]. This is because the restoring agent is the same mineral product as the substrate it is applied to, importantly, obtained by mimicking the natural process responsible for calcareous stone formation. This warrants the excellent compatibility of the two materials and the fixation of the newly formed CaCO3 crystals on the outer and inner surfaces of the stone. Since its first real-site application in 1993 in Thouars, France, on the tower of Saint Médard Church [12], several procedures for the bio-based restoration of limestone have been developed by various academic institutions, such as the University of Nantes (France)-Historic Monuments Research Laboratory-Calcite Bioconcept [50], the University of Granada (Spain) [73], the University of Ghent (Belgium) [79] as well as the Biobrush consortium (UK) [12]. The developed procedures, optimized experimentally with use of different limestone samples in different conditions, have been mainly implemented for the biodeposition of the protective layers on building facades, of historic monuments in particular. Until now, the biodeposition treatments have been most commonly applied in France on several historic monuments across the country [12].
Another prospective application of biocementation is that applied to restore cementitious materials, i.e. concrete and mortar [80], [81], [82], [83], [84]. Cheap, easily available and convenient to cast, they are one of the most common man-made building materials worldwide, however, highly susceptible to weathering and cracking. Resulting from aging and freeze/thaw cycles, cracks and fissures in concrete are detrimental, as they allow penetration of water and ions, particularly chloride and acids, an effect that leads to corrosion of reinforcing steel. Importantly, in the biocementing of concrete care must be taken to protect the activity of bacteria/free urease from the high pH characteristic of cement, reaching the values ca. 11–13. One way to accomplish this protection is by using the immobilized bacteria/urease [56]. Also to avoid the impact of Cl− ions, the use of calcium acetate was advised [8], [80]. Incidentally, in the successful biodeposition of CaCO3, the observed thicknesses of the deposited layers were 2–5 μm on limestone [12], [50] and 10–40 μm on concrete [12].
The estimated total costs of biodeposition treatments comprising both the price of the raw materials (bacteria 2.2–3.3 €/m2; nutrients 7–15 €/m2 (ca. 80% of the raw materials costs)) and the number of applications, amount typically to 23–28 €/m2 and to 35–40 €/m2 for heavily degraded surfaces [12]. Given these data, the biodeposition treatments have been judged to be unable to outcompete the traditional surface treatments [12]. However, as in the case of biogrouting, if the costs of nutrients could be lowered, e.g. by using corn steep liquor (with the price 1.5 €/L compared to the standard nutrient 180 €/kg) [82], the costs of the raw materials could be radically reduced, indicating the substantial economization of the process.
Remediation of groundwater (removal of toxic metals, radionuclides and excess Ca2+)
Contamination of the environment with toxic metals and radionuclides constitute a serious hazard to human health and the environment. The presence of toxic metals in soil and groundwater, arsenic, copper, chromium, cadmium, mercury, lead and others, derives from various industries, mining and smelting in particular. Conventional remediation methods include phytoremediation, on-site chemical leaching of contaminants from soil, or bioremediation with toxic metal-tolerant bacteria [8], [10], [11], [16], in addition to physico-chemical methods, such as chemical precipitation, evaporation, adsorption, ion exchange and membrane separation [11]. All these treatments are judged expensive and insufficient for long terms [8], [10], [11], [16]. Likewise, the traditional methods using pump and treat for the removal of radionuclides, e.g. 90Sr2+, UO22+, 60Co2+, have been found ineffective [8], [10], [11], [16].
In this situation, urease-induced CaCO3 biomineralization was proposed for an effective and eco-friendly water clean-up of toxic metals and radionuclides through solid-phase capture [8], [10], [11], [16], [57]. Basically, the biomineralization of these metals consists of a competitive co-precipitation, in which the cations are incorporated into the CaCO3 lattice by substituting Ca2+ ions. This incorporation immobilizes the ions within the calcite structure thereby slowing their transport in the environment. However attractive, the process can be seriously hindered by the inactivating effect of heavy metal ions and other substances present in waters, on the activity of bacteria/free urease [39], [40], the use of immobilized urease being a solution to this problem [34], [47], [85], [86], [87].
