Abstract
Background
This research assessed the in vitro antidiabetic activity and phytochemical constituents of the traditionally used medicinal plants, Psiadia punctulata and Meriandra bengalensis.
Method
The leaves of both plants were subjected to cold extraction method using 70% ethanol and hot Soxhlet extraction using n-hexane, chloroform, methanol, and distilled water. The extracts were studied for their effect on glucose transport across yeast cells and inhibition of α-amylase and α-glucosidase enzyme activities. Thin-layer chromatographic analysis of ethanol extract was also undertaken.
Results
The results of yeast glucose uptake assay revealed that extracts from both plants had a maximum increase in glucose uptake at the 25mM glucose concentration with a maximum dose of 2000μg/ml plant extract. The ethanol extract of P. punctulata and aqueous extract of M. bengalensis showed a high activity of 68% and 96%, respectively, at 25mM and 2000μg/ml of glucose and extract concentration. P. punctulata exerted peak inhibition activity of α-amylase of 37.5 ± 3% mg/dl (IC50 = 0.523 mg/dl) for methanol and distilled water extract at 0.5 mg/dl, respectively. M. bengalensis methanol extract exhibited the highest inhibition activity of 38 ± 8 % mg/dl (IC50 = 0.543 mg/dl) at 0.5 mg/dl. In the α-glucosidase inhibition assay, the methanolic extract of P. punctulata exhibited the highest inhibitory activity of 17.29 ± 9% mg/dl (IC50 = 0.761 mg/dl) at 0.5mg/dl. The chloroform extract of M. bengalensis had the highest inhibitory activity of 30 ± 5% mg/dl (IC50 = 0.6mg/dl) at 0.5 mg/dL. Phytochemical analysis of the different extracts of P. punctulata and M. bengalensis revealed the presence of flavonoids, alkaloids, tannins, saponins, phytosterols, and carbohydrates. Thin-layer chromatography analysis of ethanolic extract of both plants indicated presence of 15 and 17 spots for P. punctulata and M. bengalensis respectively.
Conclusion
P. punctulata and M. bengalensis extracts have moderate inhibitory activity against pancreatic α-amylase and relatively low inhibitory activities against α-glucosidase. The observed effects may be associated with the presence of flavonoids, saponins, and alkaloids. Additional in vivo analysis, toxicological studies, isolation, and structural characterization of the phytomolecules identified in this study and molecular docking studies should be undertaken.
1. Introduction
Diabetes Mellitus (DM) is a complex metabolic disorder characterized by abnormal secretion and/or activity of insulin. Typical abnormalities of the disease include alterations of carbohydrate, protein, and lipid metabolism which manifest in a variety of complications including chronic hyperglycaemia, weight loss, polydipsia, polyuria, lethargy, and several macrovascular and microvascular complications [1]
At present, Diabetes Mellitus figures among the top ten killers worldwide. According to the International Diabetes Federation (IDF), the disease affected 285 million people in 2010, a figure projected to increase to 439 million by 2030 [2, 3]. The increasing prevalence presents a challenge because most of the pharmacotherapeutics/medicines indicated for the disease are not cures. In addition, allopathic drugs are costly, have limited tolerability, and have a range of adverse effects such as hypoglycemia and weight gain with sulfonylureas, potential liver toxicity with thiazolidinediones, and skin rash with insulin injection [4].
These shortcomings have encouraged a search for alternatives with herbal formulations gaining prominence, a fact underscored by a 1980 recommendation by the World Health Organization (WHO) on the need for scientific research on traditional herbal medicines [5]. To date, ethnobotanical studies of traditional herbal remedies with antidiabetic activity have identified more than 1200 plant species [6]. Documented activities of phytocompounds include stimulation of insulin release from pancreatic ß-cells, inhibition of the activity of glucose absorption in the gut, modulation of glucose, and reduction of end-stage glycation products, among others [7–9].
The fact that ethnopharmacology can be leveraged to direct and optimize the search for plant derived antidiabetics is evidenced further by the fact that Metformin, a first-line antidiabetic drug, was developed from a biguanide synthesized from Galega officinalis (French lilac) [10]. In addition, multiple studies have demonstrated that specific phytocompounds have comparable activity to α-amylase and α-glucosidase inhibitors such as Acarbose, Voglibose, and Miglitol [8–10].
In this study, we assessed the in vitro antidiabetic activity, specifically inhibition of α-amylase and α-glucosidase, of the plants Psiadia punctulata (DC) Vatke (family: Asteraceae) (locally named as Tsehay ferhet) and Meriandra bengalensis (Koenig ex Roxb.) Benth (family: Lamiaceae) (locally named as Mezaguf /nhba). Psiadia punctulata is mostly found in some east African countries including Eritrea, Saudi Arabia, and North East India. The plant is used in Eritrea primarily to treat ‘Gonfi' (febrile disease) but recently its antidiabetic effect has been reported in some literature [11]. In Eritrean pharmacopeia, Meriandra bengalensis (M. bengalensis) is indicated for hypertension, infection, hepatitis, malaria, and diabetes [12]. The plant is mostly found in the Southern region around Degerra Valley, near Segenaiti in Eritrea. Qualitative analysis of phytocompounds was also undertaken.
