Abstract
The integrin α6β4, a laminin receptor that stabilizes epithelial cell adhesion to the basement membrane (BM) through its association with cytokeratins, can stimulate the formation and stabilization of actin-rich protrusions in carcinoma cells. An important, unresolved issue, however, is whether this integrin can transmit forces to the substrate generated by the acto-myosin system. Using a traction-force detection assay, we detected forces exerted through α6β4 on either laminin-1 or on an anti-α6 antibody, demonstrating that this integrin can transmit forces without the need to engage other integrins. These α6β4-dependent traction forces were organized into a compression machine localized to the base of lamellae. We hypothesized that the compression forces generated by α6β4 result in the remodeling of BMs because this integrin plays a major role in the interaction of epithelial and carcinoma cells with such structures. Indeed, we observed that carcinoma cells are able to remodel a reconstituted BM through α6β4-mediated compression forces by a process that involves the packing of BM material under the cells and the mechanical removal of BM from adjacent areas. The distinct signaling functions of α6β4, which activate phosphoinositide 3-OH kinase and RhoA, also contribute to remodeling. Importantly, we demonstrate remodeling of a native BM by epithelial cells and the involvement of α6β4 in this remodeling. Our findings have important implications for the mechanism of both BM organization and tumor invasion.
INTRODUCTION
The α6β4 integrin, which is expressed primarily on the basal surface of most epithelia and in most carcinoma cells, is a structural and functional anomaly among the integrin family of receptors. This integrin is defined as an adhesion receptor for most of the known basement membrane laminins. The distinguishing structural feature of α6β4 is the atypical cytoplasmic domain of the β4 subunit. A primary function of α6β4, revealed by studies on knockout and transgenic mice, is to maintain the integrity of epithelia. This critical role for α6β4 derives from its ability to mediate the formation of stable adhesive structures termed hemidesmosomes on the basal cell surface that link the cytokeratin network with laminins in the basement membrane (Green and Jones, 1996; Shaw et al., 1997).
Although this involvement of α6β4 in hemidesmosome organization and function has dominated the study of this integrin, recent studies have revealed novel and important functions for this integrin in epithelial wound healing and in the migration and invasion of carcinoma cells. Many studies have observed that expression of α6β4 is maintained or often increased in invasive and metastatic carcinomas and that α6β4 expression levels actually correlate with the progression of these carcinomas (reviewed in Rabinovitz and Mercurio, 1996). Although such studies have provided evidence to implicate α6β4 in the invasive process, they do not explain how an integrin, which associates with intermediate filaments and forms stable adhesive contacts, could promote the dynamic processes of migration and invasion. Indeed, the presence of α6β4-containing hemidesmosomes would impede invasion. A significant breakthrough, therefore, was our finding that α6β4 actually mediates the migration of carcinoma cells through its ability to associate with the actin cytoskeleton and promote the formation and stabilization of filopodia and lamellae (Rabinovitz and Mercurio, 1997; O'Connor et al., 1998). This finding implied that the function and cytoskeletal association of α6β4 in invasive carcinoma cells is distinct from its established role of anchoring epithelial cells to the basement membrane (BM) through its association with cytokeratins. A second significant finding that provided a mechanistic basis for the involvement of α6β4 in invasion was that this integrin stimulates the activity of the enzyme phosphoinositide 3-OH kinase (PI3-K) in invasive carcinoma cells and that PI3-K is essential for migration and invasion (Shaw et al., 1997).
Our current interest is to understand the mechanisms by which the α6β4 integrin contributes to the migration process and to relate such mechanisms to invasion. The migration of many cells in culture involves actin polymerization that generates protrusions at the edge of the cell and the contraction of the actin network by associated myosin motors (Lauffenburger and Horwitz, 1996; Mitchison and Cramer, 1996). It has become apparent from studies on fish keratocytes and fibroblasts that adhesion to the substrate in the lamellar area provides the necessary traction to support the propulsive forces generated by the cytoskeleton to haul the rest of the cell (Lee et al., 1994; Burton et al., 1999). Given this knowledge, one possible mechanism by which α6β4 stimulates migration is to enhance the generation of traction by lamellae. We have shown that α6β4 is localized to the base of lamellae in carcinoma cells migrating on laminin (Rabinovitz and Mercurio, 1997). Moreover, the formation of actin bundles parallel to the lamella in such cells, which is considered to be crucial for the generation of traction in the keratocyte and fibroblast models, is dependent on α6β4 (Rabinovitz and Mercurio, 1997). The ability of α6β4 to generate traction forces created by the actomyosin cytoskeleton has not been determined, in part, because most studies on this integrin have focused on its association with the cytokeratin cytoskeleton. Moreover, the consequences of α6β4-mediated traction forces on BM organization and invasion have not been considered.
In this study, we used a well characterized traction-force detection assay (Dembo and Wang, 1999; Pelham and Wang, 1999) to establish that such forces are exerted through the α6β4 integrin. These α6β4-dependent traction forces are organized into a compression machine localized to the base of lamellae. Moreover, we observed that carcinoma cells are able to remodel a reconstituted BM through α6β4-mediated compression forces by a process that involves the packing of BM material under the cells and the mechanical removal of BM from adjacent areas. Importantly, we also demonstrate that the signaling properties of α6β4 can stimulate BM remodeling. Finally, we provide evidence for the remodeling of a natural BM by epithelial cells and for the involvement of α6β4 in this process. Our findings have important implications for the mechanism of both BM organization and tumor invasion.
MATERIALS AND METHODS
Cells and Antibodies
The clone A cell line was isolated from a human, poorly differentiated colon adenocarcinoma (Lotz et al., 1990). The clone A integrin receptors have been described previously (Lotz et al., 1990). Stable subclones of MDA-MB-435 human breast carcinoma cells were used that had been transfected with either the expression vector alone (mock transfectants) or a full-length β4 cDNA (MDA/β4 transfectants). The characterization of these transfectants has been described previously (Shaw et al., 1997).
The following three monoclonal antibodies (mAbs) were used in this study: GoH3, a rat mAb specific for the integrin α6 subunit (Immunotech, Westbrook, ME); MC-13, a mouse mAb specific for the integrin β1 subunit (provided by Dr. Steven Akiyama); and anti-LDL receptor mAb (Oncogene Science, Cambridge, MA).
Reagents
Laminin-1, prepared from the EHS sarcoma, was provided by Dr. Hynda Kleinman (National Institutes of Health, Bethesda, MD). Fluorescein isothiocyanate (FITC)-labeled laminin was provided by Dr. Peter D. Yurchenco (Robert Wood Johnson Medical School, Piscataway, NJ). Matrigel was purchased from BD Biosciences (San Jose, CA). Cytochalasin B, nocodazole, butanedione monoxime (BDM), and phorbol-12-myristate-13-acetate (PMA) were obtained from Sigma (St. Louis, MO). The Rho kinase inhibitor Y27632 was purchased from U.S. Biochemical (Cleveland, OH) and the PI3-K inhibitor LY294002 was purchased from Calbiochem (San Diego, CA). Fluorescent beads (2-μm fluospheres) were obtained from Molecular Probes (Eugene, OR), and gold colloid particles (5 nm) were obtained from Ted Pella (Redding, CA).
Traction-Detection System
A flexible, polyacrylamide gel containing fluorescent beads and coated with either laminin (100 μg/ml) or goat anti-rat/mouse antibodies (100 μg/ml) was prepared following a published protocol (Dembo and Wang, 1999; Pelham and Wang, 1999). Cells were plated on the laminin substrate and incubated for 1 h at 37°C in a CO2 incubator. Before plating the cells on the antibody substrates, the cells were incubated for 20 min in the presence of either the rat GoH3 antibody or anti-LDL receptor antibody, and the unbound antibody was removed by two cycles of centrifugation and resuspension in medium. The dishes were sealed with Parafilm (American National Can, Menasha, WI). Random cells were filmed digitally using time lapse video-microscopy on a Nikon Diaphot 300 inverted microscope equipped with a heated stage, by using both phase contrast and fluorescence optics. This microscope was connected to a charge-coupled device camera (Dage-MTI, Michigan City, IN), a frame-grabber (Scion, Frederick, MD), and a G3 Power Macintosh computer to capture the images. Images were collected and analyzed with IPlab Spectrum image analysis software (Scanalytics, Fairfax, VA). Both phase contrast and fluorescence images were registered for each point in time. The phase contrast and fluorescent images were merged and frame sequences were animated using the image analysis software.
