Graphical abstract
Keywords: Schiff base, Metal complex, Urea, Urease, Urease inhibitor, Helicobacter pylori, Canavalia ensiformis
Abstract
Schiff bases, an aldehyde- or ketone-like compounds in which the carbonyl group is replaced by an imine or azomethine, are some of the most widely used organic compounds. Indeed, they are widely used for industrial purposes and also exhibit a broad range of biological activities, including anti-urease activity. Ureases, enzymes that catalyze urea hydrolysis, have received considerable attention for their impact on living organisms’ health, since the persistence of urease activity in human and animal cells can be the cause of some diseases and pathogen infections. This short review compiles examples of the most antiurease Schiff bases (0.23 μM < IC50 < 37.00 μM) and their metal complexes (0.03 μM < IC50 < 100 μM). Emphasis is given to ureases of Helicobacter pylori and Canavalia ensiformis, although the active site of this class of hydrolases is conserved among living organisms.
Introduction
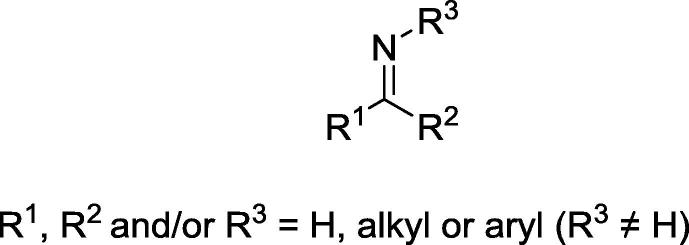
Schiff bases are a well-known class of compounds with the general structure R1R2C=NR3 (with R3 ≠ H) (Fig. 1) [1], and they are named in honor to Hugo Schiff, the scientist who first synthesized members of this class of substances in 1864 [2], [3]. Schiff bases are some of the most widely used organic compounds. They serve as pigments and dyes, catalysts, intermediates in organic synthesis, and polymer stabilizers [4], [5]. Schiff bases also exhibit a wide variety of biological activities, including antifungal, antibacterial, antitumor, anti-inflammatory, trypanocidal, anti-HIV, antimalarial, and anti-urease activities (reviewed by [1], [6], [7], [8], [9], [10], [11]). Indeed, the imine group present in these compounds is critical for their biological activities [12], and thus that moiety has been extensively explored for the development of new bioactive substances [13], [14], [15], [16], [17].
Fig. 1.
General structure of Schiff base.
Urease, a natural enzyme strictly dependent on nickel ions (Ni2+), is widely distributed among plants, fungi and bacteria and belongs to the family of amidohydrolases [18], [19]. This type of hydrolase accelerates the rate of urea hydrolysis to ammonia (NH3) and carbon dioxide (CO2) one-hundred-trillion-fold [18], [20], [21]. Increasing the pH of the medium by the generation of NH3 is a urease trait of tremendous medical importance. For instance, urine and/or gastrointestinal infections by ureolytic bacteria can cause health complications in humans and animals including kidney stone formation, pyelonephritis, hepatic encephalopathy, and ultimately hepatic coma [21], [22]. Therefore, there are major public health problems related to Helicobacter pylori, which is able to survive in the acid environment of the stomach (pH = 1–2) by excreting urease to the medium and consequently increasing the pH by the accumulation of NH3, making its microenvironment more favorable for its growth and development [19], [23]. Indeed, urease represents 10% of the total protein mass in H. pylori [24]. Consequently, H. pylori infection can induce gastric inflammation and increase the risk for the development of duodenal and gastric ulcers, gastric adenocarcinoma and gastric lymphoma [3], [19]. Urease is also produced by most strains of Proteus mirabilis and Staphylococcus saprophyticus and by some plasmid-containing strains of Escherichia coli [25]. These bacteria are some of the most primary etiological agents related to urinary tract infections, and urease is a key virulence factor that determines the severity of the urinary tract infection [26], [27], [28].
Due the tremendous medical importance of ureases, these enzymes have become important therapeutic targets for the treatment of disease caused by urease-dependent pathogenic microorganisms. Here, we present examples of Schiff bases as well as their metal complexes that possesses anti-urease activity, highlighting the most representative compounds/complexes belonging to this class of substances.
Schiff base as urease inhibitors
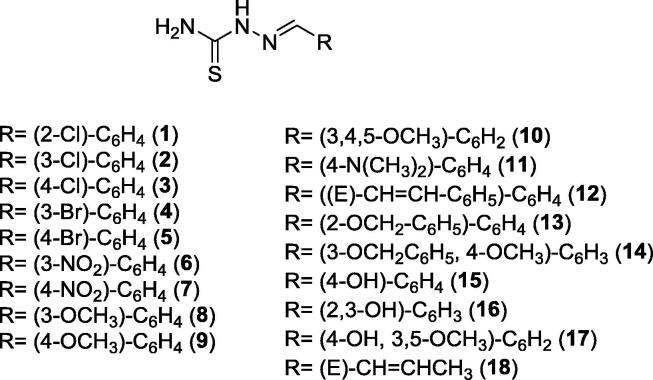
Although Schiff bases are known to have a variety of biological properties, few examples of this class of substances have been described as potent anti-urease agents. In 2011, Aslam and co-workers described the synthesis and in vitro anti-urease activity of 18 Schiff base hydrazone derivatives (Fig. 2). All synthesized compounds exhibited significant urease inhibition, but compound 6 showed the most potent activity (IC50 = 0.102 μM) followed by compounds 8 and 11 (IC50 = 0.177 and 0.127 μM, respectively). Schiff base hydrazone derivatives 1, 13, 14, 16 and 17 exhibited moderate activity, while analogs 2, 3, 4, 5, 7, 9, 10, 12, 15 and 18 showed little effect on urease activity. In general, Schiff base hydrazone derivatives with electron-withdrawing substituents on the aromatic ring showed stronger anti-urease activities than those with electron-donating substituents. Aslam and co-workers also disclosed that compound 6, which bears an electron-withdrawing group (NO2) at the meta position, exhibited competitively inhibition against urease [29].
Fig. 2.
Schiff bases hydrazone derivatives 1–18 synthesized by Aslam and co-workers [29].
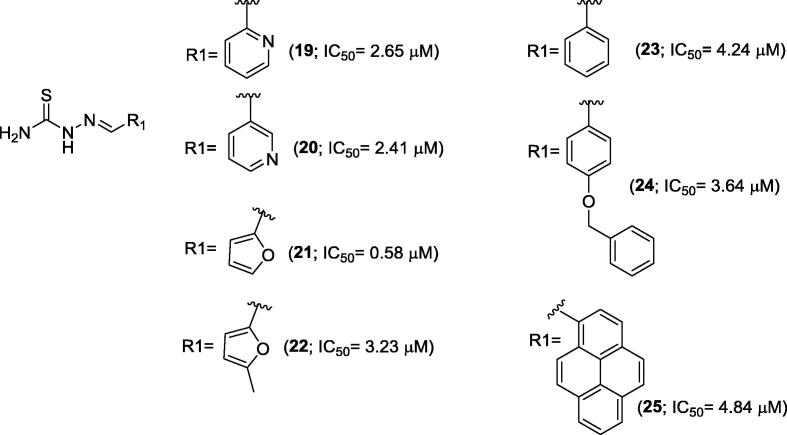
In 2014, Saeed and co-workers described the inhibition of purified urease from jack bean by Schiff base thiosemicarbazide derivatives. Out of a series of thirteen compounds, seven of them presented promising abilities to inhibit urease enzyme (Fig. 3; compounds 19–25). The range of IC50 values for Schiff base thiosemicarbazide derivatives 19–25 was 0.58–4.84 µM, and all of them were more potent than thiourea (IC50 = 21 µM), a positive control used in the urease inhibitory assay [30].
Fig. 3.
Schiff bases thiosemicarbazide derivatives 18–25 synthesized by Saeed and co-workers [30].
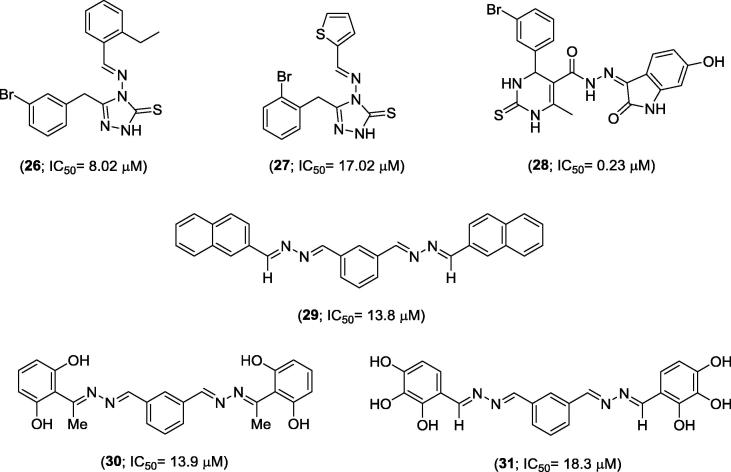
Rafiq and co-workers (2017) reported the preparation of eleven Schiff bases containing 1,2,4-triazole cores and their inhibitory effects on urease activity [31]. Out of this series of Schiff bases, compounds 26 and 27 (Fig. 4) were the most potent with IC50 values of 8.02 µM and 17.02 µM, respectively (31)].
Fig. 4.
Chemical structures of some Schiff bases 26–31.
Other Schiff bases have also been recognized as potential urease inhibitors. For instance, Iftikhar [32] and co-workers (2017) described dihydropyrimidine (DHPM) 28 as the most potent jack bean urease inhibitor (IC50 = 0.23 µM) [32]. Rahim and co-workers showed that bis-Schiff bases 29, 30 and 31 (Fig. 4), derived from isophthalaldehyde, were able to inhibit urease with IC50 values of 13.8 µM, 13.9 µM and 18.3 µM, respectively. According to Rahim and co-workers, the urease inhibition by Schiff bases 30 and 31 is due to possible hydrogen bonding between the hydroxyl group present in the Schiff bases and an amino acid residue in the active site of the urease, while the inhibitory effect of 29 might be due to an arene interaction with an amino acid residue [33].
