Abstract
Background/Aims
Bone mineral density (BMD) is often low in patients with Crohn's disease (CD). This study aimed to evaluate the association between nutritional factors and BMD in a group of CD patients.
Methods
CD patients 18 years of age or older were included. The body mass index (BMI), waist circumference (WC) and dietary intake were evaluated during two 24-hour recalls. Bone densitometry was performed by dual-energy X-ray absorptiometry of the full body to assess body composition and of the lumbar vertebrae and femoral neck to assess BMD.
Results
In the 60 patients evaluated, there was no association between BMD and disease activity or between BMD and disease duration. We observed moderate correlations between BMD in at least one of the evaluated sites and BMI, lean mass, WC, and protein, calcium, phosphorus and magnesium dietary intakes (P<0.05). In the linear regression analysis for spinal BMD, only BMI and calcium dietary intake remained associated (P<0.05). In the linear regression analysis for femoral BMD, WC and phosphorus intake continued to be significant in the final model, although they had low explanatory power for BMD (P<0.05).
Conclusions
The prevalence of low BMD was high in CD patients. BMI, WC, calcium and phosphorus dietary intake were positively correlated with BMD.
Keywords: Crohn disease, Bone density, Body mass index, Waist circumference, Nutritional features
INTRODUCTION
Bone mineral density (BMD) is often low in patients with CD. It is believed that the etiology of decreased BMD in CD patients is multifactorial and that patient age, steroid use, low physical activity, and the chronic inflammatory state may be some of the factors that have deleterious effects on bone mass.1
Nutritional characteristics such as weight and BMI, body composition and dietary intake are some of the modifiable factors involved in the etiology of bone disease in healthy people.2,3 In CD patients, BMD is also associated with BMI and lean mass.4,5
Malnutrition is reported in 20% to 85% of CD patients, and despite recent research showing an increase in the frequency of overweight in this population, nutritional deficiencies occur even in individuals with adequate or elevated BMIs; decreased dietary intake of the nutrients associated with BMD has been observed.6,7,8,9
Several nutrients play important roles in bone health. In addition to calcium and vitamin D, protein, fat, vitamins C and K and minerals such as phosphorus, potassium and magnesium are involved in bone health.10,11,12,13 These nutrients have been linked, positively or negatively, with bone health indicators in healthy populations and in IBD patients, although the association between these characteristics and BMD is controversial in CD patients.14,15 The aim of this study was to evaluate the association between BMD and anthropometry, body composition and nutrient intake in CD patients.
METHODS
This study was a cross-sectional study with a convenience sample formed by patients 18 years of age or older with clinical, radiologic, endoscopic and histological diagnoses of CD. The patients were selected from 2 referral centers from a capital in northeastern Brazil from July 2012 to January 2013.
The following patients were excluded: those with a history of cancer or other diseases that could induce changes in bone metabolism (chronic renal failure, chronic obstructive pulmonary disease, thyroid disease, liver disease and lupus erythematosus); pregnant, menopausal or post-menopausal women or those using estrogen therapy; and patients with limitations impacting anthropometry.
The Harvey-Bradshaw index16 was used to determine disease activity. The age at diagnosis, location and disease behavior were described using the Montreal classification.17 Data regarding the duration and extent of disease were collected from medical records.
The complete evaluation of each participant was completed within 15 days.