Bead displacement was quantified as described (Wang and Pelham, 1998; Pelham and Wang, 1999). Briefly, cells and fluorescent beads were photographed after a 1-h incubation, and the cells were then treated for 15 min with either trypsin/EDTA (for cells on laminin substrate) or for 1 h with proteinase K (1 mg/ml phosphate-buffered saline; for cells on antibody substrate) to relax the elastic substrate. A second photograph was taken at this time point and compared with the first photograph. In this way, vector maps indicating the magnitude and direction of bead displacement were built and analyzed using image analysis software. For the comparative purposes of our study, we estimated traction forces only in terms of relative bead displacement, because displacement is related proportionally to the traction force according to Hooke's law. It was apparent that groups of beads in defined regions were being displaced coherently in a similar direction. To facilitate quantification of the total area displaced by each cell as an estimate of traction, regions of coherent bead displacement were delineated. The displacement of individual beads inside each of these regions was calculated and the values obtained were averaged. The average displacement of beads was then multiplied by the area of the region containing the beads to obtain the total region displacement. The total area displaced per cell was obtained by the sum of all region displacement. A total of 10 cells was analyzed for each assay.
Remodeling of Reconstituted BM
Matrigel was mixed with fluorescent beads and reconstituted on coverslips that had been glued to “punched” plastic Petri dishes. Clone A or MDA-MB-435 cells were plated on the top of the gel and incubated for 1–8 h inside a CO2 incubator, the time depending on the nature of experiment. In some experiments, the dishes were sealed with parafilm, and analyzed using time-lapse videomicroscopy as described above. For each point in time, both cells and fluorescent beads were photographed using phase contrast or fluorescence microscopy, respectively. Function-blocking antibodies, PMA, or cytoskeleton-related inhibitors were added at the concentrations and times described in the corresponding figures. In some experiments, the cells were assayed on Matrigel prepared without fluorescent beads, and then the preparations were fixed with methanol and stained with Coomassie Blue. In other experiments, FITC-labeled laminin (instead of fluorescent beads) was mixed with Matrigel and reconstituted as described above before plating the cells. A semiquantitative analysis of Matrigel compression was done by determining the distance between specific pairs of beads filmed before, during, and after they were traversed by lamellae. Ten pairs of beads were tracked for each assay. In some experiments, a digital analysis of the fluorescence intensity produced by the clustered beads around the cells was done to assess Matrigel compression. As the beads concentrate in small areas, they also increase the net fluorescence of the area. The background produced by nonclustered beads was removed by thresholding the images above a certain intensity value.
Analysis of Corneal BMs
Rabbit corneas were used to obtain stroma with preserved BMs as well as sheets of corneal epithelium. The method to remove epithelia from their underlying stroma and to obtain viable epithelial sheets has been described previously (Gipson and Grill, 1982; Gipson et al., 1983). The separated stroma containing the BM was transferred to a gold colloid suspension (5-nm particles; adjusted to pH 9) and incubated at room temperature for 30 min. The gold particles bound to the stroma were stabilized by removing the gold colloid suspension and incubating the samples in Hanks' balanced salt solution containing 1% bovine serum albumin for 10 min. The tissue was rinsed extensively using the bovine serum albumin/Hanks' balanced salt solution buffer. This corneal substrate was cut in quarters. The epithelial sheets obtained from other corneas were cut smaller than the portion of gold labeled stroma to which it was to be applied to provide a leading edge because it has been shown previously that epithelia in these circumstances can migrate along the substrate and retard the formation of hemidesmosomes (Gipson et al., 1983). Before recombining with the substrate, the epithelial sheet was incubated or not with GoH3 or anti-rabbit major histocompatibility complex (MHC)-I antibody (10 μg/ml) for 20 min. The GoH3 antibody has been previously shown to recognize the α6 integrin of rabbit corneas (Stepp et al., 1990). MHC-I is also present in cornea (Gipson, unpublished data). The epithelial sheet was recombined with the substrate and incubated for 3–6 h in minimal essential medium (supplemented as described previously; Gipson et al., 1983). The tissues were fixed and processed for electron microscopy analysis by using standard techniques (Gipson et al., 1983). Five to 10 fields were photographed for each block sectioned at two different depths.
The photographic negatives were scanned into G3 PowerMac. The distribution of gold particles was performed by counting and sizing clusters of gold particles by using IPLab spectrum image analysis software.
Online Supplemental Material
All videos are merged composites of phase contrast (red: cells) and fluorescent images (green: beads). Frame intervals: 2 min. Figure 1b.mov: Formation of a lamella (arrow) produces bead displacement on a laminin-coated elastic substrate. Figure 1c.mov: Filopodium (arrow) produces the displacement of a few beads. Figure 6.mov: Filopodia (arrows) efficiently pull Matrigel/beads toward the cell body. Figure 7.mov: clone A cell stimulated with PMA compresses Matrigel/beads (gray scale).
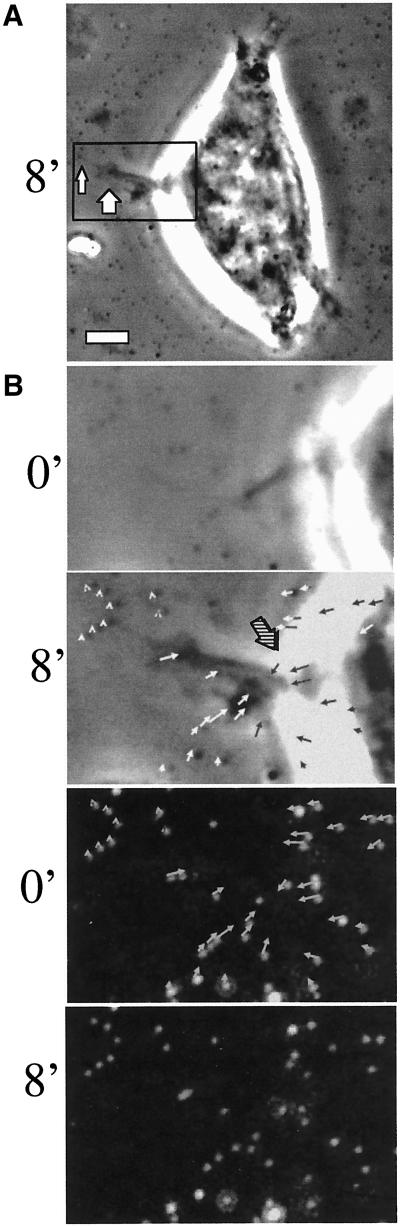
Figure 1.