The ureolytic biomineralization of CaCO3 was also explored for the removal of calcium from calcium-rich wastewater (500–1500 mg/L) [88]. Resulting from industries, such as paper recycling, bone processing and citric acid production, this condition provokes excessive precipitation of calcium salts, causing severe scaling in pipelines and reactors.
Sealing/plugging geological formations (geologic CO2 sequestration; secondary oil recovery)
Geologic CO2 sequestration, also termed carbon capture and storage, is a process aimed at mitigating the release of CO2 into the atmosphere to resolve the contribution of fossil fuel emissions to global warming and ocean acidification [59], [60], [61]. In the process, waste CO2 is captured from large point sources, e.g. fossil fuel power plants, compressed to a supercritical fluid and transported to a place of storage, where it is injected underground, typically ca. 1 km, into the formations, such as depleted oil and gas reservoirs, saline aquifers or un-minable coal beds, where it is trapped in pores and spaces in the rock structure. The site chosen for CO2 storage ideally should have the structure with high porosity and permeability; however, to prevent the leakage of CO2 it must be capped by a layer with low permeability (cap rock), which acts as a seal. Also the installations and the cementing materials need to be gas-tight. It is in this context that ureolysis-driven CaCO3 precipitation was proposed and studied to be employed in situ as a sealant for treating fractures and high permeability areas in cap rocks, well bore cement and installations [59], [61]. Besides, it is seen as a potential means to enhance the durability of CO2 storage by inducing the transformation of CO2 into a solid carbonate phase [60]. Importantly for this application, it was shown that neither Sporosarcina pasteurii [89] nor the free enzyme [23] had their activities incapacitated by the pressure and temperature conditions corresponding to the CO2 storage sites (P > 8.9 MPa, T ≥ 32 °C) [59], [89].
Another special application of CaCO3 bio-precipitation in the management of geological formations, is the selective plugging of oil reservoir bedrocks. The plugging is done for enhancing secondary oil recovery [62], [63]. This is because the secondary oil recovery is performed with water flooding, and to enable water to reach oil in the small pores of bedrocks, the bigger ones, from which oil has been already recovered by primary oil production, have to be blocked.
Other applications
In addition to the presented applications, the bio-process of Ca-carbonate formation has been also considered for other purposes. For instance, the formation of CaCO3 coating to encapsulate polychlorinated biphenyls (PCB) was proposed as a means to clean concrete surfaces contaminated with PCB-containing oil [90]. Other potential innovative applications were envisaged for the bacterially-synthesized fluorescent calcite as a filler in rubber and plastics, fluorescent particles in stationery ink, and a fluorescent marker for biochemistry applications [91]. Further to that, there are also novel concepts to exploit CaCO3 biomineralization in producing low energy building materials and in dealing with their industrial by-products [10].
Conclusions and future perspectives
The concept of using urease-aided calcium carbonate mineralization as a strengthening, consolidating or sealing/plugging technique, represents a promising development in the processes of remediation, cementation and deposition, employed in various engineering fields, notably geotechnical, construction and environmental, also importantly in the conservation of historic stone cultural heritage. The significance of the technique as compared to the present conventional techniques, lies in several facts. (i) The technique avoids using polymers and resins as cementing agents, which are alien to the materials they are applied to in respect of mechanical and thermal properties, (ii) it avoids using organic solvents, (iii) both the cementing agents and the solvents applied may be toxic/harmful to the environment, and finally, (iv) it avoids using cement, a material whose production greatly contributes to the emission of CO2, Quite to the contrary, (v) the technique employs aqueous mixtures of the reacting components (ureolytic bacteria/free urease, urea and Ca salt), (vi) the cementing agent is CaCO3, the natural mineral perfectly compatible with the materials under treatment, (vii) the cementing agent is not mechanically injected to the material, but formed in situ inside the material following the same reaction scheme as in nature, (viii) the process is carried through in mild conditions. These facts classify the technique as an environmentally-friendly and sustainability-promoting approach to geotechnical and construction engineering applications.