2. Materials and Methods
2.1. Reagents and Chemicals
The following chemicals were used in the study: chloroform (Blulux laboratories Product No: CO1120, Batch No: 35563), n-hexane (BDH laboratory Supplies Lot I 957476036, Prod 284884U), methanol (VWR International Ltd., Lot K36105570 626, Prod 101585A), ethanol (El Nasr Pharmaceutical Chemicals Co., E0058111), distilled water (National Health Laboratory, Asmara, Eritrea), DMSO (BDH Lab Supplies, Prod 282164K, Lot K31165384-289), α-amylase, α-glucosidase, and 2-deoxy-d-glucose (2-DG) (Sigma Aldrich Chemical Co., USA). Unless stated otherwise, all the chemicals were obtained from Sigma Aldrich.
2.2. Collection and Preparation of Plant Extracts
The selected plants P. punctulata and M. bengalensis were collected from the outskirts of Asmara on the way to Flfl-Solomuna, 40 Km North of Asmara. All the samples were authenticated by a botanist from the Eritrean Institute of Technology (EIT). All plant samples were deposited in the Department of Clinical Laboratory Sciences, (CLS) Asmara College of Health Sciences (ACHS). The leaves of both plants were shade-dried and powdered mechanically with a pestle and mortar.
Hot extraction method was carried out using hot continuous Soxhlet apparatus involving increasing polarity of solvents namely n-hexane, chloroform, methanol, and distilled water. The cold extraction used 70% ethanol.
The solvents were removed under reduced pressure and controlled temperature by rotary evaporator (Heidolph).The extracts were dried and stored in a clean glass bottle and kept at 4-6°C for further use of in vitro antidiabetic assays.
2.3. Antidiabetic Activity
The different plant extracts were tested for their effects on glucose uptake by yeast cells and their inhibitory effect against digestive enzymes (α-amylase and α-glucosidase).
2.4. Glucose Uptake in Yeast Cells
A 10% (v/v) suspension was prepared in distilled water after repeated washing of commercial baker's yeast. Into 1ml glucose solution different concentrations of plant extract were added and incubated together at 37 degrees Celsius. After 10 min of incubation to initiate the reaction, a yeast suspension was added; this was mixed by vortexing. The reaction mixture was further incubated for 60 minutes at 37 degrees Celsius. Then, the tubes were centrifuged and glucose was estimated in the supernatant spectrophotometrically. Metronidazole was taken as standard drug. The percentage increase in glucose uptake by yeast cells was calculated using the following formula:
| (1) |
where Abccontrol is the absorbance of the control reaction (containing all reagents except the test sample) and Abssample is the absorbance of the test sample [13, 14].
2.5. Inhibition of α-Amylase Enzyme
This assay was carried out using a modified procedure of McCue and Shetty [15]. The plant extract was placed in a test tube and 0.02M sodium phosphate buffer (pH 6.9) containing α-amylase solution was added. This solution was preincubated at 25 degrees Celsius for 10min; later 1% starch solution in 0.02M sodium phosphate buffer (pH 6.9) was added and then further incubated at 25 degrees Celsius for 10min. The reaction was terminated by adding 2 ml, dinitrosalicylic acid (DNSA) reagent (40 mM, K+-Na+ tartrate 1 M, NaOH 0.4 M). The test tubes were then incubated in boiling water for 5 min and cooled to room temperature. The reaction mixture was diluted with distilled water and the absorbance was measured at 25 degrees Celsius at 540 nm using a spectrophotometer.
A control was prepared using the same procedure replacing the plant extract with distilled water. The α-amylase inhibitory activity was calculated as percentage inhibition:
| (2) |
where Abccontrol is the absorbance of the control and Abssample is the absorbance of the sample
2.6. Inhibition of α-Glucosidase Enzyme
The inhibitory activity was determined by incubating a solution of maltose substrate with Tris buffer pH 8.0 and various concentrations of plant extracts at 35 degrees Celsius. The reaction was initiated by adding α-glucosidase enzyme into the reaction mixture followed by incubation at 35 degrees Celsius. Then the reaction was determined by the addition of a colorimetric reagent (DNSA). A control was prepared using the same procedure replacing the plant extract with distilled water. The intensity of the color was measured at 540nm. Percentage inhibition (I %) was calculated by
| (3) |
where Abccontrol is the absorbance of the control and Abssample is the absorbance of the sample.
2.7. Phytochemical Analysis and Thin-Layer Chromatography (TLC)
Qualitative phytochemical analysis of n-hexane, chloroform, methanol, distilled water, and ethanolic extracts was undertaken according to the method described by Prashant Tiwari and colleagues [16]. Because ethanol showed a comparatively higher percentage yield, thin-layer chromatography (TLC) was used as a qualitative means of separating the various compounds present in the plant extract.