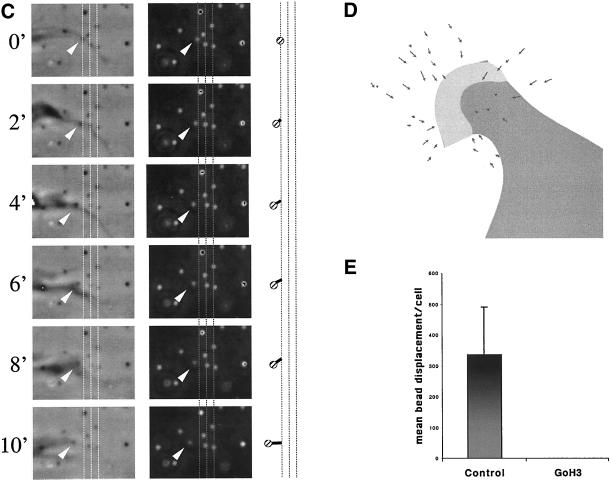
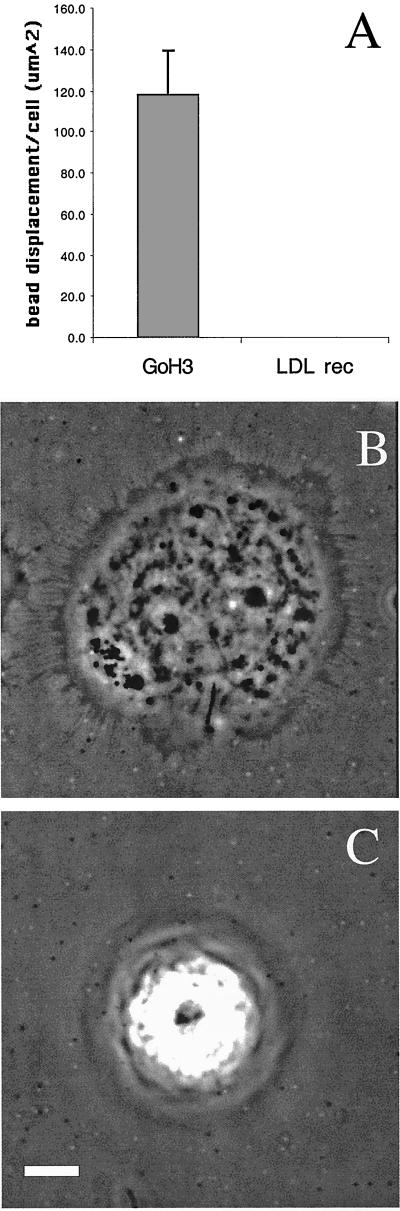
Integrin α6β4 mediates traction forces on laminin. Clone A colon carcinoma cells were plated on a polyacrylamide-flexible substrate containing fluorescent beads (2 μm diameter) and coated with laminin-1. (A and B) Cells were incubated for 1 h at 37°C and analyzed by time-lapse video microscopy by using phase contrast and fluorescent illumination. (A) Typical morphology of cell plated on a flexible laminin substrate. Notice that small lamellae (thick arrow) and filopodia (thin arrow) are formed. Bar, 10 μm. (B) Frame sequence of a protruding lamella magnified from the rectangular area in A. The upper two panels show a phase contrast image of the protruding lamella, whereas the lower ones show the corresponding fluorescent image of the underlying beads. Times 0′ an 8′ are shown. The entire frame sequence can be observed in the accompanying video (Figure 1b.mov). A vector map of bead displacement was built using both phase contrast and fluorescent images (middle two panels) by connecting the initial and final positions of each bead at the beginning and end of the frame sequence; the arrows indicate the direction of displacement and the thick-hatched arrow indicates the region were opposing forces are focused. (C) Frame sequence of discrete traction forces produced by filopodia. The columns of frames in the left and middle panels were photographed using phase contrast and fluorescence optics, respectively. The graphic on the right column represents the displacement of a small number of beads produced by the filopodia in the left columns. Grid lines are spatial references. Video Figure 1c.mov contains the corresponding frame sequence. (D and E) Cells were incubated 1 h at 37°C in the presence of GoH3 antibody or rat IgG control, and photographed using phase contrast and fluorescence illumination. The cells were treated with trypsin/EDTA to produce a relaxation of the substrate. The position of beads before and after trypsin/EDTA treatment was registered and a map of vectors representing the magnitude and direction of displacement was built for every cell. (D) Example of a vector map of such bead displacement. In E, the average bead displacement/cell (μm2) was calculated for cells in the presence or absence of the GoH3 antibody (see MATERIALS AND METHODS for calculation procedure).
Figure 6.
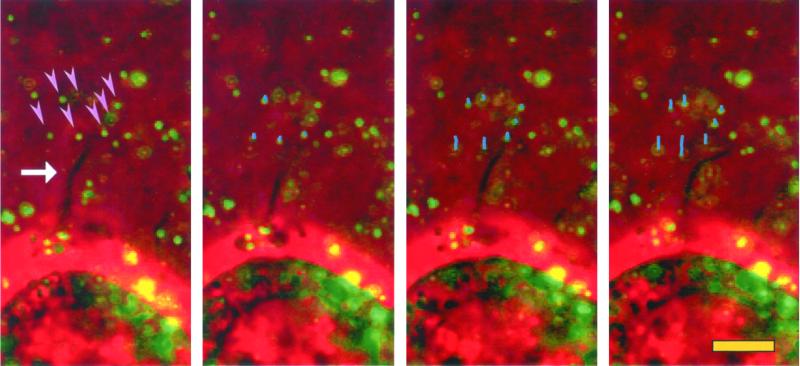
Remodeling of reconstituted BM by filopodia. Clone A cells were plated on Matrigel containing fluorescent beads. The cells were analyzed by video microscopy with both phase contrast and fluorescence illumination. The frame sequence shown was taken at 2-min intervals. Only a few beads (arrowheads) from a small area surrounding a filopodium (arrow) are pulled toward the cell at the bottom. The lines in the second and subsequent frames represent the movement tracks of the beads indicated in the first frame. Beads distal to filopodia show little movement. In the video (Figure 6.mov), the arrows point to the area where filopodia activity is occurring.
Figure 7.
Fan-shaped lamellae are powerful compressors of reconstituted BM. Time-lapse analysis of clone A cells stimulated with PMA (25 ng/ml) by using phase contrast (left) or fluorescent (right) illumination. Panels shown represent frames taken at 15-min intervals. Bar, 10 μm. In Figure 7.mov, the phase contrast and fluorescent images frames were captured 2-min intervals and then merged. Notice the strong compression that occurs under the lamella, relaxing partially after the base of the lamella passes over the compressed region. Also, note that although most of the direction of compression occurs perpendicular to the lamella, strong compression parallel to the lamella is evident at the end of the video.
RESULTS
α6β4 Integrin Can Mediate Traction Forces onto a Laminin Substrate
A well-characterized force-detection system was used to assess the ability of the α6β4 integrin to mediate traction forces (Wang and Pelham, 1998; Pelham and Wang, 1999). The system consisted of an elastic polyacrylamide sheet that was embedded with fluorescent beads and coated with laminin-1 on its surface. Elastic deformation of the gel by the cell produces displacement of the beads, proportional to the forces generated by the acto-myosin system and transmitted to the substrate via adhesive interactions (Wang and Pelham, 1998; Pelham and Wang, 1999). For these initial studies, we used either clone A colon or A431 squamous carcinoma cells because we and others have established that these cells couple α6 exclusively with β4, and that α6β4 mediates the migration of these cells on laminin (Falcioni et al., 1988; Lotz et al., 1990; Lee et al., 1992; Rabinovitz and Mercurio, 1997; Rabinovitz et al., 1999). The cells plated on these elastic substrates formed small lamellae and filopodia (Figure 1A, arrows).
Traction forces were analyzed indirectly by measuring the deformation of the elastic substrate produced by the cells. Bead positions were registered before and after relaxing the substrate by using trypsin, which abolishes cell traction by eliminating adhesion (Pelham and Wang, 1999). The arrows in Figure 1D represent the direction and distance a bead is displaced before and after treatment with trypsin. Subsequently, we assessed the importance of α6β4 in the transmission of traction forces by quantifying the average bead displacement in the presence or absence of an α6 function-blocking antibody (Figure 1E). This antibody eliminated most of the traction on the substrate generated by the cells. These data provide evidence that α6β4 can transmit traction forces onto a laminin substrate.
In previous work studying the behavior of clone A cells and other carcinoma cells on laminin (Rabinovitz and Mercurio, 1997; Rabinovitz et al., 1999), we demonstrated that α6β4 is present in lamellae and that their formation depends on this integrin. We had also observed that α6β4 is present at sites of filopodia anchorage (Rabinovitz and Mercurio, 1997). Consistent with a role of α6β4 in the transmission of traction forces at these sites, the analysis of bead displacement by time-lapse videomicroscopy revealed that small lamellae produced the strongest deformation (Figure 1, A and B, and Figure 1a.mov). Vector maps of bead displacement, which were built by connecting the initial and final positions of each bead at the beginning and end of the frame sequence (Figure 1B, middle), revealed that these small lamellae frequently compressed the substrate. These vectors focused their compressive action to the base of the lamellae (Figure 1b.mov, and Figure 1, B and D), an area where α6β4 expression is concentrated (Rabinovitz and Mercurio, 1997). Filopodia exerted discrete, small forces on the substrate, as indicated by limited displacement of one or a few beads in areas where filopodia attachment was apparent (Figure 1C and Figure 1c.mov). This was observed frequently in cells that presented filopodia. Other cell areas, such as the nuclear area, showed little traction activity. These data suggest that α6β4 mediates transmission of traction forces at cell protrusions and that these forces comprise a substrate-compression machine.