Advantageous as it is, the urease-based technique also features several limitations. These include: (i) the production of by-products: ammonia and an anion (most commonly Cl−), both being harmful, the former for health and the environment, the latter for the treated materials, (ii) the longevity of bacteria implanted into the materials that may evolve into biofilm, (iii) limited reliability of the catalytic action of bacteria/urease, deriving from their susceptibility to inhibitions, (iv) limited cost effectiveness brought in mainly by the bacterial nutrients, (v) the complexity of the process that relies on multiplicity of bio-, chem- and geo-parameters, all of them difficult for comprehensive modelling.
Already in use in the protection of historic buildings, in many respects the technique remains under laboratory experimentation. To become fully implementable as a reliable, functional and economic technique, it still needs much exploration involving the resolution of the limitations, the parametrical optimization, and importantly, up-scaling and life-size field experiments. These, as an interdisciplinary effort of geologists, microbiologists, chemists and biochemists, civil engineers and conservators of historic monuments, will finally bring this environmentally safe, energy-saving and convenient technology from laboratory to field applications, and will advance this innovative branch of engineering to commercial scale.
Acknowledgments
Conflict of Interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
This work was supported by DS WCh/43 from Faculty of Chemistry of the Jagiellonian University, Kraków, Poland.
Biography
 Barbara Krajewska, Dr. Habil, PhD, has received her academic degrees in chemistry from the Faculty of Chemistry of the Jagiellonian University in Kraków, Poland. Her research interests evolved from membrane separation processes, through (bio)polymers as biomedical materials, to enzymes free and immobilized. The polymer and enzyme she concentrates on are chitosan and urease. She studies chitosan as a biomaterial and as a urease immobilization support, also native and immobilized urease, interfacial phonemena taking place in the urease system, and importantly, applications of both forms of urease.
Barbara Krajewska, Dr. Habil, PhD, has received her academic degrees in chemistry from the Faculty of Chemistry of the Jagiellonian University in Kraków, Poland. Her research interests evolved from membrane separation processes, through (bio)polymers as biomedical materials, to enzymes free and immobilized. The polymer and enzyme she concentrates on are chitosan and urease. She studies chitosan as a biomaterial and as a urease immobilization support, also native and immobilized urease, interfacial phonemena taking place in the urease system, and importantly, applications of both forms of urease.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Fearon W.R. Urease. Part I. The chemical changes involved in the zymolysis of urea. Biochem J. 1923;17:84–93. doi: 10.1042/bj0170084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sumner J.B. The isolation and crystallization of the enzyme urease. J Biol Chem. 1926;69:435–441. [Google Scholar]
- 3.Dixon N.E., Gazzola C., Blakeley R.L., Zerner B. Jack bean urease (EC 3.5.1.5). A metalloenzyme. A simple biological role for nickel. J Am Chem Soc. 1975;97:4131–4133. doi: 10.1021/ja00847a045. [DOI] [PubMed] [Google Scholar]
- 4.Mazzei L., Musiani F., Ciurli S. Urease. RSC Metallobiology. 2017;10:60–97. [Google Scholar]
- 5.Krajewska B. Ureases. I. Functional, kinetic and catalytic properties: a review. J Mol Catal B: Enzym. 2009;59:9–21. [Google Scholar]
