Abstract
In the past two decades, besides conventional adenoma pathway, a subset of colonic lesions, including hyperplastic polyps, sessile serrated adenoma/polyps, and traditional serrated adenomas have been suggested as precancerous lesions via the alternative serrated neoplasia pathway. Major molecular alterations of sessile serrated neoplasia include BRAF mutation, high CpG island methylator phenotype, and escape of cellular senescence and progression via methylation of tumor suppressor genes or mismatch repair genes. With increasing information of the morphologic and molecular features of serrated lesions, one major challenge is how to reflect this knowledge in clinical practice, such as pathologic and endoscopic diagnosis, and guidelines for treatment and surveillance.
Keywords: Serrated neoplasia, Colorectal neoplasm, Carcinogenesis, Hyperplastic polyp
INTRODUCTION
Colorectal cancer (CRC) holds the third rank in cancer incidence and the major cause of cancer-related death in the world.1 In Asia, the incidence of CRC rapidly increased between 1998 and 2007, and CRC became the leading cause of cancer-related death.2
For two decades, conventional adenomas were considered the only precancerous lesions of CRC.3 However, other type of colonic lesions have been considered to have malignant potential which progress via the alternative serrated neoplasia pathway.4,5,6,7,8,9 Circumstantial evidence has shown that the serrated neoplasia pathway contributes to the development of 15% to 30% of all CRCs.4,10,11 The latest World Health Organization (WHO) guideline published in 2010 described sporadic serrated polyp lesions have a typical serrated (“saw-tooth” or stellate) architecture of the crypt lining epithelium. Sporadic serrated polyps are subdivided into sessile serrated adenoma/polyps (SSA/Ps), traditional serrated adenomas (TSAs), and hyperplastic polyps (HPs).12
Clinically, a large portion of post-colonoscopy interval cancers are suspected of developing from serrated polyps.13,14,15,16 The development of high resolution endoscopic equipment and an improvement of colonoscopy quality has enhanced recognition of serrated lesions, such as right-sided flat lesions with indiscrete margins.17
The purpose of this review is an overview of the clinicopathological and molecular features of alternative serrated pathway in colorectal carcinogenesis to promote better management and clinical outcomes.
CLASSIFICATION OF SERRATED PRECURSORS
1. Hyperplastic Polyps
HPs are the most prevalent (60%–75%) serrated lesions.18,19 Twenty-five percent of the average risk individuals have 1 or more HPs, which are located in left side colon.18 HPs are generally equal to or smaller than 5 mm with a flat or sessile endoscopic feature.20 Histopathologic characteristics of HPs include elongated crypts with proliferation and serration of the upper crypts without cytological atypia.21
HPs can be subclassified on the basis of mucin type into microvesicular HPs (MVHPs), goblet-cell-rich HPs (GCHPs), and mucin-poor HPs (MPHPs).12 MVHP is the most common subtype, accounting for 60% of HPs.20 Histologically, MVHPs are characterized by columnar cells with lots of microvesicular mucin and stellate crypt openings.7,22
Because MVHPs commonly exhibit BRAF mutations (80%) and occasionally present increased CpG island methylation, 23,24 especially in proximal lesions, they are considered precursors of SSA/Ps. When they are large and located in the right colon, it is difficult to distinguish MVHPs from SSA/Ps.
