Abstract
Objectives
The aims of this study were to create a new surface topography using simulated body fluids (SBF) and Gold Nanoparticles (GNPs) and then to assess the influence of UV Photofunctionalization (PhF) on the osteogenic capacity of these surfaces.
Materials and methods
Titanium plates were divided into six groups All were acid etched with 67% Sulfuric acid, 4 were immersed in SBF and 2 of these were treated with 10 nm GNPs. Half of the TiO2 plates were photofunctionalized to be compared with the non-PhF ones. Rat's bone marrow stem cells were seeded into the plates and then CCK8 assay, cell viability assay, immunofluorescence, and Scanning electron microscopy (SEM) were done after 24 hours. Gene expression analysis was done using real time quantitative PCR (qPCR) one week later to check for the mRNA expression of Collagen-1, Osteopontin and Osteocalcin. Alkaline phosphatase (ALP) activity was assessed after 2 weeks of cell seeding.
Results
Our new topography has shown remarkable osteogenic potential. The new surface was the most biocompatible, and the 10 nm GNPs did not show any cytotoxicity. There was a significant increase in bioactivity, enhanced gene expressions and ALP activity.
Conclusions
GNPs enhances osteogenic differentiation of stem cells and Photofunctionalizing GNPs highly increases this. We have further created a novel highly efficient topography which highly enhances the speed and extent of osseointegration. This may have great potential for improving treatment outcomes for implant, maxillofacial as well as orthopedic patients.
Keywords: Nanotechnology, Biomedical engineering, Dentistry
1. Introduction
Titanium has become the material of choice in many treatment modalities in the dental field [1]. For dental implants, osseointegration (OI) of bone to the implant surface is considered the major criteria of implant's treatment success [2, 3, 4, 5]. Titanium has a high osseointegrative capacity compared to other biomaterials. It can achieve high levels of bone anchorage due to their biocompatibility and excellent mechanical properties, and extensive research has been done to enhance these surface properties. In this study, we combine some of the most recent trends of titanium surface modifications to assess their effect on the OI process.
1.1. Photofunctionalization
Although titanium is the most biocompatible and the most clinically used alloy for orthopedic and dental implants, it was found to be subjected to “biological ageing” in which they encounter time-related degradation of their bioactivity leading to a decrease in their osteoconductivity over time [6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24]. One of the approaches that have been done to overcome this issue was Photofunctionalization (PhF), a concept described by Takahiro Ogawa [11] in which he used ultraviolet light to alter the physiochemical properties of titanium surfaces and increase the bone-implant contact (BIC) to almost 100%, a phenomenon termed “Superosseointegration”. Ultraviolet application to titanium surfaces was shown to effectively reverse the ageing process of titanium caused by the deposition of hydrocarbon on its surface. It also restored the surface charge back to positive and remarkably increased the hydrophilicity of the surface.
On the molecular level, protein absorption, and osteoblastic function becomes highly enhanced leading to an almost ideal surface contact, and therefore better primary stability and substantial therapeutic significance. Photofunctionalization have shown to decreases morbidity and improves treatment outcome by highly increasing the Bone-implant contact leading to a 3-fold increase in the strength of primary stability. It therefore allows for a quicker loading protocol and a decreased overall treatment time. It also permits the use of shorter and smaller implants in complex cases without compromising the success rate [17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68]. Photofunctionalization resulted in similar effects when applied on other alloys as well as non-alloy materials such as zirconium, opening possibilities to further research applications in the field of dental materials. Moreover, this new technology has been applied with different other surface modification techniques such as nanotechnology and stem cell tissue engineering and has shown promising results [32, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97].
1.2. Gold nanoparticles (GNPs)
The unique physical and chemical properties and the high biocompatibility of Gold nanoparticles (GNPs) have made them highly attractive for the use in different medical fields such as targeted gene and drug delivery, diagnostics as well as tissue engineering research [98, 99, 100, 101, 102, 103, 104].
Cellular uptake of GNPs causes them to bind to cytoplasmic proteins and act as osteogenic agents to enhance bone tissue regeneration [105]. Following cellular endocytosis [106, 107], GNPs act as a mechanical stimuli and lead to activation of the p38 mitogen-activated protein kinase signaling pathway (MAPK) which up-regulates the transcription factor (Runx-2) in the nucleus. Runx-2 is the main gene responsible for directing and enhancing osteogenic differentiation of mesenchymal stem cells into osteoprogenitor cells. It induces the expression of other osteoblastic specific genes such as Osteopontin (OPN), Osteocalcin (OCN) and Collagen type I, alpha 1(Col-1) [108, 109, 110, 111] (Fig. 1).
Fig. 1.
Endocytosis of GNPs causes them to activate the p38 MAPK pathway which stimulates of the Runx-2 gene leading to osteogenic differentiation.
Previous studies have shown that beside these osteogenic capabilities, GNPs also inhibit osteoclast formation [112] as well as adipogenic differentiation [113]. GNPs can therefore be very useful if it is applied on titanium surfaces as a method of surface modification as it can stimulate bone formation and hence, faster implant osseointegration and better primary stability [75]. One recent study found that 28 nm sized GNPs enhances osteogenic differentiation of Adipose Derived Stem Cells and increases the expression of specific markers related to osseous growth. These concluded that these findings suggested that Titanium-GNP (Ti-GNP) have an increased osteoconductive capabilities [114]. Nevertheless, Others have found that the smaller the size of GNPs, the more significant impact on osteogenic differentiation [115].
In this study, 10 nm diameter spherical GNPs were used in order to assess whether this size will promote more osteogenic differentiation than those previously mentioned in the literature.
1.3. Simulated body fluid (SBF)
In order to bond to living bone, it is essential for any biomaterial to form a biomimetic bone-like apatite on the material surface. Simulated body fluid (SBF) is a solution which has an ion concentration close to that found in human blood plasma [116, 117]. The precipitation of calcium and phosphate ions found in SBF causes spontaneous growth of apatite with a similar molecular structure to bone on the surface of biomaterials. Different materials have been immersed in SBF, and it was shown that their in vitro bone bioactivity could be predicted by the extent of resulting apatite formation [118]. Several studies have reported that SBF application on titanium leads to biomimetic deposition of apatite crystals on their surfaces [119, 120, 121]; this formed hydroxyapatite layer highly increases the osteoconductivity of titanium surface and hence, improves the osseointegration process [122, 123]. In our study, we have used SBF alone and with gold nanoparticles and with or without UV light application to assess the extent of tissue growth on the titanium plates.
1.4. Mesenchymal stem cells (MSCs)
Stem cells are promising tools in tissue engineering; they are characterised by their ability of self-renewal and their potential to differentiate into various cell lineages from the three germ layers. Mesenchymal stem cells (MSCs) are multipotent cells and have the potential to differentiate into mesodermal cells to form bone, cartilage, skeletal muscles or adipose tissue [124, 125] This process of differentiation is a basic step in promoting regeneration [126]. The abundance of MSCs at the site of bone injury and their ability to suppress the immune response and stimulate bone regeneration [127] makes these cells very influential in the process of osseointegration.
The bone marrow contains a considerable population of MSCs capable of differentiating into bone. The differentiation of these cells into osteogenic progenitor cells can be influenced by many factors [128, 129]. In this in vivo study, we have used rat's bone marrow stem cells (rBMSCs) to evaluate the growth of MSCs on different titanium surface treatment modalities. Cellular differentiation was evaluated by the expression of col-1, OPN and OCN as well as the expression of the Alkaline phosphatase (ALP) activity of the cells.
2. Materials and methods
2.1. Experiment outline
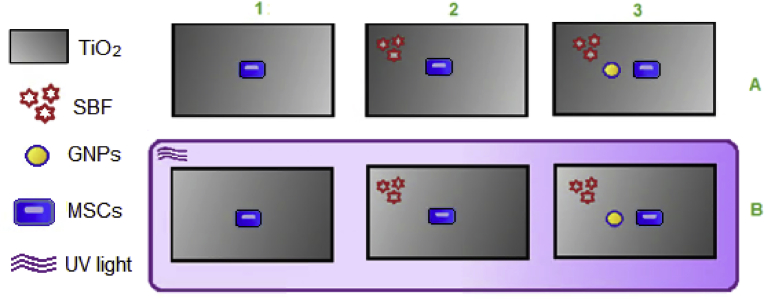
The objective of this study was to assess the cell growth of mesenchymal stem cells (MSCs) obtained from rat's bone marrow stem cells on the surface of titanium (TiO2) in different conditions. SBF, GNPs and UV PhF were applied to the TiO2 plates. The main outline of the experiment consisted of six groups of titanium plates, each group has a different modification modality. The groups were named 1A, 2A, 3A, 1B, 2B and 3B. Their surface characteristics were prepared as the following (Fig. 2):
Group A:
1- (TiO2 + MSCs),
2- (TiO2 + MSCs + SBF),
3- (TiO2 + MSCs + SBF + GNPs)
Group B:
1- (TiO2 + MSCs + PhF),
2- (TiO2 + MSCs + SBF + Ph)F,
3- (TiO2 + MSCs + SBF + GNPs + PhF)
Fig. 2.
TiO2 discs were grouped as follows: 1. MSCs, 2. MSCs and SBF, 3. MSC, SBF and GNPs, A. Non-photofunctionalized group, B. Photofunctionalized group.
2.2. Processing methods
Commercially pure (99.7%) Titanium grade 4 disks (20*20 mm, 1.5 mm thickness) were obtained (Baoji Rare Titanium Nickel co, Guangzhou, P.R. China). The sequence of processing was acid etching for 30 minutes, SBF for 24 hours, PhF for 48 hours, GNPs for 24 hours, followed by MSCs application on the TiO2 plates to evaluate their osteogenic differentiation and growth.
2.2.1. Acid etching
All titanium plates were sterilized using a regular autoclave, and then acid-etched with 67% Sulfuric acid (H2SO4) at 120 °C for 30 minutes. All plates were then disinfected, washed twice with distilled water and then put into the 6 well cell culture plate.
2.2.2. Photofunctionalization
Group B titanium plates were photofunctionalized by closely exposing them to UV light for 48 hours in a dark box using a 15 W bactericidal lamp; (Toshiba, Tokyo, Japan) with a combined peak intensity of 0.05 mW/cm2 (λ = 360 ± 20 nm) and 2 mW/cm2 (λ = 250 ± 20 nm).
2.2.3. Biomimetic hydroxyapatite deposition and characterization
Simulated Body Fluids from Beijing LEAGENE Biotechnology Co., Ltd (CZ0400), was prepared to mimic the ion concentrations found in human plasma. Titanium plates in groups 2A, 3A, 2B and 3B were soaked in SBF (pH 7.40) at 37 °C for 24 hours prior to the experiment. The disks were then removed and gently washed three times in distilled water for 30 seconds and then air-dried on a clean bench. Surface morphology was then assessed using scanning electron microscopy (SEM).
2.2.4. Titanium plates preparation
TiO2 plates from groups 3A and 3B were immersed in hexane acetone, ethanol and deionized water. They were then ultrasonically cleaned for 15 minutes, dried with nitrogen, soaked in sodium hydroxide solution for 24 hours, washed thoroughly with deionized water and ultrasonically cleaned again. TiO2 plates were then immersed in toluene solution containing 2% MPTPs at 60 °C for 24 hours, disinfected with ethanol, washed with deionized water and dried with nitrogen. Finally, the plates were immersed in 10 nm GNP solution from Shanghai carboxene biopharmaceutical Technology Co., Ltd (Au01002) at 37 °C for 24 hours, thoroughly cleaned with deionized water and then put into the 6-well plate.
2.2.5. Rat bone marrow stem cells (rBMSCs) isolation and culture
All procedures employed in this study involving animals and their care were conducted in accordance to the National Institutes of Health guidelines (NIH Publication No. 85–23, revised 1996) and were approved by the Wuhan University Animal Care and Use Committee.
2.2.5.1. Isolation
Healthy 6–8 week old Sprague Dawley (SD) rats (4 SPF) were purchased from animal experimental center of Zhongnan Hospital of Wuhan University; L-DMEM medium (Dulbecco's Modified Eagle's medium), fetal bovine serum, trypsin, penicillin and streptomycin from (Procell); 100 mm plastic cell culture dishes from (Corning, USA); Inverted phase contrast microscope from (Olympus, Japan).