Schiff base metal complexes as urease inhibitors
Schiff base copper complexes
Copper is an essential element that is necessary for a wide variety of metabolic processes. A broad range of Cu-containing enzymes are known, and they all serve as redox catalysts or as dioxygen carriers. Copper is classified as a transition metal, and it has three oxidation states: Cu0, Cu1+ and Cu2+. Copper is also classified as a heavy metal since its density is greater than 5 g cm−3 [34], [35], [36]. Copper (II) (electronic configuration 3d9), present in most complexes that have urease inhibitory activity, is an ion that exhibits a wide range of stereochemistries, such as tetra-, penta-, and hexa-coordinate geometries [37]. The great interest in copper complexes as urease inhibitors might be due to the strong Lewis acid properties of its metal ions [38]. In a study conducted using jack bean urease enzyme, Follmer and Carlini showed that copper ions can polymerize the protein by modifying it in a way that the enzyme loses its inhibitory activity and through other mechanisms, such as blocking thiol groups in the thiol-dependent domain, which contains the ureolytic active site, and by binding to histidine residues in the protein [39].
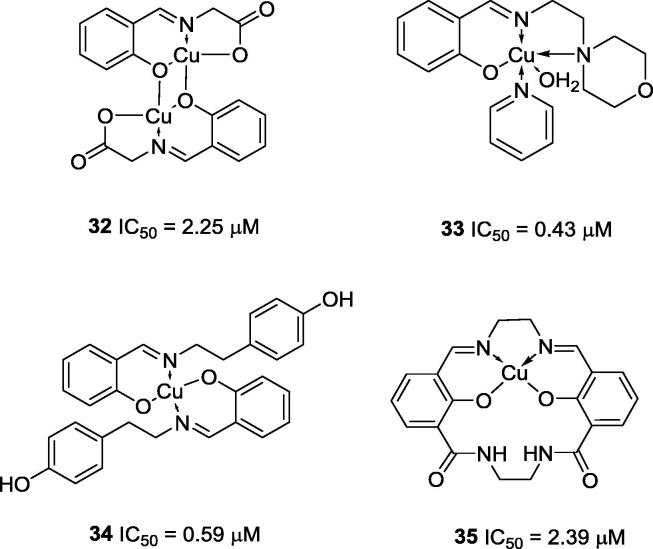
The first copper complexes synthesized and assayed as urease inhibitors were described in 2007 by Zhu’s research group (Fig. 5) [38], [40]. Zhu and co-workers (2007) synthesized three copper complexes, as well as Ni and Mn complexes, derived from Schiff bases (32–34) and evaluated their activities against urease. These authors also tested the free Cu2+, Ni2+ and Mn2+ ions as inhibitors of jack bean urease. They observed that those ions presented anti-urease activities by themselves, and Cu2+ was more effective (IC50 = 0.37 µM) than Ni2+ (IC50 = 2.87 µM), while Mn2+ did not have any effect on urease activity. Zhu and co-workers also compared copper complexes bearing three different Schiff bases as ligands. For instance, compound 32 (IC50 = 2.25 µM), a dinuclear complex, was less potent than compound 33 (IC50 = 0.43 µM), a mononuclear complex (Fig. 5). In compound 32, each copper atom exists in a square-pyramidal configuration with five coordination sites (one site is occupied by the nitrogen atom and the other four by oxygen atoms from three N-salicylideneglycinate ligands). In contrast, complex 33 is a mononuclear square-pyramidal five-coordinate complex in which the apical coordination site is occupied by water. The basal plane is occupied by one oxygen atom from a phenolate group and three nitrogen atoms from the imine group, the morpholine group and the pyridine. Compound 34 (Fig. 5; IC50 = 0.59 µM), a mononuclear tetra-coordinate complex in a trans-square-planar configuration, was more potent than compound 32. The Schiff bases discussed herein act as bidentate ligands and coordinate through the oxygen atom of the ortho-OH group and the nitrogen atom from the imine group (Fig. 5) [38]. In 2007, Zhu and coworker also reported copper complex 35, in addition to Ni, Zn and Co complexes, derived from Schiff bases as a potential urease inhibitor (IC50 = 2.39 µM). Complex 35 is a four-coordinate square-planar complex, and the ligand is coordinated to the metal through two nitrogen and two oxygen atoms from the Schiff base (Fig. 5) [40].
Fig. 5.
Chemical structures of the first copper complexes reported as urease inhibitors [38], [40].
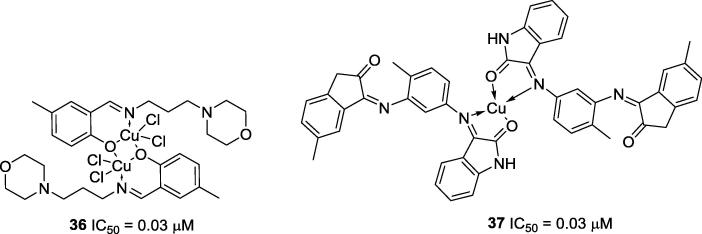
You and co-workers (2016) synthesized nine copper complexes bearing Schiff base ligands, and of those complexes, five complexes (36, 38–41) had strong activities (IC50 lower than 1 µM) against the urease of Helicobacter pylori. Complex 36 (Fig. 6), which had the best anti-urease activity (IC50 = 0.03 µM), was shown to be a mixed-competitive inhibitor (Ki = 15.0 µM) [41]. Pervez and co-workers (2016) also synthesized some copper complexes of isatin-derived bis-Schiff base ligands, and they evaluated the anti-urease activities of all synthesized complexes. Among the series of obtained complexes, compound 37 stands out due its high anti-urease activity (IC50 = 0.03 µM), which is ten-fold stronger than its own free Schiff base ligand (IC50 = 0.34 µM) (Fig. 6) [42].
Fig. 6.
Chemical structures of some Schiff bases-based Cu complexes 36 and 37[41], [42].
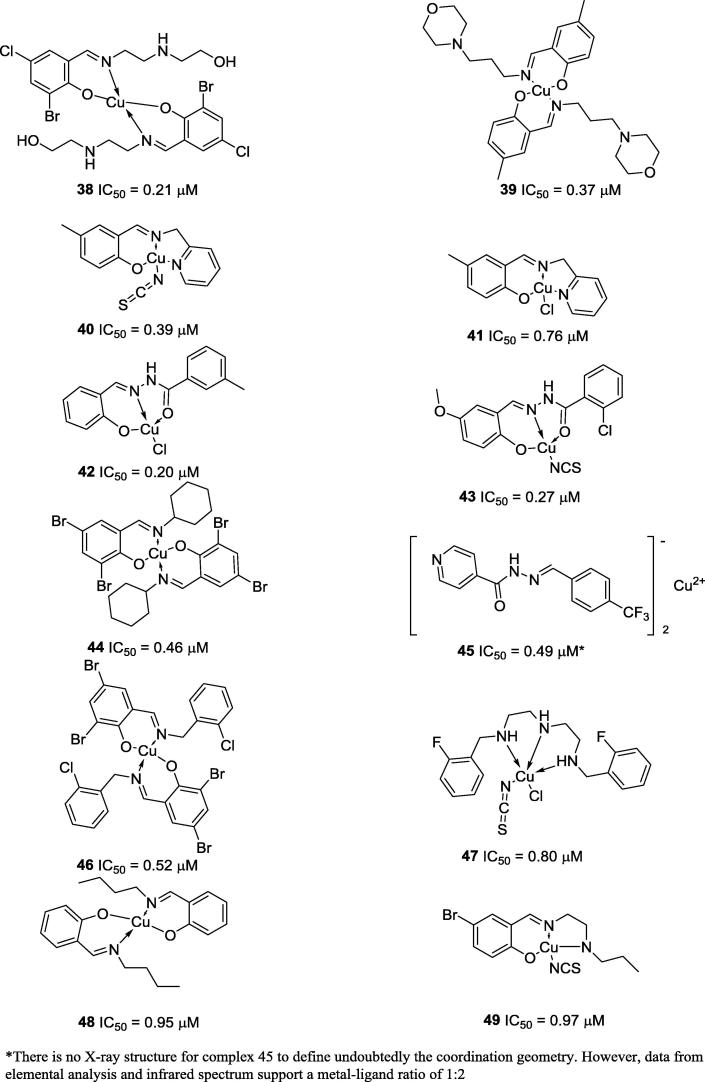
It is also important to highlight the work of Pan and co-workers (2016) (42 and 43), Chen and co-workers (44 and 46), Habala and co-workers (2016) (45), Dong and co-workers (2011) (47), Cui and co-workers (2011) (48) and You and co-workers (2010) (49), who synthesized copper complexes with IC50 values better than 1 µM, i.e., compounds with potent activities (Fig. 7) [43], [44], [45], [46], [47], [48].
Fig. 7.
Chemical structures of relevant Schiff bases-based Cu complexes that present IC50 values lower than 1.0 µM.
Schiff base zinc complexes
Zinc is the second most abundant element in biological systems. The Zn2+ ion has a closed d-shell, which makes it a redox-stable ion. Zn2+ interacts with the side chains of amino acids residues in proteins/peptides and with non-protein ligands. Zinc atoms contribute to the structure and catalytic activity of metalloproteins [49], [50], [51]. Because of the diversity of its biological functions and its low toxicity [47], zinc has been a starting point for the design of urease inhibitors based on Zn-complexes. Notably, Zn2+ by itself has no anti-urease activity [44], [52], [53], [54], [55], [56], [57]; however, when it is used in the form of a zinc-complex, this metal enhances the inhibitory activity of the ligand.
