Correction to:
https://doi.org/10.5217/ir.2018.16.2.233
Intest Res 2018;16(2):233-245; published April 30, 2018; updated July 30, 2018
In this article, the authors regret an error in the reporting of Inflammatory Bowel Disease Questionnaire (IBDQ) patient-reported outcome data in the manuscript. A data extraction error resulted in incorrect IBDQ data being presented in the publication. The error does not affect the overall conclusions regarding IBDQ as the difference between the corrected and erroneous numbers is, in general, small. The error was specific to IBDQ data; all other data have been reviewed and are correct as originally reported.
The error affected the IBDQ total scores at baseline reported in Table 1 of the manuscript, and the IBDQ data reported in Section 3. Patient-Reported Outcomes of the manuscript. The corrected IBDQ data for Table 1 and Section 3. Patient-Reported Outcomes are reported below. The corrigendum does not change the conclusion of the article.
The original text read as follows:
3. Patient-Reported Outcomes
1) Patient-Reported Outcomes in OCTAVE Induction 1 and 2
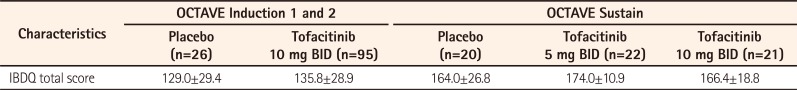
The baseline (SD) mean IBDQ total score was 129.0 (29.4) in the placebo group and 135.8 (28.9) in the tofacitinib 10 mg BID group. Treatment with tofacitinib 10 mg BID resulted in numerically greater improvements from baseline versus placebo in the IBDQ total score and in all IBDQ domain scores at weeks 4 and 8. The mean change from baseline in total IBDQ score with tofacitinib 10 mg BID was 22.5 at week 4 and 27.4 at week 8. The respective values with placebo were 11.5 and 14.9. For the Bowel Function Score, the week 8 mean change from baseline was 10.6 with tofacitinib 10 mg BID versus 6.6 with placebo (week 4: 8.5 vs. 4.7, respectively). For the Emotional Status Score, the mean changes from baseline were 7.5 versus 5.0 at week 8 and 6.6 versus 4.6 at week 4 for tofacitinib 10 mg BID and placebo, respectively. The respective changes from baseline in the Systemic Symptoms Score were 4.3 versus 2.1 at week 8 and 3.3 versus 0.9 at week 4. For the Social Function Score, the changes from baseline were 5.1 versus 1.2 at week 8 and 4.1 versus 1.3 at week 4 in the tofacitinib and placebo groups, respectively. IBDQ remission at week 8 was achieved by 56.8% of tofacitinib-treated patients versus 15.4% of patients with placebo (44.2% vs. 19.2% at week 4), and IBDQ response at week 8 was achieved by 62.1% of tofacitinib-treated patients versus 38.5% of those with placebo (55.8% vs. 34.6% at week 4).
2) Patient-Reported Outcomes in OCTAVE Sustain
The mean (SD) IBDQ score at baseline of OCTAVE Sustain was 164.0 (26.8) in the placebo group, 174.0 (10.9) in the tofacitinib 5 mg BID group, and 166.4 (18.8) in the tofacitinib 10 mg BID group. The mean changes from baseline in the total IBDQ score at week 52 of OCTAVE Sustain were 3.1 with tofacitinib 5 mg BID and 9.9 with tofacitinib 10 mg BID, compared with −1.0 with placebo. The mean changes from baseline in the Bowel Function Score at week 52 were −0.8 with tofacitinib 5 mg BID, 3.4 with tofacitinib 10 mg BID, and 0.8 with placebo. For the Emotional Status Score, the week 52 mean changes from baseline were 3.4 and 3.0 with tofacitinib 5 mg BID and 10 mg BID, respectively, versus −1.3 with placebo. For the Systemic Symptoms Score, the mean changes from baseline at week 52 were 0.3 with tofacitinib 5 mg BID, 1.5 with tofacitinib 10 mg BID, and −0.8 with placebo. For the Social Function Score, the week 52 changes from baseline were 0.1 and 2.1 with tofacitinib 5 mg BID and 10 mg BID, respectively, and 0.3 with placebo. At week 52 of OCTAVE Sustain, 50.0% of patients in the tofacitinib 5 mg BID group, 42.9% of patients in the tofacitinib 10 mg BID group, and 15.0% of patients in the placebo group achieved IBDQ remission. IBDQ response at week 52 was achieved by 40.9% and 52.4% of patients receiving tofacitinib 5 mg BID and tofacitinib 10 mg BID, respectively, versus 30.0% of patients receiving placebo.