SD rats anesthetized with 10% chloral hydrate. 75% ethanol disinfection was done for 10 minutes twice and both lower limbs and lower abdominal area were cut with sterile scissors. Lower limb skin was cut. Legs, thighs, thigh muscle and bone were removed. Femur was exposed and hip joint and knee joint were cut. Then the bilateral femur and tibia were removed under sterile conditions, followed by the bone surface muscle and periosteum. Femur and tibia were separated into a sterile petri dish and sterilized by flushing the bone three times with a sterile solution containing 5% PBS penicillin and streptomycin, and 1% mycillin. Washing was repeated by soaking in large 50 ml centrifuge tube. When the liquid was completely soaked the bones, rongeur metaphysis was applied, using a needle pulling 5 ml culture system (L-DMEM medium +100 U/mL penicillin and streptomycin + 2 mML- glutamine + 7.5% NaHCO3 + 10% FBS). Bone marrow was isolated, percussion with pipettes, made into single cell suspension adding PBS at 1600 rpm for 5 min followed by centrifugal elutriation twice. The density gradient centrifugation isolated the cell suspension in a L-DMEM1:5 dilution. Lymphocytes of rats were placed in a separate volume Liquid. The liquid layered Percoll (density 1.073 g/mL), placed in the centrifuge at 1600 r/min for 15 min. A ring with a middle brown cloudy layer of cells (mononuclear cell layer) were formed. It was washed with PBS twice, centrifuged for 10 min at 1600 r/min, supernatant was discarded, and then 10 mL L-DMEM medium was added into a single cell suspension and then inoculated in 100 mm cell culture dish at 37 °C and 5% CO2 hatch.
2.2.5.2. Cell culture
Following 48 h of primary culture, the medium was replaced by a fresh medium. Then the cells were covered with the bottom of the culture bottle and they were fused into a single layer. The density of the cells was 70%–80%. Cell passaging was carried out with the ratio of 1:2.
Cells where seeded on all titanium plates. MEM- alpha was added to 10 % FBS and 1% Penicillin-Streptomycin Solution. When the cell density reached 80%, the cells were passaged. The culture medium was then discarded and washed with PBS and 1–2 ml 0.25% trypsin. Separation of cells was observed under the microscope till they became rounded indicating complete digestion. Pancreatin was discarded and the cells were put into a single cell suspension and then passaged according to the ratio of 1:3 at 37 °C and 5% CO2 in saturated humidity conditions to expand cultivation. Light microscopy was used to assess proliferation.
2.3. Tests
2.3.1. CCK8 test and spectrophotometry
The cell counting kit (CCK-8) was performed to determine the extent of the MSCs viability after 24 hours of cell culture with the different TiO2 modification modalities. Following one day of incubation the absorbance on bare titanium with no modification (1A) was fixed at 100% as a control and spectrophotometry was done. The optical density (OD) of all groups were compared to 1A.
Cell counting kit (CCK8) was applied to each well by adding of 300 ml of the solution for 4 h, and then another 100 ul was added to each of the 5 holes in every group of titanium plates. The mortality rate was measured in each hole by OD450 standard enzyme. spectrophotometry was then done do check for the optical density.
2.3.2. Dead/live assay
Confocal laser scanning microscopy was used to examine cell viability in hBMSCs seeded onto titanium surfaces. After 24 h of culture, the cells were fixed in 10% formalin and stained using the fluorescent dye calcein-AM (Green) for live cells and Propidium iodide (red) for dead cells. Using fluorescent images, each TiO2 group were assessed for four different plates and they were then averaged together.
2.3.3. Immunofluorescence
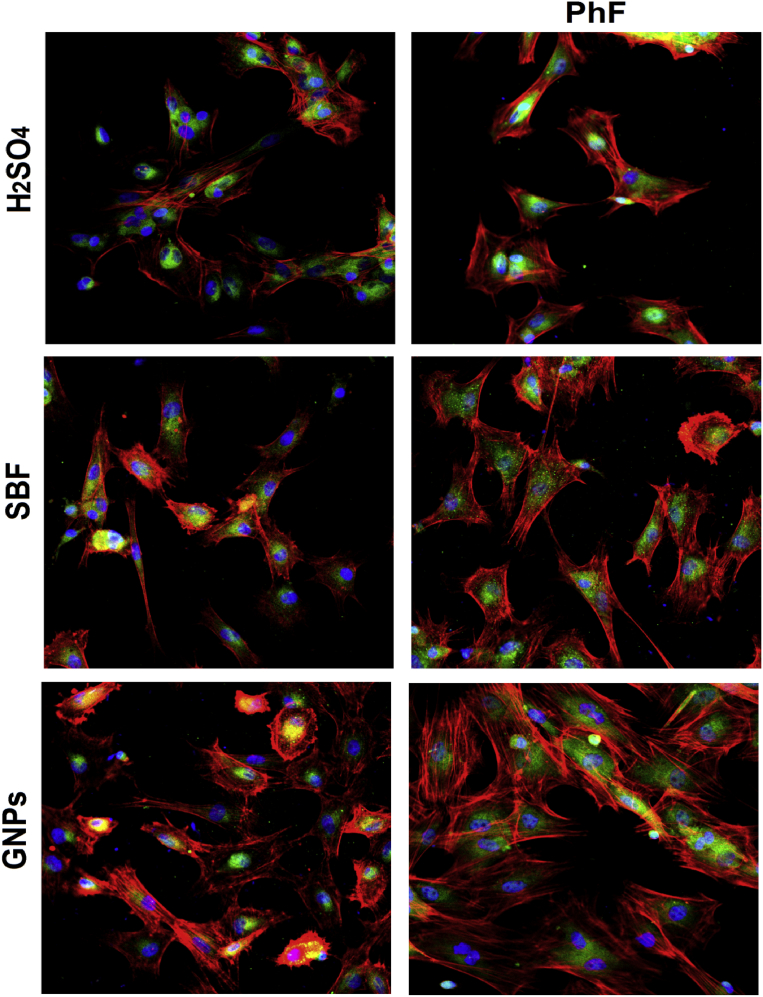
Morphology and morphometry of cells on TiO2 surfaces of all groups were examined after 24 hours following cell seeding to evaluate the cellular spreading and cytoskeletal arrangement of the cultured cells using confocal laser scanning microscopy. Cells were fixed in 10% formalin and confocal microscopic images of cells stained with 4′,6-diamidino-2-phenylindole (DAPI) to detect the nucleus (keygentec), antivinculin to detect vinculin protein within the cytoplasm (Proteintech), and rhodamine phalloidin for actin filaments (beyotime.inc).
2.3.4. Surface characterization
Scanning electron microscope (S2300, Hitachi, Japan) was used after 24 hours of seeding the cells on the plates to examine the surface topographies of the titanium plates as well as the cellular morphology in each group. 1500, 3000 and 5000 magnifications were used for observation.
2.3.5. PCR gene expressions
2.3.5.1. Real time fluorescence quantitative PCR detection mRNA
Extraction of RNA was done using TRIzol™ reagent, the excess PBS was washed off, rinsed twice, then 1 ml of TRIzol™ was added to cells followed by to 1.5 ml RNase in the EP tube for 10 min and 200 ul chloroform and mixed thoroughly several times in room temperature for 5 minutes. It was then centrifuged for 15 minutes at 12000 rpm till the RNA was visible under the third phase. The upper aqueous phase (about 400 ul) was transferred into a new 1.5 ml EP tube. 400 ul isopropyl alcohol was added, mixed well, placed at room temperature for 10 min and then centrifuged at 12000 rpm for 10 minutes. The bottom of the tube was seen white due to RNA precipitation. Excess liquid was discarded and RNase of 75% ethanol 1 ml was added, mixed and centrifuged again for 5 minutes at 10000 rpm. The RNA precipitation was air dried for 5–10 minutes and then dissolved and diluted in 20 ul DEPC solution. Optical density (OD) was calculated. The OD260, OD280 and OD260/OD280 values were determined by UV spectrophotometer, and the purity and concentration of RNA were calculated. According to the ratio of OD260/OD280, the quality of RNA was estimated, and the ratio was between 1.8 and 2.0. The absorbance value was calculated according to the following formula the concentration of the sample RNA: Total RNA concentration (ug/ul) = OD260 * 40 * 200 * 10-3. The total RNA was placed in the refrigerator at −80 °C for storage.
2.3.5.2. Reverse transcription into cDNA
Reagents: 2.576 ul RNA, 2 ul of Oligo (dT) 15 (10 uM), 2 ul of dNTP (2.5 mM) and up to 14.5 ul of ddH2O (RNase free). Reaction conditions were 70 °C for 5 min, following a brief centrifugation on the ice. All were put in the PCR tube (14.5 ul) and added with 4 ul of 5×RT buffer, 0.5 ul of RNase Inhibitor. 1 ul of M-MLV reverse transcriptase and Up to 20 ul of ddH2O (RNase free). Reaction conditions for this were 42 °C for 60 minutes and 95 °C for 5 minutes.
2.3.5.3. Semi quantitative RT-PCR detection
Reagents: 0.5 ul Reference F (10 uM), 0.5 ul Reference R (10 uM), 2 ul dNTP (2.5 mM), 0.25 ul Ex Taq, 2.5 ul 10×Ex Taq E buffer, 1 ul cDNA and Up to 25 ul ddH2O.
Reaction condition: 4 minutes at 94 °C; 30 seconds at 94 °C, 30 seconds at 56 °C, 25 seconds at 72 °C, and then 30 cycles for 4 minutes at 72 °C.
2.3.5.4. Real time fluorescent quantitative PCR detection
Reagents: 4 ul of cDNA was diluted 10 times, then added to 0.4 ul Forward Primer (100 uM), 0.4 ul Reverse Primer (100 uM), 10 ul SYBR Green/Fluorescein qPCR Master Mix (2×), and 2 ul H2O5. Reaction condition: 0 °C for 2 minutes, 95 °C for 10 minutes; 95 °C for 30 seconds, 60 °C for 30 seconds, 40 cycles. 50 °C minutes, 95 C 10 minute as; 95 °C for seconds 30, 60 °C for 30 seconds. For the dissolution curves, the final data were analyzed by 2−△△Ct.
2.3.5.5. Primer sequence table
The primer sequence for different markers is shown in (Table 1).
Table 1.
Primer sequence for Col-1, OCN and OPN.
| Name | Primer | Sequence | Size |
|---|---|---|---|
| b-actin | Forward | 5′- CACGATGGAGGGGCCGGACTCATC -3′ | 240 bp |
| Reverse | 5′- TAAAGACCTCTATGCCAACACAGT -3 | ||
| Rat Col I | Forward | 5′-TGACTGGAAGAGCGGAGAGT -3′ | 202 bp |
| Reverse | 5′-GAATCCATCGGTCATGCTCT-3 | ||
| Rat OCN | Forward | 5′- TCATGTCCAAGCAGGAGGGCAGTAA-3′ | 175 bp |
| Reverse | 5′- TTGTAGGCGTCCTGGAAGCCAATGT -3 | ||
| Rat OPN | Forward | 5′-CCCGATGCCACAGATGAG -3′ | 121 bp |
| Reverse | 5′-TCCCGTTGCTGTCCTGAT -3 |
2.3.6. Alkaline phosphatase activity assay
Alkaline phosphatase (ALP) activity was assessed after two weeks of cell seeding into the titanium plates and was evaluated as the amount of p-Nitrophenyl Phosphate (PNPP) released by the enzymatic reaction at specific areas in a photo of the titanium plates. It was measured using the use of IPP6.0 software.
2.4. Statistical analysis
Graphpad prism 5.0 was used to perform the statistical analysis in this study. The One-way analysis of variance (One-way ANOVA) was used to compare between the results of different TiO2 groups either by using Bonferroni's Multiple Comparison Test for multiple comparisons or by Dunnett's Multiple Comparison Test to compare between TiO2 results with the control group 1A. Unpaired one-tailed t-tests were performed separately for comparison between the results of two separate groups.