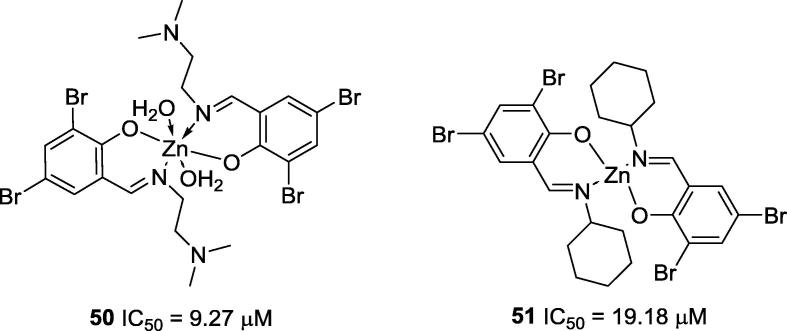
Cheng and co-workers (2007) synthesized the first zinc complex bearing Schiff base ligands, and the complex was assayed as a urease inhibitor; however, it showed no anti-ureolytic activity [40]. After Cheng's zinc complex, other zinc complexes were prepared, and they have been shown to possess promising anti-urease activities. For instance, Chen and co-workers (2010) reported two Zn2+ complexes (50, IC50 = 9.27 mM and 51, IC50 = 19.18 mM; Fig. 8) that were more potent than acetohydroxamic acid (IC50 = 42.12 µM) in the inhibition of jack bean urease. Just like Zn2+, which has no anti-urease activity, the ligands used to prepare 50 and 51 also were ineffective to inhibit such enzyme [44].
Fig. 8.
Chemical structures of Cheng's zinc complexes that present high anti-urease activities [44].
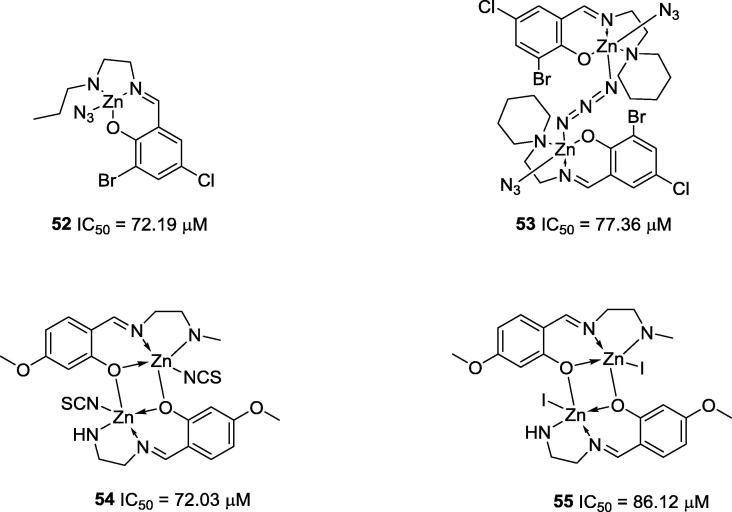
It is also important to highlight the zinc complexes obtained by You and co-workers (2009) (52 and 53) and Wang and co-workers (2012) (54 and 55); however, these complexes presented only moderate anti-urease activities (70 µM < IC50 < 100 µM) (Fig. 9) [57], [58].
Fig. 9.
Chemical structures of zinc complexes synthesized by You’s and Wang’s research groups [57], [58].
Schiff base nickel complexes
Nickel plays an important role in biological systems such as urease, a strictly nickel-dependent enzyme in biological environment [59], [60]. Nickel may exist in several oxidation states, and this will directly affect the formation of Ni complexes as well as their capacities to display biological effects. Ni2+ complexes are the most important Ni complexes in medicinal chemistry. These complexes usually adopt four- or six-coordinate three-dimensional structures (Fig. 10).
Fig. 10.
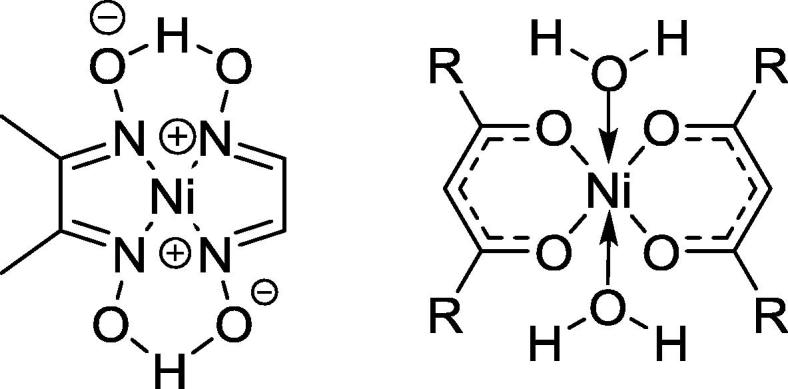
Nickel (Ni2+) complex with four or six coordinations spheres.
In 2007, Li and co-workers reported that free Ni2+ ions (IC50 = 2.87 μM) inhibited the ureolytic activity of purified jack bean urease; however, the observed inhibitory activity was influenced by the type of ligands present on the complexes studied. These authors also evaluated the anti-urease activity of complexes of Cu2+, Ni2+ and Mn2+ ions, but nickel complex 56 (Fig. 11) presented the strongest inhibition of urease [38].
Fig. 11.
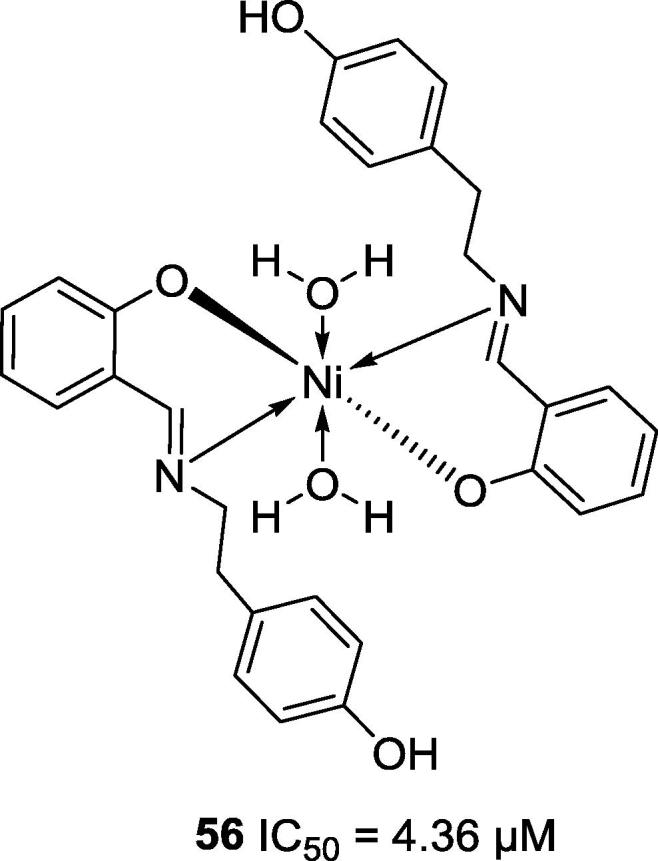
Chemical structure of the nickel complex 56.
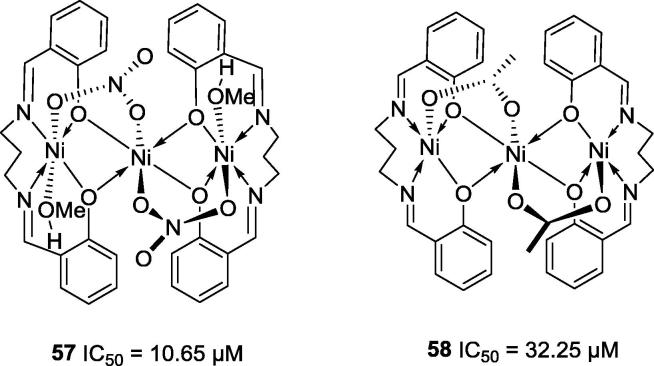
Shi and co-workers described the preparation and anti-ureolytic activity of six hexa-coordinate complexes of Ni2+, Mn2+, Co2+ and Cd2+ [61]. Nickel complexes 57 (IC50 = 32.25 µM) and 58 (IC50 = 10.65 µM) (Fig. 12) showed potent jack bean anti-urease activity with IC50 values lower than that determined for acetohydroxamic acid (IC50 = 42.12 µM), a positive control used for the enzymatic assay [61].
Fig. 12.
Chemical structures of hexa-coordinated complexes of Ni2+ synthesized by Shi and co-workers [61].
Schiff base cobalt complexes
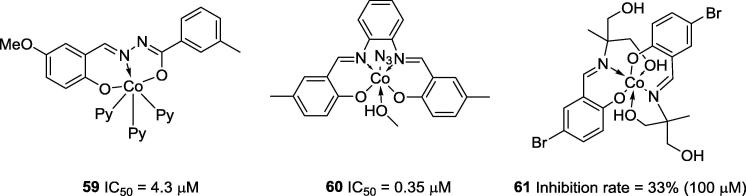
Cobalt is one of the most studied transition metals for the inhibition of the ureolytic activity of urease enzymes. Cobalt has two common oxidation states: Co2+ and Co3+. Co3+ can be found in different biological systems, such as vitamin B12, which is an essential molecule for blood cell formation and normal function of the nervous system [40], [62]. Cobalt complexes have been used to fight bacteria, viruses, fungi, and tumor cells and some of them have shown strong anti-urease activities [40], [63], [64]. The best complexes tested against the pure urease obtained from H. pylori were described by Jing and co-workers and Lu and co-workers [65], [66]. Jing’s research group showed that complexes 59 and 60 (Fig. 13; IC50 = 4.3 μM and 0.35 μM, respectively) can effectively inhibit urease enzymes, while Lu’s group reported that Co-Schiff base complex 61 (Fig. 13) was also able to inhibit urease; however, its efficacy (33% inhibition) was lower than that observed for the ligand itself (83% inhibition).
Fig. 13.
Chemical structures of active anti-urease cobalt-complexes.