- 6.Follmer C. Insights into the role and structure of plant ureases. Phytochemistry. 2008;69:18–28. doi: 10.1016/j.phytochem.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 7.Hausinger R.P., Karplus P.A. Urease. In: Wieghardt K., Huber R., Poulos T.L., Messerschmidt A., editors. Handbook of Metalloproteins. Wiley; West Sussex: 2001. pp. 867–879. [Google Scholar]
- 8.Phillips A.J., Gerlach R., Lauchnor E., Mitchell A.C., Cunningham A.B., Spangler L. Engineered applications of ureolytic biomineralization: a review. Biofouling. 2013;29:715–733. doi: 10.1080/08927014.2013.796550. [DOI] [PubMed] [Google Scholar]
- 9.Mujah D., Shahin M.A., Cheng L. State-of-the-art review of biocementation by microbially induced calcite precipitation (MICP) for soil stabilization. Geomicrobiol J. 2017;34:524–537. [Google Scholar]
- 10.Dhami N.K., Reddy M.S., Mukherjee A. Biomineralization of calcium carbonates and their engineered applications. Frontiers Microbiol. 2013;4:314. doi: 10.3389/fmicb.2013.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anbu P., Kang C.-H., Shin Y.-J., So S.-S. Formation of calcium carbonate minerals by bacteria and its multiple applications. SpringerPlus. 2016;5:250. doi: 10.1186/s40064-016-1869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Muynck W., De Belie N., Verstraete W. Microbial carbonate precipitation in construction materials: a review. Ecol Eng. 2010;36:118–136. [Google Scholar]
- 13.DeJong J.T., Mortensen B.M., Martinez B.C., Nelson D.C. Bio-mediated soil improvement. Ecol Eng. 2010;36:197–210. [Google Scholar]
- 14.Achal V., Mukherjee A., Kumari D., Zhang Q. Biomineralization for sustainable construction – A review of processes. Earth-Sci Rev. 2015;148:1–17. [Google Scholar]
- 15.Dhami N.K., Reddy M.S., Mukherjee A. Application of calcifying bacteria for remediation of stones and cultural heritage. Frontiers Microbiol. 2014;5:304. doi: 10.3389/fmicb.2014.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumari D., Qian X.-Y., Pan X., Achal V., Li Q., Gadd G.M. Microbially-induced carbonate precipitation for immobilization of toxic metals. Adv Appl Microbiol. 2016;94:79–108. doi: 10.1016/bs.aambs.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Ivanov V., Chu J. Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev Environ Sci Biotechnol. 2008;7:139–153. [Google Scholar]
- 18.Dilrukshi R.A.N., Kawasaki S. Effective use of plant-derived urease in the field of geoenvironmental/geotechnical engineering. J Civil Environ Eng. 2016;6:207. [Google Scholar]
- 19.Rodriguez-Navarro C., Jroundi F., Schiro M., Ruiz-Agudo E., Gonzalez-Muñoz M.T. Influence of substrate mineralogy on bacterial mineralization of calcium carbonate: Implications for stone conservation. Appl Environ Microbiol. 2012;78:4017–4029. doi: 10.1128/AEM.07044-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammes F., Verstraete W. Key roles of pH and calcium metabolism in microbial carbonate precipitation. Re/Views Environ Sci Bio/Technol. 2002;1:3–7. [Google Scholar]
- 21.Castanier S., Le Métayer-Levrel G., Perthuisot J.-P. Ca-carbonates precipitation and limestone genesis – the microbiogeologist point of view. Sediment Geol. 1999;126:9–23. [Google Scholar]
- 22.Benini S., Rypniewski W.R., Wilson K.S., Miletti S., Ciurli S., Mangani S. A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: why urea hydrolysis costs two nickels. Structure. 1999;7:205–216. doi: 10.1016/S0969-2126(99)80026-4. [DOI] [PubMed] [Google Scholar]
- 23.Krajewska B., van Eldik R., Brindell M. Temperature- and pressure-dependent stopped-flow kinetic studies of jack-bean urease. Implications for the catalytic mechanism. J Biol Inorg Chem. 2012;17:1123–1134. doi: 10.1007/s00775-012-0926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krajewska B. A combined temperature-pH study of urease kinetics. Assigning pKa values to ionizable groups of the active site involved in the catalytic reaction. J Mol Catal B: Enzym. 2016;124:70–76. [Google Scholar]