GCHPs account for 30% of HPs25,26,27 and have more rounded crypt openings than MVHPs, showing abundant mature goblet cells in the upper crypt with tendency of left-sided location in colon.22,28 GCHPs are linked to KRAS mutations which are presented in approximately 50% of cases,24,29 suggesting the possibility of progression to TSAs.30 MPHPs, the rarest form of HPs, lack goblet cells, and are considered a variant of MVHPs developed from reactive change with unknown clinical significance.31
2. Sessile Serrated Adenomas or Polyps
SSA/Ps are comprised of 20% to 35% of colorectal serrated lesions.18,19 SSA/Ps were first mentioned by Torlakovic et al.22,32 in 1996, but before 2003, SSA/Ps were labeled HPs.6 Current SSA/Ps were defined by WHO classification.12 However, the terminology has remained controversial. Recently, the British Society of Gastroenterology suggested that it should be renamed to “sessile serrated lesions” because it shows neither cytologic dysplasia nor polypoid morphology.33,34
The diagnosis of SSA/Ps has increased as our knowledge of this entity grows, both endoscopically and pathologically. 20,35,36 However, the prevalence of SSA/Ps is thought to be underestimated, because there was some discrepancy between expected SSA/P detection rate and actual detection rate of 16% and 12%, respectively in patients with an average CRC risk.18,37 In addition, SSA/Ps are most commonly located proximally, but 20% to 40% are also detected in the distal colon.18,38
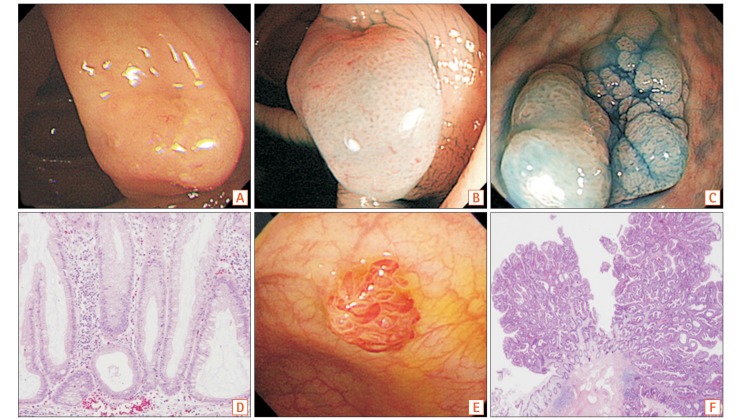
SSA/Ps are histopathologically characterized by patterns including irregular branching, dilation towards the base, and L-shaped or inverted T-shaped crypts (Fig. 1). The inverted crypts can be found below the muscularis mucosa, which is designated as displaced crypts or pseudoinvasion.12,21 In addition, SSA/Ps can have cytologic dysplasia, which is more progressive form in adenoma carcinoma sequence.12 Previous studies reported the prevalence of SSA/Ps with dysplasia as 20% to 30% of entire SSA/Ps.18,38 The median age of patients with SSA/Ps without dysplasia was 61 years, which with SSA/Ps with dysplasia was 66 years, and which with SSA/P with early cancer was 72 years. This increasing tendency of median age of prevalence indicates their serial progression.9 Meanwhile, the differentiation of SSA/P from MVHP is difficult, especially in small lesions.39 Therefore, a recent consensus recommended that a single characteristic crypt base is enough to diagnose SSA/P, and MVHPs larger than 10 mm should be considered equivalent to SSA/Ps for clinical or surveillance purposes.20
Fig. 1. Endoscopic and histologic features of sessile serrated polyps and traditional serrated adenoma. Endoscopic appearance of sessile serrated adenoma (SSA) with white light endoscopy shows smooth and indistinct surface pattern covered with mucus (A). Chromoendoscopy after indigo carmine dye spraying in SSA shows clear boundaries and characteristic pit pattern (type II-O) (B, C). Microscopic features of SSA show irregular branching and T-shaped or L-shaped basal crypt (H&E, ×400) (D). Endoscopic appearance of traditional serrated adenoma (TSA) shows protruded polypoid shape with villous surface (E), and microscopic feature of TSA shows villous serration with dysplasia (H&E, ×40) (F).
3. Traditional Serrated Adenomas