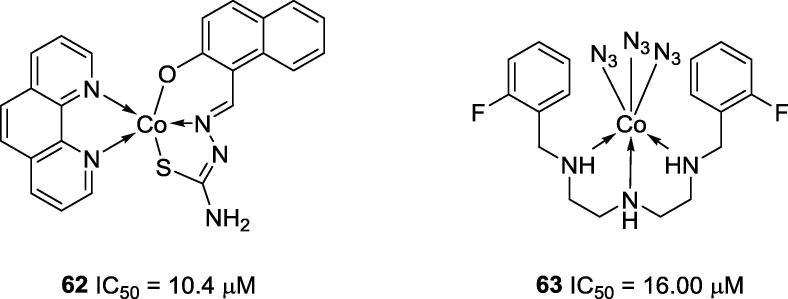
In 2013, Qiu and co-workers reported that Co2+ complex 62 (IC50 = 10.4 μM; Fig. 14), as well as the Ni2+, Cu2+ and Zn2+ complex analogues, possess high anti-urease activities. They also demonstrated that these metallic complexes interact with the sulfhydryl groups of cysteines, the nitrogen atoms of histidines and/or the oxygen atoms of glutamic acid residues of the amino acids present on the urease. According to the results obtained by Qiu and co-workers, the Co2+ and Zn2+ ions had no anti-urease activities, while Ni2+ and Cu2+ were able to inhibit ureolytic activity [53]. The anti-urease activity of the complexes decreased in the order [Cu(L)] > [Co(L)] > [Ni(L)], while the zinc complexes had no anti-urease activities [53]. Notably, the trend in the anti-urease activities observed for the metals by themselves did not match the trend in the activities of the complexes. For instance, Ni2+ and Cu2+ ions were not able to inhibit urease enzyme; however, their complexes were effective. On the other hand, Zn2+ inhibited urease, but its complexes were inactive. Dong and co-workers also synthesized highly active Co complex 63 (IC50 = 16.00 μM), and its anti-urease activity was attributed to its interaction with the metallic center and the sulfhydryl moieties of cysteine residue close to the enzyme’s active site [46].
Fig. 14.
Chemical structures of the most active cobalt complexes tested against Canavalia ensiformes urease.
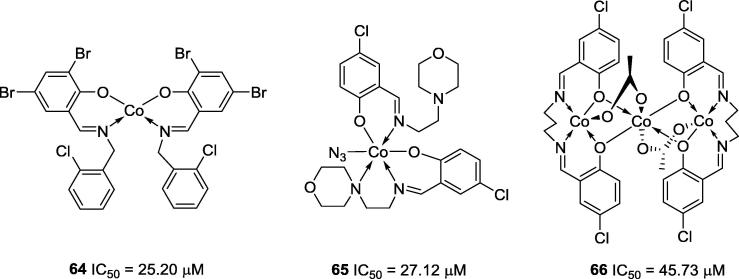
Other notable contributions to cobalt complex urease inhibitors were made by Chen and co-workers (2010) (64), Wang (2010) (65) and You and co-workers (2007) (66) (Fig. 15). Many of the cobalt complexes synthesized by these research groups were effective against urease and showed IC50 values similar to those of acetohydroxamic acid, a positive control used in the anti-urease assays [44], [67], [68].
Fig. 15.
Chemical structures of anti-urease cobalt complexes 64–66.
Vanadium complexes
Vanadium, a transitional metal, exists in oxidation states including −3, −1, 0 and +1 to +5, but +4 and +5 are the most common states in biological systems, and V ions are usually bound to proteins [69], [70]. The main biological activities of vanadium complexes are related to diabetes due its ability to enhance the production of insulin [69], [70], [71], [72]. Other bioactivities described for such complexes are antitumor [73], [74], [75], antibacterial [76], [77], antifungal [76] and antioxidant activities [77] as well as anti-urease properties, and these activities were primarily observed while aiming to develop new anti-Helicobacter pylori agents.
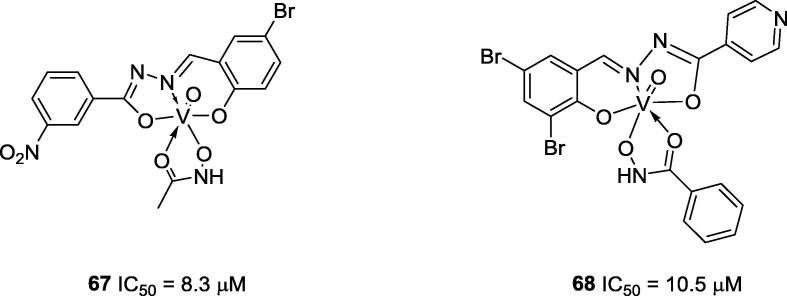
In 2014, Huo and co-workers reported the anti-urease activity (IC50 = 8.3 μM) of vanadium complex 67 and disclosed that it is a mixed inhibitor [78] (Fig. 16). In the same year, Sheng and co-workers prepared new Schiff base oxovanadium complex 68, which also showed promising urease inhibitor activity (IC50 = 10.5 μM) [79] (Fig. 16). Both complexes have the same metal coordination geometry differing only by the types of the ligands present; however, these modifications lead to slight variations in their inhibitory potency.
Fig. 16.
Chemical structures of vanadium complexes 67 and 68.
You and co-workers synthesized six vanadium complexes with Schiff base ligands that were prepared with different imines but with the same hydroxyl group present on the aldehyde. Among these complexes, 69, a mixed inhibitor (Ki = 99 μM), was the most active vanadium Schiff base complex (IC50 = 17.35 μM) [80] (Fig. 17).
Fig. 17.
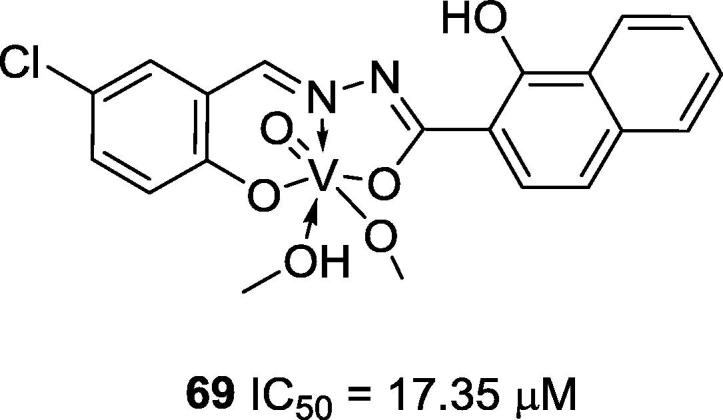
Chemical structure of vanadium complex 69, reported by You and co-workers [80].
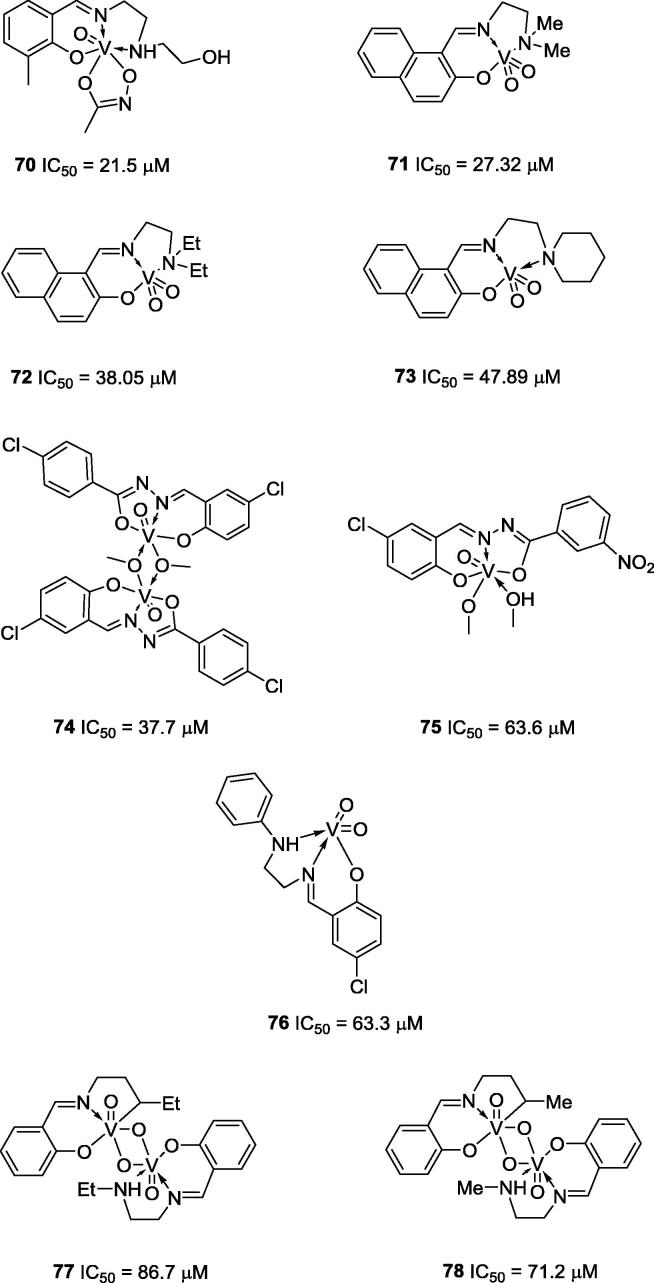
Among all vanadium Schiff base complexes described, the most active anti-urease complexes (Fig. 18) are those reported by Ren and co-workers (2014) (70; IC50 = 21.5 µM), You and co-workers (2011) (71–73; IC50 = 27.32 µM, 38.05 µM and 47.89 µM, respectively), Zhao and co-workers (2013) (74 and 75; IC50 = 37.7 µM and 63.6 µM, respectively), You and co-workers (2012) (76; IC50 = 63.3 µM) and You and co-workers (2011) (77 and 78. IC50 = 86.7 µM and 71.2 µM, respectively) [81], [82], [83], [84], [85].
Fig. 18.
Chemical structures of Schiff base vanadium complexes 70–78, which possess anti-urease activities.
Other metals complexes
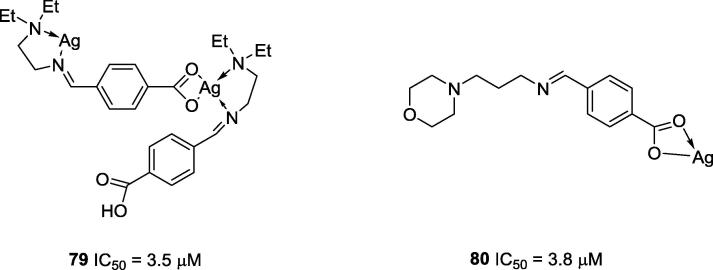
In addition to the Schiff base complexes mentioned above, other complexes bearing silver, manganese, cadmium, iron and rhodium ions have been described as potential anti-urease agents. For instance, Zhang and co-workers (2017) synthesized two silver complexes, 79 and 80 (Fig. 19), which showed potent anti-urease activities (IC50 = 3.5 and 3.8 μM, respectively) [86]. However, in both cases no improvement in the anti-urease activity was observed when the metal was present as the complex since the metal by itself showed the same level of anti-urease activity (IC50 = 3.5 μM) [86].
