Abstract
Objective
To evaluate the clinical efficacy and safety of Compound Danshen Dripping Pill (CDDP) and Isosorbide Mononitrate (ISMN) in the treatment of unstable angina pectoris (UAP) in the elderly.
Materials and Methods
CNKI, Wanfang, VIP, CBM, and PubMed databases were searched for appropriate articles without language limitations on keywords. RevMan 5.3 software was used to perform the meta-analysis.
Results
This analysis compared CDDP with ISMN of 21 randomized controlled trials (RCTs) that involved a total of 2356 patients with UAP. When the treatment lasted for four weeks, the clinical effective rate was OR = 3.97, 95% CI = 2.97, 5.30, and P < 0.00001, the ECG efficiency was OR = 3.43, 95% CI = 2.13, 5.53, and P < 0.00001, and incidence of adverse reactions was OR = 0.73, 95% CI = 0.52, 1.04, and P = 0.08 > 0.05. When the treatment lasted for eight weeks, clinical efficiency rate was OR = 4.22, 95% CI = 2.37, 3.79, and P < 0.00001, incidence of adverse reactions was OR = 0.58, 95% CI = 0.26, 1.27, and P = 0.17 > 0.05, whole blood low-cut blood viscosity was SMD = -0.61 and 95% CI -1.60, 0.38, whole blood high-cut blood viscosity was SMD = -0.38 and 95% CI -0.97, 0.21, and blood cells specific volume was SMD = -0.80 and 95% CI -2.61, 1.01.
Conclusion
Based on this meta-analysis, the CDDP was superior to ISMN with UAP in the elderly. However, there is still a need to further verify the clinical efficacy and safety of CDDP with more strictly designed RCTs with large sample and multiple centers in the future.
1. Introduction
UAP [1] is a common coronary syndrome between stable angina and acute myocardial infarction, which could lead to myocardial infarction or sudden death [2]; it is a clinically common cardiovascular disease (CVD) [3]. The pain can be induced; UAP is accompanied by accidental pains which would be induced even in the resting state [4]. It is similar to typical stable angina [5] but lasts longer. It is easy to evolve into acute myocardial infarction or sudden death, so timely diagnosis and correct treatment are needed urgently, including early onset angina pectoris, angina pectoris, spontaneous angina pectoris, X synthesis, supine angina pectoris, and postprandial angina pectoris. With the acceleration of population aging in China, the incidence of unstable angina pectoris is on the rise, threatening people's health and maybe threatening life if not treated promptly.
The CDDP is a new type of pure Chinese medicine drop that has been successfully developed based on the basic theory of traditional Chinese medicine and the use of modern medical technology [6]. Compared with the original dosage form, it has the advantages of smaller dosage, better curative effect, more prominent effect, fewer side effects, and reduced gastrointestinal irritation, and it is a commonly used traditional Chinese medicine preparation [7]. Its main components are Salvia miltiorrhiza, Panax notoginseng, and Dipterocarpaceae [8]. Salvia miltiorrhiza [9] as a traditional medicine for promoting blood circulation and stasis has the effect of promoting blood circulation, relieving blood, and relieving pain and plays an important role in the treatment of cardiovascular diseases (CVD). The main component is water-soluble danshensu [10], which has the function of dilating blood vessels, increasing coronary flow, improving microcirculation, and so forth. Notoginseng saponins extracted from Panax notoginseng can increase coronary blood flow and reduce myocardial oxygen consumption and arterial pressure. Dipterocarpaceae has anti-myocardial infarction, reduced myocardial oxygen consumption, anti-inflammatory, and analgesic effects [11]. Therefore it has been widely used for treating cardiovascular diseases and peripheral circulation disorder clinically [2]. ISMN [12] is one of the most effective and frequently used agents for treating angina pectoris [13]. The main pharmacological effect is a relaxation of the vascular smooth muscle [14], which can effectively prevent the onset of angina [15].
In recent years, more and more RCTs on the CDDP and ISMN in the treatment of angina pectoris were compared [11, 16–18]. However, UAP was rarely considered; only a few studies have put emphasis on sample clinical measurements such as the clinical efficacy and the ECG efficiency. Since some drugs have different onset times and drug efficacy duration, different dose cycles may have different effects on efficacy. In this study, the meta-analysis was performed on the research data of different clinical dosing cycles to evaluate the clinical efficacy and safety of CDDP and ISMN in the treatment of elderly patients with UAP.
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
According to the suggestions of a cardiologist, we designed the inclusion criteria as follows: (1) We selected elderly patients who meet the diagnostic criteria for UAP. (2) The study was a randomized, double-blind controlled study. (3) The patients were between 30 and 90 years of age. (4) There was no limitation of race and gender of the study subjects. (5) Patients received general treatment, according to the different drugs to be divided into treatment group and control group; the treatment group used CDDP, and the control group used ISMN; in addition to this, two groups have no other treatment measures. (6) The duration of treatment is 4 weeks or 8 weeks. Exclusion criteria were (1) unclear diagnosis, (2) unmatched treatment cycles, and (3) unsatisfactory interventions
2.2. Retrieve Information
CNKI, Wanfang, VIP, CBM, PubMed and other databases were searched to retrieve information from RCTs of the CDDP and ISMN in the treatment of UAP in the elderly in recent years. Keywords were “compound Danshen dripping pills" and “unstable angina elderly" [Title/Abstract], “isosorbide mononitrate" and “unstable angina elderly" [Title/Abstract], “unstable angina elderly" [Title/Abstract], “compound Danshen dripping pills" [Title/Abstract], “isosorbide mononitrate" [Title/Abstract], and “unstable angina" [Title/Abstract]. RCTs were examined without language limitations in order to obtain a comprehensive retrieval published before 25 December 2017. All RCTs were screened according to certain criteria. And relevant RCTs were downloaded into Endnote software (version X8, Thomson Reuters, Inc., New York, USA) for further exploring. We have made detailed records and analysis of relevant data. Duplicate records were removed. The full-text review was performed, while the title/abstract was thought to be thematic.
2.3. Quality Assessment
This meta-analysis used Review Manager 5.3 software to perform quality assessment.
It was evaluated from random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases and divided into three indexes: “high risk,” “unclear risk,” and “low risk.”
2.4. Statistical Methods
Review Manager 5.3 (Cochrane Collaboration) statistical software was used for analysis and processing. Outcome measures such as the clinical efficacy, adverse reactions, and the ECG efficiency were regarded as dichotomous variables and presented as the odds ratio (OR) with 95% confidence intervals (95%). Blood viscosity was continuous variable that was presented as the Std. mean difference (SMD) with 95% CI. Q statistic and I2 tests were applied to assess the heterogeneity among studies. If P > 0.10 and I2 ≤ 50%, the study was homogeneous, using a fixed-effects model for statistical analysis. And a random-effects model was used to analyze data with heterogeneity (P ≤ 0. 10; I2 > 50%), and the effective results are statistically significant at P < 0.05 [11]. Potential publication bias was revealed by funnel plots.