3. Results
3.1. Cell count and optical density (OD)
3.1.1. Spectrophotometry
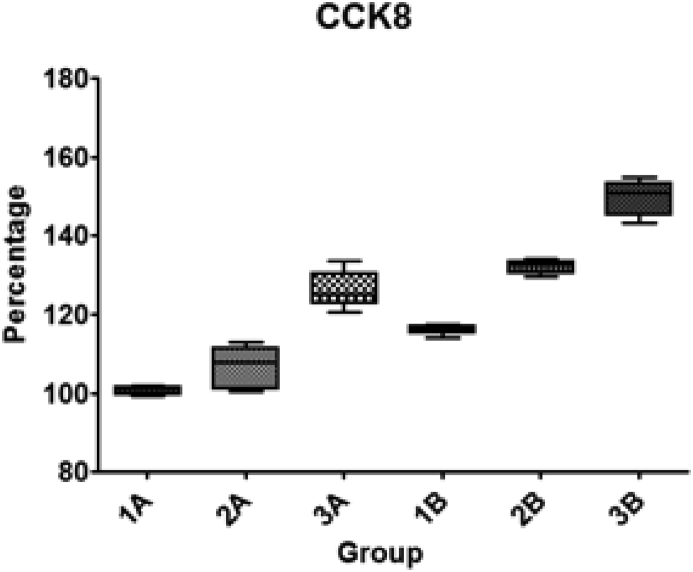
The optical density (OD) was measured and cell count was performed and then calculated in percentages compared to 1A (Table 2) and plotted in (Fig. 3).
Table 2.
Cell count was performed in 5 separate TiO2 plates of each group. 3B (GNP + SBF + PhF) showed maximum cellular proliferation compared to the other TiO2 surfaces.
| TiO2 group | 1 | 2 | 3 | 4 | 5 | Average |
|---|---|---|---|---|---|---|
| 1A | 100.00% | 101.29% | 101.29% | 101.93% | 99.36% | 100% |
| 2A | 110.30% | 101.29% | 112.87% | 100.64% | 107.72% | 106% |
| 3A | 125.10% | 133.46% | 127.67% | 125.10% | 120.59% | 126% |
| 1B | 116.73% | 114.16% | 117.37% | 116.09% | 116.09% | 116% |
| 2B | 134.11% | 131.53% | 132.82% | 129.60% | 132.82% | 132% |
| 3B | 146.98% | 152.12% | 143.11% | 150.84% | 154.70% | 149% |
Fig. 3.
Cell growth ratio and standard deviation (SD) on different TiO2 modified surfaces compared to the control group (1A) which was set at 100%. UV treated groups showed higher cellular density in general, and Group 3B showed the highest density.
3.1.1.1. Statistical analysis
One-way ANOVA was used to perform statistical analysis. Bonferroni's Multiple Comparison Test between each two groups revealed that there is a statistical significant increase in cellular count when adding SBF, GNPs and UV light treatment to the titanium plates. The only two non-significant differences were between 1A vs 2A and 3A vs 2B (Table 3).
Table 3.
Bonferroni's Multiple Comparison Test between different optical densities. Significance level is indicated by*.
| Bonferroni's Multiple Comparison Test | Mean diff. | t | Significance P < 0.05 | Summary | 95% CI of diff |
|---|---|---|---|---|---|
| 1A vs 2A | −5.790 | 2.369 | No | ns | −13.93 to 2.352 |
| 1A vs 3A | −25.61 | 10.48 | Yes | *** | −33.75 to −17.47 |
| 1A vs 1B | −15.31 | 6.265 | Yes | *** | −23.46 to −7.172 |
| 1A vs 2B | −31.40 | 12.85 | Yes | *** | −39.54 to −23.26 |
| 1A vs 3B | −48.78 | 19.95 | Yes | *** | −56.92 to −40.63 |
| 2B vs 3B | −17.37 | 7.107 | Yes | *** | −25.52 to −9.232 |
2B vs 3B: T-test revealed that there is a significantly high increase in the OD of 3B compared to 2B with P value < 0.0001.
3.2. Cell viability assay (live/dead assay)
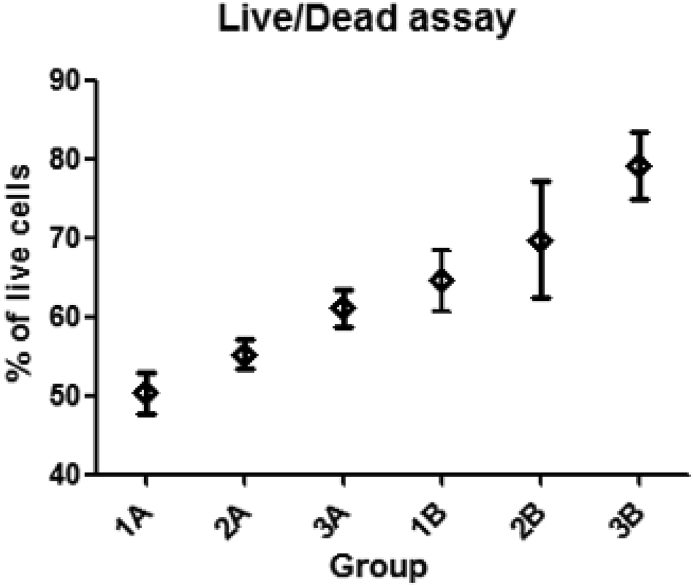
PhF and GNPs increase the cellular viability (Fig. 4), The percentage of viable cells was counted. The ratio of live cells was found to increase in the TiO2 plates in the following order 1A, 2A, 3A, 1B, 2B, 3B (Table 4). The ratio with the standard deviations of all groups was plotted in (Fig. 5).
Fig. 4.
All PhF TiO2 surfaces showed higher cellular viability when compared to the non-PhF ones. In both UV treated and non treated groups, the viability in comparison was GNPs > SBF > H2So4.
Table 4.
Live/dead assay shown by the average percentage of live cells from the total cell count.
| 1 | 2 | 3 | 4 | Average | |
|---|---|---|---|---|---|
| 1A | 48.84 | 49.06 | 54.39 | 49.18 | 50.37% |
| 2A | 53.73 | 55.10 | 54.12 | 57.89 | 55.21% |
| 3A | 60.00 | 59.26 | 64.52 | 60.61 | 61.10% |
| 1B | 70.00 | 60.71 | 63.41 | 64.15 | 64.57% |
| 2B | 78.26 | 72.22 | 67.50 | 60.98 | 69.74% |
| 3B | 82.35 | 81.82 | 79.17 | 73.33 | 79.17% |
Fig. 5.
The ratio of live cells in different TiO2 groups, with the standard deviation (SD).
3.2.1. Live/dead assay results
3.2.1.1. Statistical analysis
Statistical analysis shows that the live/dead ratio is significantly higher in UV treated groups than the non UV treated ones (Table 5).
Table 5.
Dunnett's Multiple Comparison Test for cell viability between different TiO2 groups. T test was preformed for 2B and 3B. Significance level is indicated by*.
| Dunnett's Multiple Comparison Test | Mean diff. | q | Significance P < 0.05 (t-test) | Summary | 95% CI of diff |
|---|---|---|---|---|---|
| 1A vs 2A | −4.897 | 1.677 | No | ns | −12.96 to 3.167 |
| 1A vs 3A | −10.79 | 3.693 | Yes | ** | −18.85 to −2.721 |
| 1A vs 1B | −14.26 | 4.882 | Yes | *** | −22.32 to −6.191 |
| 1A vs 2B | −19.43 | 6.653 | 0.0156 | *** | −27.49 to −11.36 |
| 1A vs 3B | −28.86 | 9.881 | 0.0147 | *** | −36.92 to −20.79 |
*B2 vs B3: Unpaired t test: shows that there is a significant increase in viability in 3B compared to 2B with a P value of 0.0330.
3.3. Morphology and morphometry of cells
The OD of Actin and vinculin are good indicators for cytoskeletal development. The pattern of actin and vinculin expression was as the following:
1B > 1A, 2B > 2A, and 3B > 3A. The expression of actin and vinculin was found to increase in the titanium plates in the following order 1A, 2A, 1B, 3A, 2B, 3B (Fig. 6).
Fig. 6.
PhF treated titanium expressed higher stress fibers development, cytoskeletal maturation as well as higher differentiation and cellular density than those with no PhF treatment. The cellular growth and development was GNPs > SBF > H2SO4.
3.3.1. Optical density (OD) calculations
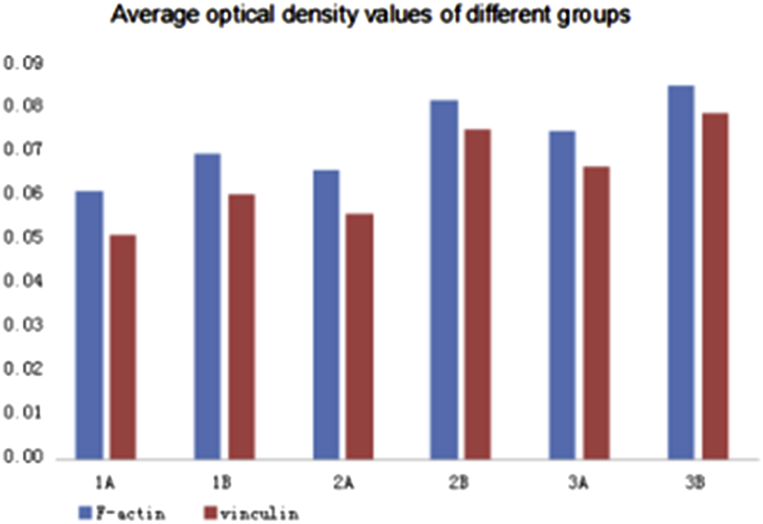
IPP6.0 software was used for the analysis of the optical density of fluorescent photographs, each group had three TiO2 plates analyzed, optical density analysis per area was calculated for the integral optical density (IOD). The average OD were then calculated (Table 6) and (Fig. 7).
Table 6.
Average optical density of actin and vinculin for different TiO2 groups.
| Group | Average OD of F-actin | Average OD of vinculin |
|---|---|---|
| 1A | 0.06132 | 0.05135 |
| 1B | 0.06958 | 0.06033 |
| 2A | 0.06608 | 0.05608 |
| 2B | 0.08210 | 0.07518 |
| 3A | 0.07508 | 0.06690 |
| 3B | 0.08518 | 0.07911 |
Fig. 7.
UV treated groups show enhanced expression of actin and vinculin.
3.4. Surface characterization using scanning electron microscopy (SEM)
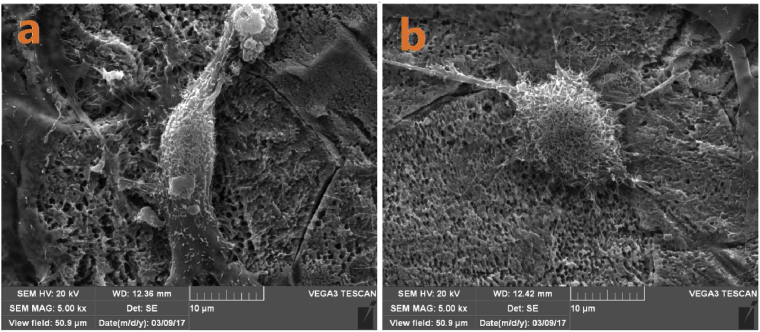
SEM was preformed 24 hours following seeding the cells onto the TiO2 plates to check for surface morphology. The acid-etched titanium plates were of uniform roughness with about 2 um pits as can be seen in 1A and 1B (Fig. 8a). SBF coated plates (2A and 2B) shows the hydroxyapatite layer at higher magnification (Fig. 8b). Both PhF treated (Fig. 9) and GNP treated (Fig. 10) SBF surfaces showed enhanced cellular differentiation. The osteoblasts showed a more defined filopodia and lamellipodia attached to the TiO2 surface. The PhF groups in general show high increase in affinity and more cellular spread on titanium indicating an increase of the hydrophilicity to the titanium surface (Fig. 11).
Fig. 8.
a) acid etched TiO2. b) SBF-modified showing the hydroxyapatite layer covering the TiO2 surface.
Fig. 9.
a) SBF-treated none PhF surface, b) SBF-treated PhF surfaces show higher cellular differentiation.
Fig. 10.
a) SBF treated surfaces, b) GNP treated surfaces at the same magnification showing more cellular maturation.
Fig. 11.
a) GNP – treated surfaces, b) GNP and UV treatment showing more pronounced pseudopodia and cytoskeletal development.
3.5. Osteogenic gene expression
The osteogenic differentiation was assessed using quantitative real-time PCR (qPCR) after one week of cell culture to measure the gene expressions of the Col-1, OPN and late osteogenic biomarker OCN was analyzed.