- 25.Kosikowska P., Berlicki Ł. Urease inhibitors as potential drugs for gastric and urinary tract infections: a patent review. Expert Opin Ther Pat. 2011;21:945–957. doi: 10.1517/13543776.2011.574615. [DOI] [PubMed] [Google Scholar]
- 26.Macegoniuk K. Inhibitors of bacterial and plant urease A review. Folia Biol Oecol. 2013;9:9–16. [Google Scholar]
- 27.Macegoniuk K., Kowalczyk R., Rudzińska A., Psurski M., Wietrzyk J., Berlicki Ł. Potent covalent inhibitors of bacterial urease identified by activity-reactivity profiling. Bioorg Med Chem Lett. 2017;27:1346–1350. doi: 10.1016/j.bmcl.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 28.Modolo L.V., de Souza A.X., Horta L.P., Araujo D.P., de Fatima A. An overview on the potential of natural products as ureases inhibitors: a review. J Adv Res. 2015;6:35–44. doi: 10.1016/j.jare.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krajewska B., Zaborska W. Jack bean urease. The effect of active-site binding inhibitors on the reactivity of enzyme thiol groups. Bioorg Chem. 2007;35:355–365. doi: 10.1016/j.bioorg.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Todd M.J., Hausinger R.P. Competitive inhibitors of Klebsiella aerogenes urease. Mechanisms of interaction with the nickel active site. J Biol Chem. 1989;264:15835–15842. [PubMed] [Google Scholar]
- 31.Benini S., Rypniewski W.R., Wilson K.S., Ciurli S., Mangani S. The complex of Bacillus pasteurii with β-mercaptoethanol from X-ray data at 1.65 Å resolution. J Biol Inorg Chem. 1998;3:268–273. doi: 10.1007/s007750050014. [DOI] [PubMed] [Google Scholar]
- 32.Krajewska B., Brindell M. Thermodynamic study of competitive inhibitors’ binding to urease. J Therm Anal Calorim. 2016;123:2427–2439. [Google Scholar]
- 33.Benini S., Rypniewski W.R., Wilson K.S., Miletti S., Ciurli S., Mangani S. The complex of Bacillus pasteurii with acetohydroxamate anion from X-ray data at 1.55 Å resolution. J Biol Inorg Chem. 2000;5:110–118. doi: 10.1007/s007750050014. [DOI] [PubMed] [Google Scholar]
- 34.Krajewska B., Zaborska W., Leszko M. Inhibition of chitosan-immobilized urease by slow binding inhibitors: Ni2+, F− and acetohydroxamic acid. J Mol Catal B: Enzym. 2001;14:101–109. [Google Scholar]
- 35.Vassiliou S., Grabowiecka A., Kosikowska P., Yiotakis A., Kafarski P., Berlicki Ł. Design, synthesis, and evaluation of novel organophosphorus inhibitors of bacterial ureases. J Med Chem. 2008;51:5736–5744. doi: 10.1021/jm800570q. [DOI] [PubMed] [Google Scholar]
- 36.Krajewska B., Zaborska W., Leszko M., Brzózka Z. Inhibition of jack bean urease by a mixture of boric acid and phosphate buffer pH 6.96. Polish J Chem. 1999;73:359–366. [Google Scholar]
- 37.Krajewska B., Zaborska W. The effect of phosphate buffer in the range of pH 5.80-8.07 on jack bean urease activity. J Mol Catal B: Enzym. 1999;6:75–81. [Google Scholar]
- 38.Krajewska B., Ciurli S. Jack bean (Canavalia ensiformis) urease. Probing acid-base groups of the active site by pH-variation. Plant Physiol Biochem. 2005;43:651–658. doi: 10.1016/j.plaphy.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Zaborska W., Krajewska B., Olech Z. Heavy metal ions inhibition of jack bean urease: potential for rapid contaminant probing. J Enzyme Inhib Med Chem. 2004;19:65–69. doi: 10.1080/14756360310001650237. [DOI] [PubMed] [Google Scholar]