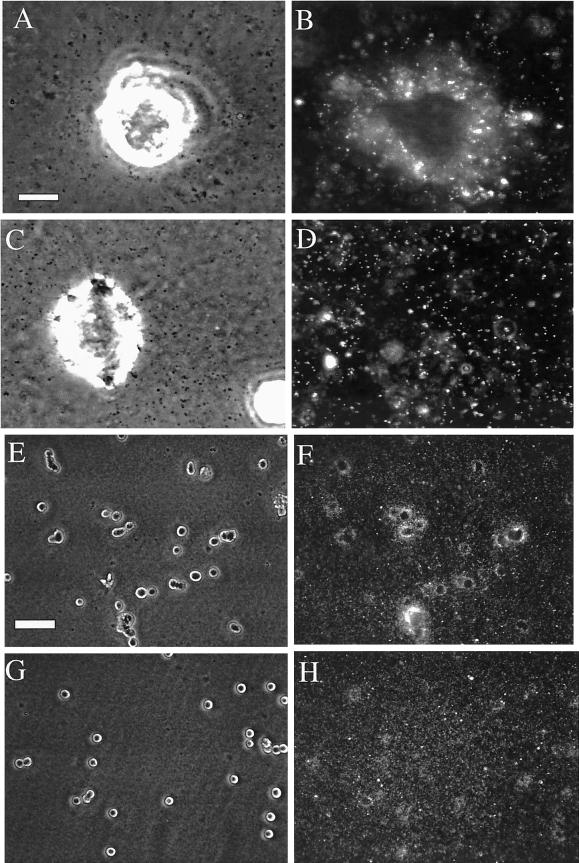
The α6β4 integrin can stimulate intracellular signaling (Shaw et al., 1997; O'Connor et al., 2000). The possibility existed, therefore, that the inhibition of traction forces by α6β4 function blocking antibodies resulted from the inhibition of α6β4-dependent signaling events that impact the mechanical transmission of traction by laminin receptors other than α6β4. To establish more definitively that α6β4 can transmit traction forces onto the substrate directly, we modified the traction-detection system substrate so that it would engage cells exclusively through the α6β4 integrin. For this purpose, we cross-linked the polyacrylamide gel with an anti-rat antibody to link α6β4 from A431 cells that had been coated with the rat GoH3 antibody and analyzed the traction generated. Any traction observed in this system must be transmitted through α6β4. As a specificity control, we coated the cells with a mouse anti-LDL receptor mAb and cross-linked the polyacrylamide with an anti-mouse antibody. The GoH3-coated cells partially spread on the polyacrylamide gel and produced a substantial number of filopodia and lamellar protrusions. Importantly, a significant amount of bead displacement was observed under these conditions (Figure 2). In contrast, the anti-LDL mAb-coated cells did not spread and they produced no protrusions or bead displacement. These data establish that α6β4 can mechanically transmit traction forces onto a laminin substrate.
Figure 2.
α6β4 integrin can relay forces directly onto the substrate. A polyacrylamide-flexible substrate containing fluorescent beads (2 μm diameter) was cross-linked with either anti-rat or anti-mouse antibodies. A431 cells were coated with the rat GoH3 mAb or the mouse anti-LDL receptor antibody, plated on the gel, and incubated for 1 h at 37°C. (A) Average bead displacement/cell (μm2) was analyzed by time-lapse video-microscopy by using phase contrast and fluorescent illumination as described in MATERIALS AND METHODS. The cells coated with the GoH3 antibody spread on the gel and produced numerous filopodia and lamellae (B), in contrast to the cell coated with the anti-LDL receptor antibody (C).
Traction Forces Mediated by α6β4 Integrin Remodel Reconstituted BM
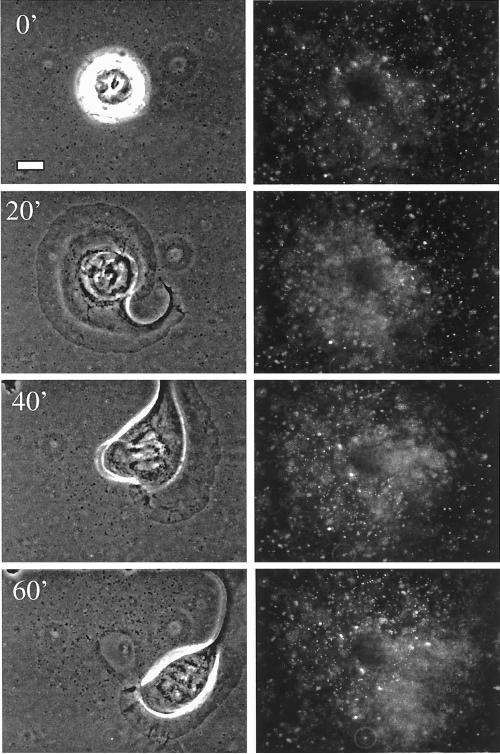
Our finding that the α6β4 integrin can mediate traction forces on laminin and deform this matrix raised the possibility that cells that express this integrin could also deform or remodel BMs. To examine this possibility, fluorescent beads (0.2 um) were embedded in reconstituted BM (Matrigel) and allowed to solidify into a thick gel (40 μm thick). Clone A cells were plated on the Matrigel and the system was videotaped using both phase contrast and fluorescence microscopy. This video analysis revealed that clone A cells rapidly pull in the adjacent Matrigel, as indicated by the inward (centripetal) movement and increasing concentration of beads around the cells (Figure 3, A and B, and videos; Figure 6.mov; and Figure 7.mov).
Figure 3.
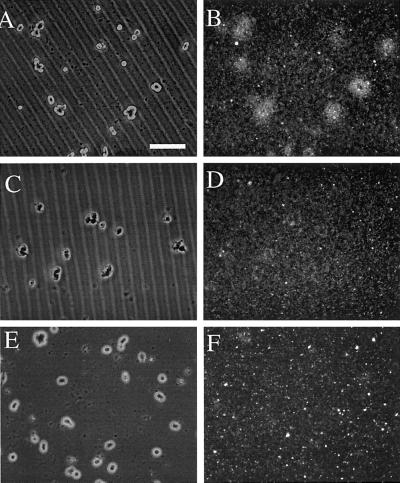
Mechanical remodeling of reconstituted BM is dependent on the α6β4 integrin. Clone A cells were plated on a film of Matrigel containing fluorescent beads and incubated in the presence of GoH3 (C and D and G and H) or control antibody (A and B and E and F) for 4 h at 37°C. The cells were photographed at high (A–D) and low (E–H) magnifications by using phase contrast (A and C) or fluorescence (B and D) microscopy. Notice the inhibition of bead concentration around the cells in the presence of the GoH3 antibody. Bars, 10 μm (A–D), 100 μm (E–H).
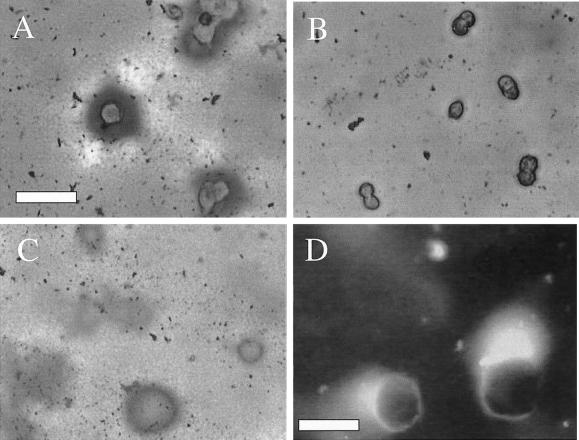
To demonstrate that the observed concentration of beads around the cells reflected a concentration of Matrigel protein, cells were stained with Coomassie Blue to detect protein concentration. The intense Coomassie Blue staining around the cells and diminished staining in adjacent areas (Figure 4A) indicate that clone A cells induce condensation of Matrigel protein around them by sequestering Matrigel from adjacent areas that are devoid of cells. A similar pattern was observed by indirect immunofluorescence by using an anti-laminin antibody (our unpublished data). The possibility that these observations reflect the de novo secretion of laminin by clone A cells was excluded by incorporating FITC-laminin in the Matrigel. As shown in Figure 4D, the FITC-laminin was concentrated around the cell in a manner consistent with Coomassie and laminin stains, as well as the observed bead movement. Together, these data demonstrate that clone A cells can remodel reconstituted BM by concentrating BM proteins around them.
Figure 4.