1. Anthropometric Assessment and Body Composition
The weight (in kilograms) was measured in duplicate using a scale (Filizola, São Paulo, Brazil) with a capacity of 150 kg and an interval of 100 g. The height (in centimeters) was evaluated with a stadiometer coupled with a 0.5 cm enlarged scale.18 The BMI was calculated using these data and rated according to the guidelines of the World Health Organization (WHO).19 The waist circumference (WC) was obtained by measuring the circumference at the midpoint between the last rib and the iliac crest using an inelastic tape measure (TBW, São Paulo, Brazil) while the individual was standing. The WC was considered an indication of central obesity when the WC was ≥80 cm for women and ≥90 cm for men.19
Total body bone densitometry was performed to assess body composition as measured by dual-energy X-ray absorptiometry (DEXA) using a Hologic QDR1000 densitometer (GE Medical Systems, USA). Only one physician analyzed the data of all the patients. The values obtained were the percentage of body fat and lean body mass and total fat in grams. The percentage of body fat was considered high when it was above 25% for men or above 30% for women.19
2. Assessment of Food Intake
A 24-hour diet recall (R-24 h) survey was used to assess the food intake. Each participant completed two R-24 h surveys. A photo album of food was used to assist in the characterization of portions. DietWin Personal version 1.0 (DietWin, Porto Alegre, Brazil) was used to calculate the participants' individual average intake of energy, protein, total fat, calcium, phosphorus, magnesium, potassium, vitamin D, vitamin K and vitamin C. The limits proposed by the Dietary Reference Intakes: Estimated Average Requirements (EAR) suggested by the Institute of Medicine were used as references to ensure adequate dietary intake.20,21,22 Among the 60 patients included, 10 responded to only one of the R-24 h surveys and were therefore excluded from this analysis.
3. Blood Tests
The CRP level and ESR were measured after fasting for 4 hours as indicators of inflammatory activity. Also, serum calcium and ionic calcium levels were evaluated. All samples were collected and analyzed by the same laboratory. The method used to measure CRP was immunoturbidimetry, and ESR was measured using the Westergren method.
4. Bone Densitometry
The bone densitometry was performed using the same DEXA machine and the same physician for all patients. BMD was assessed by bone densitometry at the lumbar spine (lumbar vertebrae L1-L4) and femoral neck. The patients were classified according to the SD (T-Score) as recommended by the WHO23 with normal being within 1 SD, osteopenia being between −1 SD and −2.5 SD and osteoporosis being less than or equal to −2.5 SD. The scores were considered normal BMD (T-score within 1 SD) or low BMD (T-score < −1.0 SD) for data analysis.
5. Statistical Analysis
Verification of the normal distribution of the variables was performed using the Kolmogorov-Smirnov test. Descriptive analyses of the sample proportions were used for categorical variables, and the mean (mean±SD) was used for continuous variables. Categorical variables were analyzed using the chi-square test or Fisher exact test, and continuous variables were analyzed using Student t-test or the Mann-Whitney test. Pearson's or Spearman's correlations were used to evaluate the degree of association between continuous measures. Multiple linear regressions were performed. The SPSS version 21.0 (IBM Corp., Armonk, NY, USA) was used for the tabulation of data and data analysis. Differences were considered statistically significant when the probability of type 1 error was <0.05.
6. Ethical Aspects
This paper was submitted to the ethics committee for research at the Professor Edgar Santos University Hospital Complex as opinion No. 117/2011. All participants gave consent after being informed about the procedures. The test results were delivered to the patients. Nutritional counseling was provided. Patients were referred to a rheumatology specialist for medical care in cases of osteopenia and osteoporosis.
RESULTS
We evaluated 60 CD patients, equally distributed between gender, with a mean age of approximately 37 years (SD, 8.2). Most of the patients were in remission (75.0%). The median HBI was 3 (interquartile range, 1–4) and showed simultaneous involvement of the ileum and colon segments (53.3%). Seventy percent of the patients did not have complications such as a fistula, a fissure or an abscess at the time of the assessment, however 51.7% of the patients presented more aggressive forms of the disease; penetrating or structuring disease were identified in 21.7% and 30.0% of the patients, respectively. A low BMD was also observed in a high proportion of the patients (53.3%), however there was no association between low BMD and either disease activity or disease duration (Table 1).