3. Results
3.1. Literature Search
A total of 21 RCTs involving 2356 patients were included in this systematic review. Two people were retrieved and cross-checked when there are disagreements; the third person participates in the discussion. Studies selection process is shown in Figure 1.
Figure 1.
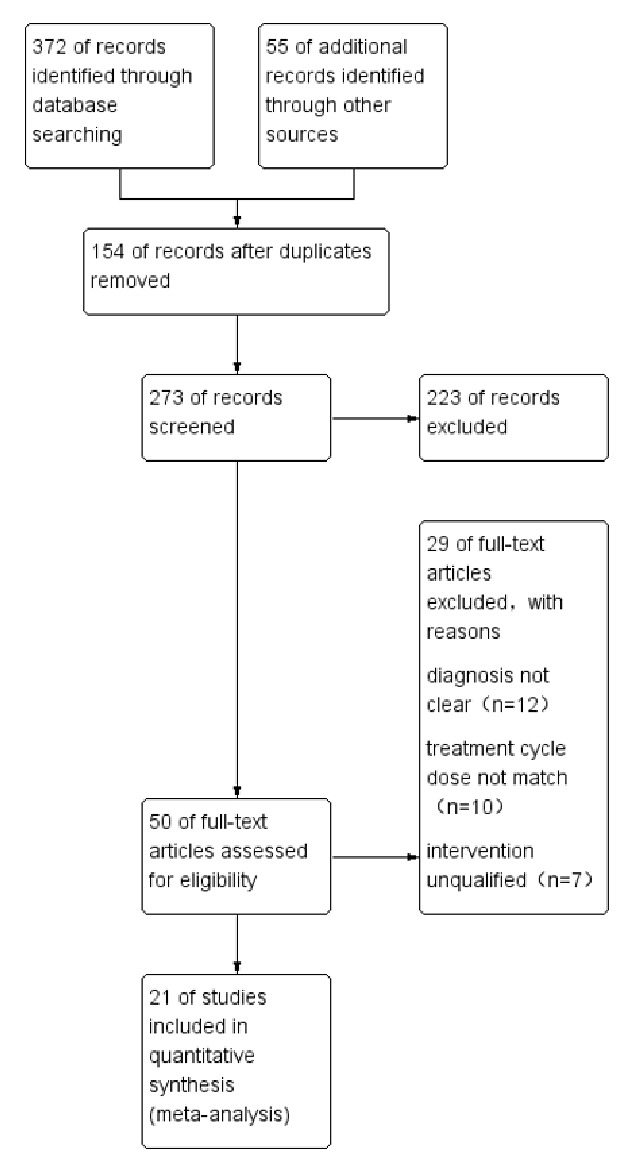
Studies selection process.
3.2. Risk of Bias Assessment
According to the Cochrane risk of bias estimation, six RCTs referred to “random number table method" or similar methods [19–24]. Therefore, selection bias was evaluated as “low risk.” Although the remaining RCTs mentioned “random," they did not describe specific methods; selection bias was evaluated as “unclear risk.” Ten RCTs selection bias was remarked as “high risk” in allocation concealment [19, 21, 23, 25–31]. Three RCTs were remarked as “unclear risk” [20, 24, 32] and eight RCTs were “low risk” [22, 33–39]. Blinding of participants and outcome assessment of all RCTs were not mentioned, so performance bias and detection bias were deemed as “unclear risk.” There is no shortage of cases or selective reports, so the attrition bias and reporting bias were assessed as “low risk.” Although no other biases were found in these trials, considering their poor methodological quality, we decided to assign an “unclear risk” of bias to all the included trials.
3.3. Literature Screening
According to the above search terms, a total of 427 RCTs were consulted. Depending on the inclusion criteria and exclusion criteria, including unclear diagnosis, unmatched treatment cycles, and unsatisfactory interventions, only 21 RCTs match the standards. Patients in experimental group received CDDP therapy, whereas patients in control group received ISMN therapy only. Observed indicators include clinical effective rate, ECG changes, blood viscosity improvement, and adverse reactions. The detailed characteristics of the included 21 studies are shown in Tables 1 and 2.
Table 1.
Characteristics of included studies.
| Author, year | Cases (T/C) | Diagnostic standard | Age (years) | Sex | Dosage |
|---|---|---|---|---|---|
| Range, mean | Male/female | T/C | |||
| Ren, 2013 | 32/23 | DCWHO | T: 45~67, 56.2 C: 39~66, 54.8 |
23/9 | T: 10 capsules/time, 3 times/d, oral C: 30 mg/time, 1 time/d, oral |
|
| |||||
| Liu, 2005 | 33/32 | DCWHO | T: 43~65, 52.3 ± 7.5 C: 41~64, 53.6 ± 8.4 |
T: 18/15 C: 19/13 |
T: 10 capsules/time, 3 times/d, oral C: 20 mg/time, 3 times/d, oral |
|
| |||||
| Shi, 2013 | 40/40 | DCWHO | 60~83, 6.75 ± 6.32 | 43/37 | T: 10 capsules/time, 3 times/d, oral C: 50 mg/time, 1 time/d, oral |
|
| |||||
| Zhang, 2016 | 45/45 | DCWHO | T: 55~83, 64.9 ± 6.2 C: 56~82, 67.3 ± 6.4 |
T: 30/15 C: 32/13 |
T: 10 capsules/time, 3 times/d, oral C: 10 mg/time, 3 times/d, oral |
|
| |||||
| Zhang, 2014 | 80/80 | NR | 32~78,65.32 ± 3.10 | 87/73 | T: 10 capsules/time, 3 times/d, oral C: NR |
|
| |||||
| Jing, 2014 | 84/84 | DCWHO | 62~84,68.8 ± 5.48 | 88/80 | T: 10 capsules/time, 3 times/d, oral C: 50 mg/time, 1 time/d, oral |
|
| |||||
| Li, 2012 | 60/52 | DCWHO | NR | NR | T: 10 capsules/time, 3 times/d, 5% glucose 250 ml, oral C: 10 mg/time, 3 times/d, 5% glucose 250 ml, oral |
|
| |||||
| Li, 2016 | 43/42 | NR | T: 61~84, 69.5 ± 5.6 C: 60~83, 69.3 ± 5.4 |
T: 26/17 C: 25/17 |
T: 10 capsules/time, 3 times/d, oral C: 50 mg/time, 1 time/d, oral |
|
| |||||
| Yang, 2015 | 32/32 | DCWHO | T: 62~84, 69.4 ± 5.8 C: 60~81, 68.2 ± 5.4 |
T: 18/14 C: 19/13 |
T: 3 tablets /time, 3 times/d, oral C: 50 mg/time, 1 time/d, oral |
|
| |||||
| Wang, 2014 | 75/75 | DCWHO | T: 60~82, 68.8 ± 6.5 C: 61~80, 66.7 ± 6.3 |
T: 40/35 C: 38/37 |
T: 10 capsules/time, 3 times/d, oral C: 50 mg/time, 1 time/d, oral |
|
| |||||
| Sheng, 2015 | 40/40 | NR | T: 58~80, 65.9 ± 3.6 C: 57~82, 66.8 ± 4.1 |
T: 17/23 C: 16/24 |
T: 10 capsules/time, 3 times/d, oral C: 10 mg/time, 3 times/d, oral |
|
| |||||
| Su, 2015 | 106/106 | NR | 58~83, 67.3 ± 6.9 | 127/85 | T: 10 capsules/time, 3 times/d, oral C: 50 mg/time, 1 time/d, oral |
|
| |||||