Remodeling of reconstituted BM involves condensation of material gathered from adjacent areas and is dependent on the integrin α6β4. (A and B) Clone A cells were plated on a film of Matrigel in the presence of either GoH3 (B) or control (A) antibody and incubated for 4 h. The cells were fixed and stained with Coomassie Blue. Notice the condensation of matrix material that forms around the cells and the clearing of material that occurs in adjacent areas. Bar, 75 μm. (C) At a short time of incubation (1 h), the cells have already produced a condensation ring around them. In this photograph, the cells were removed using EDTA to reveal the underlying matrix. (D) Fluorescence image of Clone A cells that were plated on a film of Matrigel containing FITC-conjugated laminin and incubated for 8 h. Notice that the fluorescent laminin condenses around the cells. Some intact Matrigel can be seen as a light veil at the top of this image. Bar, 25 μm.
Next, we assessed the contribution of the α6β4 integrin to BM remodeling by using initially a function-blocking antibody. Treatment of clone A cells with this antibody significantly reduced the centripetal movement of beads toward cells (Figure 3C) and the condensation of Matrigel proteins around cells (Figure 4B). Importantly, antibody inhibition of α6β4 did not detach cells from Matrigel; it only prevented their ability to remodel this matrix. The use of a function-blocking β1 integrin antibody, in contrast, did perturb clone A attachment to Matrigel (our unpublished data). Thus, it is likely that remodeling involves the concerted action of both α6β4 and β1 integrins and that these integrins mediate distinct functions, a scenario that we postulated for clone A migration on laminin (Rabinovitz and Mercurio, 1997).
Stimulation of Breast Carcinoma Invasion by α6β4 Expression Is Coincident with Increased BM Remodeling
Additional evidence to establish a distinct role for α6β4 in BM remodeling was obtained using a different cell system. MDA-MB-435 breast carcinoma cells do not express this integrin (Shaw et al., 1997). Stable transfectants of these cells that express α6β4 exhibit a marked increase in their ability to invade Matrigel (Shaw et al., 1997). As shown in Figure 5, the MDA-MB-435/α6β4 transfectants were able to remodel Matrigel to a much greater extent than the mock transfectants as evidenced by bead compression around the cells.
Figure 5.
Expression of the α6β4 integrin in breast carcinoma cells increases their ability to remodel reconstituted BM. MDA-MB-435 transfectants expressing the α6β4 integrin (C and D) or mock transfectants (A and B) were plated on Matrigel containing fluorescent beads and incubated at 37°C for 4 h. The cells were photographed using phase contrast (A and C) or fluorescence (B and D) microscopy. Note the intense concentration of beads surrounding the α6β4 transfectants in comparison with the mock transfectants. Bar, 10 μm.
Remodeling of Reconstituted BM Results from Compressive Forces Exerted by Lamellae and Filopodia
The dynamics of Matrigel remodeling by clone A cells and the cell structures involved in this process were studied using time-lapse videomicroscopy. At early times after plating on Matrigel, cells extended small lamellae and filopodia in all directions and formed a ring of Matrigel condensation around them (Figure 4D), suggesting that compression forces occur mostly at the cell edges and exclude the central area of the cell. Using the Matrigel/fluorescent bead method described above to monitor movement of BM material, we observed that the beads moved toward cells in areas where lamellae were protruded. At distal sites, much of the bead pulling was done by filopodia (Figure 6 and Figure 6.mov).
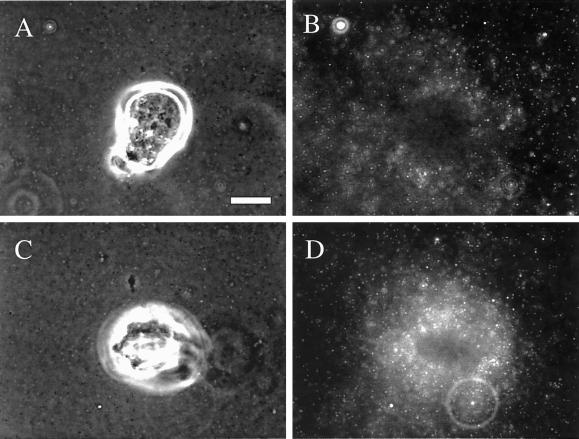
To examine the involvement of lamellae in remodeling in more detail, we stimulated clone A cells on Matrigel with PMA, which results in the formation of large, fan-shaped lamellae. PMA stimulation increased the remodeling of Matrigel significantly as evidenced by an increase in bead concentration under the cells compared with unstimulated cells (Figure 7 and Figure 7.mov). By analyzing bead compression, which is defined as the distance between specific beads before, during, and after the cell passes over them, we noted that Matrigel is compressed to 50% of its original dimension and recovers to only 65% of its original size (our unpublished data). Importantly, compression occurs specifically under lamellae and terminates behind the flat portion of lamellae (Figure 8 and Figure 8.mov). No bead movement was observed at the rear of the cell or at the rear of the “wings” of the lamellae, suggesting that most of the force is compressive only at the sites where Matrigel is in contact with lamellae. This phenomenon was particularly evident at the wings of lamellae, where there is a small distance between the lamella and the rear of the cell (Figure 7.mov). Soon after the wing of a lamella passed over an area of Matrigel, a modest, but significant relaxation of the Matrigel was seen (Figure 8). Thus, it is clear from these images that most of the compression is achieved, not between the front and rear of the cell, but in the area beneath the lamellae, before the bulk of cytoplasm begins.
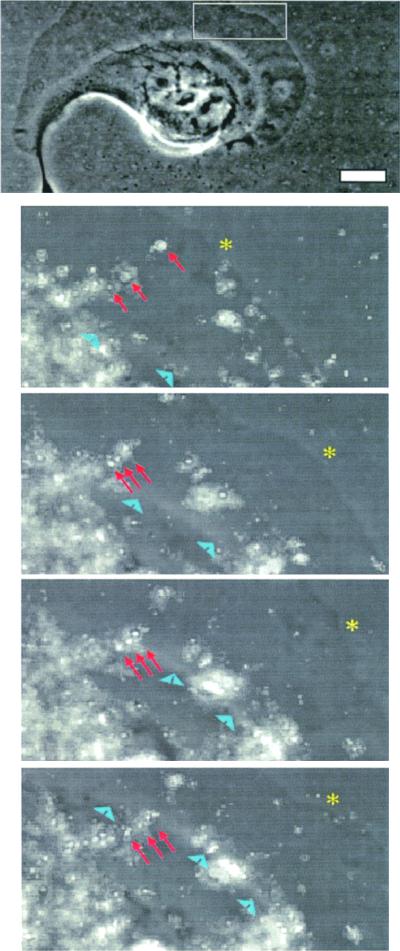
Figure 8.
Matrigel compression ends at the base of lamellae. Clone A cells were stimulated with PMA (25 ng/ml) and analyzed using time-lapse video microscopy. Both phase contrast and fluorescent images were taken at 2-min intervals and merged digitally. Top panel shows a phase contrast image of the cell and bottom panels show a magnified frame sequence of the region specified by the rectangle in the top panel. The frame sequence shows the compression of a group of beads (arrows) until the base of the lamellae (arrowheads) passes over them, a time at which the beads partially relax (bottom). This observation was commonly seen for other beads undergoing compression beneath the lamella. Asterisk denotes the leading edge. Bar, 10 μm.
Our results indicate that BM remodeling is clearly a phenomenon driven by traction. The massive movements of Matrigel associated with cell protrusions that occur in such short times support this conclusion. Furthermore, remodeling requires the acto-myosin system because it is inhibited by either cytochalasin B or BDM (our unpublished data).
Signaling Pathways Stimulated by α6β4 Are Involved in BM Remodeling
Based on the findings that α6β4 stimulates the activity of signaling molecules involved in regulating actin dynamics in carcinoma cells (Shaw et al., 1997; O'Connor et al., 2000), it was important to assess whether these signaling pathways are involved in BM remodeling. Specifically, α6β4 has been shown to stimulate the activity of both PI3-K and RhoA. We assessed the participation of these molecules in the remodeling activity of clone A cells by using pharmacological inhibitors. As shown in Figure 9 both LY294002 (PI3-K) and Y27632 (Rho kinase) impeded the remodeling process. These data indicate an important role for PI3-K and RhoA in BM remodeling, and they suggest an important role for α6β4-mediated signaling in addition to its ability to mediate traction forces in the remodeling process.
Figure 9.
Signaling pathways regulated by the α6β4 integrin are necessary for BM remodeling. Clone A cells were plated on Matrigel containing fluorescent beads and incubated for 4 h in the presence of either DMSO (A and B); Y27632, a Rho-kinase inhibitor (30 μM) (C and D); or LY294002, a PI3-K inhibitor (20 μm). The cells were photographed using either phase contrast (A, C, and E) and fluorescence (B, D, and F) microscopy. Bar, 100 μm. Notice that the inhibitors inhibit the remodeling process strongly.
Evidence for Remodeling of Native BMs by α6β4 Integrin
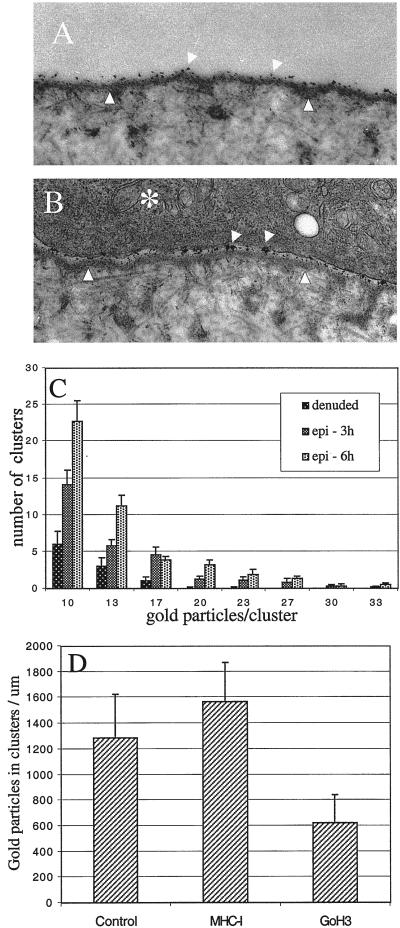
Given our findings that the α6β4 integrin can mediate traction forces and remodel reconstituted BMs, it was important to establish whether these events occurred with native BMs. For this purpose, we used the BM of the rabbit cornea. The corneal epithelium can be removed to yield a “denuded” BM (Gipson et al., 1983). An intact epithelial-stromal structure can be reconstituted by the addition of a freshly isolated epithelium to the denuded BM. The size of the epithelium that is added back is smaller than the area of the BM, a situation that promotes cell migration in the direction of the free edge. In our experiments, we coated the denuded BM with gold colloid particles (5 nm) that bound to the BM in a homogeneous pattern (Figure 10A). Subsequently, these denuded BMs were recombined with corneal epithelia. Our assumption was that a mechanical displacement or remodeling of the BM by the epithelium would alter the distribution pattern of the gold particles. For example, a local compression of BM material by the epithelial cells would result in the formation of a more densely packed group of gold particles. Indeed, using this approach, we observed that the epithelium did induce a redistribution of gold particles into more densely packed clusters (Figure 10B). Quantitative analysis of this particle redistribution revealed a significant increase in clustering at 3 h and an additional increase at 6 h (Figure 10C). At these time points, mature hemidesmosomes were not apparent, an observation that is consistent with previous evidence showing that migratory epithelia do not form hemidesmosomes (Gipson et al., 1993).
Figure 10.
α6β4 integrin functions in the remodeling of corneal BM. Deepithelialized corneas were labeled with gold colloid particles (5 nm) and recombined with fresh cornea epithelium as described in MATERIALS AND METHODS. The recombined corneas were incubated at 37°C for either 3 or 6 h, in the either presence or absence of the GoH3 mAb. (A) Electron micrograph of a recombined cornea showing areas of BM (arrowheads) either denuded (A) or covered with epithelium (B, asterisk). Notice that the distribution of the gold particles on the BM is homogeneous in A and aggregated in B (arrows). Bar, 0.5 μm. (C and D) Quantitative analysis of gold particle distribution. (C) Density of aggregates per unit length of BM (μm), was estimated at 3 and 6 h after recombining the epithelium with the denuded BM. Shown is the distribution of different size aggregates (D) Number of particles present in aggregates (>10 gold particles) per unit area of BM was quantified in recombined corneas that were incubated for 6 h in the presence or absence of the GoH3 mAb or anti-rabbit MHC-I antibody.
Next, we assessed whether the α6β4 integrin contributes to the remodeling of the corneal BM. The corneal epithelium expresses α6β4 but not α6β1 (Stepp et al., 1993). An α6β4 function-blocking antibody was incubated with isolated epithelium before and after recombining this epithelium with denuded BM. Importantly, this antibody treatment did not perturb the attachment of the epithelium to the BM, an observation consistent with recent findings that BM attachment is mediated by β1 integrins (Raghavan et al., 2000). We did observe, however, that this antibody treatment reduced the formation of gold particle aggregates significantly (Figure 10D). As a specificity control, we used an antibody specific for the rabbit MHC-I protein, which showed no inhibitory activity (Figure 10D). These data provide evidence that the α6β4 integrin participates in the remodeling of a natural BM.
DISCUSSION
The integrin α6β4, a laminin receptor that stabilizes epithelial cell adhesion to the BM through its association with cytokeratins, can stimulate the formation and stabilization of actin-rich protrusions in carcinoma cells (Rabinovitz and Mercurio, 1997; Rabinovitz et al., 1999). An important, unresolved issue, however, was whether this integrin could transmit forces to the substrate generated by the acto-myosin system. Although α6β4 can associate with the actin cytoskeleton (Rabinovitz and Mercurio, 1997), this association does not imply necessarily that it can transmit forces to a substrate directly. Moreover, the distinct signaling functions of α6β4 could have a significant impact on actin dynamics and acto-myosin contraction. Using a well-characterized traction-force detection assay, we were able to detect forces exerted through the α6β4 integrin on either laminin-1, a matrix ligand, or on an anti-α6β4 antibody, demonstrating that this integrin can transmit forces without the need to engage other integrins. These α6β4-dependent traction forces were organized into a compression machine localized to the base of lamellae. We hypothesized that the compression forces generated by α6β4 result in the remodeling of BMs because this integrin plays a major role in the interaction of epithelial and carcinoma cells with such structures. Indeed, we observed that carcinoma cells are able to remodel a reconstituted BM through α6β4-mediated compression forces by a process that involves the packing of BM material under the cells and the mechanical removal of BM from adjacent areas. Importantly, we also provide evidence for the remodeling of a native BM by epithelial cells and for the involvement of α6β4 in this remodeling. In addition, we demonstrate that the distinct signaling functions of α6β4 also stimulate remodeling. Our findings have important implications for the mechanism of both BM organization and tumor invasion.
Traction forces play an important role in cell migration and in remodeling of the extracellular membrane (ECM) (Stopak and Harris, 1982; Lee et al., 1994). These forces are generated by myosin motors, which act on the actin cytoskeleton, to transmit tension to the ECM through cell adhesion receptors (Mitchison and Cramer, 1996). Studies in this area, however, have focused primarily on the ability of β1 integrins, which associate only with F-actin, to mediate traction forces (Lee and Jacobson, 1997). The α6β4 integrin, in contrast, is a unique receptor that was defined originally as the only integrin that associates with cytokeratins and not with F-actin (Green and Jones, 1996). To date, no studies have demonstrated that cytokeratins generate traction forces. Importantly, therefore, our observation that α6β4 is able to mediate traction forces strengthens our hypothesis that this integrin can interact with F-actin, as well as cytokeratins, and that its association with F-actin can be a significant factor in cell movement. The ability of α6β4 to mediate traction forces generated by the acto-myosin cytoskeleton provides an important functional consequence of its interaction with F-actin because such forces may play a critical role in cell migration and BM remodeling.
The organization of the α6β4-dependent traction forces in carcinoma cells is consistent with the description of these forces in other extensively studied models, such as fish keratocytes and fibroblasts (Lee et al., 1994; Dembo et al., 1996; Svitkina et al., 1997; Burton et al., 1999; Oliver et al., 1999; Pelham and Wang, 1999). Fish keratocytes have a distinct compressive component that is parallel to the lamella and probably functions to release rear attachments allowing the cell to move forward efficiently (Lee et al., 1994; Oliver et al., 1995). The “dynamic contraction network” model has been proposed to explain this compression pattern (Svitkina et al., 1997; Burton et al., 1999). In this model, compression forces increase gradually from the edge of the cell and achieve a maximum at the base of the lamella. Force vectors from the cell's edge are directed to the base of the lamella, whereas beyond this point the vectors can reverse direction. Interestingly, the dynamic contraction network may account for the α6β4-dependent traction forces that we observed. Specifically, the vector maps of bead displacement we generated suggest a concentration of forces from the edge of the cell toward the base of the lamella that reverses direction after this point. In addition, we noted that cells “pull” Matrigel inward toward the base of the lamella where compression is maximal and that the compressed Matrigel relaxes after this point. Additional evidence in support of this model is provided by our previous finding that a gradient of actin filament bundles is present parallel to the lamella in colon carcinoma cells that concentrate at the base of this structure (Rabinovitz and Mercurio, 1997). The enhanced localization of α6β4 at the base of lamellae (Rabinovitz and Mercurio, 1997; Rabinovitz et al., 1999) is compatible with the idea that α6β4 is localized at sites of traction to provide the necessary attachment. Interestingly, however, α6β4 is also localized in retraction fibers at the rear of migrating cells (Rabinovitz and Mercurio, 1997) but we observed that little traction is generated by these structures. These observation suggest that the ability of α6β4 to generate traction is a function of its localization within the cell.
A likely possibility is that the α6β4-dependent traction forces account for the ability of this integrin to mediate both compression and migration on laminin matrices. Forces that lead to compression may pull the cell body forward if some release of rear attachments occurs. Indeed, our observation that the lamella of PMA-stimulated clone A cells produces both compression and forward movement is consistent with the idea that both compression and movement can occur at the same time (Figure 7.mov). The mechanism of rear detachment in clone A cells involves little frictional force, as indicated by the absence of bead movement behind the lamella. This rear detachment may result from lateral forces such as those observed in fish keratocytes, which generate minimal frictional force (Oliver et al., 1998, 1999). These forces probably result from the high concentration and orientation of stress fibers at the base of the lamella. As mentioned above, clone A cells exhibit a concentration of stress fibers parallel to the base of the lamella (Rabinovitz and Mercurio, 1997). Importantly, we observed significant bead movement parallel and toward to the wings of lamellae in PMA-stimulated cells (Figure 7.mov), and relaxation of the gel behind the base of the lamella, in agreement with the mechanism of keratocyte movement.
A key finding in this study is that the compressive component of α6β4-mediated traction forces results in the remodeling of BMs. In fact, few studies have focused on the possibility that cells alter BM organization and that this reorganization is an integrin-mediated process. The salient example is the remodeling of Matrigel by endothelial cells to form tubule structures, a process that is dependent on β1 integrins (Vernon et al., 1992; Davis and Camarillo, 1995). Our findings are significant not only because they extend this concept of remodeling but also because they link the biophysical properties of an integrin that plays a preeminent role in the interaction of both epithelial and carcinoma cells with BMs in this remodeling function. Moreover, an important and novel aspect of our study is the finding that epithelial cells can remodel native BMs mechanically and that the α6β4 integrin contributes to this remodeling. Specifically, we demonstrated, using the well-established cornea reconstitution model (Gipson and Grill, 1982; Gipson et al., 1983), that the corneal epithelium is able to remodel corneal BMs that had been coated with gold particles as evidenced by the redistribution of these particles. Supporting the mechanical nature of this redistribution, a function-blocking antibody specific for α6β4 was able to inhibit the redistribution of gold particles significantly. We conclude from these results that BM remodeling by epithelial and carcinoma cells is not limited to reconstituted basement membranes.
The distinct signaling properties of α6β4 also contribute to BM remodeling by this integrin. At least two key signaling molecules, PI3-K and RhoA, are activated by α6β4 in carcinoma cells (Shaw et al., 1997; O'Connor et al., 2000). Importantly, the α6β4-mediated activation of these signaling molecules has been shown to be essential for the ability of this integrin to promote carcinoma migration and invasion (Shaw et al., 1997; O'Connor et al., 1998). Given our findings in the present study, a link between α6β4-mediated compression and α6β4-mediated signaling becomes apparent. Specifically, the contractile forces that are converted to traction by α6β4 under lamellae could arise from α6β4-mediated activation of Rho and the consequent Rho stimulation of actin myosin contraction. This α6β4-mediated signaling of contraction may also be an important component of invasion that occurs by α6β4-dependent BM remodeling as we postulated above. In support of this possibility, we observed that inhibition of Rho kinase activity disrupts the α6β4-mediated compression of Matrigel. Along the same lines, α6β4-stimulation of PI3-K and consequent changes in actin dynamics may contribute to the formation of filopodial and lamellar protrusions, processes that are also facilitated by the engagement of α6β4 with laminins in the matrix. Together, the studies on α6β4 exemplify the fruitfulness of integrating signaling studies with studies on integrin-mediated traction forces and ECM remodeling.
Our findings on BM remodeling by the α6β4 integrin may have important implications for BM organization, especially in light of recent studies on integrin knockout mice. It is apparent from analysis of the β4 knockout mice that the α6β4 integrin is not necessary for the formation of the BM itself (Dowling et al., 1996; Dipersio et al., 1997). Interestingly, however, we noticed in the published electron micrographs of the epidermis from these mice that the BM is atypically smooth and homogeneous (Dowling et al., 1996). In contrast, similar electron microscopy images obtained from wild-type mice, as well as other BMs, indicate an increased concentration of BM material adjacent to the hemidesmosomes and a relatively “thin” BM in areas between BMs (Gipson et al., 1983; Rousselle et al., 1991). The hypothesis can be derived from these images, in concert with our data, that the α6β4 integrin “sweeps” or compresses BM as part of the process of hemidesmosome formation. Such localized compression of BM would be consistent with our observation that homogeneously distributed gold particles attached to the cornea BM are gathered into discrete clusters by the epithelium, suggesting that compression occurs toward discrete, small areas. Although α6β4 may play a distinct role in BM remodeling, other integrins probably contribute to this process. Evidence to support a role for β1 integrins, for example, in BM organization comes from the analysis of β1 integrin knockout mice. The BMs of these mice are disorganized compared with wild-type mice (Raghavan et al., 2000). Interestingly, although the BMs of the α3 integrin knockout mice appear to be discontinuous, stretches of BMs corresponding to areas of hemidesmosome localization are preserved (Dipersio et al., 1997). One explanation for these observations based on our results is that the α6β4 integrin compresses BM toward the hemidesmosome and the α3β1 integrin functions to maintain a continuous structure, perhaps by contributing to BM formation. This notion is consistent with the report that the α7β1 integrin, a laminin receptor on muscle cells, can nucleate laminin polymerization (Colognato et al., 1999).
A major implication of BM remodeling is tumor invasion. Although remodeling as a mechanism of BM breaching during tumor invasion has not been considered previously, our findings support such a mechanism. Specifically, compression of BM in certain areas would generate gaps in adjacent areas through which tumor cells could escape. Interestingly, expression of the α6β4 integrin in MDA-MB-435 breast carcinoma cells increases their ability to invade Matrigel in a standard Boyden chamber assay dramatically (Shaw et al., 1997). In addition, we observed in the present study that α6β4 expression in these cells enhanced their ability to remodel Matrigel substantially. A likely possibility is that the stimulation of invasion that is coincident with α6β4 expression derives from an increase in Matrigel remodeling. It is also worth noting that the α6β4-mediated remodeling of Matrigel is not blocked by protease inhibitors (our unpublished data). Although these findings do not discount a role for proteases in tumor invasion, they do suggest that force-dependent remodeling should be considered as a mechanism of invasion. In fact, ECM remodeling as a component of tumor progression has been suggested previously. For example, a correlation between ECM remodeling and melanoma invasion has been observed (Klein et al., 1991). Similar studies observed that highly invasive melanoma cells can remodel three-dimensional matrices, although partial matrix degradation was also observed (Friedl et al., 1997). Most likely, both remodeling activity and protease activity are needed to maximize invasion (Silletti et al., 1998)
Supplementary Material
ACKNOWLEDGMENTS
We thank Steve Akiyama, Hynda Kleinman, and Peter Yurchenco for reagents. We also thank Dongmei Cheng, Ann Tisdale, Pat Pearson, and Sandra Spurr-Michaud for technical assistance. This work was supported by National Institutes of Health grants CA-88919 (to I.R.), CA-80789 (to A.M.M.), and EY-03306 (to I.K.G.) and by the Harvard Digestive Diseases Center.