- 40.Krajewska B. Mono- (Ag, Hg) and di- (Cu, Hg) valent metal ions effects on the activity of jack bean urease. Probing the modes of metal binding to the enzyme. J Enzyme Inhib Med Chem. 2008;23:535–542. doi: 10.1080/14756360701743051. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L., Mulrooney S.B., Leung A.F.K., Zeng Y., Ko B.B.C., Hausinger R.P. Inhibition of urease by bismuth(III): implications for the mechanism of action of bismuth drugs. Biometals. 2006;19:503–511. doi: 10.1007/s10534-005-5449-0. [DOI] [PubMed] [Google Scholar]
- 42.Zaborska W., Krajewska B., Kot M., Karcz W. Quinone-induced inhibition of urease. Elucidation of its mechanisms by probing thiol groups of the enzyme. Bioorg Chem. 2007;35:233–242. doi: 10.1016/j.bioorg.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Krajewska B., Zaborska W. Double mode of inhibition-inducing interactions of 1,4-naphthoquinone with urease. Arylation vs oxidation of enzyme thiols. Bioorg Med Chem. 2007;15:4144–4151. doi: 10.1016/j.bmc.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 44.Mazzei L., Cianci M., Musiani F., Ciurli S. Inactivation of urease by 1,4-benzoquinone: chemistry at the protein surface. Dalton Trans. 2016;45:5455–5459. doi: 10.1039/c6dt00652c. [DOI] [PubMed] [Google Scholar]
- 45.Benini S., Cianci M., Mazzei L., Ciurli S. Fluoride inhibition of Sporosarcina pasteurii urease: structure and thermodynamics. J Biol Inorg Chem. 2014;19:1243–1261. doi: 10.1007/s00775-014-1182-x. [DOI] [PubMed] [Google Scholar]
- 46.Krajewska B. Ureases. II. Properties and their customizing by enzyme immobilizations: a review. J Mol Catal B: Enzym. 2009;59:22–40. [Google Scholar]
- 47.Krajewska B. Urease immobilized on chitosan membrane. Inactivation by heavy metal ions. J Chem Tech Biotechnol. 1991;52:157–162. [Google Scholar]
- 48.De Muynck W., Verbeken K., De Belie N., Verstraete W. Influence of urea and calcium dosage on the effectiveness of bacterially induced carbonate precipitation on limestone. Ecol Eng. 2010;36:99–111. [Google Scholar]
- 49.Stocks-Fischer S., Galinat J.K., Bang S.S. Microbiological precipitation of CaCO3. Soil Biol Biochem. 1999;31:1563–1571. [Google Scholar]
- 50.Le Métayer-Levrel G., Castanier S., Orial G., Loubière J.-F., Perthuisot J.-P. Applications of bacterial carbonatogenesis to the protection and regeneration of limestones in buildings and historic patrimony. Sediment Geol. 1999;126:25–34. [Google Scholar]
- 51.Whiffin V.S., van Paassen L.A., Harkes M.P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol J. 2007;24:417–423. [Google Scholar]
- 52.Harkes M.P., van Paassen L.A., Booster J.L., Whiffin V.S., van Loosdrecht M.C.M. Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol Eng. 2010;36:112–117. [Google Scholar]
- 53.van Paassen L.A., Ghose R., van der Linden T.J.M., van der Star W.R.L., van Loosdrecht M.C.M. Quantifying biomediated ground improvement by ureolysis: large-scale biogrout experiment. J Geotech Geoenviron Eng. 2010;136:1721–1728. [Google Scholar]
- 54.Lauchnor E.G., Topp D.M., Parker A.E., Gerlach R. Whole cell kinetics of ureolysis by Sporosarcina pasteurii. J Appl Microbiol. 2015;118:1321–1332. doi: 10.1111/jam.12804. [DOI] [PubMed] [Google Scholar]
- 55.Okwadha G.D.O., Li J. Optimum conditions for microbial carbonate precipitation. Chemosphere. 2010;81:1143–1148. doi: 10.1016/j.chemosphere.2010.09.066. [DOI] [PubMed] [Google Scholar]
- 56.Bachmeier K.L., Williams A.E., Warmington J.R., Bang S.S. Urease activity in microbiologically-induced calcite precipitation. J Biotechnol. 2002;93:171–181. doi: 10.1016/s0168-1656(01)00393-5. [DOI] [PubMed] [Google Scholar]
- 57.Fujita Y., Ferris F.G., Lawson R.D., Colwell F.S., Smith R.W. Calcium carbonate precipitation by ureolytic subsurface bacteria. Geomicrobiol J. 2000;17:305–318. [Google Scholar]