Beta-actin was used as the reference gene, it was used as a positive control for the expression of Col-1, OPN and OCN (Table 7).
Table 7.
B-actin relative gene expression for different TiO2 groups.
| 1A | 2A | 3A | 1B | 2B | 3B | |
|---|---|---|---|---|---|---|
| Rat B-actin | 19.071 | 18.286 | 18.701 | 18.636 | 19.204 | 18.722 |
| 19.083 | 18.198 | 18.612 | 18.474 | 19.184 | 18.802 | |
| 19.066 | 18.341 | 18.588 | 18.681 | 19.339 | 18.843 |
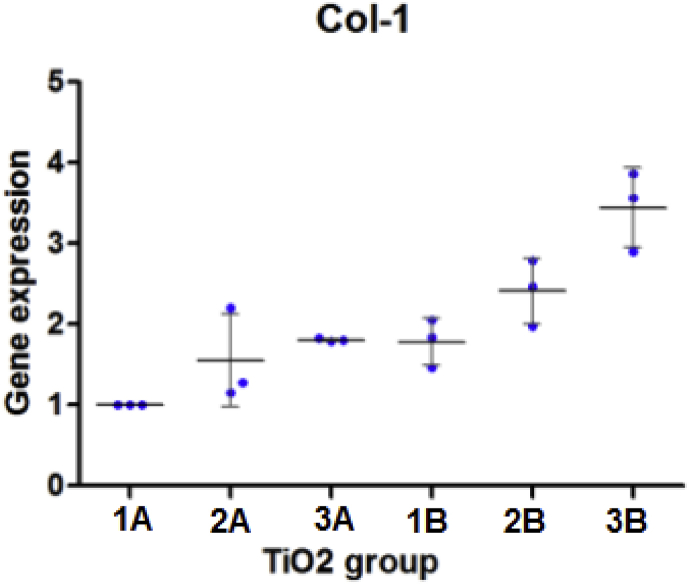
3.5.1. Collagen 1
The average relative gene expression of Col-1 protein was calculated from the PCR results are plotted in (Fig. 12). 1A was considered the control group. Statistical analysis is shown in (Table 8).
-
-
Analysis of relative gene expression of Col-1:
Fig. 12.
Col-1 expression with the standard deviation (SD).
The mean Col-1 expression of different groups was 1A (1.000), 2A (1.547), 3A (1.807). 1B (1.781), 2B (2.412), 3B (3.446).
Col-1 expression after one day of cell culture on the titanium plates was higher on the UV treated group (group B) than that of group A. 1B showed more Col-1 expression than 1A, 2B showed more expression than B1 and 3B also showed more expression of Col-1 than 3A. Titanium plates with SBF coating showed more expression of this biomarker than the plates with no SBF treatment, while the TiO2 plates modified with GNPs were the highest to express this gene.
3.5.1.1. Statistical analysis
Table 8.
Dunnett's Multiple Comparison Test shows significant increase of Col-1 between 2B and 3B compared to the control. *indicates the level of significance. Separate t-tests shows that 3B is more significant.
| Dunnett's Multiple Comparison Test | Mean diff. | q | Significance P < 0.05 (t- test) | Summary | 95% CI of diff |
|---|---|---|---|---|---|
| 1A vs 2A | −0.5467 | 1.811 | No | ns | −1.422 to 0.3289 |
| 1A vs 3A | −0.8070 | 2.674 | No | ns | −1.683 to 0.06860 |
| 1A vs 1B | −0.7813 | 2.589 | No | ns | −1.657 to 0.09427 |
| 1A vs 2B | −1.412 | 4.679 | 0.0263 | ** | −2.288 to −0.5364 |
| 1A vs 3B | −2.446 | 8.104 | 0.0065 | *** | −3.321 to −1.570 |
*2B vs 3B: Paired t test showed that there is a significant increase in Col-1 Expression in 3B compared to 2B with a P value of 0.0238.
3.5.2. Osteopontin
The average relative gene expression of OPN protein was calculated from the PCR results are plotted in (Fig. 13). 1A was considered the control group. Statistical analysis is shown in (Table 9).
-
-
Analysis of relative gene expression of osteopontin:
Fig. 13.
OPN expression with the standard deviation (SD).
The mean OPN expression of different groups was 1A (1.000), 2A (1.202), 3A (1.850). 1B (1.365), 2B (2.616), 3B (3.843).
qPCR analysis of OPN showed that the non-PhF surfaces expressed less OPN than the UV treated groups.
3.5.2.1. Statistical analysis
Table 9.
Dunnett's Multiple Comparison Test shows significant increase of OPN between 3A, 2B and 2B with the control. * indicates the level of significance. Separate t-tests shows the P values of the significant groups.
| Dunnett's Multiple Comparison Test | Mean diff. | q | Significance P < 0.05 (t test) | Summary | 95% CI of diff |
|---|---|---|---|---|---|
| 1A vs 2A | −0.2017 | 1.326 | No | ns | −0.6429 to 0.2395 |
| 1A vs 3A | −0.8497 | 5.587 | yes | *** | −1.291 to −0.4085 |
| 1A vs 1B | −0.3653 | 2.402 | No | ns | −0.8065 to 0.07587 |
| 1A vs 2B | −1.616 | 10.63 | 0.0067 | *** | −2.057 to −1.175 |
| 1A vs 3B | −2.843 | 18.69 | 0.0006 | *** | −3.284 to −2.401 |
*2B vs 3B: t test showed that there is a significant increase in OPN Expression in 3B compared to 2B with a P value of 0.0023.
3.5.3. Osteocalcein
The average relative gene expression of OCN protein was calculated from the PCR results are plotted in (Fig. 14). 1A was considered the control group. Statistical analysis is shown in (Table 10).
-
-
Analysis of relative gene expression of osteocalcin:
Fig. 14.
OCN expression with the standard deviation (SD).
The mean OCN expression of different groups was 1A (1.000), 2A (1.735), 3A (2.037). 1B (1.848), 2B (2.834), 3B (3.885).
The expression of the OCN was similar in pattern to those expressed by Col-1 and OPN. The UV treated surfaces had a noticeably higher gene expressions than their counter groups in the none-PhF surfaces.
3.5.3.1. Statistical analysis
Table 10.
Dunnett's Multiple Comparison Test shows significant increase of OCN in all groups. * indicates the level of significance. Separate t-tests shows the P values of the significant groups.
| Dunnett's Multiple Comparison Test | Mean diff. | q | Significance P < 0.05 (t test) | Summary | 95% CI of diff |
|---|---|---|---|---|---|
| A1 vs A2 | −0.7523 | 3.941 | Yes | * | −1.323 to −0.1815 |
| A1 vs A3 | −1.037 | 5.434 | Yes | ** | −1.608 to −0.4665 |
| A1 vs B1 | −0.8473 | 4.439 | Yes | ** | −1.418 to −0.2765 |
| A1 vs B2 | −1.834 | 9.607 | 0.0120 | *** | −2.405 to −1.263 |
| A1 vs B3 | −2.886 | 15.12 | 0.0002 | *** | −3.457 to −2.315 |
*2B vs 3B: t test showed that there is a significant increase in OCN Expression in 3B compared to 2B with a P value of 0.0038.
3.6. ALP activity
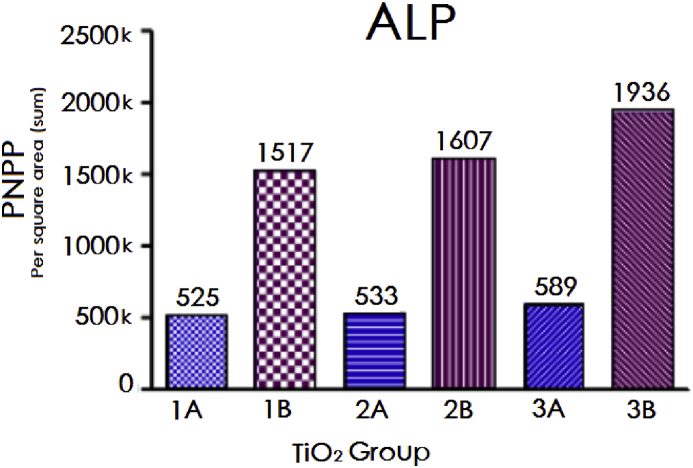
ALP activity was measured two weeks after cell seeding onto the TiO2 plates. The p-Nitrophenyl Phosphate (PNPP) was used a single-point photospectrometry assay via calculating the catalytic ability of ALP to hydrolyse the PNPP. It was measured by the amount of hydrolysis per square area on the TiO2 surfaces (Fig. 15) using the IPP6.0 software.
Fig. 15.
ALP expression after 2 weeks of cell seeding, showing much higher results in the PhF TiO2 groups.
ALP activity results following two weeks of cell seeding showed remarkable differences between the PhF TiO2 surfaces and the non-PhF ones (Fig. 14). 3B had the highest expression among all surfaces with 20% more ALP expression than B2.
4. Discussion
The implant's surface topography can highly affect the osseointegrative capacity and enhance the level of cellular attachment and spreading. Rougher surfaces for instance show higher osteoblastic proliferation and collagen synthesis than smooth surfaces. The biological properties can also significantly influence the cellular reactions toward the surface of the implant. For example, hydrophilic surfaces enhances osteoblastic differentiation than hydrophobic surfaces [130, 131].
Takahiro Ogawa et al. [11] used UV light to photofunctionalize the titanium surface and convert it from hydrophobic to what he called “superhydrophilic”, the UV modification have also to change the surface charge from negative to positive as well as removing the surface carbons that accumulates on the TiO2 surface resulting in a significant increase in protein absorption, osteoblastic differentiation and mineralization. It was shown that UV PhF can increase the BIC from 55% to over 98%, translating into a significant increase in primary stability and decrease in healing time. Simulated body fluid (SBF) was reported in the literature to form a hybrid micro-nano TiO2 surface. Its ability to form a bone-like apatite layer has proved to enhance both the mechanical and biological properties [132]. Similarly, many other reports have also suggested that the increase in surface energy (hydrophilicity) does not only improve cellular function, but also leads to more expression of osteogenic biomarkers such as Col-1, OPN and ALP [133, 134, 135]. Makiko Saita et al. photofunctionalized SBF-modified titanium and found that PhF accelerated the deposition of nanoscale apatite on titanium, and the function of osteoblasts were further enhanced on the apatite-coated titanium [136]. Dohan Ehrenfest et al. found that titanium surface nanomodification has a remarkable impact on the osseointegration process by altering the molecular level via the expression of higher surface energy leading to an increase in the hydrophilicity of the surface and therefore enhancing the cellular and protein interactions on the TiO2 surface [137]. In another study, Dong Nyoung et al. demonstrated that gold nanoparticles enhances the osteogenic differentiation of adipose derived stem cells and shows high expression of osteogenic differentiation specific markers such as COL1, Runx2, OCN, as well as high levels of alkaline phosphatase activity [138].
Collagen type I (COL-1) is the major matrix protein produced during the proliferation of osteoblasts and it is the most abundant protein in bone matrix [139, 140]. It is a good parameter of cellular differentiation but is not exclusive to osteoblastic cells, therefore, quantifying the expression of osteopontin (OPN), osteocalcin (OCN) and alkaline phosphatase (ALP) is essential. These biomarkers are more specific to bone formation and mineralization [141]. Osteopontin is a multifunctional protein linked mainly to bone metabolism and remodeling. It is produced by osteoblasts and osteoclasts and it is involved in the remodeling of bone matrix and in tissue calcification [142, 143]. Osteocalcin is produced only by osteoblasts and is involved in bone metabolism [144] It is one of the main biomarkers indicating activity of osteoblasts [145]. ALP, although not specific to osteoblasts, is an essential early marker for osteoblast differentiation. It also plays a major role in the mineralization process [146].
4.1. Optical density
In our study we have created a hybrid micro-nano topography on the TiO2 surface. The titanium plates in group 3B had all the surface treatment modalities we proposed. Results have shown a significant increase in OD for 3B group compared to the only acid etched groups, except for 2A which increased by only 6%, indicating that the hydroxyapatite layer alone has less impact on cellular differentiation and proliferation than GNPs and PhF. The OD increased by 20% in 3A compared to the control group indicating higher biocompatibility of nanoscale surfaces over not only microtopographies, but also Phf ones. The PhF-SBF plates (2B) showed higher results than those non-PhF nanotopographies indicating higher impact of UV light, 3B had a significantly more OD when compared to 2B with a p value < 0.0001, which also puts an emphasis on the GNPs effect on the cellular differentiation.