2.8. Preparation of the TLC Plates
TLC plates of thickness 0.25mm were placed in an oven at 100 degrees Celsius for 30 minutes to activate the silica gel. The plates were taken from the oven and kept at room temperature for 15 minutes. The dried filtrate, obtained from both plants, was dissolved in ethanol to a ratio of 1mg/ml using a calibrated microcapillary tube; a small drop of the ethanol extract of the plants was placed on the TLC plate, 3 cm (above the bottom). This spot was allowed to dry and the TLC plate was placed into the TLC chamber which was saturated with chloroform solvent carefully to have uniform solvent level. When the solvent reached 2 cm below the top, the plates were taken out of the chamber and detected with UV spectrophotometry. Rf values of the spots were calculated by:
| (4) |
3. Data Analysis
All analysis was undertaken in triplicate and each experiment was repeated three times. Quantitative values were presented as means ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to evaluate the statistical differences between different inhibitory concentrations followed by Tukey HSD post hoc test. Probit regression modelling was used to estimate the inhibitory concentration 50 (IC50). Statistical analysis was performed using statistical package for social sciences (SPSS), version 20.0 software, and Microsoft 2007) and SPSS version 16.0 for Windows 7. Differences at P < 0.05 were considered significant.
4. Result
4.1. Percentage Yield
The percentage yields of crude extracts are given in Table 1. Both plants M. bengalensis and P. punctulata extracts showed highest yield in their cold extracts with a yield of 23.7% and 22%, respectively.
Table 1.
Percentage yield in hot and cold extraction for different extracting solvents.
| Materials | Hot extraction | Cold extraction | |||
|---|---|---|---|---|---|
| Extracting solvents | |||||
| n-Hexane | Chloroform | Methanol | Dist.H 2 O | Ethanol | |
| Weight of dried and powdered plant leaves |
30gm %w/w |
30gm %w/w |
30gm %w/w |
30gm %w/w |
50gm %w/w |
|
| |||||
| P. punctulata | 10.4% | 13.3% | 15.9% | 20.8% | 22.00% |
|
| |||||
| M. bengalensis | 9.6% | 12.5% | 16.1% | 21.2% | 23.7% |
4.2. Glucose Uptake in Yeast Cells
The current research tried to assess the in vitro antidiabetic potential of two medicinal plants used in Eritrea for treating Diabetes mellitus.
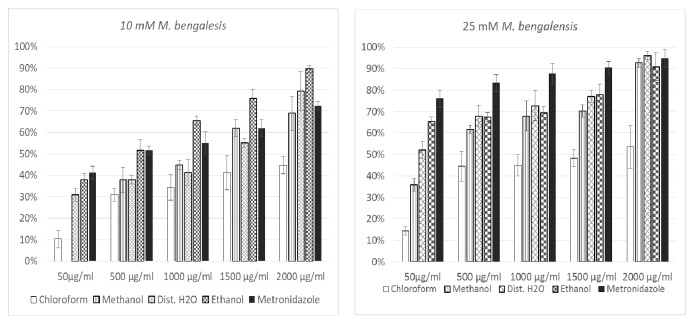
The selected plant extracts were studied for their effect on glucose uptake in yeast cells using different concentrations of glucose solutions. Yeast cells were incubated in glucose solution containing different concentrations of the two plants extracts and the glucose uptake was assessed. The different solvent extracts of both plants showed an increased activity of glucose uptake in yeast cells. As shown in Figure 1, the glucose uptake in yeast cells increased significantly with an increase in glucose concentration (50 μg/ml, 500 μg/ml, 1000 μg/ml, 1500 μg/ml, and 2000 μg/ml) and plant extract concentration (10 mM and 25 mM).
Figure 1.
Glucose uptake in different concentration of plants extracts.
The crude extracts for P. punctulata, however, showed almost no activity at 5 mM of glucose concentration. The n-hexane and chloroform extracts did not show any activity across all glucose concentrations measured.
The plant M. bengalensis exhibited an increased glucose uptake activity for the five different extracts at the four different glucose concentrations measured (Figure 2).
Figure 2.
Glucose uptake in different concentration of plants extracts.
At 25 mM of glucose concentration, all the four different plant extracts exhibited an increased activity ranging from 14.35 to 96.17% with the different doses of plant extracts ranging from (50 μg/ml, 500 μg/ml, 1000 μg/ml, 1500 μg/ml, and 2000 μg/ml). The highest activity at 25mM of glucose concentration was recorded for the aqueous extract which was 96.17% at a maximum dose of 2000μg/ml.
4.3. Inhibition of α-Amylase
The in vitroα-amylase inhibitory activities of the five solvent extracts of P. punctulata and M. bengalensis were assayed. The results indicated that at the lowest concentrations of 0.1 mg/dl and 0.2mg/dl there was no inhibition activity (Figure 3).
Figure 3.

Inhibitory potency of P. punctulata extracts against α-amylase activity. The values are expressed as means ± SD., n=3. α and β compare the effect of different concentrations of a particular extraction solvent on α-amylase inhibitory activity and subsequent bars designated by different letters signify significant different inhibitory activity p < 0.05 (Tukey HSD test).