Fig. 19.
Chemical structures of silver complexes 79 and 80 reported by Zhang and co-workers [86].
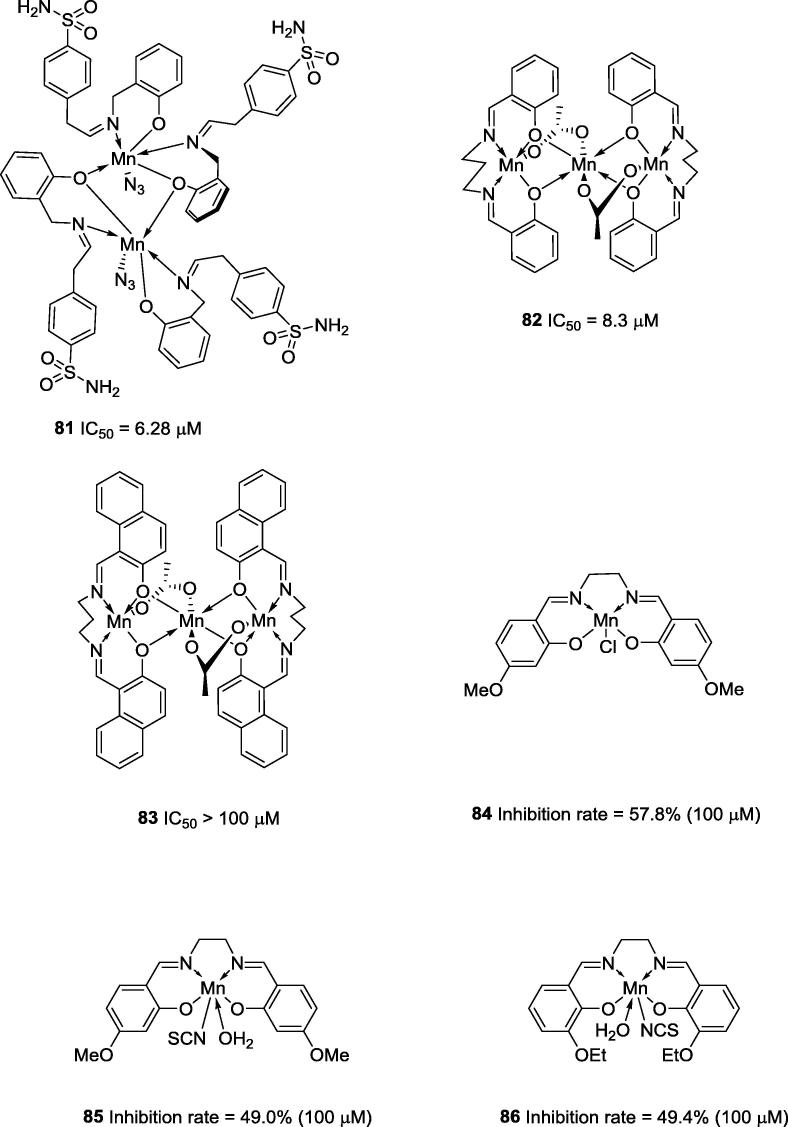
Some examples of manganese complexes with anti-urease activities were described by Li and co-workers (2007). According to these authors, bimetallic manganese complex 81 (Fig. 20) was the most active showing an IC50 value of 6.28 μM (IC50 = 42.12 μM for acetohydroxamic acid, the positive control) (38)]. In the same year, Shi and co-workers reported trimetallic manganese complexes 82 (IC50 = 8.3 μM) and 83 (IC50 > 100 μM) (Fig. 20; the IC50 for the positive control was 42.12 μM) [61]. Other manganese complexes (84–86; Fig. 20) were also reported as urease inhibitors; however, these complexes showed low potencies (inhibition rates less than 60% at 100 μM) [87], [88].
Fig. 20.
Chemical structures of penta and hexa-coordinated Schiff base manganese complexes 81–86.
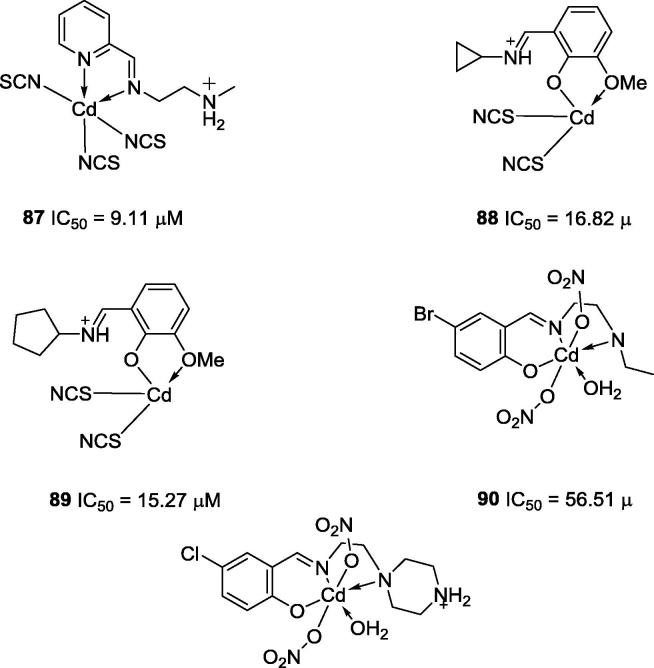
In the case of the cadmium, notable complexes include 87, 88 and 89 (Fig. 21), which were reported by You and co-workers (2008). These complexes showed IC50 values equal to 9.1 μM, 16.8 μM and 15.3 μM, respectively. However, in those cases, the cadmium salt by itself was also a very potent urease inhibitor (IC50 = 19.3 μM) [89]. Other Schiff base cadmium complexes (90 and 91, Fig. 21) were reported by Shi and co-workers (2010) [90]; however, they showed lower activities than acetohydroxamic acid (IC50 = 42.12 μM), a positive control used as a urease inhibitor.
Fig. 21.
Chemical structures of tetra, penta and hexa-coordinated Schiff base cadmium complexes 87–91.
The iron Schiff base complexes are among the least explored as urease inhibitors. The most active iron complex that has been described is 92 (Fig. 22), but it showed an inhibition rate of only 39.5% at 100 μM. Because of its low activity, the IC50 value for 92 was not determined [87].
Fig. 22.
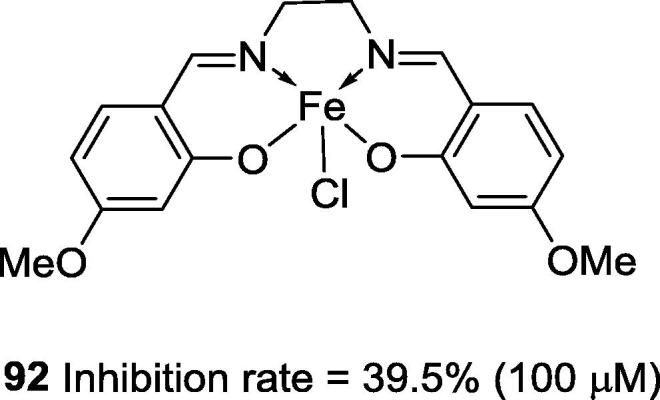
Chemical structure of Schiff base iron complex reported by Shi et al., 2012 as urease inhibitor.
Patents of Schiff bases as urease inhibitors
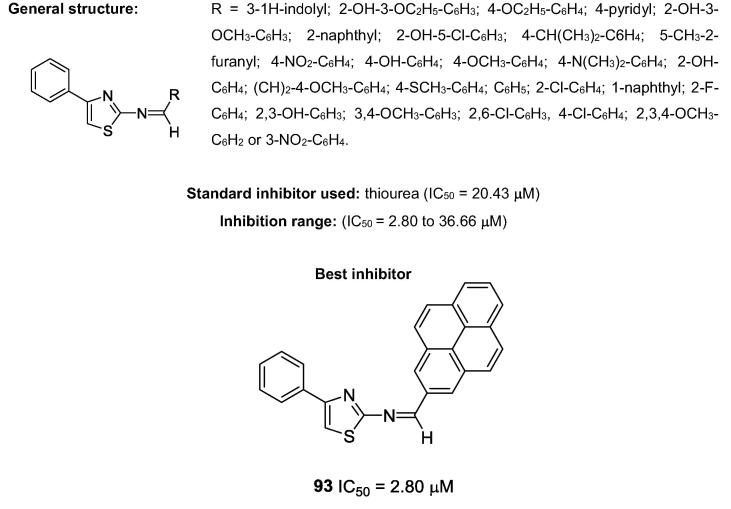
In 2015, a series of 27 thiazole Schiff bases (Fig. 23) was found to exhibit anti-urease activity and was patented by Choudhary and co-workers [91], [92]. These authors described a complete study, which included a kinetic analysis of the 10 most potent thiazole Schiff base derivatives. Of all the evaluated substances, the most potent inhibitor was thiazole 93, which presented an IC50 value of 2.80 µM [91].
Fig. 23.
Chemical structures of Schiff bases, synthesized by de Choudhary’s research group, which possess anti-urease activities.
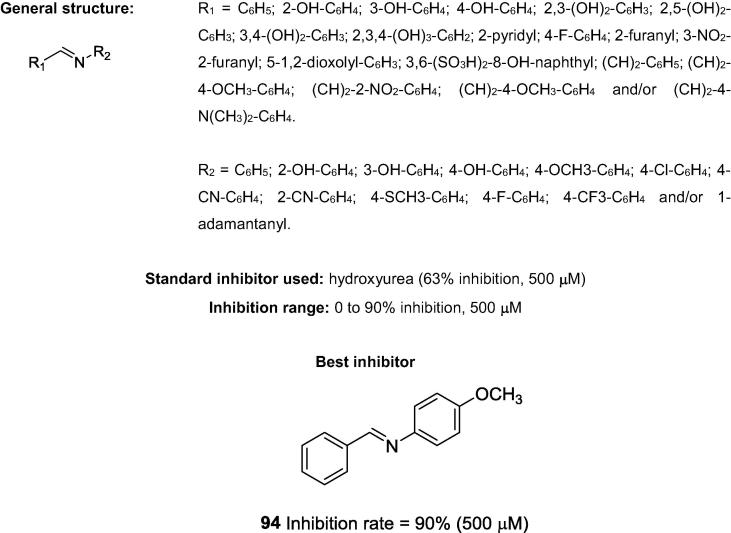
In addition to Choudhary’s patent, there is only one other relevant patent; that patent is from de Fátima’s research group in 2016, and it describes the inhibitory activities of 71 Schiff bases (Fig. 24) against urease. de Fátima and co-workers also described a method for producing urea pearls combined with aldimines (Schiff bases), which were used to inhibit soil urease to enhance the growth and development of crops by using urea-based fertilizers [93]. Among the tested Schiff bases, 94 showed the best activity against a urease purified from Canavalia ensiformis.
Fig. 24.
Chemical structures of Schiff bases, synthesized by de Fátima's research group, which possess anti-urease activities.
Conclusions and future perspectives
Schiff bases have been widely explored for medical and industrial applications. However, the antiurease activities of this class of compounds deserves more investigation. As herein highlighted, substances bearing conjugated unsaturated systems and/or heteroatoms play an important role on the urease inhibitors efficacy. In addition, it seems that, within the scope of our review, the coordination of Schiff bases with metals results in improvement of the potency of the free bases. Copper(II) is the most widely studied metal and showed the best IC50 values for urease inhibition. Despite the previously reported promising anti-urease activities described for Schiff bases and Schiff base metal complexes, the research on this subject is incipient. The number of reports disclosing the effects of Schiff bases and/or their metal complexes on purified urease from Canavalia ensiformis has increased; however, the effects of such substances on urease from H. pylori require further investigation. The study of Schiff bases and/or their metal complexes has proven in the past decades be a golden mine of effective anti-ureolytic agents with potential to treat diseases caused by urease-dependent pathogenic microorganisms. However, advances in this field will require analyses of Schiff base structure-activity relationships, particularly for the Schiff base metal complexes, as well as the mechanism of action of these compounds.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
This work was made possible by the Network for the Development of Novel Urease Inhibitors (www.redniu.org) which is financially supported by the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). AdF is recipient of research fellowships from CNPq.
Biographies

Ângelo de Fátima received his PhD in Science in 2005 from the State University of Campinas (SP, Brazil). He is currently Associate Professor of the Department of Chemistry at the Federal University of Minas Gerais (MG, Brazil). Dr. de Fátima is the coordinator of the Network for the Development of Novel Urease Inhibitors (www.redniu.org) and Group of Studies on Organic and Biological Chemistry. His research interests include the synthesis of molecules with biological, functional profile and the evaluation of their activities against cancer cells, fungi, bacteria and virus of clinical interest.

Camila P. Pereira is a graduate student in technological chemistry at the Federal University of Minas Gerais. She is a scientific initiation fellow and works on the development of novel urease inhibitors, under the mentoring of Dr. Ângelo de Fátima.

Carolina Raquel Said Dau Gonçalves Olímpio was born in 1992. She is currently doing her graduate course in Lic. Chemistry at the Federal University of Minas Gerais (MG, Brazil). She joined Dr. de Fátima’s group in 2015 when she started her scientific initiation studies in Organic Chemistry. Her primary interest includes Organic Synthesis and Biological Chemistry.

Breno Germano de Freitas Oliveira was born in 1990. He earned her BSc. degree in Chemistry in 2014 at the Federal University of Minas Gerais (MG, Brazil). He received his MSc. degree in Chemistry at the same institution in 2016. He is currently PhD student in Chemistry under the mentoring of Dr. Ângelo de Fátima. His research interests are in the fields of Organic Synthesis and Biological Chemistry applied to agriculture.

Lucas Lopardi Franco received his PhD in Science in 2015 from the Federal University of Minas Gerais (MG, Brazil), focused on carbohydrate synthesis research. Dr. Franco did one post doc in the group of bioactive complexes, focused on radiotherapy research and another on in the Group of Studies on Organic and Biological Chemistry, supervised for Professor Dr. Angelo De Fátima. He is currently Assistant Professor of Department of Food and Drugs of Pharmaceutical Sciences Faculty at Federal University of Alfenas (Unifal-MG, Brazil).

Pedro Henrique Corrêa da Silva was born in 1993. He is currently studying for his BSc. degree in Pharmacy at the Federal University of Minas Gerais (MG, Brazil). He joined Dr. de Fátima’s research group in 2013, when he started his scientific initiation studies in Organic Chemistry. His research interests are in the field of Organic and Medicinal Chemistry.
Footnotes
This work was made possible partly by the Network for the Development of Novel Urease Inhibitors (www.redniu.org).
Peer review under responsibility of Cairo University.
References
- 1.da Silva C.M., da Silva D.L., Modolo L.V., Alves R.B., de Resende M.A., Martins C.V.B. Schiff bases: a short review of their antimicrobial activities. J Adv Res. 2011;2(1):1–8. [Google Scholar]
- 2.Schiff H. Eine neue reihe organischer diamine. Eur J Org Chem. 1866;140(1):92–137. [Google Scholar]
- 3.Schiff H. Mittheilungen aus dem Universitätslaboratorium in Pisa: Eine neue Reihe organischer Basen. Liebigs Ann Chem. 1864;131(1):118–119. [Google Scholar]
- 4.Dhar D.N., Taploo C.L. Schiff-bases and their applications. J Sci Ind Res India. 1982;41(8):501–506. [Google Scholar]
- 5.Abdel-Rahman L.H., Abu-Dief A.M., Adam M.S.S., Hamdan S.K. Some new nano-sized mononuclear Cu(II) Schiff base complexes: design, characterization, molecular modeling and catalytic potentials in benzyl alcohol oxidation. Catal Lett. 2016;146(8):1373–1396. [Google Scholar]
- 6.Sztanke K., Maziarka A., Osinka A., Sztanke M. An insight into synthetic Schiff bases revealing antiproliferative activities in vitro. Bioorgan Med Chem. 2013;21(13):3648–3666. doi: 10.1016/j.bmc.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 7.Utreja D., Singh S., Kaur M. Schiff bases and their metal complexes as anti-cancer agents: a review. Curr Bioact Compd. 2015;11(4):215–230. [Google Scholar]
- 8.Abdel-Rahman L.H., El-Khatib R.M., Nassr L.A.E., Abu-Dief A.M., Lashin F.E.-D. Design, characterization, teratogenicity testing, antibacterial, antifungal and DNA interaction of few high spin Fe(II) Schiff base amino acid complexes. Spectrochim Acta A. 2013;111:266–276. doi: 10.1016/j.saa.2013.03.061. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Rahman L.H., El-Khatib R.M., Nassr L.A.E., Abu-Dief A.M., Ismael M., Seleem A.A. Metal based pharmacologically active agents: Synthesis, structural characterization, molecular modeling, CT-DNA binding studies and in vitro antimicrobial screening of iron(II) bromosalicylidene amino acid chelates. Spectrochim Acta A. 2014;117:366–378. doi: 10.1016/j.saa.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Rahman L.H., Abu-Dief A.M., El-Khatib R.M., Abdel-Fatah S.M. Sonochemical synthesis, DNA binding, antimicrobial evaluation and in vitro anticancer activity of three new nano-sized Cu(II), Co(II) and Ni(II) chelates based on tri-dentate NOO imine ligands as precursors for metal oxides. J Photochem Photobiol B. 2016;162:298–308. doi: 10.1016/j.jphotobiol.2016.06.052. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Rahman L.H., Abu-Dief A.M., Aboelez M.O., Hassan Abdel-Mawgoud A.A. DNA interaction, antimicrobial, anticancer activities and molecular docking study of some new VO(II), Cr(III), Mn(II) and Ni(II) mononuclear chelates encompassing quaridentate imine ligand. J Photochem Photobiol B. 2017;170:271–285. doi: 10.1016/j.jphotobiol.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Kajal A., Bala S., Kamboj S., Sharma N., Saini V. Schiff bases: a versatile pharmacophore. J Catal. 2013;2013 [Google Scholar]
- 13.Bringmann G., Dreyer M., Faber J.H., Dalsgaard P.W., Stærk D., Jaroszewski J.W. Ancistrotanzanine C and Related 5, 1 ‘-and 7, 3 ‘-Coupled Naphthylisoquinoline Alkaloids from Ancistrocladus tanzaniensis. J Nat Prod. 2004;67(5):743–748. doi: 10.1021/np0340549. [DOI] [PubMed] [Google Scholar]
- 14.Souza AOd, Galetti F., Silva C.L., Bicalho B., Parma M.M., Fonseca S.F. Antimycobacterial and cytotoxicity activity of synthetic and natural compounds. Quim Nova. 2007;30(7):1563–1566. [Google Scholar]
- 15.Guo Z., Xing R., Liu S., Zhong Z., Ji X., Wang L. Antifungal properties of Schiff bases of chitosan, N-substituted chitosan and quaternized chitosan. Carbohyd Res. 2007;342(10):1329–1332. doi: 10.1016/j.carres.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 16.da Silva C.M., Silva M.M., Reis F.S., Ruiz A.L.T.G., de Carvalho J.E., Santos J.C.C. Studies on free radical scavenging, cancer cell antiproliferation, and calf thymus DNA interaction of Schiff bases. J Photochem Photobiol B. 2017;172:129–138. doi: 10.1016/j.jphotobiol.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Martins D.A., Bomfim Filho L.F., Silva C.Md., Fátima Âd., Louro S.R.W., Batista D.G.J. Copper (II) nitroaromatic schiff base complexes: synthesis, biological activity and their interaction with DNA and albumins. J Brazil Chem Soc. 2017;28(1):87–97. [Google Scholar]
- 18.Krajewska B., Ureases I. Functional, catalytic and kinetic properties: a review. J Mol Catal B-Enzym. 2009;59(1):9–21. [Google Scholar]
- 19.Modolo L.V., de Souza A.X., Horta L.P., Araujo D.P., de Fátima Â. An overview on the potential of natural products as ureases inhibitors: a review. J Adv Res. 2015;6(1):35–44. doi: 10.1016/j.jare.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Follmer C. Ureases as a target for the treatment of gastric and urinary infections. J Clin Pathol. 2010;63(5):424–430. doi: 10.1136/jcp.2009.072595. [DOI] [PubMed] [Google Scholar]
- 21.Maroney M.J., Ciurli S. Nonredox nickel enzymes. Chem Rev. 2014;114(8):4206–4228. doi: 10.1021/cr4004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boer J.L., Mulrooney S.B., Hausinger R.P. Nickel-dependent metalloenzymes. Arch Biochem Biophys. 2014;544:142–152. doi: 10.1016/j.abb.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amtul Z., Siddiqui R.A., Choudhary M.I. Chemistry and mechanism of urease inhibition. Curr Med Chem. 2002;9(14):1323–1348. doi: 10.2174/0929867023369853. [DOI] [PubMed] [Google Scholar]
- 24.van Vliet A.H.M., Kuipers E.J., Waidner B., Davies B.J., de Vries N., Penn C.W. Nickel-responsive induction of urease expression in helicobacter pylori is mediated at the transcriptional level. Infect Immun. 2001;69(8):4891–4897. doi: 10.1128/IAI.69.8.4891-4897.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mobley H.L., Hausinger R.P. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989;53(1):85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raz R., Colodner R., Kunin C.M. Who are you—Staphylococcus saprophyticus? Clin Infect Dis. 2005;40(6):896–898. doi: 10.1086/428353. [DOI] [PubMed] [Google Scholar]
- 27.Nielubowicz G.R., Mobley H.L.T. Host–pathogen interactions in urinary tract infection. Nat Rev Uro. 2010;7(8):430–441. doi: 10.1038/nrurol.2010.101. [DOI] [PubMed] [Google Scholar]
- 28.Flores-Mireles A.L., Walker J.N., Caparon M., Hultgren S.J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aslam M.A.S., Mahmood S.-u., Shahid M., Saeed A., Iqbal J. Synthesis, biological assay in vitro and molecular docking studies of new Schiff base derivatives as potential urease inhibitors. Eur J Med Chem. 2011;46(11):5473–5479. doi: 10.1016/j.ejmech.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Saeed A., Imran A., Channar P.A., Shahid M., Mahmood W., Iqbal J. 2-(Hetero(aryl)methylene)hydrazine-1-carbothioamides as Potent Urease Inhibitors. Chem Biol Drug Des. 2015;85(2):225–230. doi: 10.1111/cbdd.12379. [DOI] [PubMed] [Google Scholar]
- 31.Rafiq M., Saleem M., Jabeen F., Hanif M., Seo S.-Y., Kang S.K. Facile synthesis, biological evaluation and molecular docking studies of novel substituted azole derivatives. J Mol Struct. 2017;1138:177–191. [Google Scholar]
- 32.Iftikhar F., Ali Y., Kiani F.A., Hassan S.F., Fatima T., Khan A. Design, synthesis, in vitro Evaluation and docking studies on dihydropyrimidine-based urease inhibitors. Bioorg Chem. 2017;74:53–65. doi: 10.1016/j.bioorg.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Rahim F., Shehzad M., Khan A., Taha M., Quereshi M.T., Tauseef I. Synthesis and antiurease & antioxidant activities of Bis-Schiff bases of isophthalaldehyde. Asian J Chem. 2016;28(1):39–42. [Google Scholar]
- 34.Weser U., Schubotz L.M., Younes M. Health effects. In: Nriagu J.O., editor. Copper in the environment part II. Wiley; Toronto: 1979. pp. 197–240. [Google Scholar]
- 35.Forstner U., Wittmann G.T.W. Springer-Verlag; Berlin: 1979. Metalpollution in the aquatic environment. [Google Scholar]
- 36.Flemming C.A., Trevors J.T. Copper toxicity and chemistry in the environment: a review. Water, Air, Soil Pollut. 1989;44(1):143–158. [Google Scholar]
- 37.Garribba E., Micera G. The determination of the geometry of Cu(II) complexes: an EPR spectroscopy experiment. J Chem Educ. 2006;83(8):1229–1232. [Google Scholar]
- 38.Li Y.-G., Shi D.-H., Zhu H.-L., Yan H., Ng S.W. Transition metal complexes (M = Cu, Ni and Mn) of Schiff-base ligands: syntheses, crystal structures, and inhibitory bioactivities against urease and xanthine oxidase. Inorg Chim Acta. 2007;360(9):2881–2889. [Google Scholar]
- 39.Follmer C., Carlini C.R. Effect of chemical modification of histidines on the copper-induced oligomerization of jack bean urease (EC 3.5.1.5) Arch Biochem Biophys. 2005;435(1):15–20. doi: 10.1016/j.abb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Cheng K., You Z.-L., Zhu H.-L. New method for the synthesis of a mononucleating cyclic peptide ligand, crystal structures of its Ni, Zn, Cu, and Co complexes, and their inhibitory bioactivity against urease. Aust J Chem. 2007;60(5):375–379. [Google Scholar]
- 41.You Z., Liu M., Wang C., Sheng G., Zhao X., Qu D. Inhibition studies of Helicobacter pylori urease with Schiff base copper(ii) complexes. RSC Adv. 2016;6(20):16679–16690. [Google Scholar]
- 42.Pervez H., Ahmad M., Zaib S., Yaqub M., Naseer M.M., Iqbal J. Synthesis, cytotoxic and urease inhibitory activities of some novel isatin-derived bis-Schiff bases and their copper(ii) complexes. MedChemCommun. 2016;5:914–923. [Google Scholar]
- 43.Pan L., Wang C., Yan K., Zhao K., Sheng G., Zhu H. Synthesis, structures and Helicobacter pylori urease inhibitory activity of copper(II) complexes with tridentate aroylhydrazone ligands. J Inorg Biochem. 2016;159:22–28. doi: 10.1016/j.jinorgbio.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 44.Chen W., Li Y., Cui Y., Zhang X., Zhu H.-L., Zeng Q. Synthesis, molecular docking and biological evaluation of Schiff base transition metal complexes as potential urease inhibitors. Eur J Med Chem. 2010;45(10):4473–4478. doi: 10.1016/j.ejmech.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Habala L., Varényi S., Bilková A., Herich P., Valentová J., Kožíšek J. Antimicrobial activity and urease inhibition of schiff bases derived from isoniazid and fluorinated benzaldehydes and of their copper(II) complexes. Molecules. 2016;21(12):1742. doi: 10.3390/molecules21121742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong X., Li Y., Li Z., Gong D., Zhu H.-L. Synthesis, crystal structure, and urease inhibition studies of copper(II) and cobalt(III) complexes with bi(2-fluorobenzylaminoethyl)amine. Transit Metal Chem. 2011;36(3):319–324. [Google Scholar]
- 47.Cui Y.M., Li Y., Cai Y.J., Chen W., Zhu H.L. Synthesis, molecular docking, and activity of Schiff-base copper(II) complex with N-n-butylsalicylaldiminate as Helicobacter pylori urease inhibitor. J Coord Chem. 2011;64(4):610–616. [Google Scholar]
- 48.You Z.-L., Zhang L., Shi D.-H., Wang X.-L., Li X.-F., Ma Y.-P. Synthesis, crystal structures and urease inhibitory activity of copper(II) complexes with Schiff bases. Inorg Chem Commun. 2010;13(8):996–998. [Google Scholar]
- 49.Berg J.M., Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science. 1996;271(5252):1081–1085. doi: 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- 50.Auld D.S. Handbook of metalloproteins. Wiley; Cambridge (MA): 2004. Structural zinc sites; pp. 403–415. [Google Scholar]
- 51.Patel K., Kumar A., Durani S. Analysis of the structural consensus of the zinc coordination centers of metalloprotein structures. Bba-Proteins Proteom. 2007;1774(10):1247–1253. doi: 10.1016/j.bbapap.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 52.Wang C.-Y. Syntheses, crystal structures, and urease inhibitory properties of copper (II) and zinc (II) complexes with 2-bromo-4-chloro-6-[(2-dimethylaminoethylimino) methyl] phenol. J Coord Chem. 2009;62(17):2860–2868. [Google Scholar]
- 53.Qiu X., Wang J., Shi D., Li S., Zhang F., Zhang F. Syntheses, urease inhibition activities, and fluorescent properties of transition metal complexes. J Coord Chem. 2013;66(9):1616–1625. [Google Scholar]
- 54.Shi D.H., You Z.L. Synthesis, characterization, and crystal structures of two Schiff base zinc (II) complexes with urease inhibitory activities. Russ J Coord Chem. 2010;36(7):535–540. [Google Scholar]
- 55.Shi D.H., Cao Z.L., Liu W.W., Xu R.B., Gao L.L., Zhang Q. Synthesis, crystal structures, and biological activity of zinc (II) complexes derived from 4-bromo-2-[(3-diethylaminopropylimino) methyl] phenol. Russ J Coord Chem. 2013;39(3):297–300. [Google Scholar]
- 56.Shi D.-H., Wang X.-L., Liu W.-W., Jin H. Synthesis, crystal structures, and biological activity of Schiff base zinc (II) complexes derived from (2-piperidin-1-ylethyl)-(1-pyridin-2-ylethylidene) amine. Synth React Inorg M. 2012;42(4):480–484. [Google Scholar]