- 58.Ferris F., Phoenix V., Fujita Y., Smith R. Kinetics of calcium precipitation induced by ureolytic bacteria at 10 and 20°C in artificial groundwater. Geochim Cosmochim Acta. 2003;68:1701–1710. [Google Scholar]
- 59.Mitchell A.C., Dideriksen K., Spangler L., Cunningham A., Gerlach R. Microbially enhanced carbon capture and storage by mineral-trapping and solubility-trapping. Environ Sci Technol. 2010;44:5270–5276. doi: 10.1021/es903270w. [DOI] [PubMed] [Google Scholar]
- 60.Dupraz S., Parmentier M., Ménez B., Guyot F. Experimental and numerical modeling of bacterially induced pH increase and calcite precipitation in saline aquifers. Chem Geol. 2009;265:44–53. [Google Scholar]
- 61.Phillips A.J., Lauchnor E., Eldring J., Esposito R., Mitchell A.C., Gerlach R. Potential CO2 leakage reduction through biofilm-induced calcium carbonate precipitation. Environ Sci Technol. 2013;47:142–149. doi: 10.1021/es301294q. [DOI] [PubMed] [Google Scholar]
- 62.Nemati M., Voordouw G. Modification of porous media permeability, using calcium carbonate produced enzymatically in situ. Enzyme Microb Tech. 2003;33:635–642. [Google Scholar]
- 63.Nemati M., Greene E.A., Voordouw G. Permeability profile modification using bacterially formed calcium carbonate; comparison with enzymic option. Process Biochem. 2005;40:925–933. [Google Scholar]
- 64.Neupane D., Yasuhara H., Kinoshita N., Unno T. Applicability of enzymatic calcium carbonate precipitation as a soil-strengthening technique. J Geotech Geoenviron Eng. 2013;139:2201–2211. [Google Scholar]
- 65.Yasuhara H., Neupane D., Hayashi K., Okamura M. Experiments and predictions of physical properties of sand cemented by enzymatically-induced carbonate precipitation. Soils Found. 2012;52:539–549. [Google Scholar]
- 66.Sondi I., Matijević E. Homogeneous precipitation of calcium carbonates by enzyme catalyzed reaction. J Colloid Interf Sci. 2001;238:208–214. doi: 10.1006/jcis.2001.7516. [DOI] [PubMed] [Google Scholar]
- 67.Sondi I., Salopek-Sondi B. Influence of the primary structure of enzymes on the formation of CaCO3 polymorphs: a comparison of plant (Canavalia ensiformis) and bacterial (Bacillus pasteurii) ureases. Langmuir. 2005;21:8876–8882. doi: 10.1021/la051129v. [DOI] [PubMed] [Google Scholar]
- 68.Nam I.H., Chon C.M., Yung K.-Y., Choi S.-G., Choi H., Park S.S. Calcite precipitation by ureolytic plant (Canavalia ensiformis) extracts as effective biomaterials. KSCE J Civil Eng. 2015;19:1620–1625. [Google Scholar]
- 69.Park S.-S., Choi S.-G., Nam I.-H. Effect of plant-induced calcite precipitation on the strength of sand. J Mat Civ Eng. 2014;26:1–5. [Google Scholar]
- 70.Cheng L., Cord-Ruwisch R. Selective enrichment and production of highly urease active bacteria by non-sterile (open) chemostat culture. J Ind Microbiol Biotech. 2013;40:1095–1104. doi: 10.1007/s10295-013-1310-6. [DOI] [PubMed] [Google Scholar]
- 71.Blakeley R.L., Zerner B. Jack bean urease. The first nickel enzyme. J Mol Catal. 1984;23:263–292. [Google Scholar]
- 72.Hamdan N., Kavazanjian E., Jr, O’Donnell S. Carbonate cementation via plant derived urease. In: Delage P., Desrues J., Frank R., Puech A., Schlosser F., editors. Proceedings of the 18th International Conference on Soil Mechanics and Geotechnical Engineering; 2013 Sep 2-6; Paris, France. Presses des ponts; 2013. pp. 2489–2492. [Google Scholar]
- 73.Rodriguez-Navarro C., Rodriguez-Gallego M., Chekroun K.B., Gonzalez-Muñoz M.T. Conservation of ornamental stone by Myxococcus xantus-induced carbonate biomineralization. Appl Environ Microbiol. 2003;69:2182–2193. doi: 10.1128/AEM.69.4.2182-2193.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tiano P., Biagiotti L., Mastromatei G. Bacterial bio-mediated calcite precipitation for monumental stone conservation: methods of evaluation. J Microbiol Meth. 1999;36:139–145. doi: 10.1016/s0167-7012(99)00019-6. [DOI] [PubMed] [Google Scholar]