The magnitude of inhibition depended on the nature of the extract and concentration used with group comparisons including negative control (NC) exhibiting a value < 0.05 for all extracts. The methanol and distilled water extracts of P. punctulata exerted peak inhibition activity of α-amylase of 37.5 ± 3% and 37.5 ± 5% for methanol and distilled water extract at 0.5 mg/dl, respectively. The concentrations at 0.4 mg/ml and 0.5 mg/ml differed significantly for both methanol (p < 0.023) and distilled water (p < 0.004) extracts. A lower inhibition rate of 16 ± 3% was observed for the same enzyme for ethanol extract. The IC50 values of methanol and distilled water extracts were 0.523 mg/dl and 0.543 mg/dl, respectively. (Table 2).
Table 2.
Estimated inhibitory concentration 50 (IC50) values for α-glucosidase and amylase inhibition for Psiadia punctulata and Meriandra bengalensis.
| Analyte | Inhibitory Concentration, IC50 ( µ g/ml) | |||
|---|---|---|---|---|
| Psiadia punctulata | Meriandra bengalensis | |||
| α-glucosidase | α-amylase | α-glucosidase | α-amylase | |
| Chloroform | 0.874 | - | 0.6 | - |
|
| ||||
| Methanol | 0.761 | 0.523 | - | 0.543 |
|
| ||||
| Distilled H 2 O | - | 0.543 | - | 0.572 |
|
| ||||
| Ethanol | - | - | - | 0.599 |
Inhibition of α-amylase by M. bengalensis extract increased with concentration and depended on the nature of the extract. Methanol extract exhibited the highest inhibition activity of 38 ± 4 % at 0.5 mg/dl. Ethanol and distilled water extracts demonstrated highest inhibition activities of 25 ± 2 % and 19 ± 4 %, respectively, at 0.5 mg/dl.
The inhibition activities for the other concentrations of the extracts employed in the study are as shown in Figure 4. The inhibitory activity at 0.4 mg/dl versus 0.5 mg/dl for methanol and distilled water extracts differed significantly, p < 0.001 and p < 0.021, respectively, as shown in Figure 4. The IC50 for methanol, ethanol, and distilled water extracts was 0.543 mg/dl, 0.572 mg/dl, and 0.6 mg/dl, respectively (Table 2).
Figure 4.
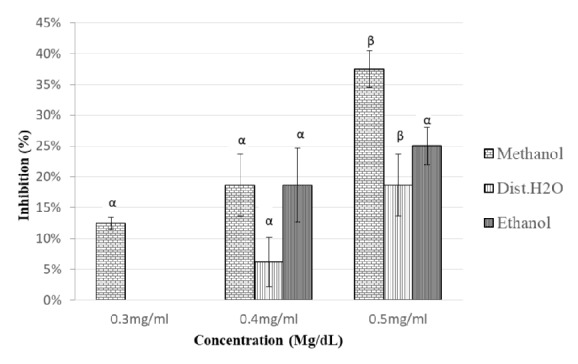
Inhibitory potency of M. bengalensis extracts against α-amylase activity. The values are expressed as means ± SD., n=3. α and β compare the effect of different concentrations of a particular extraction solvent on α-amylase inhibitory activity and subsequent bars designated by different letters signify significant different inhibitory activity p < 0.05 (Tukey HSD test).
4.4. Inhibition of α-Glucosidase
In the current research, the different extracts of both plants were able to demonstrate some inhibitory activity against α-glucosidase when compared to NC. In the P. punctulata assay; the methanolic extract exhibited the highest inhibitory activity of 17.29 ± 9% at 0.5mg/dl. The chloroform extract had an inhibitory activity of 16% ± 5%. The inhibitory activity differed significantly at concentration 0.3 mg/ml and 0.4 mg/ml for chloroform and methanol extracts, p < 0.014 and p < 0.021, respectively. The IC50 for methanol and chloroform extract was 0.761 mg/dl and 0.874 mg/dl, respectively. The other extracts had concentrations less than 10% (Figure 5).
Figure 5.
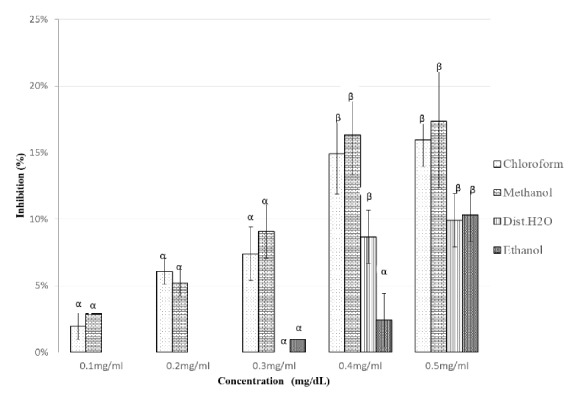
Inhibitory potency of P. Punctulata extracts against α-amylase activity. The values are expressed as means ± SD., n=3. α and β compare the effect of different concentrations of a particular extraction solvent on α-amylase inhibitory activity and subsequent bars designated by different letters signify significant different inhibitory activity p < 0.05 (Tukey HSD test).