Table 1. Demographic and Clinical Characteristics of CD Patients (n=60).
| Characteristics | Value |
|---|---|
| Age (yr) | 37.4±8.2 |
| Sex | |
| Male | 30 (50.0) |
| Female | 30 (50.0) |
| Age at diagnosis (yr) | |
| <17 | 04 (6.7) |
| 17–40 | 47 (78.3) |
| >40 | 9 (15.0) |
| Disease duration (yr) | 6.8±5.3 |
| Bone mineral density | |
| Normal | 28 (46.6) |
| Osteopenia | 25 (41.7) |
| Osteoporosis | 7 (11.7) |
| Disease activity | |
| Remission | 45 (75.0) |
| Mild/moderate activity | 12 (20.0) |
| Severy activity | 3 (5.0) |
| Location of disease | |
| Terminal ileum | 9 (15.0) |
| Colon | 19 (31.7) |
| Ileocolon | 32 (53.3) |
| Behavior of CD | |
| Nonstricturing, nonpenetrating | 29 (48.3) |
| Stricturing | 13 (21.7) |
| Penetrating | 18 (30.0) |
| Perianal involvement | |
| No | 40 (66.7) |
| Yes | 20 (33.3) |
| Complications | |
| Fissure | 6 (10.0) |
| Fistula | 12 (20.0) |
Values are presented as mean±SD or number (%).
Eight patients (13.3%) had received steroid therapy in the past year. The mean cumulative dose was 1,890 mg (SD, 1,017), ranging from 330 to 3,285 mg, and the average duration was 4.5 months (SD, 3.2), ranging from 2 to 12 months. Surgery was reported by 35.8% of the CD patients. Partial colectomy had been performed in 63.2% of the patients, small intestine and colonic resection had been performed in 26.3%, and only small intestine resection in 10.5%. No patients presented with short bowel syndrome.
1. Anthropometric Indicators and Body Composition
Most of the patients (55.0%) were eutrophic. However, overweight was observed in a high proportion of the study population (30.0%), and malnutrition was observed in 15.0% of the patients. Increased WC was observed in 26.7% of the patients. In addition, the percentage of mean body fat was found to be 28.9% (SD, 12.4), and 51.7% of the individuals presented with values above the recommended values (data not shown).
2. Food Intake
For vitamins D and K and the minerals calcium, potassium and magnesium, we observed intake values below the EAR in more than 75.0% of the studied patients, reaching 100% for vitamin D. For protein, 10.2% of the patients had intake levels below the EAR (data not shown).
Table 2 shows the mean energy and nutrition intake. Most of the patients reported a caloric intake below 30 kcal/kg.
Table 2. Energy and Nutrient Intake in CD Patients.
| Energy and nutrients | Mean±SD | Median | Minimum | Maximum |
|---|---|---|---|---|
| Energy (kcal) | 1,694.6±582.7 | 1,631.1 | 543.8 | 2,992.9 |
| Energy/weight (kcal/kg) | 27.1±9.7 | 26.9 | 8.3 | 45.1 |
| Protein (g) | 72.3±28.7 | 70.7 | 20.7 | 152.5 |
| Lipid (g) | 7.6±18.8 | 45.3 | 9.8 | 92.5 |
| Calcium (mg) | 493.1±224.7 | 529.6 | 78.6 | 925.4 |
| Phosphor (mg) | 1,012.7±369.1 | 1,033.1 | 292.2 | 1,744.4 |
| Potassium (mg) | 2,363.0±108.4 | 2,180.4 | 706.5 | 5,313.2 |
| Magnesium (mg) | 246.0±108.4 | 229.9 | 71.6 | 549.4 |
| Vitamin D (mcg) | 4.3±9.7 | 1.4 | 0.0 | 59.6 |
| Vitamin K (mcg) | 27.5±58.1 | 5.9 | 0.3 | 327.4 |
| Vitamin C (mcg) | 293.5±597.0 | 81.1 | 6.5 | 3,248.6 |
Reported nutritional supplements in the dietary recall were quantified in the consumer analysis. Only 4 patients (6.6%) reported taking a calcium supplement, vitamin D or a multivitamin. The mean BMD among the patients using a nutrient supplement was not significantly different from that of the patients not using a supplement.
Most patients had changed their intake of milk and dairy products, with 13.3% reporting a reduced intake, 21.7% reporting having excluded the products and 16.7% reporting having replaced the products. Only 1 patient avoided milk due to lactose intolerance.