- 75.Achal V., Pan X. Influence of calcium sources on microbially induced calcium carbonate precipitation by Bacillus sp. CR2. Appl Biochem Biotechnol. 2014;173:307–317. doi: 10.1007/s12010-014-0842-1. [DOI] [PubMed] [Google Scholar]
- 76.Hammes F., Boon N., de Villiers J., Verstraete W., Siciliano S.D. Strain-specific ureolytic microbial calcium carbonate precipitation. Appl Environ Microbiol. 2003;69:4901–4909. doi: 10.1128/AEM.69.8.4901-4909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Worrel E., Price L., Martin N., Hendriks C., Meida L.O. Carbon dioxide emissions from the global cement industry. Annu Rev Energy Environ. 2001;26:303–329. [Google Scholar]
- 78.Fernandes P. Applied microbiology and biotechnology in the conservation of stone cultural heritage. Appl Microbiol Biotechnol. 2006;73:291–296. doi: 10.1007/s00253-006-0599-8. [DOI] [PubMed] [Google Scholar]
- 79.Dick J., De Windt W., De Graef B., Saveyn H., Van der Meeren P., De Belie N. Bio-deposition of a calcium carbonate layer on degraded limestone by Bacillus Species. Biodegradation. 2006;17:357–367. doi: 10.1007/s10532-005-9006-x. [DOI] [PubMed] [Google Scholar]
- 80.Siddique R., Chahal N.K. Effect of ureolytic bacteria on concrete properties. Construct Build Mater. 2011;25:3791–3801. [Google Scholar]
- 81.De Muynck W., Cox K., De Belie N., Verstaete W. Bacterial carbonate precipitation as an alternative surface treatment for concrete. Constr Build Mater. 2008;22:875–885. [Google Scholar]
- 82.Achal V., Mukherjee A., Reddy M.S. Effect of calcifying bacteria on permeation properties of concrete structures. J Ind Microbiol Biotech. 2011;38:1229–1234. doi: 10.1007/s10295-010-0901-8. [DOI] [PubMed] [Google Scholar]
- 83.Achal V., Mukherjee A., Reddy M.S. Biogenic treatment improves the durability and remediates the cracks of concrete structures. Constr Build Mater. 2013;48:1–5. [Google Scholar]
- 84.Joshi S., Goyal S., Mukherjee A., Reddy M.S. Microbial healing of cracks in concrete: a review. J Ind Microbiol Biotech. 2017;44:1511–1525. doi: 10.1007/s10295-017-1978-0. [DOI] [PubMed] [Google Scholar]
- 85.Krajewska B., Zaborska W., Leszko M. Inhibition of chitosan-immobilized urease by boric acid as determined by integration methods. J Mol Catal B: Enzym. 1997;3:231–238. [Google Scholar]
- 86.Krajewska B. Chitosan membrane-immobilized urease. Kinetic behaviour in phosphate buffer in the pH range 5.76-8.19. J Bioact Comp Polym. 2000;15:155–169. [Google Scholar]
- 87.Krajewska B., Piwowarska Z. Free vs chitosan-immobilized urease. Microenvironmental effects on enzyme inhibitions. Biocatal Biotransform. 2005;23:225–232. [Google Scholar]
- 88.Hammes F., Seka A., de Knijf S., Verstraete W. A novel approach to calcium removal from calcium-rich industrial wastewater. Water Res. 2003;37:699–704. doi: 10.1016/s0043-1354(02)00308-1. [DOI] [PubMed] [Google Scholar]
- 89.Mitchell A., Phillips A., Kaszuba J., Hollis W., Cunningham A., Gerlach R. Microbially enhanced carbonate mineralization and the geologic containment of CO2. Geochim Cosmochim. Acta. 2008;72:A636. [Google Scholar]
- 90.Okwadha G.D.O., Li J. Biocontainment of polychlorinated biphenyls (PCBs) on flat concrete surfaces by microbial carbonate precipitation. J Environ Manage. 2011;92:2860–2864. doi: 10.1016/j.jenvman.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 91.Yoshida N., Higashimura E., Saeki Y. Catalytic biomineralization of fluorescent calcite by the thermophilic bacterium Geobacillus thermoglucosidasius. Appl Environ Microbiol. 2010;76:7322–7327. doi: 10.1128/AEM.01767-10. [DOI] [PMC free article] [PubMed] [Google Scholar]