Abbreviations used:
- BM
basement membrane
- ECM
extracellular, BDM, butanedione monoxime
Footnotes
Online version of this article contains video material for certain figures. Online version available at www.molbiolcell.org.
REFERENCES
- Burton K, Park JH, Taylor DL. Keratocytes generate traction forces in two phases. Mol Biol Cell. 1999;10:3745–3769. doi: 10.1091/mbc.10.11.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato H, Winkelmann DA, Yurchenco PD. Laminin polymerization induces a receptor-cytoskeleton network. J Cell Biol. 1999;145:619–631. doi: 10.1083/jcb.145.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GE, Camarillo CW. Regulation of endothelial cell morphogenesis by integrins, mechanical forces, and matrix guidance pathways. Exp Cell Res. 1995;216:113–123. doi: 10.1006/excr.1995.1015. [DOI] [PubMed] [Google Scholar]
- Dembo M, Oliver T, Ishihara A, Jacobson K. Imaging the traction stresses exerted by locomoting cells with the elastic substratum method. Biophys J. 1996;70:2008–2022. doi: 10.1016/S0006-3495(96)79767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J. 1999;76:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipersio CM, Hodivaladilke KM, Jaenisch R, Kreidberg JA, Hynes RO. Alpha-3-beta-1 integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J, Yu QC, Fuchs E. Beta-4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcioni R, Sacchi A, Resau J, Kennel SJ. Monoclonal antibody to human carcinoma-associated protein complex: quantitation in normal and tumor tissue. Cancer Res. 1988;48:816–821. [PubMed] [Google Scholar]
- Friedl P, Maaser K, Klein CE, Niggemann B, Krohne G, Zanker KS. Migration of highly aggressive MV3 melanoma cells in 3-dimensional collagen lattices results in local matrix reorganization and shedding of alpha2 and beta1 integrins and C.D44. Cancer Res. 1997;57:2061–2070. [PubMed] [Google Scholar]
- Gipson IK, Grill SM. A technique for obtaining sheets of intact rabbit corneal epithelium. Invest Ophthalmol Vis Sci. 1982;23:269–273. [PubMed] [Google Scholar]
- Gipson IK, Grill SM, Spurr SJ, Brennan SJ. Hemidesmosome formation in vitro. J Cell Biol. 1983;97:849–857. doi: 10.1083/jcb.97.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Tisdale A, Elwell J, Stepp MA. Redistribution of the hemidesmosome components alpha 6 beta 4 integrin and bullous pemphigoid antigens during epithelial wound healing. Exp Cell Res. 1993;207:86–98. doi: 10.1006/excr.1993.1166. [DOI] [PubMed] [Google Scholar]
- Green KJ, Jones JCR. Desmosomes and hemidesmosomes - structure and function of molecular components. FASEB J. 1996;10:871–881. doi: 10.1096/fasebj.10.8.8666164. [DOI] [PubMed] [Google Scholar]
- Klein CE, Dressel D, Steinmayer T, Mauch C, Eckes B, Krieg T, Bankert RB, Weber L. Integrin alpha 2 beta 1 is upregulated in fibroblasts and highly aggressive melanoma cells in three-dimensional collagen lattices and mediates the reorganization of collagen I fibrils. J Cell Biol. 1991;115:1427–1436. doi: 10.1083/jcb.115.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration - a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Lee J, Jacobson K. The composition and dynamics of cell-substratum adhesions in locomoting fish keratocytes. J Cell Sci. 1997;110:2833–2844. doi: 10.1242/jcs.110.22.2833. [DOI] [PubMed] [Google Scholar]
- Lee EC, Lotz MM, Steele GD, Jr, Mercurio AM. The integrin alpha 6 beta 4 is a laminin receptor. J Cell Biol. 1992;117:671–678. doi: 10.1083/jcb.117.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Leonard M, Oliver T, Ishihara A, Jacobson K. Traction forces generated by locomoting keratocytes. J Cell Biol. 1994;127:1957–1964. doi: 10.1083/jcb.127.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz MM, Korzelius CA, Mercurio AM. Human colon carcinoma cells use multiple receptors to adhere to laminin: involvement of alpha 6 beta 4 and alpha 2 beta 1 integrins. Cell Regul. 1990;1:249–257. doi: 10.1091/mbc.1.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- O'Connor KL, Nguyen BK, Mercurio AM. RhoA function in lamellae formation and migration is regulated by the alpha6beta4 integrin and cAMP metabolism. J Cell Biol. 2000;148:253–258. doi: 10.1083/jcb.148.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor KL, Shaw LM, Mercurio AM. Release of cAMP gating by the alpha 6 beta 4 integrin stimulates lamellae formation and the chemotactic migration of invasive carcinoma cells. J Cell Biol. 1998;143:1749–1760. doi: 10.1083/jcb.143.6.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver T, Dembo M, Jacobson K. Measurement of traction forces in cells locomoting along a substratum. Cell Motil Cytoskeleton. 1998;39:342–344. [PubMed] [Google Scholar]
- Oliver T, Dembo M, Jacobson K. Traction forces in locomoting cells. Cell Motil Cytoskeleton. 1995;31:225–240. doi: 10.1002/cm.970310306. [DOI] [PubMed] [Google Scholar]
- Oliver T, Dembo M, Jacobson K. Separation of propulsive and adhesive traction stresses in locomoting keratocytes. J Cell Biol. 1999;145:589–604. doi: 10.1083/jcb.145.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham RJ, Jr, Wang Y. High resolution detection of mechanical forces exerted by locomoting fibroblasts on the substrate. Mol Biol Cell. 1999;10:935–945. doi: 10.1091/mbc.10.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitz I, Mercurio AM. The integrin alpha-6-beta-4 and the biology of carcinoma. Biochem Cell Biol. 1996;74:811–821. doi: 10.1139/o96-087. [DOI] [PubMed] [Google Scholar]
- Rabinovitz I, Mercurio AM. The integrin alpha6beta4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J Cell Biol. 1997;139:1873–1884. doi: 10.1083/jcb.139.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitz I, Toker A, Mercurio AM. Protein kinase C-dependent mobilization of the alpha6beta4 integrin from hemidesmosomes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J Cell Biol. 1999;146:1147–1160. doi: 10.1083/jcb.146.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li QQ, Fuchs E. Conditional ablation of beta 1 integrin in skin: severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle P, Lunstrum GP, Keene DR, Burgeson RE. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991;114:567–576. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Rabinovitz I, Wang HH, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- Silletti S, Paku S, Raz A. Autocrine motility factor and the extracellular matrix. II. Degradation or remodeling of substratum components directs the motile response of tumor cells. Int J Cancer. 1998;76:129–135. doi: 10.1002/(sici)1097-0215(19980330)76:1<129::aid-ijc20>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Spurr-Michaud S, Gipson IK. Integrins in the wounded and unwounded stratified squamous epithelium of the cornea. Invest Ophthalmol Vis Sci. 1993;34:1829–1844. [PubMed] [Google Scholar]
- Stepp MA, Spurr-Michaud S, Tisdale A, Elwell J, Gipson IK. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad Sci USA. 1990;87:8970–8974. doi: 10.1073/pnas.87.22.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopak D, Harris AK. Connective tissue morphogenesis by fibroblast traction. I. Tissue culture observations. Dev Biol. 1982;90:383–398. doi: 10.1016/0012-1606(82)90388-8. [DOI] [PubMed] [Google Scholar]
- Svitkina TM, Verkhovsky AB, McQuade KM, Borisy GG. Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J Cell Biol. 1997;139:397–415. doi: 10.1083/jcb.139.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon R, Angello J, Iruela-Arispe M, Lane T, Sage E. Reorganization of basement membrane matrices by cellular traction promotes the formation of cellular networks in vitro. Lab Invest. 1992;66:536–547. [PubMed] [Google Scholar]
- Wang YL, Pelham RJ., Jr Preparation of a flexible, porous polyacrylamide substrate for mechanical studies of cultured cells. Methods Enzymol. 1998;298:489–496. doi: 10.1016/s0076-6879(98)98041-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.