3. BMD and Clinical Characteristics
Comparing the patients with normal BMD to the patients with low BMD, there were no statistically significant differences in age, physical activity, CRP, ESR, serum calcium, ionic calcium or clinical variables, such as duration, stage, location and behavior of the disease and prior surgical resection (Table 3).
Table 3. Clinical Characteristics of CD Patients According to the Classification of Bone Mineral Density.
| Characteristics | Normal BMD | Low BMD | P-value |
|---|---|---|---|
| Age (yr) | 35.8±7.1 | 38.6±9.0 | 0.18 |
| Disease duration (yr) | 6.5±4.3 | 7.1±7.0 | 0.72 |
| Disease activity | 0.78 | ||
| Remission | 21 (47.7) | 23 (52.3) | |
| Activity | 7 (43.8) | 9 (56.2) | |
| CPR (mg/L) | 4.8±7.7 | 9.5±21.4 | 0.26 |
| ESR (mm/hr) | 18.0±20.4 | 20.8±23.5 | 0.62 |
| Serum calcium (mg/dL) | 2.3±0.2 | 2.3±0.1 | 0.42 |
| Ionic calcium (mg/dL) | 1.3±0.1 | 1.3±0.1 | 0.82 |
| Age at diagnosis (yr) | 0.15 | ||
| >40 | 2 (22.2) | 7 (77.8) | |
| ≤40 | 26 (51.0) | 25 (49.0) | |
| Ileum involvement | 0.94 | ||
| No | 9 (47.4) | 10 (52.6) | |
| Yes | 20 (47.6) | 22 (53.7) | |
| Physical exercise | 0.37 | ||
| No | 20 (43.5) | 26 (56.5) | |
| Yes | 8 (57.1) | 6 (42.9) |
Values are presented as mean±SD or number (%).
BMD, bone mineral density.
No patient had serum calcium or ionic calcium levels outside the reference values.
4. BMD versus Nutritional Characteristics
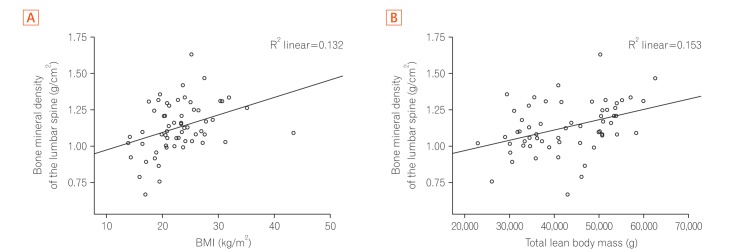
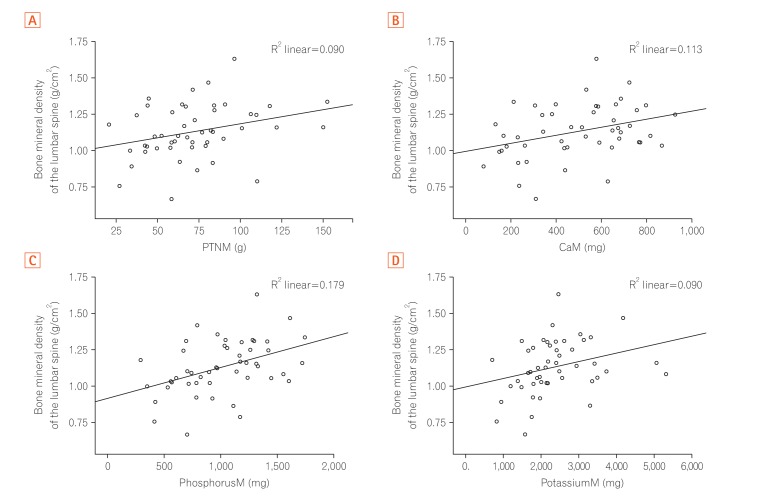
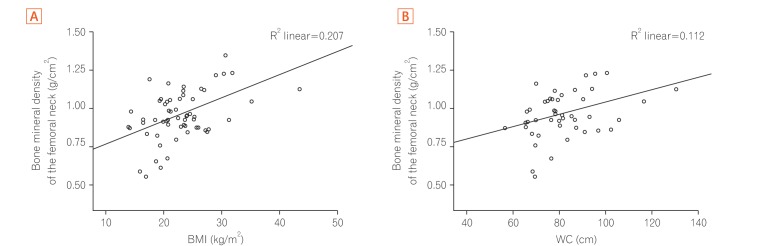
BMI, WC, total lean mass, and phosphorus intake showed moderate positive correlations with BMD in the spine and the femur (P<0.05), whereas for protein, calcium and potassium intake, the correlation was valid only for spinal BMD (Table 4). The increase in the values of these variables was accompanied by an increase in BMD values (Figs 1,2,3).