TSAs, the rarest subtype of serrated lesions,18,19,29 are usually pedunculated in shape, and located in the left colon and rectum (Fig. 1).20,40,41 Histologic differentiation of TSAs from tubulovillous adenomas is more difficult than histologic differentiation of SSA/Ps.12,21 TSAs are serrated and show villous or tubulovillous configuration that is composed of columnar cells with intensively and diffusely eosinophilic cytoplasm (Fig. 1).20,21,42 Ectopic crypt foci is another important finding of TSAs, which are small aberrant crypts that develop horizontally without anchoring to the underlying muscularis mucosa.21 Cytologic dysplasia (90% low-grade and 10% high-grade) is also commonly presented in TSAs.12,21
Filiform serrated adenoma is a rare, less aggressive variant form of TSAs that is characterized by thin and elongated projection lined by neoplastic epithelium.43,44
SERRATED NEOPLASIA PATHWAY
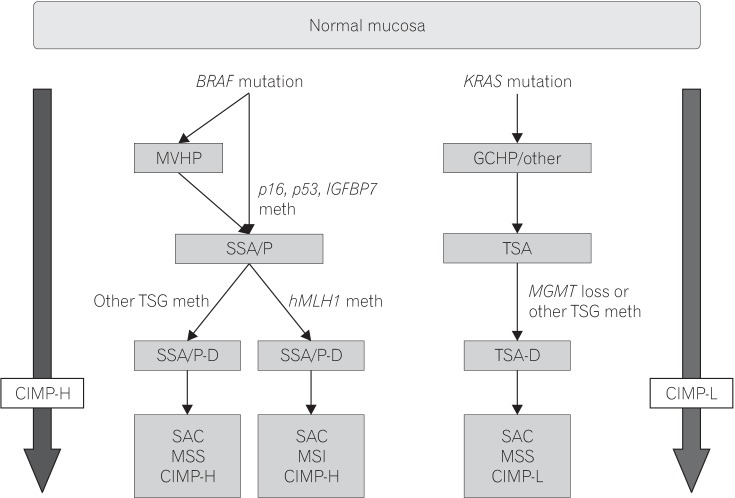
Three molecular pathways of CRC carcinogenesis have been established: (1) chromosomal instability (CIN); (2) microsatellite instability (MSI); and (3) CpG island methylator phenotype (CIMP), which is also referred to the serrated neoplasia pathway or epigenetic instability pathway. These pathways are related with each other in complex way in carcinogenesis, and the serrated neoplasia pathway is considered to be separated from traditional pathway (Fig. 2).4,26,45
Fig. 2. Simplified models of the sessile and traditional serrated pathways. MVHP, microvesicular hyperplastic polyp; GCHP, goblet cell-rich hyperplastic polyp; IGFBT7, insulin-like growth factor-binding protein 7; SSA/P, sessile serrated adenoma/polyp; TSA, traditional serrated adenoma; TSG, tumor suppressor gene; hMLH1, human MutL homolog 1; MGMT, O-6-methylguanine-DNA methyltransferase; SSA/P-D, SSA/polyp with dysplasia; TSA-D, TSA with dysplasia; SAC, serrated adenocarcinoma; meth, methylation; MSS, microsatellite stable; CIMP-H, CpG island methylator phenotype-high; MSI, microsatellite instability; CIMP-L, CpG island methylator phenotype-low.
Hypermethylation of CpG islands on the promoter regions of tumor suppressor genes and subsequent silencing is common in CRC. For example, silencing of hMLH1 leads to mismatch repair (MMR) dysfunction, which results in sporadic MSI. This CIMP, was observed in 20% to 30% of CRC and is one of the major molecular characteristics of the serrated neoplasia pathway.46,47 In a previous study, CIMP was detected in 11% of MVHPs and 40% of SSA/Ps.48 CIMP was found not only in serrated polyps, but also in histologically normal mucosa of patients with hyperplastic polyposis syndrome, which supports that CIMP is an important early step of the serrated pathway.49,50,51 In addition, hypermethylation of hMLH1 gene, a DNA MMR gene, was detected in 40% of sporadic CIMP+ CRC. When it is inactivated by hypermethylation, high frequency MSI (MSI-high) is induced, and these lesions tend to develop additional mutations, including BAX, PTEN, MSH3, MSH6, and IGF2R.52 As a result of these mutations, rapid development of cytologic dysplasia and carcinomatous transformation may occur. Relatively rapid development of CRC by the serrated pathway is suggested in the literature, comparable with carcinogenesis in patients with Lynch syndrome.53 In addition, Bettington et al.54 reported 74.5% loss of hMLH1 in SSA/Ps containing dysplasia/carcinoma, showing its relationship with older age, female gender, proximal location, CIMP, and lack of aberrant p53.
Prior to CIMP, a mutation in the BRAF proto-oncogene is the most pronounced key feature in the initial phase of the serrated neoplastic pathway (MSI high and/or CIMP high CRC), which activates the mitogen activated protein kinase (MAPK) cascade. This BRAF mutation results in uncontrolled cell proliferation, similar to KRAS mutation in adenomas. Incidence of BRAF mutation has been reported as 50% to 72% of MVHPs, 70% to 80% of SSA/Ps, and only 1% of tubular adenomas.25,55 Because of the exclusive mutation between BRAF and KRAS, however, KRAS mutations were reported to have low prevalence in CIMP-high CRCs.56
Mutations of BRAF or KRAS activate cell proliferation, which is followed by cell senescence.57 If tumor suppressor genes are silenced, as with methylation of p16INK4a or p53 mutations, BRAF or KRAS-induced senescence can be avoided with progression of carcinogenesis.58,59,60 Silencing by methylation of insulin-like growth factor binding protein 7 (IGFBP7), an important mediator of p53 induced senescence, also induces an escape from cell senescence.61
Furthermore, the Wnt signaling pathway, a major signaling pathway of CIN+ CRC, could be involved in the serrated neoplastic pathway. For example, some reports showed that aberrant β-catenin accumulation was detected in 0 out of 19 HPs and 9 out of 22 (41%) SSA/Ps.62 Another study reported aberrant β-catenin accumulation in 8 out of 27 (29%) SSA/Ps without dysplasia and 27 out of 27 (100%) SSA/Ps with dysplasia, suggesting that the Wnt pathway is involved in progression of SSA/Ps, rather than early change of SSA/Ps.56 The mechanism and role of the Wnt signaling in the serrated pathway is not well understood, but silencing by methylation of mutated in colorectal cancer gene (MMC), which correlates with BRAF mutation and CIMP, might have an important role because MMC suppress Wnt signaling via interactions with β-catenin.24
The molecular pathway of TSA development is not well understood. However, it is characterized by promoter hypermethylation and subsequent silencing of the methylguanine methyltransferase (MGMT), KRAS mutation, and CIMP low tumor.42,63,64
ENDOSCOPIC DIAGNOSIS AND MANAGEMENT
Clinically, serrated polyps are considered to be related with the development of interval CRCs. Therefore, endoscopic detection and complete removal is important.65 Endoscopic detection rates of SSA/Ps vary because these lesions are frequently flat and resemble folds of the proximal colon. Magnifying chromoendoscopy and narrow-band imaging (NBI) can facilitate distinction of serrated lesions, but it is not enough to differentiate between SSA/Ps and HPs.66
For detection and diagnosis, it is important to know the distinct features of SSA/Ps (Fig. 1). HPs are usually smaller than 5 mm, and have a pale color and a stellate type II Kudo pit pattern on chromoendoscopy or NBI.67,68 The endoscopic features of SSA/Ps are sessile or flat morphology, pale color, indistinct borders with mucus capping, and rim of bubbles or debris.69,70,71,72 With NBI, a cloud-like surface, dark spots inside crypts, and type II-O or open Kudo pit pattern, which is wider and more rounded than type II pit pattern, are highly predictive of SSA/Ps.72 Meanwhile, TSAs show protuberant and/or pedunculated shape with a type IV pit pattern and a fern-like feature.73,74 In addition, HPs and TSAs tend to locate in the distal part of colon, while SSA/Ps are more frequently observed in the proximal colon.9,19,29,75
There is no conclusive evidence-based guideline on management of serrated polyps, but new guidelines recommend that all serrated polyps should be endoscopically removed, except small and diminutive HPs in the rectosigmoid area. SSA/Ps are reported to have higher incomplete resection rates due to their flat shape and indiscrete borders.76 Chromoendoscopy contrast dye can be used to define the border of these lesions,6,30 and lifting of the lesion is helpful in identifying accurate margins. Therefore endoscopic mucosal resection is an appropriate method for endoscopic teatment.6 If the lesion is not suitable for endoscopic resection due to difficult location or huge size, segmental colectomy is advised.77
As for surveillance after removal of serrated polyp, U.S. Multi-Society Task Force recommended 5-year surveillance interval for SSA/Ps smaller than 10 mm without dysplasia,78 which is shorter than 10 years recommended by European Society of Gastrointestinal Endoscopy guideline.79 Most guidelines recommended 3-year interval for SSA/Ps equal or larger than 10 mm, SSA/Ps with dysplasia, and TSAs.78,79,80 After piecemeal resection of large SSA/Ps, follow-up within 6 months is recommended, consistently.
CONCLUSIONS
In the past two decades, as our knowledge of the morphologic and molecular features and clinical meaning of serrated lesions increased, its detection and diagnosis rate has been improved. Now, we should consider about how to reflect this knowledge in clinical practice, such as pathologic and endoscopic diagnosis, and guidelines for treatment and surveillance. Further investigations about the molecular mechanisms, natural history, and management strategies are needed.
Footnotes
FINANCIAL SUPPORT: The authors received no financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION: Conceptualization, writing - review and editing, approval of final manuscript: Tae Il Kim. Writing - original draft: Soon Young Kim.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Tsoi KKF, Hirai HW, Chan FC, Griffiths S, Sung JJ. predicted increases in incidence of colorectal cancer in developed and developing regions, in association with ageing populations. Clin Gastroenterol Hepatol. 2017;15:892–900. doi: 10.1016/j.cgh.2016.09.155. [DOI] [PubMed] [Google Scholar]
- 3.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251–2270. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- 4.Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42:1–10. doi: 10.1016/j.humpath.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Boparai KS, Dekker E, Polak MM, Musler AR, van Eeden S, van Noesel CJ. A serrated colorectal cancer pathway predominates over the classic WNT pathway in patients with hyperplastic polyposis syndrome. Am J Pathol. 2011;178:2700–2707. doi: 10.1016/j.ajpath.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.East JE, Saunders BP, Jass JR. Sporadic and syndromic hyperplastic polyps and serrated adenomas of the colon: classification, molecular genetics, natural history, and clinical management. Gastroenterol Clin North Am. 2008;37:25–46. doi: 10.1016/j.gtc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Bettington M, Walker N, Clouston A, Brown I, Leggett B, Whitehall V. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367–386. doi: 10.1111/his.12055. [DOI] [PubMed] [Google Scholar]
- 8.Oono Y, Fu K, Nakamura H, et al. Progression of a sessile serrated adenoma to an early invasive cancer within 8 months. Dig Dis Sci. 2009;54:906–909. doi: 10.1007/s10620-008-0407-7. [DOI] [PubMed] [Google Scholar]
- 9.Lash RH, Genta RM, Schuler CM. Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol. 2010;63:681–686. doi: 10.1136/jcp.2010.075507. [DOI] [PubMed] [Google Scholar]
- 10.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 11.Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci U S A. 1999;96:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snover D. Serrated polyps of the colon and rectum and serrated polyposis. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC; 2010. pp. 160–165. [Google Scholar]
- 13.Burgess NG, Tutticci NJ, Pellise M, Bourke MJ. Sessile serrated adenomas/polyps with cytologic dysplasia: a triple threat for interval cancer. Gastrointest Endosc. 2014;80:307–310. doi: 10.1016/j.gie.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 14.Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol. 2010;105:1189–1195. doi: 10.1038/ajg.2009.699. [DOI] [PubMed] [Google Scholar]
- 15.le Clercq CM, Sanduleanu S. Interval colorectal cancers: what and why. Curr Gastroenterol Rep. 2014;16:375. doi: 10.1007/s11894-014-0375-3. [DOI] [PubMed] [Google Scholar]
- 16.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–1105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arthur JF. Structure and significance of metaplastic nodules in the rectal mucosa. J Clin Pathol. 1968;21:735–743. doi: 10.1136/jcp.21.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazewinkel Y, de Wijkerslooth TR, Stoop EM, et al. Prevalence of serrated polyps and association with synchronous advanced neoplasia in screening colonoscopy. Endoscopy. 2014;46:219–224. doi: 10.1055/s-0033-1358800. [DOI] [PubMed] [Google Scholar]
- 19.Carr NJ, Mahajan H, Tan KL, Hawkins NJ, Ward RL. Serrated and non-serrated polyps of the colorectum: their prevalence in an unselected case series and correlation of BRAF mutation analysis with the diagnosis of sessile serrated adenoma. J Clin Pathol. 2009;62:516–518. doi: 10.1136/jcp.2008.061960. [DOI] [PubMed] [Google Scholar]
- 20.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315–1329. doi: 10.1038/ajg.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aust DE, Baretton GB Members of the Working Group GI-Pathology of the German Society of Pathology. Serrated polyps of the colon and rectum (hyperplastic polyps, sessile serrated adenomas, traditional serrated adenomas, and mixed polyps)-proposal for diagnostic criteria. Virchows Arch. 2010;457:291–297. doi: 10.1007/s00428-010-0945-1. [DOI] [PubMed] [Google Scholar]