4.2. Cytotoxicity
In order to investigate the cytotoxicity of different modification modalities on the MSCs, a live/dead assay was performed. and the results showed an increased cellular viability in the following matter 1A < 2A < 3A < 1B < 2B < 3B with the number of live cells compared to total cells is roughly 50%, 55%, 60%, 65%, 70% and 80% respectively. Following 24 hours of cell seeding into TiO2 plates, there was roughly 5% increase of the number of living cells per each modification except for a 10% increase from 2B to B3 indicating that the increased protein absorption due to PhF probably enhances the GNPs role in cellular differentiation as well. It can also be suggested that the rapid differentiation of MSCs might may contribute to the increasing ratio of live cells. Our results are in accordance to previous studies which suggested that reported cytotoxicity of GNPs can be related to their surface ligand rather than from the nanoparticles themselves [147, 148, 149]. Based on our results, we suggest that our 10 nm GNPs has less cytotoxicity than other groups and it also seemed to highly improve cellular viability.
4.3. Immunofluorescence
Confocal laser scanning microscopy was done after 24 hours of cell seeding and the immunofluorescence images showed the various osteoblast differentiation on the different titanium surfaces. PhF surfaces expressed 13% more actin fibers and 17% more vinculin than 1A which can be considered a representative of typical “as received” commercial implants. There was a higher cytoskeletal development and more organized stress fibers in the SBF coated plates 2B than 2A, and in the GNP-modified 3B than 2B. This confirms the positive physiochemical effect of the UV on reversing the biological ageing of the titanium surface. Our findings comes in accordance with many previous studies regarding the ageing of TiO2 surfaces and the reversing of this phenomena with PhF [8, 11, 12, 13, 14].
The increased cellular attachment on UV treated plates can be linked to the removal of the hydrocarbon layer on the surface of TiO2 and to the increase in the surface energy which has been previously reported to accelerate both osteogenic differentiation of stem cells and the mineralization process [142]. The enhanced cellular spreading on all groups of TiO2 compared to the none UV treated groups is also indicative of the newly acquired “Superhydrophilicity” of the surface.
Other treatment modalities also showed noticeable differences in cellular behavior. SBF immunofluorescent images showed higher cellular proliferation, more actin and vinculin expression and more cytoskeletal development than the none-SBF coated TiO2 plates due to the precipitation of calcium and phosphate ions from the simulated body fluid onto the titanium to form a layer of hydroxyapatite on its surface. These findings came in accordance to the previous studies which concluded that SBF coating enhances both the biological and the mechanical properties and therefore enhances the osteoblastic differentiation [133]. The GNP modified group of titanium surfaces on the other hand showed the fastest rates of cellular differentiation, cytoskeletal maturation and associated actin and vinculin fibers expression, with 3B expressing just below 40% more actin and more than 50% vinculin compared to the control group. More mitotic activity was detected in the GNP modified surfaces. The positive biological effect of GNPs on cellular behaviour was shown to further enhance the physiochemical effect of PhF.
4.4. Surface characterization
The scanning electron microscopy (SEM) images were also taken 24 hours after the stem cells were seeded onto the titanium plates. UV treated plates showed considerable increase in the cellular activity and extracellular matrix (ECM) production compared to the none UV treated ones indicating that PhF induced an increase in the affinity of cellular and protein absorption to the TiO2 surfaces. The hydroxyapatite coating on the SBF modified plates can be seen clearly covering the titanium micropits indicating the precipitation of the calcium and phosphate ions on the surface. This bone-like surface participates in the enhancement of the mechanical and biological properties, resulting in an enhanced cellular attachment and more cytoskeletal differentiation. 2B (UV treated) showed much more spreading due to the superhydrophilic TiO2 surfaces. The SEM images of the GNP treated TiO2 surfaces (3A, 3B) revealed more pronounced cellular extensions with 3B having more spreading and attachment across the titanium surfaces due to the PhF effect. Previous studies have shown that the increased roughness of TiO2 surface up-regulates the ALP activity and enhances osteoblastic differentiation but at the same time it reduces the proliferation rate compared to smooth surfaces while smooth surfaces have less effect on differentiation and more on proliferation [143, 144, 145, 146, 147, 148, 149]. This suggests that the higher cellular attachment and proliferation seen in the GNP modified TiO2 plates in our study is not actually due to the hybrid micro-nano topography which was formed by the acid etched-hydroxyapatite coated layer but it is rather due to the GNP and the UV light effect.
The size of GNPs is reported in the literature to have various effects on cellular differentiation. D. Zhang et al. investigated the osteogenic effect of 20 nm and 40 nm GNPs differentiation and mineralization and they concluded that “size matters” and that the smaller GNPs show higher ALP activity than the larger ones [114]. Wan-Kyu and colleagues also investigated other sizes ranging from 15 to 100 nm GNPs on adipose derived stem cells and they said 30–50 nm GNPs were the most efficient [115].
We used 10 nm GNPs in our experiment, which is one of the smallest to be used for growing cells on a titanium surface and could be a key parameter for catalyzing the cellular differentiation process. The results showed that the 10 nm GNPs are very effective in promoting cellular differentiation and adhesion via increasing the protein adsorption at the nanoscale surface. Some studies have shown that nanosurfaces can interact with the osteoblasts and activate the integrin singling pathway to produce more extracellular matrix (ECM), and can also induce surface binding proteins to expose more membrane anchoring sites in the osteoblasts leading to enhanced cellular differentiation [150, 151, 152, 153, 154, 155]. Although results were more pronounced in UV treated surfaces (3B), 3A confirms the effectiveness of GNPs alone in promoting cellular differentiation is by cellular intake of the colloidal gold.
4.5. Gene expression
Previous studies have shown that GNPs can specifically and highly promote the mitogen-activated protein kinases (MAPK) pathway leading to upregulation of the Runt-related transcription factor 2 (RunX-2) which is considered the major gene in the process of osteoblastic differentiation and in turn directly promotes the expression of other osteoblastic specific genes such as Col-1, OPN and OCN [114]. This is consistent with our ECM and immunofluorescence findings which show increased ECM and pseudopodia development suggesting enhanced osteoblastic differentiation and maturation.
The results of the real time quantitative PCR have shown different levels of mRNA expression of Col-1, OPN and OCN. 1A had the least mRNA expression for the three genes and was considered the control group. All the other titanium groups showed an increase in all gene expressions indicating involvement of the MAP kinase pathway and the up-regulation of the expression of the osteogenic gene Runx-2. The expression of the genes was significantly higher than the control group in the UV treated hydroxyapatite coated TiO2 plates 2B and 3B (GNP-treated). This can be related to the increased serum protein adsorption due to the PhF effect and the GNPs combined. Previous reports highlighted that the incubation of nanomaterials results in an increased adsorption of serum proteins on the surface of the cells, and induces cellular influx of these nanoparticles through receptor-mediated endocytosis, causing them to aggregate in peri-nuclear compartments, interact with cytoplasmic proteins and activate metabolic signaling pathways [156, 157]. Despite the lack of visual detection of our GNPs due to their size we can still suggest based on these previous reports that the absorbed serum proteins on the surface of our GNPs have acted in a similar manner. This is supported by the increase in the expression of the three tested genes in group 3A indicating internalization of the 10 nm GNPs and activation of the MAPK pathway. The PCR results were further supported by the results of the ALP expression done one week after cell incubation.
4.6. Alkaline phosphatase activity
ALP is a considered an early marker of osteogenic differentiation [158] and is indicative of an ongoing mineralization process [141]. The ALP assay in our experiment was performed 2 weeks after cell incubation and it revealed remarkable differences between the PhF TiO2 plates and the none-UV treated ones. The molecular response to UV light demonstrated by around a 3-fold increase in ALP activity in all TiO2 groups. Therefore, we can conclude that although GNPs are very effective in triggering and enhancing the cellular differentiation cascade, the absorption and cellular internalization of GNPs largely depends on the UV enhanced protein absorption capacity of the TiO2 surface. These PhF surfaces showed much higher cellular activity which confirms that the ageing of titanium and the resulting accumulation of surface carbon on the surface, loss of positive surface charge and hydrophilicity indeed significantly affects the speed and extent of cellular behavior as well as GNP internalization. Nevertheless, our novel hybrid micro-nanoscale hydroxyapatite-coated GNP-modified UV-treated TiO2 surface seems to be the clear winner regarding the speed and extent of differentiation, spread, osteogenic gene expressions and even mineralization which is indicated by this high alkaline phosphatase activity.
Our attempt was to combine different treatment modalities in order to get the advantages of each and get the most biocompatible TiO2 surface. The results show that we have largely succeeded in doing that. The new surface (3B) that we have created have shown the most cellular differentiation and proliferation within the first 24 hours, it showed the highest expression of osteogenic biomarkers after one week and the highest ALP expression two weeks after seeding the TiO2 plates with the MSCs. Cellular morphology and function were significantly enhanced in 3B even when compared to the closest modality (2B). This indicates that GNPs played a vital role in this osteogenic cascade. Though we did not test for the p38-MAP Kinase signaling pathway, based on the levels of mRNA expression of the osteogenic biomarkers, we believe that the GNPs triggered the differentiation process, and it was enhanced by the increased protein absorption caused by the effect of the UV rays.
Our method showed highly enhanced cellular differentiation rates on a titanium. The new topography could further enhance the already existing “Superosseointergration” phenomenon, meaning that it can improve the bone to implant contact (BIC) ratio to more than 98% in a very short time, leading to better primary stability, less healing time, elimination of the stability dip, better anchorage for short implants and better predictability and prognosis. It could also open new treatment options in the fields of orthopedics and maxillofacial surgery for the use of screws, plates or any titanium surfaces. Further investigations should be done to evaluate the effect of this new surface on osseointegration in vivo.
5. Conclusions
We have created a novel hybrid micro-nano titanium surface with a remarkable osteogenic potential. 10 nm GNPs did not show any cytotoxicity. Immunofluorescence and SEM results showed that it highly increased the rate of differentiation, proliferation, attachment, spreading and mineralization. PCR results showed enhanced gene expressions of Col-1, OPN and OCN osteogenic markers. ALP activity was also higher than the other groups. GNPs plays an important role in cellular activity and growth. Photofunctionalizing GNPs can highly improve its ostogenic capabilities. The new topography seems to have a very high potential in enhancing osseointegration and improving outcomes for implant, maxillofacial and orthopedic patients.