Table 4. Correlation between Anthropometric Measurements, Body Composition, Food Intake and BMD in CD Patients.
| Characteristics | Lumbar spine BMD | Femur BMD | ||
|---|---|---|---|---|
| r | P-value | r | P-value | |
| BMI (kg/m2) | 0.36 | 0.004a | 0.45 | 0.000a |
| Waist circumference (cm) | 0.29 | 0.040a | 0.33 | 0.020a |
| Lean body mass (g) | 0.39 | 0.002a | 0.38 | 0.002a |
| % Body fat | 0.12 | 0.340 | 0.10 | 0.420 |
| Protein (g) | 0.30 | 0.030a | 0.20 | 0.170 |
| Lipid (g) | 0.17 | 0.220 | −0.02 | 0.800 |
| Calcium (mg) | 0.34 | 0.018a | 0.14 | 0.320 |
| Phosphor (mg) | 0.42 | 0.002a | 0.32 | 0.020a |
| Potassium (mg) | 0.30 | 0.030a | 0.26 | 0.060 |
| Magnesium (mg) | 0.25 | 0.070 | 0.23 | 0.110 |
| Vitamin D (mcg) | 0.06 | 0.630 | −0.09 | 0.530 |
| Vitamin K (mcg) | 0.11 | 0.450 | 0.03 | 0.830 |
| Vitamin C (mcg) | 0.28 | 0.040a | 0.18 | 0.210 |
aP<0.05.
BMD, bone mineral density.
Fig. 1. Correlation between bone mineral density of the lumbar spine and anthropometric indicators. (A) Bone mineral density of the lumbar spine (g/cm2) versus BMI (kg/m2). (B) Bone mineral density of the lumbar spine (g/cm2) versus the total lean body mass (g).
Fig. 2. Correlation between bone mineral density of the lumbar spine and the intake of secondary nutrients. (A) Bone mineral density of the lumbar spine (g/cm2) versus protein intake (pTNM, g). (B) Bone mineral density of the lumbar spine (g/cm2) versus calcium intake (CaM, mg). (C) Bone mineral density of the lumbar spine (g/cm2) versus phosphorus intake (PhosphorusM, mg). (D) Bone mineral density of lumbar spine (g/cm2) versus potassium intake (PotassiumM, mg).
Fig. 3. Correlation between bone mineral density of the femoral neck and anthropometric indicators. (A) Bone mineral density of the femoral neck (g/cm2) versus BMI (kg/m2). (B) Bone mineral density of the femoral neck (g/cm2) versus WC (cm). WC, waist circumference.
For vitamin C, the correlation was weak despite the statistically significant value (Table 4).
Linear regression analyses were performed for the BMD of the spine and the femur. The dependent variables included the number of models of nutritional characteristics (BMI, lean mass and food intake) that best correlated with BMD and total energy intake; phased out models were utilized for those variables that were not statistically significant. The final model (Table 5) shows that among the characteristics evaluated, BMI, WC, calcium and phosphorus showed some predictive value for BMD. These modifiable factors explain 24% of the variability in BMD.
Table 5. Final Model Multivariate Linear Regression Analysis.
| Final model | Lumbar spine BMD | Femur BMD | ||||
|---|---|---|---|---|---|---|
| β | P-value | Adjusted R2 | β | P-value | Adjusted R2 | |
| BMI (kg/m2) | 0.0130 | 0.0020 | 0.24 | |||
| Calcium (mg) | 0.0003 | 0.0006 | ||||
| WC (cm) | 0.0040 | 0.01 | 0.24 | |||
| Phosphor (mg) | 0.0002 | 0.01 | ||||
BMD, bone mineral density; WC, waist circumference.
DISCUSSION
Osteoporosis and osteopenia are common BMD changes in CD patients. There was a positive correlation between BMI, WC, lean mass, calcium intake and BMD.
In our findings, 11.7% of the participants had osteoporosis, and 41.7% had osteopenia, which is consistent with the results described previously, with frequencies varying from 4.3% to 17.0% and from 19.0% to 50.0% for osteoporosis and osteopenia, respectively.1,24 It is interesting to note that the variability of the results in several studies may be due to differences between the included subjects; the sample in this study consists of outpatients, the majority of whom were in remission.
The pathogenesis of bone disorders in IBD is considered multifactorial; however, the etiologic factors remain under discussion, especially regarding the importance of nutritional status and food intake for this complication of CD.25,26 In a previous study from our group, low BMD was associated with penetrating and perianal disease, age at diagnosis >40 years and male gender in CD patients.27
In this study, differences in serum markers of inflammation (CRP and ESR) between patients with normal or low BMD were not observed, which may have been influenced by the fact that most patients were in remission.
Low BMI is already known to be an important predictor of bone disease in IBD patients.28,29,30 However, evidence of the association between WC and BMD in IBD patients is scarce, and therefore more research is required. In individuals with metabolic syndromes, the results are inconsistent.10
There have been few studies that have evaluated the isolated effect of lean and fat mass in IBD patients. In the studies by Lee et al.4 and Leslie et al.,5 it was observed that lean mass was an independent predictive factor for low BMD and that the correlation between lean mass and low BMD was stronger than between fat mass and BMD.
Few studies have evaluated the association between nutrient intake and BMD in IBD patients. No correlation was found in CD patients between protein intake and BMD,14 and there were no differences in the protein intake in patients with and without osteoporosis.25
Although the average intake values of vitamins D and K were below the recommended values in our study population, none of these vitamins correlated with low BMD. However, Reed et al.14 observed lower intake levels of vitamins D and K in CD patients with low BMD.
Among the minerals investigated, the potassium intake was moderately correlated with the BMD of the lumbar spine, which corroborates the findings of studies in healthy populations.31,32,33 A positive correlation between phosphorus and BMD was found; however, Reed et al.14 found no relationship between the intake of nutrients and low BMD in CD patients, despite a similar mean intake (1,088 mg/day) in both studies. In addition, Reed et al.14 found an average intake of magnesium (228 mg/day) similar to the level observed in our study, but with only a weak correlation.
Abitbol et al.15 evaluated the effects of supplemental therapy with calcium and vitamin D (1 g and 800 IU, respectively) in IBD patients and found an increase in BMD. However, the calcium intake was not associated with BMD in other studies of CD patients.14,24,25 In these studies, however, the average intake of the nutrient was not supplied or was above the average found in our study (493.1 mg/day).
Despite the low predictive capacity of nutritional variables for the BMD of these CD patients, when we evaluated the difference between the mean intake of nutrients such as calcium and the intake recommendations, which was associated with low BMD in the linear regression analysis, we estimate that the importance of these nutrients for BMD is greater than presented here.
Our article has some limitations, such as the absence of a control group, serum ALP, intact parathyroid hormone and vitamin D. We believe that the long-term effects of restrictive diets and inadequate nutritional status, especially among those with associated risk factors such as older age, longer disease duration, the presence of inflammatory activity and complications may be more harmful than we determined in our study. A longitudinal study may be needed to better determine the association between these nutritional factors and decreased BMD.
In conclusion, low BMD is common in CD patients, even those in remission. BMD was positively correlated with BMI and WC as well as with calcium and phosphorus intake. Although only a minimal influence on low BMD was found in this study, it suggests that the persistence of a compromised nutritional status and dietary intake over a long period may be an associated factor for osteoporosis and osteopenia in these patients.
ACKNOWLEDGEMENTS
At Dr. Jairo Brandão for their support in carrying out the examinations and at students Clarissa Factum and Luize Sales for the support in data collection.
Footnotes
FINANCIAL SUPPORT: Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB, grant numbers 025/2010).
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION: Conceptualization: FGC, RR, GOS. Methodology: FGC, RR, GOS. Formal analysis: FGC, RR, GOS. Funding acquisition: FGC, RR, GOS. Project Administration: RR, GOS. Visualization: all authors. Writing-original draft: FGC, RR. Writing-review and editing: all authors. Approval of final manuscript: all authors
References
- 1.Siffledeen JS, Fedorak RN, Siminoski K, et al. Bones and Crohn's: risk factors associated with low bone mineral density in patients with Crohn's disease. Inflamm Bowel Dis. 2004;10:220–228. doi: 10.1097/00054725-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Drake MT, Murad MH, Mauck KF, et al. Clinical review: risk factors for low bone mass-related fractures in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97:1861–1870. doi: 10.1210/jc.2011-3058. [DOI] [PubMed] [Google Scholar]
- 3.Genaro PS, Pereira GA, Pinheiro MM, Szejnfeld VL, Martini LA. Influence of body composition on bone mass in postmenopausal osteoporotic women. Arch Gerontol Geriatr. 2010;51:295–298. doi: 10.1016/j.archger.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Lee N, Radford-Smith GL, Forwood M, Wong J, Taaffe DR. Body composition and muscle strength as predictors of bone mineral density in Crohn's disease. J Bone Miner Metab. 2009;27:456–463. doi: 10.1007/s00774-009-0059-5. [DOI] [PubMed] [Google Scholar]
- 5.Leslie WD, Miller N, Rogala L, Bernstein CN. Body mass and composition affect bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD Cohort Study. Inflamm Bowel Dis. 2009;15:39–46. doi: 10.1002/ibd.20541. [DOI] [PubMed] [Google Scholar]
- 6.Alastair F, Emma G, Emma P. Nutrition in inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2011;35:571–580. doi: 10.1177/0148607111413599. [DOI] [PubMed] [Google Scholar]
- 7.Nascimento AT, Rocha R, Coqueiro FG, Santana GO, Lyra AC. Does obesity complicate inflammatory bowel diseases? J Crohns Colitis. 2012;6:1041. doi: 10.1016/j.crohns.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Hartman C, Eliakim R, Shamir R. Nutritional status and nutritional therapy in inflammatory bowel diseases. World J Gastroenterol. 2009;15:2570–2578. doi: 10.3748/wjg.15.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sousa Guerreiro C, Cravo M, Costa AR, et al. A comprehensive approach to evaluate nutritional status in Crohn's patients in the era of biologic therapy: a case-control study. Am J Gastroenterol. 2007;102:2551–2556. doi: 10.1111/j.1572-0241.2007.01439.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Kim B, Lee H, Choi H, Won C. The relationship between prevalence of osteoporosis and proportion of daily protein intake. Korean J Fam Med. 2013;34:43–48. doi: 10.4082/kjfm.2013.34.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:2504–2512. doi: 10.1359/jbmr.2000.15.12.2504. [DOI] [PubMed] [Google Scholar]
- 12.Nieves JW. Osteoporosis: the role of micronutrients. Am J Clin Nutr. 2005;81:1232S–1239S. doi: 10.1093/ajcn/81.5.1232. [DOI] [PubMed] [Google Scholar]
- 13.Bonjour JP, Guéguen L, Palacios C, Shearer MJ, Weaver CM. Minerals and vitamins in bone health: the potential value of dietary enhancement. Br J Nutr. 2009;101:1581–1596. doi: 10.1017/S0007114509311721. [DOI] [PubMed] [Google Scholar]
- 14.Reed CA, Nichols DL, Bonnick SL, DiMarco NM. Bone mineral density and dietary intake in patients with Crohn's disease. J Clin Densitom. 1998;1:33–40. doi: 10.1385/jcd:1:1:33. [DOI] [PubMed] [Google Scholar]
- 15.Abitbol V, Mary JY, Roux C, et al. Osteoporosis in inflammatory bowel disease: effect of calcium and vitamin D with or without fluoride. Aliment Pharmacol Ther. 2002;16:919–927. doi: 10.1046/j.1365-2036.2002.01247.x. [DOI] [PubMed] [Google Scholar]
- 16.Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet. 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 17.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5A–36A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 18.Leslie M, Mikanowicz C. Assessment of body composition in the healthy adult. J Am Acad Nurse Pract. 1997;9:123–127. doi: 10.1111/j.1745-7599.1997.tb00709.x. [DOI] [PubMed] [Google Scholar]
- 19.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 20.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary ReferenceIntakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academies Press (US); 1997. [PubMed] [Google Scholar]
- 21.Trumbo P, Schlicker S, Yates AA, Poos M Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102:1621–1630. doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- 22.Ross AC, Manson JE, Abrams SA, et al. The 2011 Dietary Reference Intakes for calcium and vitamin D: what dietetics practitioners need to know. J Am Diet Assoc. 2011;111:524–527. doi: 10.1016/j.jada.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4:368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 24.Habtezion A, Silverberg MS, Parkes R, Mikolainis S, Steinhart AH. Risk factors for low bone density in Crohn's disease. Inflamm Bowel Dis. 2002;8:87–92. doi: 10.1097/00054725-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Cravo M, Guerreiro CS, dos Santos PM, et al. Risk factors for metabolic bone disease in Crohn's disease patients. Inflamm Bowel Dis. 2010;16:2117–2124. doi: 10.1002/ibd.21297. [DOI] [PubMed] [Google Scholar]
- 26.Ezzat Y, Hamdy K. The frequency of low bone mineral density and its associated risk factors in patients with inflammatory bowel diseases. Int J Rheum Dis. 2010;13:259–265. doi: 10.1111/j.1756-185X.2010.01542.x. [DOI] [PubMed] [Google Scholar]
- 27.Lima CA, Lyra AC, Mendes CM, et al. Bone mineral density and inflammatory bowel disease severity. Braz J Med Biol Res. 2017;50:e6374. doi: 10.1590/1414-431X20176374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koutroubakis IE, Zavos C, Damilakis J, et al. Low bone mineral density in Greek patients with inflammatory bowel disease: prevalence and risk factors. Ann Gastroenterol. 2011;24:41–46. [PMC free article] [PubMed] [Google Scholar]
- 29.Atreja A, Aggarwal A, Licata AA, Lashner BA. Low body mass index can identify majority of osteoporotic inflammatory bowel disease patients missed by current guidelines. ScientificWorldJournal. 2012;2012:807438. doi: 10.1100/2012/807438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Targownik LE, Leslie WD, Carr R, et al. Longitudinal change in bone mineral density in a population-based cohort of patients with inflammatory bowel disease. Calcif Tissue Int. 2012;91:356–363. doi: 10.1007/s00223-012-9650-1. [DOI] [PubMed] [Google Scholar]
- 31.Farrell VA, Harris M, Lohman TG, et al. Comparison between dietary assessment methods for determining associations between nutrient intakes and bone mineral density in postmenopausal women. J Am Diet Assoc. 2009;109:899–904. doi: 10.1016/j.jada.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.New SA, Robins SP, Campbell MK, et al. Dietary influences on bone mass and bone metabolism: further evidence of a positive link between fruit and vegetable consumption and bone health? Am J Clin Nutr. 2000;71:142–151. doi: 10.1093/ajcn/71.1.142. [DOI] [PubMed] [Google Scholar]
- 33.Rondanelli M, Opizzi A, Perna S, Faliva MA. Update on nutrients involved in maintaining healthy bone. Endocrinol Nutr. 2013;60:197–210. doi: 10.1016/j.endonu.2012.09.006. [DOI] [PubMed] [Google Scholar]