- 22.Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65–81. doi: 10.1097/00000478-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 23.O'Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol. 2006;30:1491–1501. doi: 10.1097/01.pas.0000213313.36306.85. [DOI] [PubMed] [Google Scholar]
- 24.Burnett-Hartman AN, Newcomb PA, Phipps AI, et al. Colorectal endoscopy, advanced adenomas, and sessile serrated polyps: implications for proximal colon cancer. Am J Gastroenterol. 2012;107:1213–1219. doi: 10.1038/ajg.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernando WC, Miranda MS, Worthley DL, et al. The CIMP phenotype in BRAF mutant serrated polyps from a prospective colonoscopy patient cohort. Gastroenterol Res Pract. 2014;2014:374926. doi: 10.1155/2014/374926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138:2088–2100. doi: 10.1053/j.gastro.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 27.Rosty C, Hewett DG, Brown IS, Leggett BA, Whitehall VL. Serrated polyps of the large intestine: current understanding of diagnosis, pathogenesis, and clinical management. J Gastroenterol. 2013;48:287–302. doi: 10.1007/s00535-012-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg DW, Yang S, Pleau DC, et al. Mutations in BRAF and KRAS differentially distinguish serrated versus non-serrated hyperplastic aberrant crypt foci in humans. Cancer Res. 2007;67:3551–3554. doi: 10.1158/0008-5472.CAN-07-0343. [DOI] [PubMed] [Google Scholar]
- 29.Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131:1400–1407. doi: 10.1053/j.gastro.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 30.Huang CS, Farraye FA, Yang S, O'Brien MJ. The clinical significance of serrated polyps. Am J Gastroenterol. 2011;106:229–240. doi: 10.1038/ajg.2010.429. [DOI] [PubMed] [Google Scholar]
- 31.Arnold CA, Montgomery E, Iacobuzio-Donahue CA. The serrated pathway of neoplasia: new insights into an evolving concept. Diagn Histopathol. 2011;17:367–375. [Google Scholar]
- 32.Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology. 1996;110:748–755. doi: 10.1053/gast.1996.v110.pm8608884. [DOI] [PubMed] [Google Scholar]
- 33.Vieth M, Quirke P, Lambert R, von Karsa L, Risio M. Annex to Quirke et al. Quality assurance in pathology in colorectal cancer screening and diagnosis: annotations of colorectal lesions. Virchows Arch. 2011;458:21–30. doi: 10.1007/s00428-010-0997-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.East JE, Atkin WS, Bateman AC, et al. British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut. 2017;66:1181–1196. doi: 10.1136/gutjnl-2017-314005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahi CJ, Li X, Eckert GJ, Rex DK. High colonoscopic prevalence of proximal colon serrated polyps in average-risk men and women. Gastrointest Endosc. 2012;75:515–520. doi: 10.1016/j.gie.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 36.Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol. 2010;105:2656–2664. doi: 10.1038/ajg.2010.315. [DOI] [PubMed] [Google Scholar]
- 37.Abdeljawad K, Vemulapalli KC, Kahi CJ, Cummings OW, Snover DC, Rex DK. Sessile serrated polyp prevalence determined by a colonoscopist with a high lesion detection rate and an experienced pathologist. Gastrointest Endosc. 2015;81:517–524. doi: 10.1016/j.gie.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 38.Bouwens MW, van Herwaarden YJ, Winkens B, et al. Endoscopic characterization of sessile serrated adenomas/polyps with and without dysplasia. Endoscopy. 2014;46:225–235. doi: 10.1055/s-0034-1364936. [DOI] [PubMed] [Google Scholar]
- 39.Chung SM, Chen YT, Panczykowski A, Schamberg N, Klimstra DS, Yantiss RK. Serrated polyps with “intermediate features” of sessile serrated polyp and microvesicular hyperplastic polyp: a practical approach to the classification of nondysplastic serrated polyps. Am J Surg Pathol. 2008;32:407–412. doi: 10.1097/PAS.0b013e318158dde2. [DOI] [PubMed] [Google Scholar]
- 40.Wiland HO, 4th, Shadrach B, Allende D, et al. Morphologic and molecular characterization of traditional serrated adenomas of the distal colon and rectum. Am J Surg Pathol. 2014;38:1290–1297. doi: 10.1097/PAS.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 41.Jaramillo E, Tamura S, Mitomi H. Endoscopic appearance of serrated adenomas in the colon. Endoscopy. 2005;37:254–260. doi: 10.1055/s-2005-861007. [DOI] [PubMed] [Google Scholar]