In the M. bengalensis assay, inhibitory activity against α-glucosidase increased significantly with increasing concentration (0.1 – 0.5 mg/dL) for the chloroform extract with highest inhibitory activity of 30 ± 5% mg/dl observed at 0.5 mg/dL. A significant difference in the inhibitory activity of various extracts was observed at disparate concentrations of chloroform extract (0.4 mg/dl and 0.5 mg/dl, p < 0.001). An inhibitory activity of 13 ± 2% was observed for the distilled water extract (Figure 6). The IC50 for the chloroform extract was 0.599 mg/dl.
Figure 6.
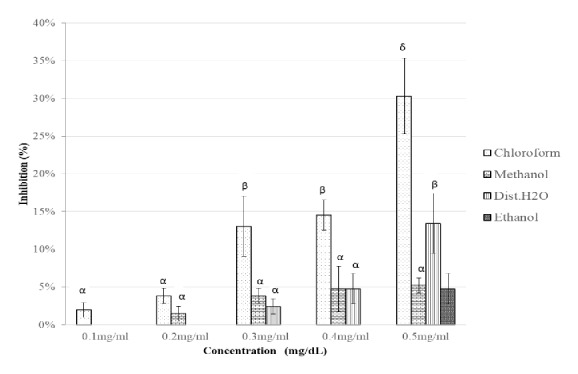
Inhibitory potency of M. bengalensis extracts against α-amylase activity. The values are expressed as means ± SD., n=3. α, β, and δ compare the effect of different concentrations of a particular extraction solvent on α-amylase inhibitory activity and subsequent bars designated by different letters signify significant different inhibitory activity p < 0.05 (Tukey HSD test).
Both plants showed a weaker α-glucosidase inhibition compared to the results reported for inhibition of α-amylase enzyme activity.
4.5. Phytochemical Analysis
Preliminary phytochemical screening on the five different solvent extracts of P. punctulata and M. bengalensis demonstrated presence of different chemical entities. The aqueous extract of P. punctulata showed presence of maximum compounds like alkaloids, tannins, saponins, flavonoids, and carbohydrates. For the plant M. bengalensis, the highest number of chemical entities such as alkaloids, tannins, saponins, flavonoids, carbohydrates, and phytosterols were detected for the methanolic, aqueous, and ethanolic extracts (Table 3).
Table 3.
Results of the qualitative analysis of the presence of specific phytoconstituents in difference extracting solvents.
| Phytomolecules | Method | Extracting solvents | ||||
|---|---|---|---|---|---|---|
| n-hexane | Chloroform | Methanol | Dist.H 2 O | Ethanol | ||
| Psiadia punctulata | ||||||
|
| ||||||
| Alkaloids | Wagner's test | - | - | + | + | + |
|
| ||||||
| Tannins | Ferric chloride test | - | - | + | + | + |
|
| ||||||
| Saponins | Foam test | - | - | - | + | + |
|
| ||||||
| Flavonoids | Alkaline reagent test | - | + | + | + | + |
|
| ||||||
| Phytosterols | Modified Liebermann-Burchard's test | + | - | - | - | - |
|
| ||||||
| Meriandra bengalensis | ||||||
|
| ||||||
| Alkaloids | Wagner's test | + | + | + | + | + |
|
| ||||||
| Tannins | Ferric chloride test | - | - | + | + | + |
|
| ||||||
| Saponins | Foam test | - | - | - | + | + |
|
| ||||||
| Flavonoids | Alkaline reagent test | - | + | + | + | + |
|
| ||||||
| Phytosterols | Modified Liebermann-Burchard's test | + | + | + | - | - |
(+) sign denotes the presence of corresponding phytoconstituents and (-) denotes the absence of corresponding phytoconstituents.
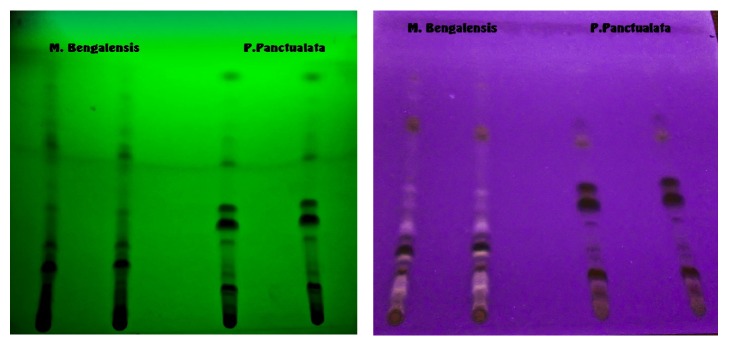
Thin-layer chromatography (TLC) was performed on methanolic extract of both plants to assist in tracking the unknown phytoconstituents. The results indicated presence of 15 and 17 spots for P. punctulata and M. bengalensis, respectively, indicating the presence of a number of phytoconstituents (Figure 7).
Figure 7.
TLC reading at 254 and 336 nm of the plants M. bengalensis and P. punctulata.