| Shao, 2013 | 40/40 | DCWHO | 60~80, 67.75 ± 6.32 | 43/37 | T: 10 capsules/time, 3 times/d, oral C: 10 mg/time, 3 times/d, oral |
|
| |||||
| Guo, 2013 | 129/107 | DCWHO | 42~70, 51.9 ± 13.6 | 161/75 | T: 10 capsules/time, 3 times/d, oral C: 20 mg, intravenous drip, 5% glucose 250 ml |
|
| |||||
| Chen, 2009 | 46/46 | ACC/AHA | T: 41~69, 48.5 ± 12.9 C: 45~72, 49.3 ± 11.1 |
T: 32/14 C: 31/15 |
T: 10 capsules/time, 3 times/d, oral C: 20 mg/time, 3 times/d, oral |
|
| |||||
| Ma, 2012 | 70/70 | CMACB | 61~78, 67.1 ± 6.4 | 103/37 | T: 10 capsules/time, 3 times/d, oral C: 30 mg/time, 2 times/d, oral |
|
| |||||
| He, 2011 | 45/45 | DCWHO | T: 66.4 C: 65.7 |
T: 25/20 C: 24/21 |
T: 10 capsules/time, 3 times/d, oral C: 30 mg/time, 1 time/d, oral |
|
| |||||
| Zeng, 2014 | 50/50 | DCWHO | 60~76, 67.8 ± 6.5 | 66/34 | T: 10 capsules/time, 3 times/d, oral C: 20 mg/time, 2 times/d, oral |
|
| |||||
| Geng, 2015 | 63/63 | DCWHO | 44~75, 53.5 ± 7.6 | 65/61 | T: 10 capsules/time, 3 times/d, oral C: NR |
|
| |||||
| Ma, 2014 | 42/41 | DCWHO | 55~73, 62.5 ± 4.2 | 50/33 | T: 10 capsules/time, 3 times/d, oral C: 30 mg/time, 1 time/d, oral |
|
| |||||
| Gao, 2013 | 44/44 | DCWHO | T: 52~76, 60.8 ± 7 C: 53~78, 61.5 ± 8 |
T: 27/17 C: 29/15 |
T: 10 capsules/time, 3 times/d, oral C: 10 mg/time, oral |
Table 2.
Clinical efficacy, ECG efficiency, and adverse reaction information.
| Author, year | Treatment/week | Effective clinical efficacy | Efficacy of ECG | Adverse reaction rate | Outcome measures | |||
|---|---|---|---|---|---|---|---|---|
| (Effective/total) | (Effective/total) | (Effective/total) | ||||||
| Treatment control | Treatment control | Treatment control | ||||||
| Ren, 2013 | 4 | 29/32 | 17/23 | 28/32 | 16/32 | NR | ||
|
| ||||||||
| Liu, 2005 | 4 | 29/33 | 24/32 | 25/33 | 19/32 | NR | ||
|
| ||||||||
| Shi, 2013 | 4 | 38/40 | 32/40 | NR | 9/40 | 10/40 | Blood viscosity | |
|
| ||||||||
| Zhang, 2016 | 4 | 42/45 | 27/45 | NR | 6/45 | 13/45 | ||
|
| ||||||||
| Zhang, 2014 | 4 | 78/80 | 69/80 | NR | NR | Blood viscosity | ||
|
| ||||||||
| Jing, 2014 | 4 | 75/84 | 61/84 | NR | 9/84 | 22/84 | ||
|
| ||||||||
| Li 2012 | 4 | 54/60 | 38/52 | NR | NR | |||
|
| ||||||||
| Li, 2016 | 4 | 40/43 | 30/42 | NR | 5/43 | 5/42 | Blood viscosity | |
|
| ||||||||
| Yang, 2015 | 4 | 29/32 | 22/32 | NR | NR | Angina pectoris frequency and attack time | ||
|
| ||||||||
| Wang, 2014 | 4 | 72/75 | 59/75 | NR | 16/75 | 17/75 | Blood viscosity | |
|
| ||||||||
| Sheng, 2015 | 4 | 37/40 | 30/40 | NR | NR | |||
|
| ||||||||
| Su, 2015 | 4 | 99/106 | 86/106 | NR | 14/106 | 11/106 | Blood viscosity | |
|
| ||||||||
| Shao, 2013 | 4 | 38/40 | 30/40 | NR | 9/40 | 10/40 | ||
|
| ||||||||
| Guo, 2013 | 4 | 117/129 | 81/107 | 118/129 | 94/129 | NR | Cardiac function index (SV, CO, LEVF) | |
|
| ||||||||
| Chen, 2009 | 4 | 43/46 | 38/46 | NR | 2/46 | 2/46 | Cardiac function index (SV, CO, LEVF) | |
|
| ||||||||
| Ma, 2012 | 4 | 65/70 | 53/70 | 64/70 | 51/70 | NR | Angina pectoris frequency and attack time | |
|
| ||||||||
| He, 2011 | 8 | 43/45 | 39/45 | NR | NR | Angina pectoris frequency and attack time | ||
|
| ||||||||
| Zeng, 2014 | 8 | 46/50 | 34/50 | NR | 7/50 | 15/50 | ||
|
| ||||||||
| Geng, 2015 | 8 | 57/63 | 47/63 | 59//63 | 45/63 | 5/63 | 4/63 | |
|
| ||||||||
| Ma, 2014 | 8 | 40/42 | 28/41 | NR | NR | Angina pectoris frequency and attack time | ||
|
| ||||||||
| Gao, 2013 | 8 | 41/44 | 37/44 | NR | NR | Angina pectoris frequency and attack time | ||
3.4. Subgroup Analysis
In the 21 RCTs, the treatment cycle of 16 RCTs was four weeks, while that of 5 RCTs was eight weeks. Among them, after four weeks of treatment and the administration of the drug, besides the clinical curative effect, four articles reported the efficacy of electrocardiogram, eight articles reported the adverse reactions, and four articles reported the blood viscosity. After eight weeks of treatment, in addition to the clinical efficacy, only one article reported the efficacy of ECG [24] and two reported adverse reactions. Indicators will not be analyzed for they were mentioned in few studies.