- 57.Wang C.-Y., Li J.-F., Zhang Z.-S., Liu Y., Yi L., Sheng S.-J. Synthesis, crystal structures, and urease inhibitory properties of two isostructural dinuclear zinc(II) complexes with schiff base 5-methoxy-2-[(2-methylaminoethylimino)methyl]phenol. Synth React Inorg M. 2012;42(10):1405–1409. [Google Scholar]
- 58.You Z.-L., Hou P., Ni L.-L., Chen S. Influence of the steric effects of the Schiff bases and the hydrogen bonds on the bridging modes of the azide groups: Syntheses and crystal structures of three azide-bridged Schiff base zinc(II) complexes. Inorg Chem Commun. 2009;12(5):444–446. [Google Scholar]
- 59.Carlini C.R., Ligabue-Braun R. Ureases as multifunctional toxic proteins: a review. Toxicon. 2016;110:90–109. doi: 10.1016/j.toxicon.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 60.Carter E.L., Flugga N., Boer J.L., Mulrooney S.B., Hausinger R.P. Interplay of metal ions and urease. Metallomics. 2009;1(3):207–221. doi: 10.1039/b903311d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi D.-H., You Z.-L., Xu C., Zhang Q., Zhu H.-L. Synthesis, crystal structure and urease inhibitory activities of Schiff base metal complexes. Inorg Chem Commun. 2007;10(4):404–406. [Google Scholar]
- 62.Dillard J.G., Crowther D.L., Murray J.W. The oxidation states of cobalt and selected metals in Pacific ferromanganese nodules. Geochim Cosmochim Acta. 1982;46(5):755–759. [Google Scholar]
- 63.Shamma A.A., Ali H.A., Kamel S. Synthesis, characterization and biological properties of mixed ligand complexes of cobalt(II/III) valproate with 2,9-dimethyl-1,10-phenanthroline and 1,10-phenanthroline. Appl Organomet Chem. 2018;32(1):3904. [Google Scholar]
- 64.Lv J., Liu T., Cai S., Wang X., Liu L., Wang Y. Synthesis, structure and biological activity of cobalt(II) and copper(II) complexes of valine-derived schiff bases. J Inorg Biochem. 2006;100(11):1888–1896. doi: 10.1016/j.jinorgbio.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 65.Lu Y., Shi D.-H., You Z.-L., Zhou X.-S., Li K. Synthesis, structures, and urease inhibition of nickel(II), zinc(II), and cobalt(II) complexes with similar hydroxy-rich Schiff bases. J Coord Chem. 2012;65(2):339–352. [Google Scholar]
- 66.Jing C., Wang C., Yan K., Zhao K., Sheng G., Qu D. Synthesis, structures and urease inhibitory activity of cobalt(III) complexes with Schiff bases. Bioorgan Med Chem. 2016;24(2):270–276. doi: 10.1016/j.bmc.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 67.Wang C.Y. Synthesis and crystal structures of cobalt(III) and zinc(II) complexes derived from 4-chloro-2-[(2-morpholin-4-ylethylimino)methyl]phenol with urease inhibitory activity. Russ J Coord Chem. 2010;36(3):177–182. [Google Scholar]
- 68.You Z.-L., Zhou P. New method for the synthesis of bis-Schiff base trinuclear cobalt(II) complex with urease inhibitory activity. Inorg Chem Commun. 2007;10(11):1273–1275. [Google Scholar]
- 69.Verma S., Cam M.C., McNeill J.H. Nutritional factors that can favorably influence the glucose/insulin system: vanadium. J Am Colloids Nutr. 1998;17(1):11–18. doi: 10.1080/07315724.1998.10718730. [DOI] [PubMed] [Google Scholar]
- 70.Goc A. Biological activity of vanadium compounds. Cent Eur J Biol. 2006;1(3):314–332. [Google Scholar]
- 71.Ashiq U., Ara R., Mahroof-Tahir M., Maqsood Z.T., Khan K.M., Khan S.N. Synthesis, spectroscopy, and biological properties of vanadium(IV)–hydrazide complexes. Chem Biodivers. 2008;5(1):82–92. doi: 10.1002/cbdv.200890016. [DOI] [PubMed] [Google Scholar]
- 72.Sanna D., Ugone V., Serra M., Garribba E. Speciation of potential anti-diabetic vanadium complexes in real serum samples. J Inorg Biochem. 2017;173:52–65. doi: 10.1016/j.jinorgbio.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 73.Kioseoglou E., Petanidis S., Gabriel C., Salifoglou A. The chemistry and biology of vanadium compounds in cancer therapeutics. Coordin Chem Rev. 2015;301–302:87–105. [Google Scholar]
- 74.Leon I.E., Cadavid-Vargas J.F., Di Virgilio A.L., Etcheverry S.B. Vanadium, ruthenium and copper compounds: a new class of nonplatinum metallodrugs with anticancer activity. Curr Med Chem. 2017;24(2):112–148. doi: 10.2174/0929867323666160824162546. [DOI] [PubMed] [Google Scholar]
- 75.Sinha A., Banerjee K., Banerjee A., Sarkar A., Ahir M., Adhikary A. Induction of apoptosis in human colorectal cancer cell line, HCT-116 by a vanadium- Schiff base complex. Biomed Pharmacother. 2017;92:509–518. doi: 10.1016/j.biopha.2017.05.108. [DOI] [PubMed] [Google Scholar]
- 76.Chohan Z.H., Sumrra S.H., Youssoufi M.H., Hadda T.B. Metal based biologically active compounds: design, synthesis, and antibacterial/antifungal/cytotoxic properties of triazole-derived Schiff bases and their oxovanadium(IV) complexes. Eur J Med Chem. 2010;45(7):2739–2747. doi: 10.1016/j.ejmech.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 77.Ebrahimipour S.Y., Mohamadi M., Sheikhshoaie I., Suárez S., Baggio R., Khaleghi M. A novel oxido-vanadium(V) Schiff base complex: synthesis, spectral characterization, crystal structure, electrochemical evaluation, and biological activity. Res Chem Intermediat. 2016;42(2):611–623. [Google Scholar]
- 78.Huo Y., Ye Y.-T., Cheng X.-S., You Z.-L. Synthesis, characterization and urease inhibition of a novel acetylhydroxamate-coordinated oxovanadium(V) complex with hydrazone ligand. Inorg Chem Commun. 2014;45:131–134. [Google Scholar]
- 79.Sheng G.H., You Z., Zhu H.L. Synthesis, crystal structure, and urease inhibition of [N′-(3,5-dibromo-2-hydroxybenzylidene)isonicotinohydrazido]-(benzohydroxamato)oxovanadium(V) Russ J Coord Chem. 2014;40(7):505–509. [Google Scholar]
- 80.You Z.-L., Shi D.-H., Zhang J.-C., Ma Y.-P., Wang C., Li K. Synthesis, structures, and urease inhibitory activities of oxovanadium(V) complexes with Schiff bases. Irnoganica Chim Acta. 2012;384:54–61. [Google Scholar]
- 81.Ren J.Q., Jiao Q.Z., Wang Y.N., Xu F.Y., Cheng X.S., You Z.L. Synthesis, structures and Helicobacter pylori urease inhibition of schiff base vanadium complexes containing acetohydroxamate ligands. Chin J Inorg Chem. 2014;30:640–648. [Google Scholar]
- 82.You Z.-L., Cui Y.-M., Ma Y.-P., Wang C., Zhou X.-S., Li K. Synthesis, characterization and urease inhibitory activity of oxovanadium(V) complexes with similar Schiff bases. Inorg Chem Commun. 2011;14(5):636–640. [Google Scholar]
- 83.Zhao Y., Han X., Zhou X.-X.Z., Hai-Hua L., Zhong-Lu Y. Synthesis, structures, and Helicobacter pylori urease inhibition of oxovanadium(V) complexes with hydrazones. Chin J Inorg Chem. 2013;29:867–874. [Google Scholar]
- 84.You Z.-L., Xian D.M., Zhang M., Sun H., Li H.H. Synthesis and structures of dioxovanadium(V) complexes with Schiff bases and their inhibition studies on Helicobacter pylori urease. Chin J Inorg Chem. 2012;28(6):1271–1278. [Google Scholar]
- 85.You Z.-L., Sun H., Ding B.-W., Ma Y.-P., Zhang M., Xian D.-M. Preparation and structural characterization of oxovanadium(V) complexes with Schiff bases and their inhibition studies on Helicobacter pylori urease. J Coord Chem. 2011;64(20):3510–3520. [Google Scholar]
- 86.Zhang Y., Liu Q., Jing H., Cai Y., Wang Q., Li Y. Synthesis, characterization, and antimicrobial activity of two Schiff base silver(I) complexes derived from 4-carboxybenzaldehyde. J Coord Chem. 2017;70(6):1066–1076. [Google Scholar]
- 87.Shi D.-H., Zhang N., Liu W.-W., Gao L.-L., Zhang Q., You Z.-L. Synthesis, crystal structures, and biological activity of Cu(II), Mn(III), and Fe(III) complexes derived from N,N′-bis(4-methoxysalicylidene)ethylenediamine. Synth React Inorg Met-Org Nano-Metal Chem. 2012;42(8):1177–1182. [Google Scholar]
- 88.Zhang N., Huang C.-Y., Shi D.-H., You Z.-L. Unprecedented preparation of bis-Schiff bases and their manganese(III) complexes with urease inhibitory activity. Inorg Chem Commun. 2011;14(10):1636–1639. [Google Scholar]
- 89.You Z.-L., Han X., Zhang G.-N. Synthesis, crystal structures, and urease inhibitory activities of three novel thiocyanato-bridged polynuclear Schiff base cadmium(II) complexes. Z Anorg Allg Chem. 2008;634(1):142–146. [Google Scholar]
- 90.Shi D.-H., Zhang L., Ni L.-L., Bai S., You Z.-L. Synthesis, crystal structures, and urease inhibitory activities of two isostructural schiff base cadmium(II) complexes. Synth React Inorg Met-Org Nano-Metal Chem. 2010;40(5):359–363. [Google Scholar]
- 91.Choudhary MI, Khan A, Khan KM, Ambreen N, Wahab A-t, Rahman A-u. InventorsSchiff bases of thiazoles: a new class of ureases inhibitors. Patent # US20150368214A1, United States, 2015.
- 92.Hameed A., al-Rashida M., Uroos M., Abid Ali S., Khan K.M. Schiff bases in medicinal chemistry: a patent review (2010-2015) Expert Opin Ther Pat. 2017;27(1):63–79. doi: 10.1080/13543776.2017.1252752. [DOI] [PubMed] [Google Scholar]
- 93.Modolo LV, de Fatima A, de Souza LT, Horta LP, da Silva CM, Barboa GM, et al. Urea pearls combine with almines, method for producing, same and use thereof in agriculture, and use of aldimines for the treatment of bacterial infections. Patent # WO2016174648A1, National Institute for Industrial Property (INPI), Brazil; 2016.