Declarations
Author contribution statement
Yassir Elkhidir: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ranfa Lai, Zhiqiang Feng: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Steinemann S.G. Titanium—the material of choice? Periodontol. 2000. 1998;17:7–21. doi: 10.1111/j.1600-0757.1998.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 2.Mavrogenis A.F., Dimitriou R., Parvizi J., Babis G.C. Biology of implant osseointegration. J. Musculoskelet. Neuronal Interact. 2009;9(2):61–72. [PubMed] [Google Scholar]
- 3.Ramazanoglu M., Oshida Y. Osseointegration and bioscience of implant surfaces – current concepts at bone-implant interface; implant dentistry – a rapidly evolvong practice. InTech. 2011 [Google Scholar]
- 4.Ogawa T. Springer; New York: 2010. Photofunctionalization of TiO2 for Optimal Bone-titanium Integration: a Novel Phenomenon of Superosseointegration; pp. 699–713. (Enviromentally Benign Photocatalysts). [Google Scholar]
- 5.Davies J.E. Mechanisms of endosseous integration. Int. J. Prosthodont. 1998;11:391–401. [PubMed] [Google Scholar]
- 6.Gao Y., Liu Y., Zhou L. The effects of different wavelength UV photofunctionalization on micro-arc oxidized titanium. PLoS One. 2013;8(7):e68086. doi: 10.1371/journal.pone.0068086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niinomi M., Liu Y., Nasaki M. Biomedical titanium alloys with Young's moduli close to that of cortical bone. Regen. Biometer. 2016;3(3):173–185. doi: 10.1093/rb/rbw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J.H., Ogawa T. The biological aging of titanium implants. Implant Dent. 2012;21(5):415–421. doi: 10.1097/ID.0b013e31826a51f4. [DOI] [PubMed] [Google Scholar]
- 9.Parkash R.B., Shetty O., Tabassum R. Surface modifications of Endosseous dental implants. Int. J. Oral Implantol. Clin. Res. 2012;3(3):116–121. [Google Scholar]
- 10.Albrektsson T., Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001;2:96–101. doi: 10.1007/s005860100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogawa T. UV photofunctionalization of titanium implants. J. Craniofac. Tissue Eng. 2012;2:151–158. [Google Scholar]
- 12.Att W., Ogawa T. Biological aging of implant surfaces and their restoration with ultraviolet light treatment: a novel understanding of osseointegration. Int. J. Oral Maxillofac. Implants. 2012;27:753–761. [PubMed] [Google Scholar]
- 13.Suzuki T., Hori N., Att W. Ultraviolet treatment overcomes time-related degrading bioactivity of titanium. Tissue Eng. Part A. 2009;15:3679–3688. doi: 10.1089/ten.TEA.2008.0568. [DOI] [PubMed] [Google Scholar]
- 14.Iwasa F., Tsukimura N., Sugita Y. TiO2 micro-nano-hybrid surface to alleviate biological aging of UV-photofunctionalized titanium. Int. J. Nanomed. 2011;6:1327–1341. doi: 10.2147/IJN.S22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hori N., Ueno T., Suzuki T. Ultraviolet light-treatment for the restoration of age-related degradation of titanium bioactivity. Int. J. Oral Maxillofac. Implants. 2010;25:49–62. [PubMed] [Google Scholar]
- 16.Hori N., Ueno T., Minamikawa H. Electrostatic control of protein adsorption on UV-photofunctionalized titanium. Acta Biomater. 2010;6:4175–4180. doi: 10.1016/j.actbio.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Iwasa F., Hori N., Ueno T. Enhancement of osteoblast adhesion to UV-photofunctionalized titanium via an electrostatic mechanism. Biomaterials. 2010;31:2717–2727. doi: 10.1016/j.biomaterials.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Rapuano B.E., Lee J.J., MacDonald D.E. Titanium alloy surface oxide modulates the conformation of adsorbed fibronectin to enhance its binding to integrins in osteoblasts. Eur. J. Oral Sci. 2012;120(3):185–194. doi: 10.1111/j.1600-0722.2012.954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rapuano B.E., MacDonald D.E. Surface oxide net charge of a titanium alloy; modulation of fibronectin-activated attachment and spreading of osteogenic cells. Colloids Surf. B Biointerfaces. 2011;82(1):95–103. doi: 10.1016/j.colsurfb.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rapuano B.E., Hackshaw K.M., Schniepp H.C. Effects of coating a titanium alloy with fibronectin on the expression of osteoblast gene markers in the MC3T3 osteoprogenitor cell line. Int. J. Oral Maxillofac. Implants. 2012;27(5):1081–1090. [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai J.A., Lagumdzija A., Stark A. Albumin-bound lipids induce free cytoplasmic calcium oscillations in human osteoblast-like cells. Cell Biochem. Funct. 2007;25:245–249. doi: 10.1002/cbf.1316. [DOI] [PubMed] [Google Scholar]
- 22.Klinger A., Steinberg D., Kohavi D. Mechanism of adsorption of human albumin to titanium in vitro. J. Biomed. Mater. Res. 1997;36:387–392. doi: 10.1002/(sici)1097-4636(19970905)36:3<387::aid-jbm13>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 23.Kusakawa Y., Yoshida E., Hayakawa T. Protein adsorption to titanium and zirconia using a quartz crystal microbalance method. Biomed Res. Int. 2017:1521593. doi: 10.1155/2017/1521593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagambisa FB1, Kappert H.F., Schilli W. Cellular and molecular biological events at the implant interface. J. Craniomaxillofac. Surg. 1994;22(1):12–17. doi: 10.1016/s1010-5182(05)80290-2. [DOI] [PubMed] [Google Scholar]
- 25.Flanagan D. Photofunctionalization of dental implants. J. Oral Implantol. 2016;42(5):445–450. doi: 10.1563/aaid-joi-D-15-00145. [DOI] [PubMed] [Google Scholar]
- 26.Att W., Hori N., Iwasa F. The effect of UV-photofunctionalization on the time-related bioactivity of titanium and chromium–cobalt alloys. Biomaterials. 2009;30(26):4268–4276. doi: 10.1016/j.biomaterials.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 27.Shie J.L., Lee C.H., Chiou C.S. Photodegradation kinetics of formaldehyde using light sources of UVA, UVC and UVLED in the presence of composed silver titanium oxide photocatalyst. J. Hazard. Mater. 2008;155:164–172. doi: 10.1016/j.jhazmat.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 28.Aita H., Hori N., Takeuchi M. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials. 2009;30:1015–1110. doi: 10.1016/j.biomaterials.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki S., Kobayashi H., Ogawa T. Implant stability change and osseointegration speed of immediately loaded photofunctionalized implants. Implant Dent. 2013;22(5):481–490. doi: 10.1097/ID.0b013e31829deb62. [DOI] [PubMed] [Google Scholar]
- 30.Att W., Hori N., Takeuchi M. Time-dependent degradation of titanium osteoconductivity: an implication of biological aging of implant materials. Biomaterials. 2009;30:5352–5363. doi: 10.1016/j.biomaterials.2009.06.040. [DOI] [PubMed] [Google Scholar]
- 31.Iwasa F., Baba K., Ogawa T. Enhanced intracellular signaling pathway in osteoblasts on ultraviolet lighttreated hydrophilic titanium. Biomed. Res. 2016;37(1):1–11. doi: 10.2220/biomedres.37.1. [DOI] [PubMed] [Google Scholar]
- 32.Le Guehennec L., Soueidan A., Layrolle P. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007;23:844–854. doi: 10.1016/j.dental.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 33.Sykaras N., Iacopino A.M., Marker V.A. Implant materials, designs, and surface topographies: their effect on osseointegration. A literature review. Int. J. Oral Maxillofac. Implants. 2000;15:675–690. [PubMed] [Google Scholar]
- 34.Parsons J.T., Horwitz A.R., Schwartz M.A. Cell adhesion: integrating cytoskeletal, dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010;11(9):633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humphries J.D., Wang P., Streuli C., Geiger B., Humphries M.J., Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J. Cell Biol. 2007;179:1043–1057. doi: 10.1083/jcb.200703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen K.K., Rubenstein P.A., Demali K.A. Vinculin nucleates actin polymerization and modifies actin filament structure. J. Biol. Chem. 2009;284(44):30463–30473. doi: 10.1074/jbc.M109.021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellingsen J.E. A study on the mechanism of protein adsorption to TiO2. Biomaterials. 1991;12:593–596. doi: 10.1016/0142-9612(91)90057-h. [DOI] [PubMed] [Google Scholar]
- 38.Goldmann W.H., Ingber D.E. Intact vinculin protein is required for control of cell shape, cell mechanics, and rac-dependent lamellipodia formation. Biochem. Biophys. Res. Commun. 2002;290:749–755. doi: 10.1006/bbrc.2001.6243. [DOI] [PubMed] [Google Scholar]
- 39.Ezzell R.M., Goldmann W.H., Wang N. Vinculin promotes cell spreading by mechanically coupling integrins to the cytoskeleton. Exp. Cell Res. 1997;231:14–26. doi: 10.1006/excr.1996.3451. [DOI] [PubMed] [Google Scholar]
- 40.Mata A., Su X., Fleischman A.J. Osteoblast attachment to a textured surface in the absence of exogenous adhesion proteins. IEEE Trans. Nanobiosci. 2003;2:287–294. doi: 10.1109/tnb.2003.820268. [DOI] [PubMed] [Google Scholar]
- 41.Pols H.A., Schilte H.P., Nijweide P.J., Visser T.J., Birkenhager J.C. The influence of albumin on vitamin D metabolism in fetal chick osteoblast-like cells. Biochem. Biophys. Res. Commun. 1984;125:265–272. doi: 10.1016/s0006-291x(84)80363-0. [DOI] [PubMed] [Google Scholar]
- 42.Al-Jawad M., Fragneto G., Liu J. Fibronectin adsorption studied using neutron reflectometry and complementary techniques. Eur. Phys. J. E. Soft Matter. 2009;30:175–179. doi: 10.1140/epje/i2009-10472-0. [DOI] [PubMed] [Google Scholar]
- 43.Dewez J.L., Doren A., Schneider Y.J. Competitive adsorption of proteins: key of the relationship between substratum surface properties and adhesion of epithelial cells. Biomaterials. 1999;20:547–559. doi: 10.1016/s0142-9612(98)00207-5. [DOI] [PubMed] [Google Scholar]
- 44.Pendegrass C.J., El-Husseiny M., Blunn G.W. The development.of fibronectin-functionalised hydroxyapatite coatings to improve dermal fibroblast attachment in vitro. J. Bone Jt. Surg. Br. 2012;94:564–569. doi: 10.1302/0301-620X.94B4.27698. [DOI] [PubMed] [Google Scholar]
- 45.Mischa Z., Darren A., Morgan R.A. The role of albumin and fibronectin in the adhesion of fibroblasts to plasma polymer surfaces. Plasma Process. Polym. 2011;9:149–156. [Google Scholar]
- 46.Arima Y., Iwata H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials. 2007;28:3074–3082. doi: 10.1016/j.biomaterials.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 47.Miyata K., Takebe J. Anodized-hydrothermally treated titanium with a nanotopographic surface structure regulates integrin-α6β4 and laminin-5 gene expression in adherent murine gingival epithelial cells. J. Prosthodont. Res. 2013;57:99–108. doi: 10.1016/j.jpor.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Bik E.M., Long C.D., Amitage G.C. Bacterial diversity in the oral cavity of 10 healthy individuals. ISME J. 2010;4(8):962–1047. doi: 10.1038/ismej.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siqueira J.F., Jr., Fouad A.F., Rocas I.N. Pyrosequencing as a tool for better understanding of human microbiomes. J. Oral Microbiol. 2012:4. doi: 10.3402/jom.v4i0.10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Safii S.H., Palmer R.M., Wilson R.F. Risk of implant failure and marginal bone loss in subjects with a history of periodontitis: a systematic review and metaanalysis. Clin. Implant Dent. Relat. Res. 2010;12:165–174. doi: 10.1111/j.1708-8208.2009.00162.x. [DOI] [PubMed] [Google Scholar]
- 51.Mombelli A., Muller N., Cionca N. The epidemiology of peri-implantitis. Clin. Oral Implants Res. 2012;23(6):67–76. doi: 10.1111/j.1600-0501.2012.02541.x. [DOI] [PubMed] [Google Scholar]
- 52.Jin L., Guo W., Xue P. Quantitative assay for the colonization ability of heterogeneous bacteria on controlled nanopillar structures. Nanotechnology. 2015;26:055702. doi: 10.1088/0957-4484/26/5/055702. [DOI] [PubMed] [Google Scholar]
- 53.Cavalcanti I.M., Ricomini Filho A.P., Lucena-Ferreira S.C., Da Silva W.J., Paes Leme A.F., Senna P.M. Salivary pellicle composition and multispecies biofilm developed on titanium nitrided by cold plasma. Arch. Oral Biol. 2014;59:695–703. doi: 10.1016/j.archoralbio.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 54.Badihi Hauslich L., Sela M.N., Steinberg D. The adhesion of oral bacteria to modified titanium surfaces: role of plasma proteins and electrostatic forces. Clin. Oral Implants Res. 2013;24(A100):49–56. doi: 10.1111/j.1600-0501.2011.02364.x. [DOI] [PubMed] [Google Scholar]
- 55.Rileya D.J., Bavastrelloa V., Covanib U., Baroneb A., Nicolinia N. An in-vitro study of the sterilization of titanium dental implants using low intensity. Dent. Mater. 2005;21(8):756–760. doi: 10.1016/j.dental.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 56.Einarsson E., Svard S.G. UV irradiation responses in Giardia intestinalis. Exp. Parasitol. 2015;154:25–32. doi: 10.1016/j.exppara.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 57.Barbut F. How to eradicate Clostridium difficile from the environment. J. Hosp. Infect. 2015;89(4):287–295. doi: 10.1016/j.jhin.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 58.Enwemeka C.S., Williams D., Enwemeka S.K. Blue 470-nm light kills methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Promot. Laser Surg. 2009;27(2):221–226. doi: 10.1089/pho.2008.2413. [DOI] [PubMed] [Google Scholar]
- 59.Gallardo-Moreno A.M., Pacha-Olivenza M.A., Saldana L. In vitro biocompatibility and bacterial adhesion of physico-chemically modified Ti6Al4V surface by means of UV irradiation. Acta Biomater. 2009;5(1):181–192. doi: 10.1016/j.actbio.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 60.Yamada Y., Yamada M., Ueda T. Reduction of biofilm formation on titanium surface with ultraviolet-C pre-irradiation. Biomater. Appl. 2014;29(2):161–171. doi: 10.1177/0885328213518085. [DOI] [PubMed] [Google Scholar]
- 61.De Avila E.D., Lima B.F., Sekiya T. Effect of UV-photofunctionalization on oral bacterial attachment and biofilm formation to titanium implant material. Biomaterials. 2015;67:84–92. doi: 10.1016/j.biomaterials.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hirota M., Ozawa T., Iwai T. Implant stability development of photofunctionalized implants placed in regular and complex cases: a case-control study. Int. J. Oral Maxillofac. Implants. 2016;31(3):676–686. doi: 10.11607/jomi.4115. [DOI] [PubMed] [Google Scholar]
- 63.Funato A., Yamada M., Ogawa T. Success rate, healing time, and implant stability of photofunctionalized dental implants. Int. J. Oral Maxillofac. Implants. 2013;28(5):1261–1271. doi: 10.11607/jomi.3263. [DOI] [PubMed] [Google Scholar]
- 64.Ogawa T. Springer; 2010. Photofunctionalization of TiO2 for Optimal Integration of Titanium with Bone; pp. 699–713. (Applications of Titanium Oxide-based Materials. Benign Photocatalysts). [Google Scholar]
- 65.Meredith N., Alleyne D., Cawley P. Quantitative determination of the stability of the implant-tissue interface using resonance frequency analysis. Clin. Oral Implants Res. 1996;7:261–267. doi: 10.1034/j.1600-0501.1996.070308.x. [DOI] [PubMed] [Google Scholar]
- 66.Huang H.L., Tsai M.T., Su K.C. Relation between initial implant stability quotient and bone-implant contact percentage: an in vitro model study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013;116(5):356–361. doi: 10.1016/j.oooo.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 67.Park K.J., Kwon J.Y., Kim S.K. The relationship between implant stability quotient values and implant insertion variables: a clinical study. J. Oral Rehabil. 2012;39:151–159. doi: 10.1111/j.1365-2842.2011.02255.x. [DOI] [PubMed] [Google Scholar]
- 68.Ueno T., Yamada M., Suzuki T. Enhancement of bone-titanium integration profile with UV-photofunctionalized titanium in a gap healing model. Biomaterials. 2010;31:1546–1557. doi: 10.1016/j.biomaterials.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 69.Nishimura I., Huang Y., Butz F. Discrete deposition of hydroxyapatite nanoparticles on a titanium implant with predisposing substrate microtopography accelerated osseointegration. Nanotechnology. 2007;18:1–9. [Google Scholar]
- 70.Mendonca G., Mendonca D.B., Aragao F.J. Advancing dental implant surface technology—from micron- to nanotopography. Biomaterials. 2008;29:3822–3835. doi: 10.1016/j.biomaterials.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 71.Ikeda T1, Hagiwara Y., Hirota M. Effect of photofunctionalization on fluoride-treated nanofeatured titanium. J. Biomater. Appl. 2014;28(8):1200–1212. doi: 10.1177/0885328213501566. [DOI] [PubMed] [Google Scholar]
- 72.Tsukimura N., Ueno T., Iwasa F. Bone integration capability of alkali- and heat-treated nanobimorphic Ti-15Mo-5Zr-3Al. Acta Biomater. 2011;7:4267–4277. doi: 10.1016/j.actbio.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 73.Svanborg L.M., Andersson M., Wennerberg A. Surface characterization of commercial oral implants on the nanometer level. J. Biomed. Mater. Res. B Appl. Biomater. 2010;92:462–469. doi: 10.1002/jbm.b.31538. [DOI] [PubMed] [Google Scholar]
- 74.Kubo K., Tsukimura N., Iwasa F. Cellular behavior on TiO2 nanonodular structures in a micro-to-nanoscale hierarchy model. Biomaterials. 2009;30:5319–5329. doi: 10.1016/j.biomaterials.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 75.Heo D.N., Ko W.K., Lee H.R. Titanium dental implants surface-immobilized with gold nanoparticles as osteoinductive agents for rapid osseointegration. J. Colloid Interface Sci. 2016;469:129–137. doi: 10.1016/j.jcis.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 76.Sanchez C., Arribart H., Guille M.M. Biomimetism and bioinspiration as tools for the design of innovative materials and systems. Nat. Mater. 2005;4:277–288. doi: 10.1038/nmat1339. [DOI] [PubMed] [Google Scholar]
- 77.Spatz J.P. Nano- and micropatterning by organic–inorganic templating of hierarchical self-assembled structures. Angew Chem. Int. Ed. Engl. 2002;41:3359–3362. doi: 10.1002/1521-3773(20020916)41:18<3359::AID-ANIE3359>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 78.Dalby M.J., Gadegaard N., Curtis A.S. Nanotopographical control of human osteoprogenitor differentiation. Curr. Stem Cell Res. Ther. 2007;2:129–138. doi: 10.2174/157488807780599220. [DOI] [PubMed] [Google Scholar]
- 79.Jager M., Zilkens C., Zanger K. Significance of nano- and microtopography for cell-surface interactions in orthopaedic implants. J. Biomed. Biotechnol. 2007;8:69036. doi: 10.1155/2007/69036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ueno T., Tsukimura N., Yamada M. Enhanced boneintegration capability of alkali- and heat-treated nanopolymorphic titanium in micro-to-nanoscale hierarchy. Biomaterials. 2011;32:7297–7308. doi: 10.1016/j.biomaterials.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 81.Ogawa T., Saruwatari L., Takeuchi K. Ti nano-nodular structuring for bone integration and regeneration. J. Dent. Res. 2008;87:751–756. doi: 10.1177/154405910808700809. [DOI] [PubMed] [Google Scholar]
- 82.Li L.H., Kong Y.M., Kim H.W. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials. 2004;25:2867–2875. doi: 10.1016/j.biomaterials.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 83.Lim Y.W., Kwon S.Y., Sun D.H., Kim H.E., Kim Y.S. Enhanced cell integration to titanium alloy by surface treatment with microarc oxidation: a pilot study. Clin. Orthop. Relat. Res. 2009;467:2251–2258. doi: 10.1007/s11999-009-0879-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jeon O., Alsberg E. Photofunctionalization of Alginate hydrogels to promote adhesion and proliferation of human mesenchymal stem cells. Tissue Eng. 2013;A19:11–12. doi: 10.1089/ten.tea.2012.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aita H., Att W., Ueno T. Ultraviolet light-mediated photofunctionalization of titanium to promote human mesenchymal stem cell migration, attachment, proliferation and differentiation. Acta Biomater. 2009;5(8):3247–3257. doi: 10.1016/j.actbio.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 86.Tabuchi M., Ikeda T., Hirota M. Effect of UV photofunctionalization on biologic and anchoring capability of orthodontic miniscrews. Int. J. Oral Maxillofac. Implants. 2015;30(4):868–879. doi: 10.11607/jomi.3994. [DOI] [PubMed] [Google Scholar]
- 87.Crismani A.G., Bertl M.H., Celar A.G. Miniscrews in orthodontic treatment: review and analysis of published clinical trials. Am. J. Orthod. Dentofac. Orthop. 2010;137(1):108–113. doi: 10.1016/j.ajodo.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 88.Hirota M., Ikeda T., Tabuchi M. Effect of ultraviolet-mediated photofunctionalization for bone formation around medical titanium mesh. J. Oral Maxillofac. Surg. 2014;72(9):1691–1702. doi: 10.1016/j.joms.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 89.Matsuura K., Utoh R., Nagase K. Cell sheet approach for tissue engineering and regenerative medicine. J Contr. Release. 2014;190 C:228–239. doi: 10.1016/j.jconrel.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 90.Ishijima M., Hirota M., Park W. Osteogenic cell sheets reinforced with photofunctionalized micro-thin titanium. J. Biomater. Appl. 2015;29(10):1372–1384. doi: 10.1177/0885328214567693. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Z., Wang K., Bai C. The influence of UV irradiation on the biological properties of MAO-formed ZrO2. Colloids Surf. B Biointerfaces. 2012;89:40–47. doi: 10.1016/j.colsurfb.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 92.Sollazzo V., Pezzetti F., Scarano A. Zirconium oxide coating improves implant osseointegration in vivo. Dent. Mater. 2008;24(3):357–361. doi: 10.1016/j.dental.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 93.Tuna T., Wein M., Swain M. Influence of ultraviolet photofunctionalization on the surface characteristics of zirconia-based dental implant materials. Dent. Mater. 2015;31(2):14–24. doi: 10.1016/j.dental.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 94.Chevalier J. What future for zirconia as a biomaterial? Biomaterials. 2006;27:535–543. doi: 10.1016/j.biomaterials.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 95.Chevalier J., Loh J., Gremillard L. Low-temperature degradation in zirconia with a poroussurface. Acta Biomater. 2011;7:2986–2993. doi: 10.1016/j.actbio.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 96.Att W., Takeuchi M., Suzuki T. Enhanced osteoblast function on ultraviolet light-treated zirconia. Biomaterials. 2009;30(7):1273–1280. doi: 10.1016/j.biomaterials.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 97.Han Y., Yan Y., Lu C. Ultraviolet-enhanced bioactivity of ZrO2 films prepared by micro-arc oxidation. Thin Solid Films. 2009;517(5):1577–1581. [Google Scholar]
- 98.Giljohann D.A., Seferos D.S., Daniel W.L. Gold nanoparticles for biology and medicine. Angew Chem. Int. Ed. Engl. 2010 Apr 26;49(19):3280–3294. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dykman L., Khlebtsov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem. Soc. Rev. 2012 Mar 21;41(6):2256–2282. doi: 10.1039/c1cs15166e. [DOI] [PubMed] [Google Scholar]
- 100.Boisselier E1, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009 Jun;38(6):1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 101.Ghosh P., Han G., De M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008;60:1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 102.Dykman L.A., Khlebtsov N.G. Immunological properties of gold nanoparticles. Sci. Chem. Sci. 2017 Mar 1;8(3):1719–1735. doi: 10.1039/c6sc03631g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kumar A., Zhang X., Liang X.-J. Gold nanoparticles: emerging paradigm for targeted drug delivery system. Biotechnol. Adv. 2013;31:593–606. doi: 10.1016/j.biotechadv.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 104.Niikura K., Matsunaga T., Suzuki T. Gold nanoparticles as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo. ACS Nano. 2013;7:3926–3938. doi: 10.1021/nn3057005. [DOI] [PubMed] [Google Scholar]
- 105.Yi C., Liu D., Fong C.-C. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano. 2010;4:6439–6448. doi: 10.1021/nn101373r. [DOI] [PubMed] [Google Scholar]
- 106.Mu Q.X., Broughton D.L. Endosomal leakage and nuclear translocation of multiwalled carbon nanotubes: developing a model for cell uptake. Nano Lett. 2009;9:4370–4375. doi: 10.1021/nl902647x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Porter A.E., Gass M., Bendall J.S. Uptake of noncytotoxic acid-treated single-walled carbon nanotubes into the cytoplasm of human macrophage cells. ACS Nano. 2009;3:1485–1492. doi: 10.1021/nn900416z. [DOI] [PubMed] [Google Scholar]
- 108.Xiao G., Jiang D., Thomas P. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J. Biol. Chem. 2000;275:4453–4459. doi: 10.1074/jbc.275.6.4453. [DOI] [PubMed] [Google Scholar]
- 109.Jaiswal R.K., Jaiswal N., Bruder S.P. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J. Biol. Chem. 2000;275:9645–9652. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- 110.Ziros P.G., Gil A.P., Georgakopoulos T. The bone- specific transcriptional regulator Cbfa1 is a target of mechanical signals in osteoblastic cells. J. Biol. Chem. 2002;277:23934–23941. doi: 10.1074/jbc.M109881200. [DOI] [PubMed] [Google Scholar]
- 111.Liu D., Zhang J., Yi C. The effects of gold nanoparticles on the proliferation, differentiation, and mineralization function of MC3T3-E1 cells in vitro. Chin. Sci. Bull. 2010;55:1013–1019. [Google Scholar]
- 112.Sul O.-J., Kim J.-C., Kyung T.-W. Gold nanoparticles inhibited the receptor activator of nuclear factor-κb ligand (RANKL)-induced osteoclast formation by acting as an antioxidant. Biosci. Biotechnol. Biochem. 2010;74:2209. doi: 10.1271/bbb.100375. [DOI] [PubMed] [Google Scholar]
- 113.Yi C.Q., Fong C.C., Chen W.W. Interactions between carbon nanotubes and DNA polymerase and restriction endonucleases. Nanotechnology. 2007;18:025102. [Google Scholar]
- 114.Zhang D., Liu D., Zhang J. Gold nanoparticles stimulate differentiation and mineralization of primary osteoblasts through the ERK/MAPK signaling pathway. Mater. Sci. Eng. C. 2014;42:70–77. doi: 10.1016/j.msec.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 115.Ko W.K., Heo D.N. The effect of gold nanoparticle size on osteogenic differentiation of adipose-derived stem cells. J. Colloid Interface Sci. 2015;438:68–76. doi: 10.1016/j.jcis.2014.08.058. [DOI] [PubMed] [Google Scholar]
- 116.Miyaza T., Kim H.M., Kokubo T. Mechanism of bonelike apatite formation on bioactive tantalum metal in a simulated body fluid. Biomaterials. 2002;23(3):827–832. doi: 10.1016/s0142-9612(01)00188-0. [DOI] [PubMed] [Google Scholar]
- 117.Wang X.X., Hayakawa S., Tsuru K. A comparative study of in vitro apatite deposition on heat-, H(2)O(2)-, and NaOH-treated titanium surfaces. J. Biomed. Mater. Res. 2001;54(2):172–178. doi: 10.1002/1097-4636(200102)54:2<172::aid-jbm3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 118.Kokubo T., Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–2915. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 119.Kim H.M., Miyaji F., Kokubo T. Bonding strength of bone-like apatite layer to Ti metal substrate. J. Biomed. Mater. Res. 1997;38(2):121–127. doi: 10.1002/(sici)1097-4636(199722)38:2<121::aid-jbm6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 120.Ajami E., Aguey-Zinsou K.F. Calcium phosphate growth at electropol-ished titanium surfaces. J. Funct. Biomater. 2012;3(2):327–348. doi: 10.3390/jfb3020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Havitcioglu H., Cecen B., Pasinli A. In vivo investigation of calcium phosphate coatings on Ti6-Al-4V alloy sub-strates using lactic acid – sodium lactate buffered synthetic body fluid. Acta Orthop. Traumatol. Turc. 2013;47(6):417–422. doi: 10.3944/aott.2013.2885. [DOI] [PubMed] [Google Scholar]
- 122.Sandrini E., Giordano C., Busini V. Apatite formation and cellular response of a novel bioactive titanium. J. Mater. Sci. Mater. Med. 2007;18(6):1225–1237. doi: 10.1007/s10856-007-0122-5. [DOI] [PubMed] [Google Scholar]
- 123.Kokubo T., Kim H.M., Kawashita M. Bioactive metals: preparation and properties. J. Mater. Sci. Mater. Med. 2004;15(2):99–107. doi: 10.1023/b:jmsm.0000011809.36275.0c. [DOI] [PubMed] [Google Scholar]
- 124.Moroni L., Fornasari P.M. Human mesenchymal stem cells: a bank perspective on the isolation, characterization and potential of alternative sources for the regeneration of musculoskeletal tissues. J. Cell. Physiol. 2013;228:680–687. doi: 10.1002/jcp.24223. [DOI] [PubMed] [Google Scholar]
- 125.Wu L., Cai X., Zhang S. Regeneration of articular cartilage by adipose tissue derived mesenchymal stem cells: perspective from stem cell biology and molecular medicine. J. Cell. Physiol. 2013;228:938–944. doi: 10.1002/jcp.24255. [DOI] [PubMed] [Google Scholar]
- 126.Undale Anita H., Westendorf Jennifer J. Mesenchymal stem cells for bone repair and metabolic bone diseases. Mayo Clin. Proc. 2009 Oct;84(10):893–902. doi: 10.4065/84.10.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Qin Yunhao, Guan Junjie, Zhang Changqing. Mesenchymal stem cells: mechanisms and role in bone regeneration. Postgrad. Med. J. 2014 Nov;90(1069):643–647. doi: 10.1136/postgradmedj-2013-132387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yue Y., Yang X., Wei X. Osteogenic differentiation of adipose-derived stem cells prompted by low-intensity pulsed ultrasound. Cell Prolif. 2013;46:320–327. doi: 10.1111/cpr.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu L., Yuan W., Wang J. Mechanisms for osteogenic differentiation of human mesenchymal stem cells induced by fluid shear stress. Biomech. Model Mechanobiol. 2010;9:659–670. doi: 10.1007/s10237-010-0206-x. [DOI] [PubMed] [Google Scholar]
- 130.Wei N., Bin S., Jing Z. Influence of implant surface topography on bone-regenerative potential and mechanical retention in the human maxilla and mandible. Am. J. Dent. 2014 Jun;27(3):171–176. [PubMed] [Google Scholar]
- 131.Wennerberg A., Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin. Oral Implants Res. 2009 Sep;20(Suppl. 4):172–184. doi: 10.1111/j.1600-0501.2009.01775.x. [DOI] [PubMed] [Google Scholar]
- 132.Hao J., Li Y., Li B. Biological and mechanical effects of micro-nanostructured titanium surface on an osteoblastic cell line in vitro and osteointegration in vivo. Appl. Biochem. Biotechnol. 2017 Mar 20 doi: 10.1007/s12010-017-2444-1. [DOI] [PubMed] [Google Scholar]
- 133.Lim J.Y., Shaughnessy M.C., Zhou Z. Surface energy effects on osteoblast spatial growth and mineralization. Biomaterials. 2008;29:1776–1784. doi: 10.1016/j.biomaterials.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 134.Lai H.C., Zhuang L.F., Liu X. The influence of surface energy on early adherent events of osteoblast on titanium substrates. J. Biomed. Mater. Res. A. 2010;93:289–296. doi: 10.1002/jbm.a.32542. [DOI] [PubMed] [Google Scholar]
- 135.Qu Z., Rausch-Fan X., Wieland M. The initial attachment and subsequent behavior regulation of osteoblasts by dental implant surface modification. J. Biomed. Mater. Res. A. 2007;82:658–668. doi: 10.1002/jbm.a.31023. [DOI] [PubMed] [Google Scholar]
- 136.Saita Makiko, Ikeda Takayuki. UV photofunctionalization promotes nano-biomimetic apatite deposition on titanium. Int. J. Nanomed. 2016;11:223–234. doi: 10.2147/IJN.S95249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dohan Ehrenfest D.M., Coelho P.G., Kang B.S. Classification of osseointegrated implant surfaces: materials, chemistry and topography. Trends Biotechnol. 2010;28(4):198–206. doi: 10.1016/j.tibtech.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 138.Li J., Li J.J., Zhang J. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale. 2016 Apr 21;8(15):7992–8007. doi: 10.1039/c5nr08808a. [DOI] [PubMed] [Google Scholar]
- 139.Kadler Karl E., Holmes David F., Trotter John A. Collagen fibril formation. Biochem. J. May 15, 1996;316(1):1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mahmoudi Morteza, Lynch Iseult, Reza Ejtehadi Mohammad. Protein−nanoparticle interactions: opportunities and challenges. Chem. Rev. 2011;111(9):5610–5637. doi: 10.1021/cr100440g. [DOI] [PubMed] [Google Scholar]
- 141.Castillo Diaz Luis A., Elsawy Mohamed. Osteogenic differentiation of human mesenchymal stem cells promotes mineralization within a biodegradable peptide hydrogel. J. Tissue Eng. 2016 Jan-Dec:7. doi: 10.1177/2041731416649789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.De Fusco Carolina, Messina Antonietta. Osteopontin: relation between adipose tissue and bone homeostasis. Stem Cells Int. 2017:4045238. doi: 10.1155/2017/4045238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kothari A.N., Arffa M.L., Chang V. Osteopontin—a master regulator of epithelial-mesenchymal transition. J. Clin. Med. 2016;5(4):39. doi: 10.3390/jcm5040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee N.K., Sowa H., Hinoi E. Endocrine regulation of energy metabolism by the skeleton. Cell. Aug 2007;130(3):456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bharadwaj S., Naidu A.G., Betageri G.V. Milk ribonuclease-enriched lactoferrin induces positive effects on bone turnover markers in postmenopausal women. Osteoporos. Int. Sep 2009;20(9):1603–1611. doi: 10.1007/s00198-009-0839-8. [DOI] [PubMed] [Google Scholar]
- 146.Štefková K., Procházková J., Pacherník J. Alkaline phosphatase in stem cells. Stem Cell. Int. 2015;2015:628368. doi: 10.1155/2015/628368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Grabinski C., Schaeublin N., Wijaya A. Effect of gold nanorod surface chemistry on cellular response. ACS Nano. 2011;5:2870–2879. doi: 10.1021/nn103476x. [DOI] [PubMed] [Google Scholar]
- 148.Heo D.N., Ko W.K., Moon H.J. Inhibition of osteoclast differentiation by gold nanoparticles functionalized with cyclodextrin curcumin complexes. ACS Nano. 2014;8:12049–12062. doi: 10.1021/nn504329u. [DOI] [PubMed] [Google Scholar]
- 149.Suarasan S., Focsan M., Soritau O. One-pot, green synthesis of gold nanoparticles by gelatin and investigation of their biological effects on osteoblast cells. Colloids Surf. B. 2015;132:122–131. doi: 10.1016/j.colsurfb.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 150.Wall I., Donos N., Carlqvist K. Modified titanium surfaces promote accelerated osteogenic differentiation of mesenchymal stromal cells in vitro. Bone. 2009;45:17–26. doi: 10.1016/j.bone.2009.03.662. [DOI] [PubMed] [Google Scholar]
- 151.Kieswetter K., Schwartz Z., Hummert T.W. Surface roughness modulates the local production of growth factors and cytokines by osteoblast-like MG-63 cells. J. Biomed. Mater. Res. 1996;32:55–63. doi: 10.1002/(SICI)1097-4636(199609)32:1<55::AID-JBM7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 152.Olivares-Navarrete R., Hyzy S.L., Hutton D.L. Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials. 2010;31:2728–2735. doi: 10.1016/j.biomaterials.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schwartz Z., Lohmann C.H., Oefinger J. Implant surface characteristics modulate differentiation behavior of cells in the osteoblastic lineage. Adv. Dent. Res. 1999;13:38–48. doi: 10.1177/08959374990130011301. [DOI] [PubMed] [Google Scholar]
- 154.Lossdorfer S., Schwartz Z., Wang L. Microrough implant surface topographies increase osteogenesis by reducing osteoclast formation and activity. J. Biomed. Mater. Res. A. 2004;70:361–369. doi: 10.1002/jbm.a.30025. [DOI] [PubMed] [Google Scholar]
- 155.Lincks J., Boyan B.D., Blanchard C.R. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials. 1998;19:2219–2232. doi: 10.1016/s0142-9612(98)00144-6. [DOI] [PubMed] [Google Scholar]
- 156.Batzer R., Liu Y., Cochran D.L. Prostaglandins mediate the effects of titanium surface roughness on MG63 osteoblast-like cells and alter cell responsiveness to 1α,25-(OH)2D3. J. Biomed. Mater. Res. 1998;41:489–496. doi: 10.1002/(sici)1097-4636(19980905)41:3<489::aid-jbm20>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 157.Boyan B.D., Batzer R., Kieswetter K. Titanium surface roughness alters responsiveness of MG63 osteoblast-like cells to 1α,25-(OH)2D3. J. Biomed. Mater. Res. 1998;39:77–85. doi: 10.1002/(sici)1097-4636(199801)39:1<77::aid-jbm10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 158.Palin E., Liu H., Webster T. Mimicking the nanofeatures of bone increases bone-forming cell adhesion and proliferation. Nanotechnology. 2005;16:9. [Google Scholar]