3.4.1. When the Treatment Lasted for Four Weeks
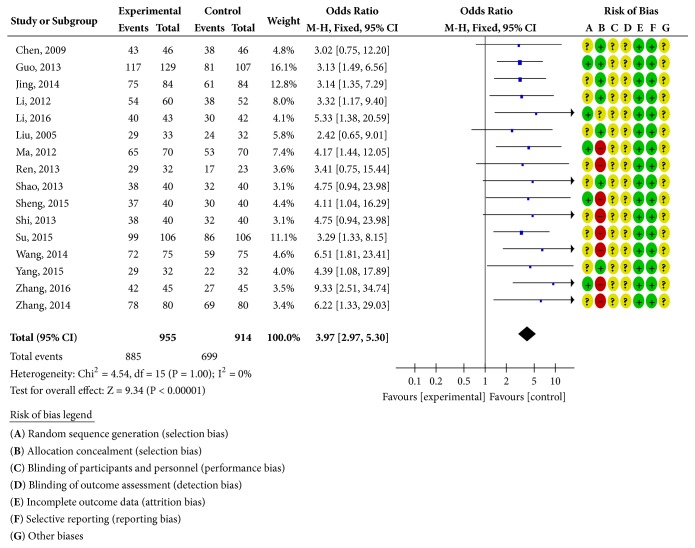
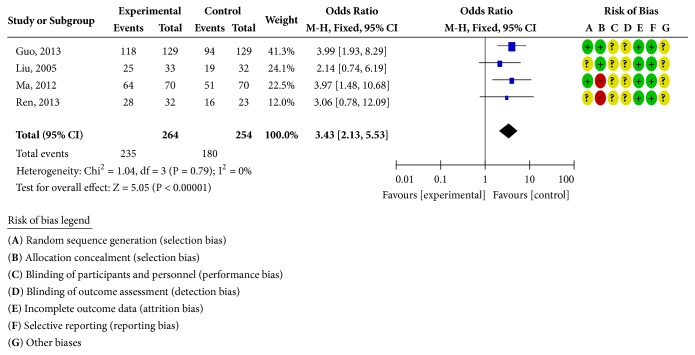
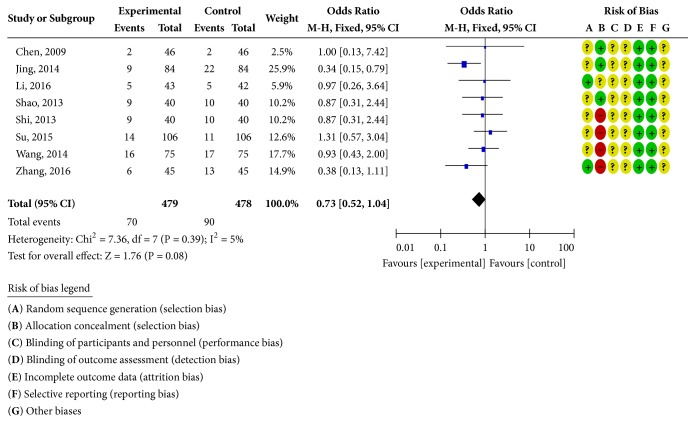
Sixteen RCTs reported clinical curative effects [19–23, 25–29, 34–39], as shown in Figure 2. There was homogeneity in each study (P = 1.00; I2 = 0%). Statistical analysis was performed using a fixed-effects model. The OR and 95% CI for clinically effective rate were OR = 3.97 and 95% CI = 2.97, 5.30) (P < 0.00001); the results showed that the clinical efficacy of the treatment group was better than the control group and the difference was statistically significant; four RCTs reported the ECG efficiency [22, 23, 25, 36], as shown in Figure 3. The study was homogeneous (P = 0.79; I2 = 0% ), using a fixed-effect model for statistical analysis; the OR and 95% CI for ECG efficiency were OR = 3.43 and 95% CI = 2.13, 5.53 (P < 0.00001); the results show that the ECG treatment group was more effective than the control group; the difference has statistical significance; eight RCTs reported adverse reactions [19, 20, 26–28, 35, 38, 39], as shown in Figure 4. There was homogeneity in each study (P = 0.39; I2 = 5%), using a fixed-effects model for statistical analysis; the OR and 95% CI for incidence of adverse reactions were OR = 0.73 and 95% CI = 0.52,1.04 (P = 0.08 > 0.05); the difference was not statistically significant and it may not be possible to draw a definite conclusion that the incidence of adverse reactions is lower than ISMN, due to the sample size and treatment cycle, and this remains to be further explored.
Figure 2.
Clinical efficacy analysis chart.
Figure 3.
ECG efficiency analysis chart.
Figure 4.
Adverse reaction rate analysis chart.
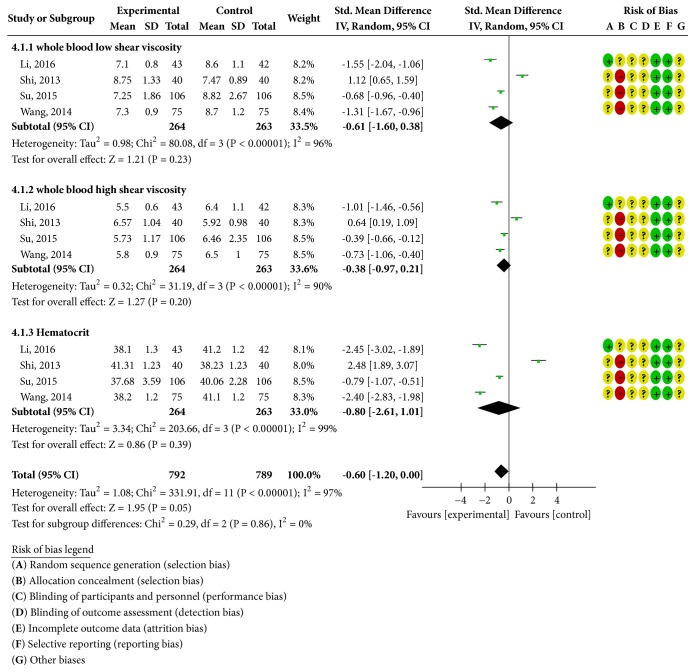
Four RCTs reported whole blood low-cut blood viscosity [20, 26–28], as shown in Figure 5. There was heterogeneity in each study (P < 0.00001; I2 = 96%); a random-effects model was used for statistical analysis; the statistical analysis of the model showed that the improvement of the low-cut viscosity of the whole blood in the experimental group was better than that of the control group. The difference was statistically significant [SMD = -0.61; 95% CI = -1.60, 0.38], and four literature studies reported that the whole blood high-cut blood viscosity was heterogeneous in each study (P < 0.00001; I2 = 90%); a random-effects model was used for statistical analysis; the improvement of whole blood hyperviscosity in the experimental group was better than that in the control group. The difference was statistically significant [SMD = -0.38; 95% CI = -0.97, 0.21]. Hematocrit was reported in four studies (P < 0.00001; I2 = 99%); a random-effects model was used for statistical analysis; the results showed that the hematocrit of the experimental group improved better than the control group. The difference was statistically significant: [SMD = -0.80; 95% CI = -2.61, 1.01].
Figure 5.
Blood viscosity change analysis chart.
3.4.2. When the Treatment Lasted for Eight Weeks
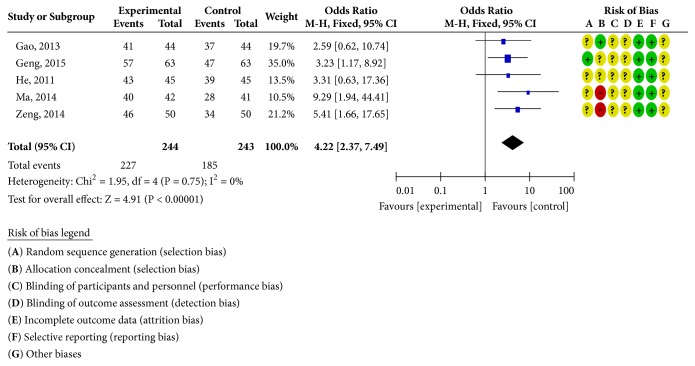
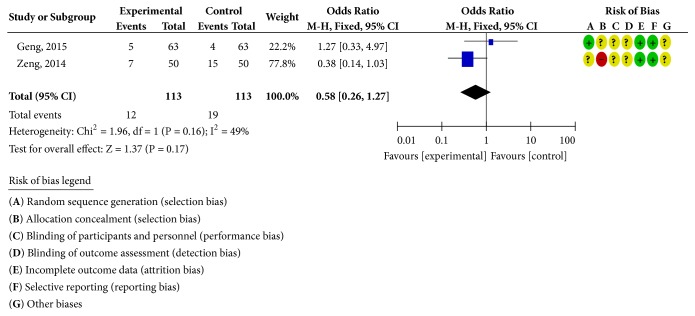
Five RCTs reported clinical outcomes [24, 30–33], as shown in Figure 6. There was homogeneity in each study (P = 0.75; I2 = 0%). Statistical analysis was performed using a fixed-effects model; the OR and 95% CI for clinically effective rate were OR = 4.22 and 95% CI = 2.37, 3.79 (P < 0.00001); the results showed that the clinical efficacy of the treatment group was better than the control group; the difference was statistically significant; two RCTs reported adverse reactions [24, 31], as shown in Figure 7. There was homogeneity in the studies (P = 0.16; I2 = 49%). Statistical analysis was performed using a fixed-effects model; the OR and 95% CI for the incidence of adverse reactions were OR = 0.58 and 95% CI = 0.26, 1.27 (P = 0.17 > 0.05). There was no statistically significant difference. It may not be possible to draw a definite conclusion that the incidence of adverse reactions is lower than ISMN, due to the sample size and the treatment cycle, and this remains to be further explored.
Figure 6.
Clinical efficacy analysis chart.
Figure 7.
Adverse reaction rate analysis chart.
3.5. Publication Bias
Funnel chart analysis of clinical efficacy found the distribution of scattered points of symmetry, indicating that the possibility of publication bias is small, as shown in Figures 8 and 9.
Figure 8.
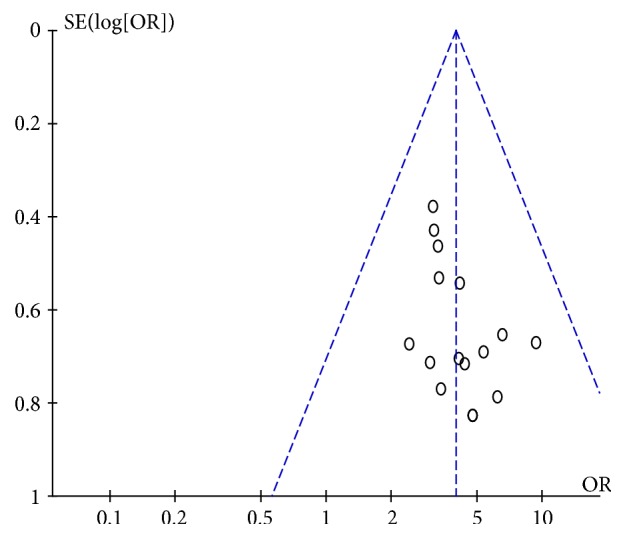
Publication bias analysis chart (4 weeks).
Figure 9.
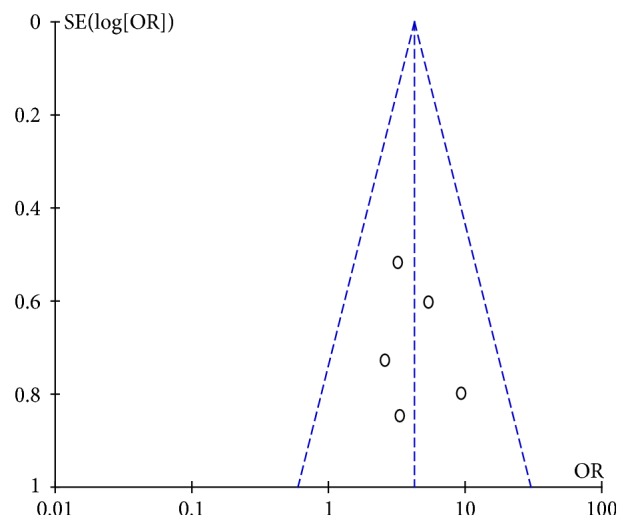
Publication bias analysis chart (8 weeks).
4. Discussion
Elderly UAP [40] is a common clinical cardiovascular disease (CVD); with the growing incidence of aging in China, the incidence of UAP in elderly patients increased year by year (nearly 300 million patients with CVD in China); increasing of the affected population has brought about huge harm to people's health. According to the survey, two out of every five deaths are cardiovascular patients in China, and CVD has become the first killer [41]. The main pathogenesis is coronary atherosclerotic plaque instability, easy to cause coronary artery obstruction and spasm after rupture, making local myocardial ischemia and hypoxia; if the treatment is not timely, the patients are likely to be attacked by acute myocardial infarction. In the theory of traditional Chinese medicine, the UAP is defined as “thoracic obstruction” and “precordial pain with cold limbs” which is involved with inward invasion of pathogenic cold, endogenous impairment due to overstrain, body deficiency due to old age, blood stasis due to cold, and Qi stagnation. ISMN is commonly used clinically as traditional antiangina drugs; its main pharmacological effects are to relax the vascular smooth muscle, dilate coronary artery, increase coronary blood flow, reduce myocardial oxygen consumption, reduce cardiac load, delay myocardial remodeling, and effectively relieve angina in patients [42].
Chinese patent medicine plays an important part in the prevention, treatment, and first aid of CVD. The CDDP, also known as the “Dantonic Pill,” is a representative Chinese patent drug with the function of activating blood circulation and removing blood stasis [43]. Its main components are Salvia miltiorrhiza, Panax notoginseng extract, and Dipterocarpaceae, which were highly dispersed in excipients such as polyethylene glycol 2000 (PEG-2000) and PEG-4000. Since it was listed in 1994, the CDDP has accumulated more than 450 million person-times and has accumulated a wealth of valuable clinical experience. Benefiting from the highly dispersed state, the active ingredients are more easily absorbed through the intestinal mucosa; then the bioavailability is enhanced significantly. Meanwhile, disadvantages of traditional tablets such as stomach injury and mucosa irritation are overcome as well [44]. Modern pharmacology studies have confirmed CDDP with coronary artery expansion, protection of vascular endothelial cells, antiplatelet aggregation, antithrombosis, improvement of microcirculation and other effects, the CDDP chemical composition including water-soluble danshensu [10], salvianolic acid B [45], protocatechuic aldehyde [46], and so on. Water-soluble danshensu can reduce platelet aggregation with anticoagulant, lipid-lowering antagonism of calcium and inhibit fibroblast proliferation and secretion of the matrix and also acts as an anti-inflammatory agent by inhibiting the adhesion molecules on the cell surface. The main component of Panax notoginseng is total Panax notoginseng saponins [47], and Dipterocarpaceae is a dispersion in the form of a solid, produced by a special process. These chemical components have the characteristics of fast dissolution, uniform dispersion, and high purity. The CDDP can also directly act on mucosal cells of patients to reduce the drug's timeliness, increase the bioavailability, and reduce stomach discomfort. Chinese medicine combination can play an antioxidant role and effectively inhibit the activation of hepatic stellate cells, thus reducing the necrosis of liver cells. In addition, its bioavailability is high; it reduces and relieves neuropeptide dysfunction caused by hypoxia after traumatic brain injury and clears the blood stasis; it is important for antithrombotic formation and anticoagulation and for reducing epileptiform discharge; it improves plasma NO concentration, reduces brain damage and brain edema, promotes brain tissue repair, and improves convulsive threshold [7]. The safety of CDDP has been demonstrated by several experiments about acute toxicity, long-term toxicity, teratogenesis, and carcinogenesis [48].
Meta-analysis is a method that uses statistical methods to analyze and summarize the numerous research data that were collected. It is essentially an observational study, but it also follows the basic principles of scientific research, including related papers search, literature inclusion and exclusion criteria, extracting data information, statistical processing, reporting results, and other basic research processes. Compared with general analytical studies, meta-analysis processes the published data instead of analyzing the raw data of each observed object in the independent study [49].
In this study, a total of 21 RCTs were screened out for meta-analysis; the data were divided into treatment cycles of four weeks and eight weeks, with ISMN as a control group and the CDDP as a treatment group to evaluate the clinical efficacy. The meta-analysis' results confirmed that the clinical efficacy and ECG efficiency of the two groups were significantly different (P < 0.05), indicating that the CDDP is better than ISMN. In terms of safety, we found that few RCTs reported relevant adverse reactions, headaches, dizziness, and facial flushing symptoms that may occur in patients; the types of adverse reactions were systemic reactions, skin and accessory reactions, and nervous system reactions [2]. However, due to the small sample size and the treatment cycle, we cannot yet draw a clear conclusion, and this remains to be further explored.
The distribution of the clinical efficacy samples around the funnel was even and symmetrical, indicating that there was a low possibility of publication bias. However, eight weeks of clinical efficacy samples were unevenly distributed, mostly concentrated on the left side, and the symmetry was not very strong. Therefore, it is more likely to be biased, but it may also be due to the small number of samples.
5. Conclusion
In summary, according to the comparison of clinical efficacy, ECG efficiency, blood viscosity, and other indicators, it can basically be concluded that CDDP is superior to ISMN in the treatment of elderly patients with UAP, and its effect is rapid and effective, although adverse reactions are mainly headache, redness, dizziness, and other symptoms; in a small amount of research nausea symptoms will appear. Therefore, CDDP is more suitable for the treatment of UAP in the elderly. However, our findings must be handled with care because of the small sample size and low quality of clinic trials cited. Other rigorous and large-scale RCTs are needed to confirm these results.
Acknowledgments
This project was supported by the Key Research and Development Program of Shaanxi Province of China (no. 2017ZDXM-SF-008). The authors would like to acknowledge the Key Laboratory of Basic and New Drug Research of Traditional Chinese Medicine in Shaanxi province.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
Yulin Liang, Junbo Zou, and Xiaofei Zhang are coauthors.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Yulin Liang, Junbo Zou, and Xiaofei Zhang searched articles in electronic databases and wrote the manuscript. Yu Wang, Jia Tai, and Dongyan Guo analyzed the data. Yulin Liang, Chunli Cui, Jing Wang, and Jiangxue Cheng performed the data extraction. Yajun Shi designed the study and amended the paper. Yulin Liang, Junbo Zou, and Xiaofei Zhang contributed equally to this work.
References
- 1.Yang F., Zou J., Li X., et al. Chinese herbal injections for unstable angina pectoris. Medicine. 2018;97(12):p. e0142. doi: 10.1097/MD.0000000000010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan D., Wu J., Zhang X., Liu S., Zhang B. Sodium Tanshinone II A Sulfonate Injection as Adjuvant Treatment for Unstable Angina Pectoris: A Meta-Analysis of 17 Randomized Controlled Trials. Chinese Journal of Integrative Medicine. 2018;24(2):156–160. doi: 10.1007/s11655-017-2424-x. [DOI] [PubMed] [Google Scholar]
- 3.Arsenault B. J., Perrot N., Puri R. Therapeutic Agents Targeting Cardiometabolic Risk for Preventing and Treating Atherosclerotic Cardiovascular Diseases. Clinical Pharmacology & Therapeutics. doi: 10.1002/cpt.1110. [DOI] [PubMed] [Google Scholar]
- 4.Hui J. K. Progress in the treatment of unstable angina. Chinese Geriatric Healthcare Journal. 2009;02:69–71. [Google Scholar]
- 5.Jensen J. M., Bøtker H. E., Mathiassen O. N., et al. Computed tomography derived fractional flow reserve testing in stable patients with typical angina pectoris: influence on downstream rate of invasive coronary angiography. European Heart Journal - Cardiovascular Imaging. 2018;19(4):405–414. doi: 10.1093/ehjci/jex068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou W., Yuan W.-F., Chen C., Wang S.-M., Liang S.-W. Study on material base and action mechanism of compound Danshen dripping pills for treatment of atherosclerosis based on modularity analysis. Journal of Ethnopharmacology. 2016;193:36–44. doi: 10.1016/j.jep.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Huang X., Kou G. J., Wang B. H. The clinical research progress of Compound Danshen Dripping Pill. Lishizhen Medicine and Materia Medica Research. 2016;05:1187–1190. [Google Scholar]
- 8.Zou H. M., Zhang B., Xu X. C., et al. Urinary metabolomic strategy to evaluate Compound Danshen Dripping Pills for myocardial ischaemia in rats. Journal of Pharmaceutical and Biomedical Analysis. 2015;112:98–105. doi: 10.1016/j.jpba.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 9.Lim C., Lim S., Lee B., Kim B., Cho S. Effect of methanol extract of Salviae miltiorrhizae Radix in high-fat diet-induced hyperlipidemic mice. Chinese Medicine. 2017;12(1) doi: 10.1186/s13020-017-0150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao H.-Y., Ding R.-L., Li M., et al. Danshensu, a major water-soluble component of Salvia miltiorrhiza, enhances the radioresponse for Lewis Lung Carcinoma xenografts in mice. Oncology Letters. 2017;13(2):605–612. doi: 10.3892/ol.2016.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue J. Z., Chen Y., Ma Z., Si X., Feng Y. Y. A meta-analysis of the efficacy of Compound Danshen Dripping Pills and Isosorbide Mononitrate in the treatment of angina pectoris of coronary heart disease. Chinese Traditional Patent Medicine. 2013;03:466–471. [Google Scholar]
- 12.Puente A., Hernández-Gea V., Graupera I., et al. Drugs plus ligation to prevent rebleeding in cirrhosis: An updated systematic review. Liver International. 2014;34(6):823–833. doi: 10.1111/liv.12452. [DOI] [PubMed] [Google Scholar]
- 13.Jia Y., Bao F., Huang F., Leung S.-W. Is tongxinluo more effective than isosorbide dinitrate in treating angina pectoris? A systematic review and meta-analysis of randomized controlled trials. The Journal of Alternative and Complementary Medicine. 2011;17(12):1109–1117. doi: 10.1089/acm.2010.0788. [DOI] [PubMed] [Google Scholar]
- 14.Kasama S., Toyama T., Hatori T., et al. Comparative effects of nicorandil with isosorbide mononitrate on cardiac sympathetic nerve activity and left ventricular function in patients with ischemic cardiomyopathy. American Heart Journal. 2005;150(3):477–e8. doi: 10.1016/j.ahj.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 15.Hasan S., Albayaty Y. N., Thierry B., Prestidge C. A., Thomas N. Mechanistic studies of the antibiofilm activity and synergy with antibiotics of isosorbide mononitrate. European Journal of Pharmaceutical Sciences. 2018;115:50–56. doi: 10.1016/j.ejps.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y. S. Treatment of angina pectoris by compound danshen concentrated pill and isosorbide mononitrate. Beijing Journal of Traditional Chinese Medicine. 2015;02:143–145. [Google Scholar]
- 17.Yao Y., Feng Y., Lin W. Systematic review and meta-analysis of randomized controlled trials comparing compound danshen dripping pills and isosorbide dinitrate in treating angina pectoris. International Journal of Cardiology. 2015;182(C):46–47. doi: 10.1016/j.ijcard.2014.12.112. [DOI] [PubMed] [Google Scholar]
- 18.Jia Y., Leung S.-W. How Efficacious is Danshen (Salvia miltiorrhiza) Dripping Pill in Treating Angina Pectoris? Evidence Assessment for Meta-Analysis of Randomized Controlled Trials. Journal of alternative and complementary medicine (New York, N.Y.) 2017;23(9):676–684. doi: 10.1089/acm.2017.0069. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J. J. The clinical efficacy and safety analysis of Isosorbide Mononitrate sustained-release tablets combined with Compound Danshen Dripping Pills in elderly patients with unstable angina pectoris. Women's Health Research. 2016;12 [Google Scholar]
- 20.Li X. The efficacy of Isosorbide Mononitrate sustained-release tablets combined with Compound Danshen Dripping Pills in the treatment of unstable angina pectoris in the elderly. Chinese Journal of Clinical Rational Drug Use. 2016;31:54–55. [Google Scholar]
- 21.Sheng Y., Liu Y. Clinical observation of elderly patients with unstable angina pectoris treated with Isosorbide Mononitrate sustained-release tablets combined with Compound Danshen Dripping Pills. China Rural Health. 2015;12:10–11. [Google Scholar]
- 22.Guo D. Z. Analysis of the effects of Compound Danshen Dripping Pills and Isosorbide Mononitrate Injection in the treatment of unstable angina. Chinese Modern Doctor. 2013;04:66–68. [Google Scholar]
- 23.Ma H. Y. Clinical analysis of unstable angina pectoris in elderly patients treated with Isosorbide Mononitrate sustained-release tablets combined with Compound Danshen Dripping Pills. Journal of Yangtze University (Nat Sci) Medicine. 2012;9(11) [Google Scholar]
- 24.Geng Y., Ma Y. M. Clinical observation of Compound Danshen Dripping Pill in treating unstable angina pectoris. Clinical Journal of Chinese Medicine. 2015;32:p. 84. [Google Scholar]
- 25.Ren Z. X. Efficacy of Compound Danshen Dripping Pills for unstable angina pectoris. The Journal of Medical Theory. 2013;19:2560–2561. [Google Scholar]
- 26.Shi L., Xu H. J. The Analysis of Curative Effect and Safety of the Combined Therapy of Isosorbide Mononitrate Sustained-release Tablets and Compound Danshen Dripping Pills To Treat the Elderly Patients with Unstable Angina Pectoris. Chinese Journal of Medicinal Guide. 2013;2013(10):p. 1662. [Google Scholar]
- 27.Su S. C. Clinical study on the treatment of elderly patients with unstable angina using Isosorbide Mononitrate and Compound Danshen Dripping Pills. Journal of Medical Informatics. 2015;28(20) [Google Scholar]
- 28.Wang X. Q. Clinical efficacy and safety analysis of Isosorbide Mononitrate sustained-release tablets combined with Compound Danshen Dripping Pills in elderly patients with unstable angina pectoris. Chinese Journal of Integrated Traditional and Western Medicine. 2016;6:50–51. [Google Scholar]
- 29.Zhang G. L. Observation on the curative effect of Compound Danshen Dripping Pills for unstable angina pectoris. Nei Mongol Journal of Traditional Chinese Medicine. 2014;18:p. 31. [Google Scholar]
- 30.Ma X. M. Isosorbide mononitrate sustained-release tablets combined with Compound Danshen Dripping Pills for elderly patients with unstable angina. The Medical Forum. 2014;23:3130–3131. [Google Scholar]
- 31.Zeng S. Z. Study on the Clinical Efficacy of Isosorbide Mononitrate Sustained Release Tablets and Compound Danshen Dripping Pills on Unstable Angina in Elder Patients. 2014;4 [Google Scholar]
- 32.He J. Efficacy of Compound Danshen Dripping Pills and Isosorbide Mononitrate in the Treatment of Unstable Angina Pectoris. Seek Medical And Ask The Medicine. 2011;10 [Google Scholar]
- 33.Gao X. F., Zheng G. Clinical efficacy of Compound Danshen Dripping Pills for unstable angina pectoris. Seek Medical And Ask The Medicine. 2013;7:294–295. [Google Scholar]
- 34.Yang X. M. Clinical analysis of Compound Danshen tablets combined with Isosorbide Mononitrate sustained-release tablets in the treatment of senile patients with unstable angina pectoris. Clinical Journal of Chinese Medicine. 2015;16:121–122. [Google Scholar]
- 35.Shao J. K. Clinical efficacy and safety analysis of isosorbide mononitrate sustained-release tablets combined with Compound Danshen Dripping Pills for elderly patients with unstable angina. Guide of China Medicine. 2013;12 [Google Scholar]
- 36.Liu L. F. Compound Danshen Dripping Pills and Lunan Xinkang in the treatment of unstable angina pectoris. Proceedings of the 3rd National Compound Danshen Dripping Pill Conference; 2005; Tianjin. p. p. 231. [Google Scholar]
- 37.Li M. Y. Efficacy of Compound Danshen Dripping Pills and Isosorbide Mononitrate Injection in the Treatment of Unstable Angina Pectoris. China Practical Medicine. 2012;16 [Google Scholar]
- 38.Jing H. Y. Clinical analysis of elderly patients with unstable angina treated with Isosorbide Mononitrate combined with Compound Danshen Dripping Pills. Health World. 2014;18:386–387. [Google Scholar]
- 39.Chen H. H. Efficacy of Isosorbide Mononitrate combined with Danshen Dripping Pills in the treatment of unstable angina pectoris. China Practical Medicine. 2012;2:185–186. [Google Scholar]
- 40.Sakaguchi M., Ehara S., Hasegawa T., et al. Coronary plaque rupture with subsequent thrombosis typifies the culprit lesion of non-ST-segment-elevation myocardial infarction, not unstable angina: non-ST-segment-elevation acute coronary syndrome study. Heart and Vessels. 2017;32(3):241–251. doi: 10.1007/s00380-016-0862-6. [DOI] [PubMed] [Google Scholar]
- 41.Cheng B., Li X. Y., Liu K. Q., Wang L., Wu W. P., Xu H. Clinical application of Compound Danshen Dripping Pills recommended by Chinese experts. Chinese Journal of Integrated Traditional and Western Medicine. Chinese Journal of Integrated Traditional. 2017;01:17–22. [Google Scholar]
- 42.Zhang X. Q., Wu T. L., Fang X. M. Clinical analysis of isosorbide mononitrate sustained-release tablets combined with Danshen Dripping Pills in elderly patients with unstable angina pectoris. Guide of China Medicine. 2017 [Google Scholar]
- 43.Luo J., Song W., Yang G., Xu H., Chen K. Compound Danshen (Salvia miltiorrhiza) dripping pill for coronary heart disease: an overview of systematic reviews. American Journal of Chinese Medicine. 2015;43(1):25–43. doi: 10.1142/s0192415x15500020. [DOI] [PubMed] [Google Scholar]
- 44.Xiao L. Advances in research on pharmacological effects and clinical application of Compound Danshen Dripping Pills. World Chinese Medicine. 2015;07:p. 1117. [Google Scholar]
- 45.Xia Z., Yuan Y., Zhang Q., Li H., Dai J., Min J. Salvianolic Acid B Suppresses Inflammatory Mediator Levels by Downregulating NF-κB in a Rat Model of Rheumatoid Arthritis. Medical Science Monitor. 2018;24:2524–2532. doi: 10.12659/MSM.907084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao L., Wu W., Dong L., et al. Protocatechuic Aldehyde Attenuates Cisplatin-Induced Acute Kidney Injury by Suppressing Nox-Mediated Oxidative Stress and Renal Inflammation. Frontiers in Pharmacology. 2016;7 doi: 10.3389/fphar.2016.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian Z., Pang H., Zhang Q., et al. Effect of aspirin on the pharmacokinetics and absorption of panax notoginseng saponins. Journal of Chromatography B. 2018;1074-1075:25–33. doi: 10.1016/j.jchromb.2017.12.033. [DOI] [PubMed] [Google Scholar]
- 48.Yuan R. Y., Li G. P. Multitarget effects of Compound Danshen Dripping Pills in the prevention and treatment of cardiovascular diseases. Chinese Community Doctors. 2010;13(17) [Google Scholar]
- 49.Wakai A., McCabe A., Kidney R., et al. Nitrates for acute heart failure syndromes. Cochrane Database of Systematic Reviews. 2013;8:p. CD005151. doi: 10.1002/14651858.CD005151.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.