5. Discussion
Multiple strategies have been devised or explored in the management of DM. Stimulation of Adenosine monophosphate-dependent protein kinase (AMPK) (Biguanides-Metformin); blockage of ATP-gated K+ channels in β cells (Sulfonylureas-Glipizide); stimulation of peroxisome proliferator–activated receptors activities (PPAR ϒ) (Thiazolidinediones-Rosiglitazone); and glucagon-like peptide-1 (GLP-1) (Exenatide-Byetta) modulation [17, 18]. The agents are directed at either enhancing insulin secretion, insulin sensitivity, or reducing glucose production by the liver.
Another important approach is directed at the management of post-prandial hyperglycemia (PPH) by inhibiting the activity of α-amylase (cleavage of 1, 4-α-D-glucosidic linkages in polysaccharides) and α-glucosidase (terminal-hydrolysis of 1, 4-α-D-glucosidic linkages in oligosaccharides). Reducing PPH is important given the fact that it can help in reducing advanced glycation end-products (AGEs) formation, a metabolite which has been identified as a major risk factor for cardiovascular complications in DM patients [19]. In particular, it has been suggested that pharmacotherapeutical inhibition of the activity of α-amylase and α-glucosidase may be beneficial to DM patients with impaired insulinotropic response, especially when used in combination with other oral hypoglycemic agents (OHA) [20]. Their potential in limiting weight gain or enhancing weight loss even in nondiabetic patients has also been proposed [21].
In the recent past, a lot of attention has been directed at elucidating the mechanism of action and phytochemistry of herbal extracts indicated for DM in traditional pharmacopeia [22]. Focus on the inhibitory activity of phytochemicals on α-amylase and α-glucosidase has particularly been popular. Interest in identifying pharmacologically active phytoconstituents which can inhibit α-amylase and α-glucosidase is premised on the claim that they have fewer side effects and are less expensive compared to synthetic pharmacotherapeutics like Acarbose and Miglitol [21, 22]. In this regard, and in keeping with this quest, we investigated two plants in vitro, Psiadia punctulata and Meriandra bengalensis, used in Eritrean pharmacopeia for DM management.
Methanolic and distilled water extracts of P. punctulata exhibited significant inhibitory activity against α-amylase at the highest concentration (0.5 mg/dl) of the extract used. The IC50 estimates were 0523 mg/dl and 0.543 mg/dl, respectively. The observed inhibitory strengths are comparable to those of similar studies [23–25]. The inhibitory activity of P. punctulata extracts on α-glucosidase was not as potent. The chloroform and methanol extracts had comparatively low inhibitory activity 16% and 17.29% and high IC50 values, 0.874 mg/dl and 0.761 mg/dl. It should be emphasized that P. punctulata is a member of Asteraceae family and some studies have shown that extracts from plants in this family can inhibit the activity of carbohydrate hydrolysing enzymes [25].
Qualitative phytochemical analysis of the analytes obtained from multiple extraction solvents detected several phytomolecules including flavonoids, saponins, tannins, alkaloids, and phytosterols. The TLC chromatogram of the methanolic extracts indicated presence of 15 and 17 spots demonstrating the presence of multiple phytomolecules. The presence of flavonoids, especially in chloroform, ethanol, methanol, and distilled water extracts, may account for the inhibitory activity observed. Flavonoids, heterogeneous group of plant polyphenols, have widely reported inhibitory activity against α-amylase and α-glucosidase in both in vitro and in vivo and in silico modelling studies [9, 26–28]. Importantly, some investigators have reported that there is a positive relationship between total flavonoid and polyphenol content and the ability to inhibit α-amylase and α-glucosidase [29]. Antiatherogenic effects of flavonoids have also been reported [30, 31]. The additional inhibitory activity observed for methanol and distilled water extracts of P. punctulata may be associated with the presence of other phytoconstituents like alkaloids and saponins. Saponins have been associated with suppression of fluid and glucose uptake at the brush borders [20]. Other studies have demonstrated that some plant extracts can enhance insulin secretion and insulin signaling in adipose and skeletal muscles [32, 33].
The inhibitory activity of extracts of M. bengalensis was also evaluated. According to the results obtained, methanol, distilled water, and ethanol extracts had significant inhibitory activity against α-amylase at 0.5 mg/dl. This fact is also evidenced by the IC50 estimates obtained 0.543 mg/dl, 0.572 mg/dl, and 0.6 mg/dl, respectively. Qualitative phytochemical analysis of the extracts obtained by the use of these solvents detected several pharmacologically active phytoconstituents, including flavonoids, tannins, and saponins. Therefore, the observed inhibitory effect may be attributed to these compounds (29-30). Further, only the chloroform extract exhibited significant inhibitory activity against α-glucosidase.
6. Conclusion
This is the first study to evaluate the inhibitory effect of P. punctulata and M. bengalensis against specific carbohydrate hydrolysing enzymes. According to the results obtained, the extracts from P. punctulata and M. bengalensis demonstrated moderate inhibitory activity against pancreatic α-amylase and relatively low inhibitory activities against intestinal α-glucosidase. Therefore, the antidiabetic effects of the two plants may be associated with the observed inhibitory activity against the specified carbohydrate hydrolysing enzymes. In particular, specific phytocompounds, including flavonoids, saponins, and alkaloids detected in the crude extracts, may be responsible for the observed activity. In this regard, the observed results may justify the traditional use of these plants in the management of post-prandial hyperglycemia in Type 2 Diabetes Mellitus (T2DM). However, it should be noted that results obtained in vitro are not necessarily confirmable by in vivo tests in appropriate animal models or in randomized clinical studies. In this regard, additional in vivo studies are warranted. The need to address toxicological issues is also pertinent. Further, elucidation of the mode of inhibition, isolation, and structural characterization of the phytomolecules and quantitative structure activity relationships (QSAR) using in silico modelling and other platforms for structural analysis should be undertaken.
Acknowledgments
The authors would like to thank Dr. Artul Kaushik, Pharmacy Department, Asmara College of Health Science (ACHS), for the supervising the extraction process. Material support was obtained from Asmara College of Health Sciences Research Committee
Data Availability
Data will be available upon reasonable request to the corresponding author.
Ethical Approval
A formal letter of approval area was obtained from ACHS Research Ethical Committee and Ministry of Health.
Conflicts of Interest
The authors have no conflicts of interest to declare
References
- 1.Stratton I. M., Adler A. I., Neil H. A. W., et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. British Medical Journal. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw J. E., Sicree R. A., Zimmet P. Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Research and Clinical Practice. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation. IDF Atlas. 7th. 2016. http://www.diabetesatlas.org. [DOI] [PubMed] [Google Scholar]
- 4.Modak M., Dixit P., Londhe J., Ghaskadbi S., Devasagayam T. P. A. Recent advances in indian herbal drug research guest editor: Thomas paul asir devasagayam indian herbs and herbal drugs used for the treatment of diabetes. Journal of Clinical Biochemistry and Nutrition. 2007;40(3):163–173. doi: 10.3164/jcbn.40.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey C. J., Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care. 1989;12(8):553–564. doi: 10.2337/diacare.12.8.553. [DOI] [PubMed] [Google Scholar]
- 6.Patel D. K., Prasad S. K., Kumar R., Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pacific Journal of Tropical Biomedicine. 2012;2(4):320–330. doi: 10.1016/S2221-1691(12)60032-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poovitha S., Parani M. In vitro and in vivo α-amylase and α-glucosidase inhibiting activities of the protein extracts from two varieties of bitter gourd (Momordica charantia L.) BMC Complementary and Alternative Medicine. 2016;16(1):185. doi: 10.1186/s12906-016-1085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sudha P., Zinjarde S. S., Bhargava S. Y., Kumar A. R. Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complementary and Alternative Medicine. 2011;11(1):5. doi: 10.1186/1472-6882-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adisakwattana S., Ruengsamran T., Kampa P., Sompong W. In vitro inhibitory effects of plant-based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Complementary and Alternative Medicine. 2012;12, article 110:1–8. doi: 10.1186/1472-6882-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M., Huang X., Ye H., et al. Randomized, Double-Blinded, Double-Dummy, Active-Controlled, and Multiple-Dose Clinical Study Comparing the Efficacy and Safety of Mulberry Twig (Ramulus Mori, Sangzhi) Alkaloid Tablet and Acarbose in Individuals with Type 2 Diabetes Mellitus. Evidence-Based Complementary and Alternative Medicine. 2016;2016 doi: 10.1155/2016/7121356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadden D. R. Goat's rue - French lilac - Italian fitch - Spanish sainfoin: gallega officinalis and metformin: the Edinburgh connection. The Journal of the Royal College of Physicians of Edinburgh. 2005;35(3):258–260. [PubMed] [Google Scholar]
- 12.Demoz M., Gachoki K., Mungai K., Negusse B. Ethnobotanical survey and preliminary phytochemical studies of plants traditionally used for diabetes in eritrea. European Journal of Medicinal Plants. 2015;9(2):1–11. doi: 10.9734/ejmp/2015/18777. [DOI] [Google Scholar]
- 13.Nair S. S., Kavrekar V., Mishra A. Evaluation of in vitro antidiabetic activity of selected plant extracts. International Journal of Pharmaceutical Science Invention. 2013;2(4):12–19. [Google Scholar]
- 14.Anam K., Widharna R. M., Kusrini D. α-glucosidase inhibitor activity of Terminalia species. International Journal of Pharmacology. 2009;5(4):277–280. doi: 10.3923/ijp.2009.277.280. [DOI] [Google Scholar]
- 15.Kalidas S. P., McCue P. Inhibitory effects of rosmarinic acid extracts on porcine pancreatic amylase in vitro. Asia Pacific Journal of Clinical Nutrition. 2004;13(1):101–106. [PubMed] [Google Scholar]
- 16.Tiwari P., Kumar B., Kaur M., Kaur G., Kaur H. Phytochemical screening and extraction: a review. Internationale Pharmaceutica Sciencia. 2011;1(1):98–106. [Google Scholar]
- 17.Chaudhury A., Duvoor C., Reddy Dendi V. S., et al. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Frontiers in Endocrinology. 2017;8(6):1–12. doi: 10.3389/fendo.2017.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alhadramy M. S. Diabetes and oral therapies: A review of oral therapies for diabetes mellitus. Journal of Taibah University Medical Sciences. 2016;11(4):317–329. doi: 10.1016/j.jtumed.2016.02.001. [DOI] [Google Scholar]
- 19.Ceriello A., Davidson J., Hanefeld M., et al. Postprandial hyperglycaemia and cardiovascular complications of diabetes: an update. Nutrition, Metabolism & Cardiovascular Diseases. 2006;16(7):453–456. doi: 10.1016/j.numecd.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Mahomoodally M. F., Subratty A. H., Gurib-Fakim A., Choudhary M. I., Nahar Khan S. Traditional medicinal herbs and food plants have the potential to inhibit key carbohydrate hydrolyzing enzymes in vitro and reduce postprandial blood glucose peaks in vivo. The Scientific World Journal. 2012;2012 doi: 10.1100/2012/285284.285284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohamed E. A. H., Siddiqui M. J. A., Ang L. F., et al. Potent α-glucosidase and α-amylase inhibitory activities of standardized 50% ethanolic extracts and sinensetin from Orthosiphon stamineus Benth as anti-diabetic mechanism. BMC Complementary and Alternative Medicine. 2012;12, article 176 doi: 10.1186/1472-6882-12-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sudha P., Zinjarde S. S., Bhargava S. Y., Kumar A. R. Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. Evidence Based Complementary and Alternative Medicine. 2011;1(11):p. 5. doi: 10.1186/1472-6882-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olaokun O. O., McGaw L. J., Eloff J. N., Naidoo V. Evaluation of the inhibition of carbohydrate hydrolysing enzymes, antioxidant activity and polyphenolic content of extracts of ten African Ficus species (Moraceae) used traditionally to treat diabetes. Evidence Based Complementary and Alternative Medicine. 2013;13(1):2–12. doi: 10.1186/1472-6882-13-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picot C. M., Subratty A. H., Mahomoodally M. F. Inhibitory potential of five traditionally used native antidiabetic medicinal plants on α-amylase, α-glucosidase, glucose entrapment, and amylolysis kinetics in vitro. Advances in Pharmacological Sciences. 2014;2014:1–7. doi: 10.1155/2014/739834.739834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spínola V., Castilho P. C. Evaluation of Asteraceae herbal extracts in the management of diabetes and obesity. Contribution of caffeoylquinic acids on the inhibition of digestive enzymes activity and formation of advanced glycation end-products (in vitro) Phytochemistry. 2017;143:29–35. doi: 10.1016/j.phytochem.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Adefegha S. A., Oboh G. In vitro inhibition activity of polyphenol-rich extracts from Syzygium aromaticum (L.) Merr. & Perry (Clove) buds against carbohydrate hydrolyzing enzymes linked to type 2 diabetes and Fe2+-induced lipid peroxidation in rat pancreas. Asian Pacific Journal of Tropical Biomedicine. 2012;2(10):774–781. doi: 10.1016/S2221-1691(12)60228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kazeem M. I., Adamson J. O., Ogunwande I. A. Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of morinda lucida benth leaf. BioMed Research International. 2013;2013:6. doi: 10.1155/2013/527570.527570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toma A., Makonnen E., Mekonnen Y., Debella A., Addisakwattana S. Intestinal α-glucosidase and some pancreatic enzymes inhibitory effect of hydroalcholic extract of Moringa stenopetala leaves. BMC Complementary and Alternative Medicine. 2014;14(1):6. doi: 10.1186/1472-6882-14-180.527570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramkumar K. M., Thayumanavan B., Palvannan T., Rajaguru P. Inhibitory effect of Gymnema montanum leaves on α-glucosidase activity and α-amylase activity and their relationship withpolyphenolic content. Medicinal Chemistry Research. 2010;19(8):948–961. doi: 10.1007/s00044-009-9241-5. [DOI] [Google Scholar]
- 30.Shabrova E. V., Tarnopolsky O., Singh A. P., Plutzky J., Vorsa N., Quadro L. Insights into the molecular mechanisms of the anti-atherogenic actions of flavonoids in normal and obese mice. PLoS ONE. 2011;6(10) doi: 10.1371/journal.pone.0024634.e24634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamisoyama H., Honda K., Tominaga Y., Yokota S., Hasegawa S. Investigation of the anti-obesity action of licorice flavonoid oil in diet-induced obese rats. Bioscience, Biotechnology, and Biochemistry. 2008;72(12):3225–3231. doi: 10.1271/bbb.80469. [DOI] [PubMed] [Google Scholar]
- 32.Elyasiyan U., Nudel A., Skalka N., et al. Anti-diabetic activity of aerial parts of Sarcopoterium spinosum. BMC Complementary and Alternative Medicine. 2017;17(1):1–12. doi: 10.1186/s12906-017-1860-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed D., Kumar V., Sharma M., Verma A. Target guided isolation, in-vitro antidiabetic, antioxidant activity and molecular docking studies of some flavonoids from Albizzia Lebbeck Benth. bark. BMC Complementary and Alternative Medicine. 2014;14(1, article 155):2–12. doi: 10.1186/1472-6882-14-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available upon reasonable request to the corresponding author.