- 42.Torlakovic EE, Gomez JD, Driman DK, et al. Sessile serrated adenoma (SSA) vs. traditional serrated adenoma (TSA) Am J Surg Pathol. 2008;32:21–29. doi: 10.1097/PAS.0b013e318157f002. [DOI] [PubMed] [Google Scholar]
- 43.Yantiss RK, Oh KY, Chen YT, Redston M, Odze RD. Filiform serrated adenomas: a clinicopathologic and immunophenotypic study of 18 cases. Am J Surg Pathol. 2007;31:1238–1245. doi: 10.1097/PAS.0b013e31802d74c0. [DOI] [PubMed] [Google Scholar]
- 44.Ha SY, Lee SM, Lee EJ, et al. Filiform serrated adenoma is an unusual, less aggressive variant of traditional serrated adenoma. Pathology. 2012;44:18–23. doi: 10.1097/PAT.0b013e32834d7bbf. [DOI] [PubMed] [Google Scholar]
- 45.Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology. 2010;138:2059–2072. doi: 10.1053/j.gastro.2009.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samowitz WS, Albertsen H, Herrick J, et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129:837–845. doi: 10.1053/j.gastro.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 47.Hawkins N, Norrie M, Cheong K, et al. CpG island methylation in sporadic colorectal cancers and its relationship to microsatellite instability. Gastroenterology. 2002;122:1376–1387. doi: 10.1053/gast.2002.32997. [DOI] [PubMed] [Google Scholar]
- 48.Yang S, Farraye FA, Mack C, Posnik O, O'Brien MJ. BRAF and KRAS Mutations in hyperplastic polyps and serrated adenomas of the colorectum: relationship to histology and CpG island methylation status. Am J Surg Pathol. 2004;28:1452–1459. doi: 10.1097/01.pas.0000141404.56839.6a. [DOI] [PubMed] [Google Scholar]
- 49.Chan AO, Issa JP, Morris JS, Hamilton SR, Rashid A. Concordant CpG island methylation in hyperplastic polyposis. Am J Pathol. 2002;160:529–536. doi: 10.1016/S0002-9440(10)64872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wynter CV, Walsh MD, Higuchi T, Leggett BA, Young J, Jass JR. Methylation patterns define two types of hyperplastic polyp associated with colorectal cancer. Gut. 2004;53:573–580. doi: 10.1136/gut.2003.030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minoo P, Baker K, Goswami R, et al. Extensive DNA methylation in normal colorectal mucosa in hyperplastic polyposis. Gut. 2006;55:1467–1474. doi: 10.1136/gut.2005.082859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shima K, Morikawa T, Yamauchi M, et al. TGFBR2 and BAX mononucleotide tract mutations, microsatellite instability, and prognosis in 1072 colorectal cancers. PLoS One. 2011;6:e25062. doi: 10.1371/journal.pone.0025062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edelstein DL, Axilbund JE, Hylind LM, et al. Serrated polyposis: rapid and relentless development of colorectal neoplasia. Gut. 2013;62:404–408. doi: 10.1136/gutjnl-2011-300514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bettington M, Walker N, Rosty C, et al. Clinicopathological and molecular features of sessile serrated adenomas with dysplasia or carcinoma. Gut. 2017;66:97–106. doi: 10.1136/gutjnl-2015-310456. [DOI] [PubMed] [Google Scholar]
- 55.Burnett-Hartman AN, Passarelli MN, Adams SV, et al. Differences in epidemiologic risk factors for colorectal adenomas and serrated polyps by lesion severity and anatomical site. Am J Epidemiol. 2013;177:625–637. doi: 10.1093/aje/kws282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kambara T, Simms LA, Whitehall VL, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53:1137–1144. doi: 10.1136/gut.2003.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campisi J. Suppressing cancer: the importance of being senescent. Science. 2005;309:886–887. doi: 10.1126/science.1116801. [DOI] [PubMed] [Google Scholar]
- 58.Collado M, Serrano M. The power and the promise of oncogene-induced senescence markers. Nat Rev Cancer. 2006;6:472–476. doi: 10.1038/nrc1884. [DOI] [PubMed] [Google Scholar]
- 59.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 60.Chen Z, Trotman LC, Shaffer D, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suzuki H, Igarashi S, Nojima M, et al. IGFBP7 is a p53-responsive gene specifically silenced in colorectal cancer with CpG island methylator phenotype. Carcinogenesis. 2010;31:342–349. doi: 10.1093/carcin/bgp179. [DOI] [PubMed] [Google Scholar]
- 62.Wu JM, Montgomery EA, Iacobuzio-Donahue CA. Frequent beta-catenin nuclear labeling in sessile serrated polyps of the colorectum with neoplastic potential. Am J Clin Pathol. 2008;129:416–423. doi: 10.1309/603UQKM7C2KELGJU. [DOI] [PubMed] [Google Scholar]
- 63.Whitehall VL, Walsh MD, Young J, Leggett BA, Jass JR. Methylation of O-6-methylguanine DNA methyltransferase characterizes a subset of colorectal cancer with low-level DNA microsatellite instability. Cancer Res. 2001;61:827–830. [PubMed] [Google Scholar]
- 64.Ogino S, Kawasaki T, Ogawa A, Kirkner GJ, Loda M, Fuchs CS. TGFBR2 mutation is correlated with CpG island methylator phenotype in microsatellite instability-high colorectal cancer. Hum Pathol. 2007;38:614–620. doi: 10.1016/j.humpath.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 65.Teriaky A, Driman DK, Chande N. Outcomes of a 5-year follow-up of patients with sessile serrated adenomas. Scand J Gastroenterol. 2012;47:178–183. doi: 10.3109/00365521.2011.645499. [DOI] [PubMed] [Google Scholar]
- 66.Kashida H, Ikehara N, Hamatani S, Kudo SE, Kudo M. Endoscopic characteristics of colorectal serrated lesions. Hepatogastroenterology. 2011;58:1163–1167. doi: 10.5754/hge10093. [DOI] [PubMed] [Google Scholar]
- 67.Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut. 1982;23:835–842. doi: 10.1136/gut.23.10.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Su MY, Hsu CM, Ho YP, Chen PC, Lin CJ, Chiu CT. Comparative study of conventional colonoscopy, chromoendoscopy, and narrow-band imaging systems in differential diagnosis of neoplastic and nonneoplastic colonic polyps. Am J Gastroenterol. 2006;101:2711–2716. doi: 10.1111/j.1572-0241.2006.00932.x. [DOI] [PubMed] [Google Scholar]
- 69.Oka S, Tanaka S, Hiyama T, et al. Clinicopathologic and endoscopic features of colorectal serrated adenoma: differences between polypoid and superficial types. Gastrointest Endosc. 2004;59:213–219. doi: 10.1016/s0016-5107(03)02693-2. [DOI] [PubMed] [Google Scholar]
- 70.Tadepalli US, Feihel D, Miller KM, et al. A morphologic analysis of sessile serrated polyps observed during routine colonoscopy (with video) Gastrointest Endosc. 2011;74:1360–1368. doi: 10.1016/j.gie.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 71.Rustagi T, Rangasamy P, Myers M, et al. Sessile serrated adenomas in the proximal colon are likely to be flat, large and occur in smokers. World J Gastroenterol. 2013;19:5271–5277. doi: 10.3748/wjg.v19.i32.5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hazewinkel Y, López-Cerón M, East JE, et al. Endoscopic features of sessile serrated adenomas: validation by international experts using high-resolution white-light endoscopy and narrow-band imaging. Gastrointest Endosc. 2013;77:916–924. doi: 10.1016/j.gie.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 73.Buda A, De Bona M, Dotti I, et al. Prevalence of different subtypes of serrated polyps and risk of synchronous advanced colorectal neoplasia in average-risk population undergoing first-time colonoscopy. Clin Transl Gastroenterol. 2012;3:e6. doi: 10.1038/ctg.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim MJ, Lee EJ, Suh JP, et al. Traditional serrated adenoma of the colorectum: clinicopathologic implications and endoscopic findings of the precursor lesions. Am J Clin Pathol. 2013;140:898–911. doi: 10.1309/AJCPDJC9VC5KTYUS. [DOI] [PubMed] [Google Scholar]
- 75.Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology. 2005;47:32–40. doi: 10.1111/j.1365-2559.2005.02180.x. [DOI] [PubMed] [Google Scholar]
- 76.IJspeert JE, Bastiaansen BA, van Leerdam ME, et al. Development and validation of the WASP classification system for optical diagnosis of adenomas, hyperplastic polyps and sessile serrated adenomas/polyps. Gut. 2016;65:963–970. doi: 10.1136/gutjnl-2014-308411. [DOI] [PubMed] [Google Scholar]
- 77.Leonard DF, Dozois EJ, Smyrk TC, et al. Endoscopic and surgical management of serrated colonic polyps. Br J Surg. 2011;98:1685–1694. doi: 10.1002/bjs.7654. [DOI] [PubMed] [Google Scholar]
- 78.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 79.Hassan C, Quintero E, Dumonceau JM, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2013;45:842–851. doi: 10.1055/s-0033-1344548. [DOI] [PubMed] [Google Scholar]
- 80.Yang DH, Hong SN, Kim YH, et al. Korean guidelines for post-polypectomy colonoscopic surveillance. Intest Res. 2012;10:89–109. doi: 10.4166/kjg.2012.59.2.99. [DOI] [PubMed] [Google Scholar]