The revised text now reads:
3. Patient-Reported Outcomes
1) Patient-Reported Outcomes in OCTAVE Induction 1 and 2
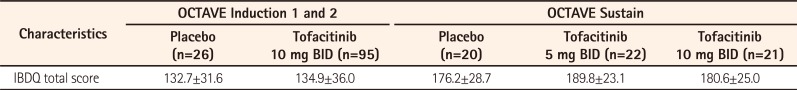
The baseline (SD) mean IBDQ total score was 132.7 (31.6) in the placebo group and 134.9 (36.0) in the tofacitinib 10 mg BID group. Treatment with tofacitinib 10 mg BID resulted in numerically greater improvements from baseline versus placebo in the IBDQ total score and in all IBDQ domain scores at weeks 4 and 8. The mean change from baseline in total IBDQ score with tofacitinib 10 mg BID was 30.1 at week 4 and 37.2 at week 8. The respective values with placebo were 11.5 and 18.2. For the Bowel Function Score, the week 8 mean change from baseline was 15.0 with tofacitinib 10 mg BID versus 6.5 with placebo (week 4: 11.9 vs 4.4, respectively). For the Emotional Status Score, the mean changes from baseline were 10.8 versus 6.3 at week 8 and 8.8 versus 3.7 at week 4 for tofacitinib 10 mg BID and placebo, respectively. The respective changes from baseline in the Systemic Symptoms Score were 5.2 versus 3.9 at week 8 and 4.1 versus 2.3 at week 4. For the Social Function Score, the changes from baseline were 6.3 versus 1.6 at week 8 and 5.3 versus 1.2 at week 4 in the tofacitinib and placebo groups, respectively. IBDQ remission at week 8 was achieved by 61.1% of tofacitinib-treated patients versus 23.1% of patients with placebo (50.5% vs. 26.9% at week 4), and IBDQ response at week 8 was achieved by 71.6% of tofacitinib-treated patients versus 42.3% of those with placebo (68.4% vs. 42.3% at week 4).
2) Patient-Reported Outcomes in OCTAVE Sustain
The mean (SD) IBDQ score at baseline of OCTAVE Sustain was 176.2 (28.7) in the placebo group, 189.8 (23.1) in the tofacitinib 5 mg BID group, and 180.6 (25.0) in the tofacitinib 10 mg BID group. The mean changes from baseline in the total IBDQ score at week 52 of OCTAVE Sustain were −0.3 with tofacitinib 5 mg BID and 11.4 with tofacitinib 10 mg BID, compared with 8.2 with placebo. The mean changes from baseline in the Bowel Function Score at week 52 were −0.8 with tofacitinib 5 mg BID, 2.6 with tofacitinib 10 mg BID, and 1.9 with placebo. For the Emotional Status Score, the week 52 mean changes from baseline were 0.3 and 4.3 with tofacitinib 5 mg BID and 10 mg BID, respectively, versus 2.4 with placebo. For the Systemic Symptoms Score, the mean changes from baseline at week 52 were −0.1 with tofacitinib 5 mg BID, 2.4 with tofacitinib 10 mg BID, and 0.4 with placebo. For the Social Function Score, the week 52 changes from baseline were 0.4 and 2.1 with tofacitinib 5 mg BID and 10 mg BID, respectively, and 3.4 with placebo. At week 52 of OCTAVE Sustain, 63.6% of patients in the tofacitinib 5 mg BID group, 61.9% of patients in the tofacitinib 10 mg BID group, and 40.0% of patients in the placebo group achieved IBDQ remission. IBDQ response at week 52 was achieved by 59.1% and 61.9% of patients receiving tofacitinib 5 mg BID and tofacitinib 10 mg BID, respectively, versus 30.0% of patients receiving placebo.
Table 1. Demographics and Baseline Disease Characteristics for the East Asian Population of Patients in OCTAVE Induction 1 and 2 and OCTAVE Sustain, by Treatment Group
The row in Table 1 originally reported the IBDQ total scores at baseline as follows:
The revised data now reads: