Abstract
Integrin-mediated cell adhesion to the extracellular matrix is required for normal cell growth. Cyclin D1 is a key regulator of G1-to-S phase progression of the cell cycle. Our previous studies have demonstrated that integrin signaling through focal adhesion kinase (FAK) plays a role in the regulation of cell cycle progression, which correlates with changes in the expression of cyclin D1 and the cdk inhibitor, p21, induced by FAK. In this report, we first investigated the roles of both cyclin D1 and p21 in the regulation of cell cycle progression by FAK. We found that overexpression of a dominant-negative FAK mutant ΔC14 suppressed cell cycle progression in p21−/− cells as effectively as in the control p21+/+ cells. Furthermore, we found that overexpression of ectopic cyclin D1 could rescue cell cycle inhibition by ΔC14. These results suggested that cyclin D1, but not p21, was the primary functional target of FAK signaling pathways in cell cycle regulation. We then investigated the mechanisms underlying the regulation of cyclin D1 expression by FAK signaling. Using Northern blotting and cyclin D1 promoter/luciferase assays, we showed that FAK signaling regulated cyclin D1 expression at the transcriptional level. Using a series of cyclin D1 promoter mutants in luciferase assays as well as electrophoretic mobility shift assays (EMSA), we showed that the EtsB binding site mediated cyclin D1 promoter regulation by FAK. Finally, we showed that FAK regulation of cyclin D1 depends on integrin-mediated cell adhesion and is likely through its activation of the Erk signaling pathway. Together, these studies demonstrate that transcriptional regulation of cyclin D1 by FAK signaling pathways contributes to the regulation of cell cycle progression in cell adhesion.
INTRODUCTION
Cell cycle transition from G1 to S phase is regulated by distinct cyclin-dependent kinases (cdks) that are regulated by various cyclins, cdk inhibitors, and phosphorylations. In mammalian cells, key cyclins involved in G1 to S transition include cyclin D, E, and A. Cyclin D assembles with cdk4/6 in early G1, cyclin E combines with cdk2 later in G1, and cyclin A associates with cdk2 at the beginning of S phase (Draetta, 1994; Sherr, 1995). The expression level of cyclin D1 has been shown to be rate-limiting in cellular proliferation induced by a variety of stimuli (Ohtsubo and Roberts, 1993; Quelle et al., 1993; Resnitzky et al., 1994; Albanese et al., 1995; Watanabe et al., 1996; Westwick et al., 1997, 1998; Joyce et al., 1999). Accumulation of the cyclin D1/cdk4/6 complex in early to mid-G1 phase leads to activation of the kinases that phosphorylate and inactivate the tumor suppressor Rb, which is necessary for cell cycle progression through the G1 to S phases. Consistent with its critical role in cell cycle progression, increased expression of cyclin D1 has been observed in several tumors (Wang et al., 1994b; Arber et al., 1996; Zhang et al., 1997; Lee et al., 1999). Likewise, ectopic overexpression of cyclin D1 in transgenic mice induced formation of tumors (Wang et al., 1994a; Nakagawa et al., 1997). The cyclin D1 null mice have shown remarkably decreased development of tumors (Robles et al., 1998). The cyclin D1 protein levels are largely controlled at the transcriptional level and by ubiquitin-mediated degradation (Diehl et al., 1997; Pestell et al., 1999). The Ras/Erk signaling cascade (Raf, MEK, and Erk) has been implicated to play an important role in the transcriptional activation of cyclin D1 gene in response to a variety of mitogenic stimuli. Recent studies have also identified that the β-catenin/LEF-1 signaling pathway can activate the cyclin D1 promoter directly through an LEF-1 responsive element (Shtutman et al., 1999; Tetsu and McCormick, 1999).
Recent studies have indicated that integrin-mediated cell adhesion controls cell cycle progression by regulating the expression and activities of cyclins, cdks, and cdk inhibitors (Assoian, 1997; Assoian and Schwartz, 2001). Cyclin D1 expression has been shown to be decreased upon cell detachment in several different cell types (Zhu et al., 1996; Day et al., 1997; Wu and Schonthal, 1997; Huang et al., 1998). Conversely, forced overexpression of cyclin D1 (Schulze et al., 1996; Zhu et al., 1996; Resnitzky, 1997) but not cyclin E (Resnitzky, 1997), induced anchorage-independent cell cycle progression of NIH3T3 and Rat1 cells. Although integrins are known to trigger a variety of intracellular signaling pathways during cell adhesion, relatively little is known at present about possible involvement of these signaling pathways in the regulation of cyclin D1 and cell cycle progression by integrins. However, recent studies by a number of laboratories have started to identify potential signaling pathways downstream of integrins that might be involved in this process. Roovers et al. (1999) have shown that integrin-mediated cell adhesion is required for sustained Erk activation and induction of cyclin D1 expression by growth factors. The integrin linked kinase (ILK) has also been shown to activate cyclin D1 gene transcription through the CreB binding site on the promoter, although it is not known whether this pathway is involved in the regulation of cyclin D1 by integrins (D'Amico et al., 2000).
Previous studies from our laboratory suggested that focal adhesion kinase (FAK) may play a role in the regulation of cell cycle progression by integrins (Zhao et al., 1998b). These studies indicated that FAK could regulate cell cycle progression by increasing cyclin D1 expression and/or decreasing the expression of the cdk inhibitor p21. In this report, we present data suggesting that cyclin D1, but not p21, was the primary functional target of FAK signaling pathways in cell cycle regulation. We also show that FAK signaling pathways regulated cyclin D1 gene transcription through the EtsB binding site in the cyclin D1 promoter. Taken together, these results imply that FAK may serve as another mediator of cyclin D1 regulation by integrins, which could contribute to cell cycle control in cell adhesion.
MATERIALS AND METHODS
Antibodies and Reagents
The mouse mAb 12CA5 (α-HA) has been described previously (Chen and Guan, 1994; Chen et al., 1995). The rabbit polyclonal α-HA antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The rabbit α-p21 and α-cyclin D1 were generous gifts from Dr. Y. Xiong (University of North Carolina, Chapel Hill, NC). The mouse mAb α-BrdU was purchased from Sigma (St. Louis, MO). Rabbit polyclonal anti-β-Gal was from 5 prime → 3 prime (Boulder, CO). Chemical inhibitors curcumin and PD98059, and the control SB202474 were from Calbiochem (San Diego, CA).
Cell Culture
Mouse p21+/+ and p21−/− fibroblasts were kind gifts from Dr. C. Deng (NIDDK, NIH) and were maintained in DMEM with 15% fetal bovine serum (FBS). HEK293T cells were maintained in DMEM with 10% FBS. NIH3T3 cells were grown in DMEM with 10% calf serum (CS). NIH3T3 cell clones with inducible expression of FAK or ΔC14 mutant were described previously (Zhao et al., 1998b).
5-Bromodeoxyuridine (BrdU) Incorporation Assay
The expression vector encoding FAK mutant ΔC14 (pKH3-ΔC14) was described previously (Zhao et al., 1998b). The p21+/+ and p21−/− fibroblasts were transiently transfected with pKH3-ΔC14 or the empty vector pKH3. Twenty-four hours after transfection, the cells were subjected to BrdU incorporation assays essentially as described previously (Zhao et al., 1998b), except that 0.5% FBS (instead of 0.5% CS) was used for serum starvation and that rabbit polyclonal α-HA antibodies (1:200) were used to identify positively transfected cells.
The expression vector encoding human cyclin D1 (pRK5-D1) was a generous gift from Dr. T. Hunter (Salk Institute, San Diego, CA). NIH3T3 cell clones with inducible expression of ΔC14 lines were transiently transfected with pRK5-D1 or the control vector pRK5 along with a plasmid encoding β-gal. Twenty-four hours after transfection, the cells were subjected to BrdU incorporation assays under induced or uninduced conditions, as described previously (Zhao et al., 1998b), with the modification that the rabbit polyclonal α-β-gal antibodies (1:200) were used to identify positively transfected cells.
Western Blotting
Cells were washed twice with ice-cold PBS and then lysed with modified radioimmunoprecipitation assay (RIPA) buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 10% glycerol, 1.5 mM MgCl2, 1 mM EGTA, 1 mM sodium vanadate, 10 mM sodium pyrophosphate, 10 mM NaF, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecylsulfate, 10 mg/ml leupeptin, 10 mg/ml aprotinin, and 1 mM PMSF). Lysates were cleared by centrifugation and total protein concentrations were determined using Bio-Rad Protein Assay (Hercules, CA). Aliquots (20 μg) of lysate proteins were mixed in SDS-PAGE sample buffer, boiled for 5 min, and resolved by SDS-PAGE. The blots were performed with α-p21 (1:1000), α-HA (1:1000), or α-cyclin D1 (1:5000), using the enhanced chemiluminescence (ECL) system (Amersham Life Science, Arlington Heights, IL), as described previously (Xing et al., 1994).
Northern Blotting
NIH3T3 cell clones with inducible expression of FAK, ΔC14 or the mock cells (Zhao et al., 1998b) were serum starved for 48 h in DMEM with 0.5% CS, 0.5 mg/ml G418, and 0.4 μg/ml tetracycline. They were then switched into DMEM plus 10% CS, 0.5 mg/ml G418 with or without 0.4 μg/ml tetracycline and incubated for 12 h. Total cellular RNAs were extracted using TRIzol Reagent (Life Technologies, Grand Island, NY) based on the manufacturer's instructions. Ten micrograms total RNAs were resolved on 1.2% formaldehyde/agarose gels, and then transferred onto nylon membrane (0.2-μm Maximum Strength Nytran; Schleicher & Schuell, Keene, NH). The membranes were probed with α-32P-labeled cyclin D1 or EF1α cDNA fragments. The membranes were subjected to autoradiography, and intensity of the hybridized bands was analyzed with ImageQuant IQMac v1.2 on a Storm 840 scanner (Molecular Dynamics, Sunnyvale, CA).
Luciferase Reporter Assays
The cyclin D1 promoter-luciferase reporter constructs −1745CD1 (−1745CD1LUC; D'Amico et al., 2000) and −962CD1 (Tetsu and McCormick, 1999) have been described previously. The mutant reporters with deletion of each putative transcription factor binding site were generous gifts of Drs. O. Tetsu and F. McCormick (UCSF, San Francisco, CA) and have been described previously (Tetsu and McCormick, 1999). The reporter constructs were cotransfected into NIH3T3 cells with expression vectors encoding FAK, its mutants ΔC14 and F397, FRNK, or the control pKH3 vector alone. Twenty-four hours after transfection, cells lysates were prepared and luciferase activity was determined using Luciferase Assay System with Reporter Lysis Buffer (Promega, Madison, WI) and liquid scintillation counter (Beckman Instruments, Fullerton, CA) according to the manufacturer's instructions. The plasmid pSV-LacZ was also included in the cotransfections and β-galactosidase assays were performed for lysates from each transfection to normalize transfection efficiencies. In some experiments, various concentrations of the chemical inhibitors Curcumin, PD98059, or the control SB202474 (Calbiochem) were added to the cells at 24 h after transfection. After additional incubation for 24 h, cell lysates were prepared and subjected to luciferase assay as described above.
Electrophoresis Mobility Shift Assay (EMSA)
The oligodeoxyribonucleotides used for EMSA are as follows: EtsB (Ets binding consensus site B), 5′-GACAAGATGAAGGAAATGCTGGCCA-3′; mEtsB (EtsB site with the consensus mutated as underlined), 5′-GACAAGATGAAAAGAATGCTGGCCA-3′; CRE (cAMP response element consensus site), 5′-TTAACAACAGTAACGTCACACGGACTA-3′; mCRE (CRE site with the consensus mutated as underlined), 5′-TTAACAACAGTATGGTCA CACGGACTA-3′, among others. NIH3T3 cell clones with inducible expression of FAK or ΔC14 were serum starved for 48 h in DMEM with 0.5% CS, 0.5 mg/ml G418, and 0.4 μg/ml tetracycline. They were then stimulated with DMEM plus 10% CS, 0.5 mg/ml G418 with (uninduced) or without (induced) 0.4 μg/ml tetracycline. At various times after stimulation, nuclear extracts were made as described previously (Zhao et al., 1998a) with slight modifications. Briefly, cells were washed with ice-cold PBS and TBS (Tris-buffered saline), incubated on ice in buffer A (10 mM HEPES, pH 7.9; 10 mM KCl; 0.1 mM EDTA; 0.1 mM EGTA; 1 mM dithiothreitol; 0.5 mM PMSF and 0.2 mM sodium orthovanadate) for 15 min, scraped, and transferred into Eppendorf tubes that contained 10% NP-40. After being vigorously vortexed, the lysates were centrifuged and the pellets were suspended in buffer B (20 mM HEPES, pH 7.9; 400 mM NaCl; 1 mM EDTA; 1 mM EGTA; 1 mM dithiothreitol; 1 mM PMSF and 0.2 mM sodium orthovanadate) and vigorously vortexed for 15 min at 4°C. After centrifugation, the supernatants were collected, quantified, aliquoted and kept at −80°C for future use in EMSA.
Two to 10 μg nuclear extracts were incubated with γ-32P-labeled oligonucleotides for 20 min at room temperature and chilled on ice for 10 min. The DNA-protein complexes were resolved on 4% PAGE in 1× TAE running buffer. The specificity of DNA-protein complex formation was verified by using the specific mutant oligonucleotides as well as the competition assays with unlabeled oligonucleotides. Dried gels were exposed to x-ray films for autoradiography and to phosphoimaging films for quantitative analysis using ImageQuant IQMac v1.2 and a Storm 840 scanner (Molecular Dynamics).
For some experiments, the serum-starved cells were replated on fibronectin (FN) or poly-l-lysine (PLL)–coated dishes and incubated for 4 h under induced or uninduced conditions before preparation of nuclear extracts. In the experiments using the chemical inhibitors, the serum-starved cells were grown in medium with various amounts of the inhibitors for 4 h before preparation of the nuclear extracts.
RESULTS
Cyclin D1 But Not p21 Mediates FAK Regulation of Cell Cycle Progression
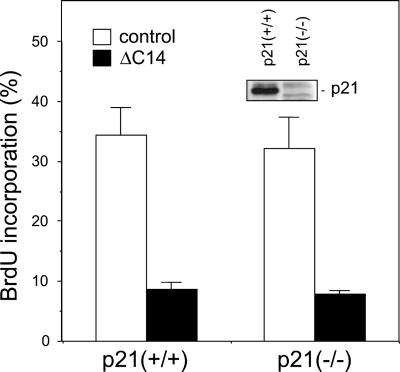
Our previous studies have suggested that FAK regulated cell cycle progression by modulating the expression of the cdk inhibitor p21 and/or cyclin D1 (Zhao et al., 1998b). Overexpression of FAK decreased p21 expression and increased cyclin D1 levels, concomitant with its stimulation of cell cycle progression. Conversely, overexpression of a FAK dominant-negative mutant, ΔC14, caused increased p21 levels and decreased cyclin D1 expression as well as inhibition of cell cycle progression. To further investigate the role of p21 in FAK-mediated cell cycle progression, we examined the effects of ΔC14 on cell cycle progression in p21−/− fibroblasts by transient transfections followed by BrdU incorporation assays, as described previously (Zhao et al., 1998b). Consistent with our previous results (Zhao et al., 1998b), Figure 1 shows that expression of ΔC14 in the control p21+/+ fibroblasts significantly inhibited cell cycle progression as measured by BrdU incorporation. Interestingly, transfection of ΔC14 into the p21−/− fibroblasts inhibited BrdU incorporation to a similar extent as the p21+/+ fibroblasts. The absence of p21 in the p21−/− fibroblasts was confirmed by Western blotting with anti-p21 antibodies (Figure 1, inset). Thus, upregulation of p21 was not required for cell cycle inhibition by ΔC14, suggesting that p21 was not playing a major role in mediating cell cycle regulation by FAK.
Figure 1.
Inhibition of cell cycle progression by ΔC14 in p21−/− cells. P21−/− or control p21+/+ fibroblasts were transiently transfected with expression vectors encoding ΔC14 or the vector alone (control), as indicated. They were then analyzed for BrdU incorporation as described in MATERIALS AND METHODS. The percentage of BrdU (+)/positively transfected cells was determined by analyzing 40–50 positively transfected cells for each transfection in multiple fields. The results show mean + SE for at least three independent experiments. Expression of p21 in p21−/− and control p21+/+ fibroblasts was analyzed by Western blotting with anti-p21 (inset).
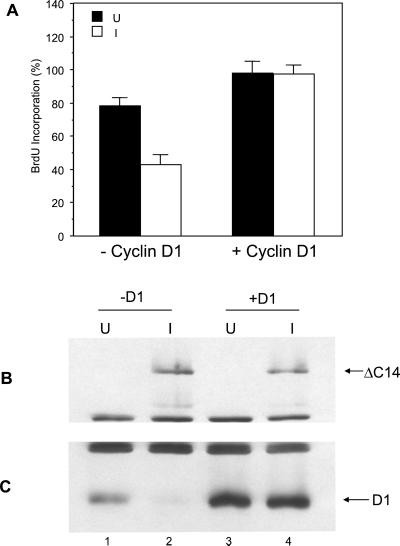
To study the role of cyclin D1, we then examined whether ectopic expression of cyclin D1 could rescue cell cycle inhibition by ΔC14. Expression vector encoding human cyclin D1 was transiently transfected into the NIH3T3 cell clone with inducible expression of ΔC14 (ΔC14 cells; Zhao et al., 1998b) along with a marker plasmid encoding β-Gal. The cells were then analyzed for BrdU incorporation under induced or uninduced conditions, as described previously (Zhao et al., 1998b), except that the positively transfected cells were identified by immunostaining with anti–β-Gal. Figure 2A shows that induction of ΔC14 expression inhibited BrdU incorporation, as expected. Expression of ectopic cyclin D1 (as marked by β-Gal + fraction of cells) rescued the inhibition of cell cycle progression by ΔC14. The induction of ΔC14 expression in these cells was verified by Western blotting with anti-HA (recognizing the HA epitope tag fused to the N-terminus of ΔC14; Figure 2B). Consistent with previous results (Zhao et al., 1998b), Figure 2C shows that induction of ΔC14 expression resulted in a significant decrease of endogenous cyclin D1 expression (cf. lanes 1 and 2). Interestingly, the expression level of the exogenous cyclin D1 was not affected by ΔC14 (cf. lanes 3 and 4). Taken together, these results suggests that cyclin D1, but not p21, is the primary functional target of FAK signaling pathways in cell cycle regulation.
Figure 2.
Ectopic expression of cyclin D1 rescues cell cycle inhibition by ΔC14. (A) ΔC14 cells were cotransfected with a plasmid encoding β-Gal and expression vector encoding cyclin D1 (+ cyclin D1) or vector alone control (− cyclin D1), as indicated. They were then analyzed for cell cycle progression under induced (I) or uninduced (U) conditions as described previously (Zhao et al., 1998b), except that the positively transfected cells were identified by immunostaining with anti-β-Gal. The percentage of BrdU (+)/β-Gal (+) cells was determined by analyzing 40–50 β-Gal (+) cells for each transfection in multiple fields. The results show mean + SE for three independent experiments. (B and C) Aliquots of cell lysates were analyzed by Western blotting with α-HA (B) or α-cyclin D1 (C) The positions of the ΔC14 and cyclin D1 are marked on the right.
Transcriptional Regulation of Cyclin D1 by FAK
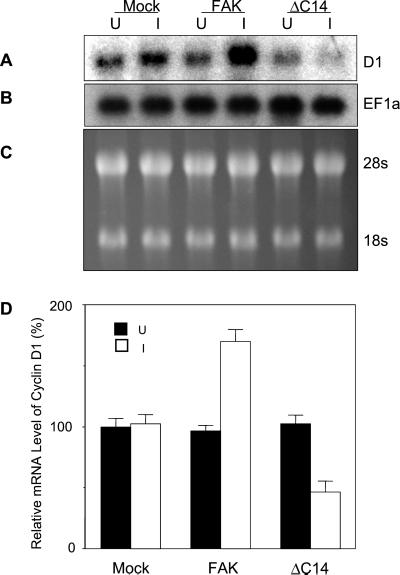
To determine the mechanisms of cyclin D1 regulation by FAK, we first examined the endogenous cyclin D1 mRNA levels in NIH3T3 cells with inducible expression of FAK or the dominant-negative mutant ΔC14. Total RNA was isolated from these cells under induced and uninduced conditions and subjected to Northern blotting analysis. As shown in Figure 3A, induction of wild-type FAK expression resulted in significant increase in cyclin D1 mRNA level, whereas induction of ΔC14 expression reduced it. Equal loading of all the lanes was verified by reprobing the membranes with EF-1α cDNA (Figure 3B) or staining of the gels for the 28S and 18S rRNA (Figure 3C). Quantitative analysis of three independent experiments showed that overexpression of FAK increased cyclin D1 mRNA levels by approximately twofold compared with uninduced cells or the mock cells under induced or uninduced conditions (Figure 3D). Expression of ΔC14 decreased the level of cyclin D1 mRNA by ∼40–50%.
Figure 3.
Regulation of Cyclin D1 mRNA level by FAK. Quiescent Mock, FAK or ΔC14 cells were stimulated with 10% CS under uninduced (U) or induced (I) conditions. Twelve hours thereafter, total RNAs were prepared using TRIzol Reagent as described in MATERIALS AND METHODS. Ten micrograms total RNAs were separated by 1.2% agarose/formaldehyde gel, transferred to nylon membrane, and blotted with 32P-labeled cyclin D1 cDNA probe (A) or EF1α cDNA probe (B). Ethidium bromide staining of the RNAs in the gel was conducted to show equal loading of samples (C). The mean + SE of relative cyclin D1 mRNA (normalized to uninduced Mock cells) from three independent experiments were obtained using PhosphoImage Analyzer (D).
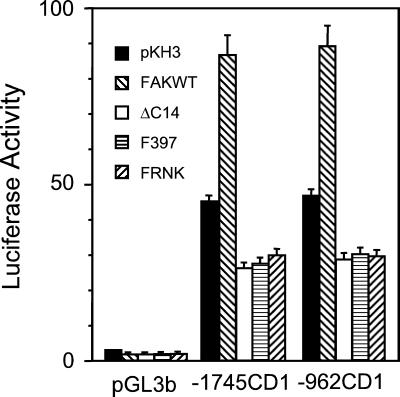
To examine whether FAK signaling increased cyclin D1 mRNA accumulation by stimulation of transcription, we studied the effects of FAK on the cyclin D1 promoter in NIH3T3 cells. Two different cyclin D1 promoter–luciferase reporter plasmids (−1745CD1 and 962CD1) were tested. The −1745CD1 reporter corresponded to the original fragment of cyclin D1 5′ sequence that was cloned from the PRAD1 breakpoint (Motokura and Arnold, 1993) and the −962CD1 reporter was used in the recent identification of the β-catenin/LEF1 binding site (Tetsu and McCormick, 1999). The luciferase reporter plasmids were cotransfected into NIH3T3 cells along with expression vectors encoding FAK, ΔC14, FAKF397, FRNK, or the empty vector. Two days after transfection, luciferase activities were determined as described previously (Shtutman et al., 1999). Figure 4 shows that expression of FAK increased the activity of both the reporter plasmids by approximately twofold. Conversely, expression of several versions of dominant-negative FAK (ΔC14, F397, or FRNK) inhibited the reporters' activities by ∼40%. Virtually no luciferase activity was detected when the pGL3b vector alone (lacking the cyclin D1 promoter sequence) was transfected into NIH3T3 cells with or without FAK constructs. These results are consistent with the Northern blotting data (Figure 3) and suggest that transcriptional activation of the cyclin D1 gene by FAK is at least partially responsible for the upregulation of cyclin D1 mRNA and proteins by FAK signaling pathways.
Figure 4.
Regulation of cyclin D1 promoter by FAK. NIH3T3 cells were cotransfected with expression vectors encoding FAK, ΔC14, FAKF397, FRNK or vector control alone (pKH3) and cyclin D1 promoter reporters −1745CD1, −962CD1, or the basic luciferase control vector (pGL3b), as indicated. A plasmid encoding β-gal was also included to normalize transfection efficiency. The ratio of reporter luciferase activity to β-gal activity is shown. The assay was performed 24 h after transfection. The results show mean + SE of at least three independent experiments.
EtsB Site Mediates Cyclin D1 Activation by FAK Signaling
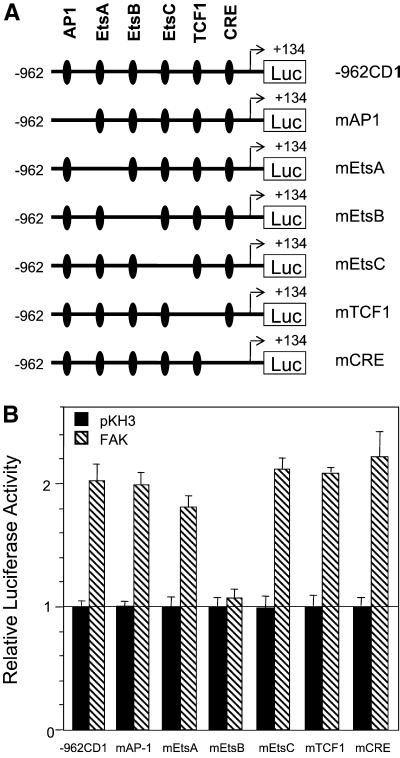
The cyclin D1 promoter contains multiple transcription factor binding sites including AP1, EtsA, EtsB, EtsC, TCF, and CRE sites (Figure 5A). Previous studies have suggested that several intracellular signaling pathways regulate cyclin D1 transcription through these sites. To determine whether these elements are required for FAK responsiveness of the cyclin D1 promoter, we examined the effect of FAK on a series of mutant cyclin D1 promoter constructs with each of these elements deleted (Figure 5A). Figure 5B shows that deletion of the AP1, EtsA, EtsC, TCF1, or CRE site did not affect regulation of the cyclin D1 promoter by FAK. In contrast, deletion of the EtsB element significantly diminished FAK responsiveness of the cyclin D1 promoter. We obtained similar results using HEK293T cells. These results suggest that FAK signaling pathways might stimulate cyclin D1 transcription through the EtsB site.
Figure 5.
EtsB site is required for FAK activation of the cyclin D1 promoter. (A) Schematic representation of the −962 cyclin D1 promoter in a luciferase reporter and deletion mutants. (B) NIH3T3 cells were cotransfected with expression vectors encoding FAK or pKH3 vector control and cyclin D1 promoter reporter −962CD1, or various mutants, as indicated. Luciferase assays were performed as described in Figure 4. The average and SD of relative luciferase activities were obtained from three independent experiments. The relative activities were normalized to pKH3 control vector transfected cells.
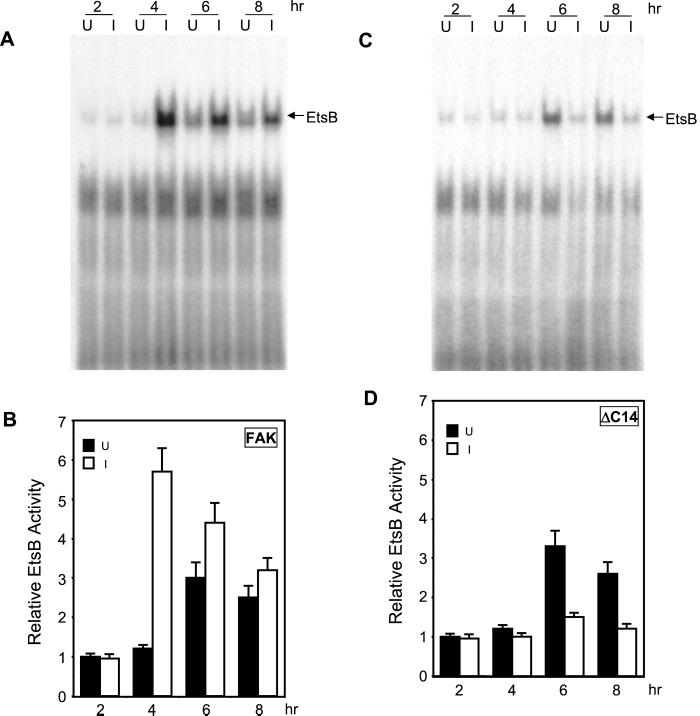
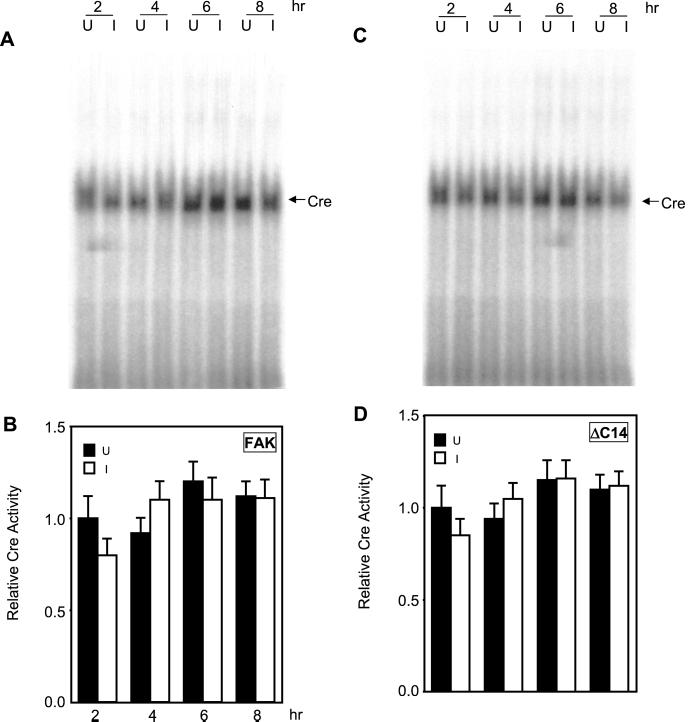
EMSA were then performed to further determine potential regulation of binding to the EtsB site by FAK signaling pathways. Nuclear extracts were prepared from NIH3T3 cells with inducible expression of FAK or ΔC14, at various times after serum stimulation under induced or uninduced conditions. They were then probed for binding activities with radiolabeled oligonucleotides corresponding to various transcription factor binding sites in the cyclin D1 promoter. Under uninduced conditions, the EtsB binding activity showed an approximately threefold increase in response to serum stimulation (Figure 6). A slight increase was detected from 4 h after stimulation, reaching the peak response at 6 h, and then showing a gradual decrease at 8 h after stimulation. Induction of FAK expression significantly enhanced the EtsB binding activity, which reached the peak of approximately sixfold increase at 4 h after serum stimulation (Figures 6, A and B). In contrast, expression of the dominant-negative mutant ΔC14 almost completely abolished the increase in EtsB binding activity after serum stimulation (Figures 6, C and D). Similar studies showed that induced expression of neither FAK nor ΔC14 affected the CRE binding activity (Figure 7) or the AP1 binding activity under the same experimental conditions. Together with data from the luciferase assays (Figure 5), these results suggest that FAK signaling pathways regulate cyclin D1 transcription primarily through their effects on the EtsB binding site.
Figure 6.
Activation of EtsB binding by FAK. Quiescent FAK (A and B) or ΔC14 cells (C and D) were stimulated with 10% CS under uninduced (U) or induced (I) conditions for various times, as indicated. Nuclear extracts were then prepared as described in MATERIALS AND METHODS. They were used for EMSA with the radiolabeled oligonucleotide corresponding to the cyclin D1 EtsB site. Panels A and C show representative results from FAK (A) and ΔC14 cells (C). The arrow indicates the cyclin D1 EtsB binding complex. (B and D) The average and SD of relative EtsB binding activities from three independent experiments of FAK (B) and ΔC14 cells (D). The relative EtsB binding activities were normalized to nuclear extracts from uninduced cells at 2 h after stimulation.
Figure 7.
Effects of FAK on the Cre binding activity. Quiescent FAK (A and B) or ΔC14 cells (C and D) were stimulated with 10% CS under uninduced (U) or induced (I) conditions for various times, as indicated. Nuclear extracts were then prepared as described in the MATERIALS AND METHODS. They were used for EMSA with the radiolabeled oligonucleotide corresponding to the cyclin D1 Cre site. (A and C) Representative results from FAK (A) and ΔC14 cells (C). The arrow indicates the cyclin D1 Cre binding complex. (B and D) The average and SD of relative Cre binding activities from three independent experiments of FAK (B) and ΔC14 cells (D). The relative Cre binding activities were normalized to nuclear extracts from uninduced cells at 2 h after stimulation.
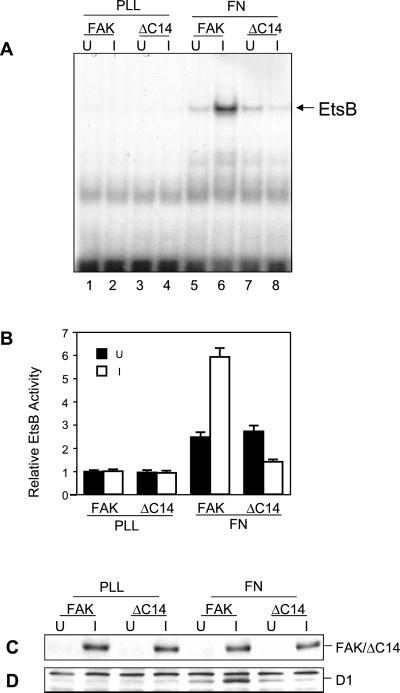
Cell Adhesion Regulates EtsB Activation
It is well documented that FAK tyrosine phosphorylation and kinase activity are regulated by integrin-mediated cell adhesion to ECM proteins such as FN (Guan and Shalloway, 1992). We therefore tested whether cell adhesion could regulate the EtsB binding activity. NIH3T3 cells with inducible expression of FAK or ΔC14 under either uninduced or induced conditions were removed from the plates and replated on either poly-l-lysine (lanes PLL) or FN (lanes FN). Nuclear extracts were then prepared and tested for FN adhesion-induced EtsB binding activity, as shown in Figure 8. Little EtsB binding activity was detected in cells plated on PLL under either induced or uninduced conditions. Cell adhesion to FN resulted in an approximatley twofold increase in the EstB binding activity, presumably due to activation of the endogenous FAK. Induction of the wild-type FAK overexpression further enhanced the EtsB binding activity, whereas expression of the dominant-negative ΔC14 inhibited FN-induced EtsB binding activity. Consistent with the effects on EtsB binding activity, induction of FAK overexpression further increased FN-induced endogenous cyclin D1 expression, whereas ΔC14 inhibited it (Figure 8, C and D). Together, these results suggested that integrin-mediated cell adhesion could activate cyclin D1 promoter through activation of EtsB binding via the FAK signaling pathways.
Figure 8.
Regulation of EtsB binding activity by cell adhesion to FN. Quiescent FAK or ΔC14 cells were suspended and replated on FN or PLL under uninduced (U) or induced (I) conditions, as indicated. After a 4-h incubation, nuclear extracts were prepared as described in MATERIALS AND METHODS. They were used for EMSA with the radiolabeled oligonucleotide corresponding to the cyclin D1 EtsB site (A). The arrow indicates the cyclin D1 EtsB binding complex. (B) The average and SD of relative EtsB binding activities from three independent experiments. The relative EtsB binding activities were normalized to nuclear extracts from FAK cells replated on PLL under uninduced condition. Cell lysates from duplicate plates under the same conditions were prepared 12 h after replating. They were then analyzed by Western blotting with α-HA (C) or α-cyclin D1 (D). The positions of the FAK or ΔC14 and cyclin D1 are marked on the right.
Role of Erk Pathway in Cyclin D1 Activation by FAK
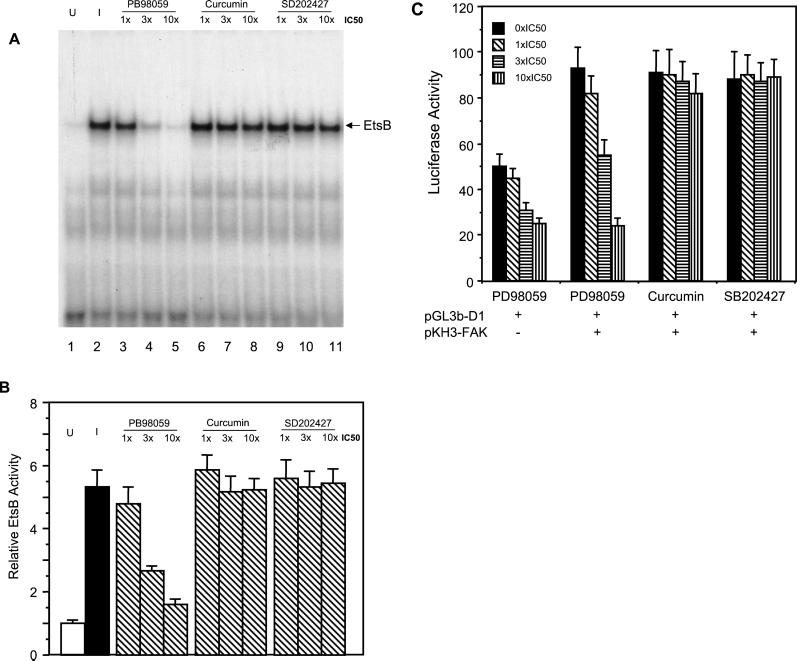
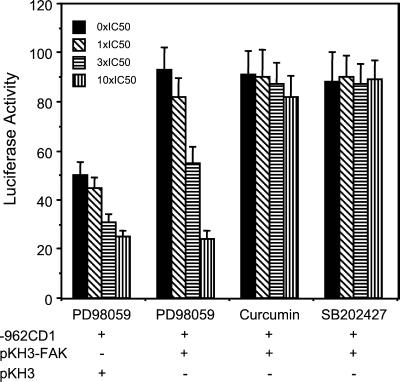
The Ets family transcription factors are specific downstream substrates of Erks (Wasylyk et al., 1998) and FAK has been suggested to activate Erks through a number of downstream pathways (Schlaepfer et al., 1999; Barberis et al., 2000; Zhao et al., 2000). Therefore, we examined the possibility that Erk signaling pathway mediates activation of EtsB binding activity by FAK by using specific chemical inhibitors for Erk and the related JNK signaling pathways. Figure 9 shows that, as observed previously, induction of FAK expression enhanced EtsB binding activity (cf. lanes 1 and 2). The specific MEK inhibitor, PD98059, reduced EtsB activation by FAK in a dose dependent manner (lanes 3–5). In contrast, the JNK inhibitor curcumin (Chen and Tan, 1998; Chen et al., 1999) or the control SB202474 had little effect, even at doses as high as 10 times the IC50 (lanes 6–11). We also tested the effects of these inhibitors on the activation of the whole cyclin D1 promoter by FAK using luciferase assays. Consistent with the EMSA results, PD98059 inhibited the FAK-activated as well as basal luciferase activities (Figure 10). Again, curcumin or SB202474 did not affect FAK induced activation of cyclin D1 promoter. Together, these data suggested that regulation of cyclin D1 transcription by FAK was primarily through the activation of Erk pathway by FAK.
Figure 9.
Effects of inhibitors for MEK or JNK on EtsB activation by FAK. Quiescent FAK cells were incubated for 4 h with 10% CS in the absence (lanes 1 and 2) or presence (lanes 3–11) of the indicated inhibitors under induced condition (lanes 2–11) or uninduced (lane 1) conditions, as indicated. Nuclear extracts were then prepared as described in MATERIALS AND METHODS. They were used for EMSA with the radiolabeled oligonucleotide corresponding to the cyclin D1 EtsB site (A). The arrow indicates the cyclin D1 EtsB binding complex. Panel B shows the average and SD of relative EtsB binding activities from three independent experiments. The relative EtsB binding activities were normalized to nuclear extracts from uninduced cells.
Figure 10.
Effects of inhibitors for MEK or JNK on activation of cyclin D1 promoter by FAK. NIH3T3 cells were cotransfected with expression vectors encoding FAK or pKH3 vector control and cyclin D1 promoter reporter −962CD1, as indicated. Twenty-four hours after transfection, various amounts of the indicated inhibitors were applied to the cells. After 24 h, luciferase assays were performed as described in Figure 4. The average and SD of luciferase activities were obtained from three independent experiments.
DISCUSSION
Integrin-mediated cell adhesion plays important roles in the regulation of cell cycle progression by affecting expression and activities of various cyclins, cdks, and cdk inhibitors (Assoian, 1997; Assoian and Schwartz, 2001). Recent studies have revealed that integrin binding to ligands lead to activation of several intracellular signaling pathways (Giancotti and Ruoslahti, 1999). However, relatively little is known about the molecular mechanisms that connect the cytoplasmic signaling pathways to cell cycle regulation by integrins. Previous studies from our laboratory and others suggest that activation of FAK and its downstream signaling pathways by integrins play an important role in mediating cell cycle regulation by integrins (Zhao et al., 1998b, 2000; Oktay et al., 1999; Reiske et al., 2000; Wang et al., 2000). In this article, we showed that FAK regulated cell cycle progression primarily through its regulation of cyclin D1 expression at the transcriptional level. We also identified the EtsB site as the regulatory component in the cyclin D1 promoter and that the MAP kinase, Erk, plays a significant role in mediating cyclin D1 expression by FAK. These results connect integrin signaling through FAK to cell cycle regulation via Erk and activation of the EtsB site on the cyclin D1 promoter.
Consistent with their ability to activate multiple signaling pathways, integrins have been shown to regulate cell cycle progression by affecting the expression and/or activities of different cell cycle regulators in various cells (Assoian and Schwartz, 2001). Our previous studies using a tet-inducible expression system in fibroblasts showed that integrin signaling through FAK affected expression levels of cyclin D1 and p21, although it did not affect expression of a number of other cell cycle regulators, suggesting that these two are potential mediators (Zhao et al., 1998b). Data presented here excluded a role for p21 because disruption of FAK signaling resulted in cell cycle inhibition in p21−/− fibroblasts (Figure 1). In contrast, overexpression of cyclin D1 rescued the cell cycle inhibition by a dominant-negative FAK mutant (Figure 2). These results demonstrate that cyclin D1 is the major mediator for FAK regulation of cell cycle progression, which is consistent with several previous reports suggesting a critical role of cyclin D1 in mediating cell cycle regulation by integrins (Resnitzky, 1997; Schulze et al., 1996; Zhu et al., 1996; Assoian and Schwartz, 2001).
Expression of cyclin D1 is controlled at both transcriptional and posttranscriptional levels by multiple intracellular signaling pathways (Pestell et al., 1999). We show here that integrin signaling through FAK could regulate transcription of cyclin D1. Transfection of wild-type FAK increased transcriptional activity of cyclin D1 promoter, whereas expression of several versions of dominant-negative FAK mutants decreased it (Figure 4). Consistent with these results, induction of FAK expression increased mRNA level of endogenous cyclin D1, whereas expression of the dominant-negative FAK mutant ΔC14 decreased it (Figure 3). Sequential activation of the Ras/Erk signaling cascade (Raf, MEK, and Erk) and the sustained activation of Erk have been shown to be required for the increased cyclin D1 expression (Lavoie et al., 1996; Aktas et al., 1997; Kerkhoff and Rapp, 1997; Weber et al., 1997; Cheng et al., 1998). Interestingly, integrin-mediated cell adhesion is known to cooperate with growth factor signaling for sustained Erk activation (Roovers et al., 1999), and FAK has been implicated in such a role by a recent study (Barberis et al., 2000). Indeed, our previous studies suggested a role for Erk in the regulation of cell cycle progression by integrin signaling through FAK (Zhao et al., 1998b, 2000). Inhibition of the Erk, but not JNK, pathway suppressed cyclin D1 promoter activation by FAK (Figures 9 and 10). Together, these results suggest that integrin signaling through FAK regulates cyclin D1 expression and cell cycle progression by affecting the transcription of cyclin D1 via sustained activation of the Erk pathway. These studies, however, do not exclude the possibility that integrin-mediated cell adhesion could also regulate cyclin D1 expression at the posttranscriptional levels.
Consistent with its critical role in cell cycle regulation, the cyclin D1 gene has multiple regulatory elements in the promoter that are targeted by various signal transduction pathways. Our analyses using a luciferase assay with promoter deletion mutants as well as EMSA showed the EtsB site as the primary mediator of cyclin D1 regulation by FAK. This site is required for the activation of the promoter by FAK (Figure 5). The EtsB binding activity is also regulated by FAK in a cell adhesion dependent manner (Figures 6 and 8). The Ets proteins are a family of transcription factors that are substrates and targets of Erk signaling pathways (Wasylyk et al., 1998). This is consistent with our observation that FAK regulates cyclin D1 transcription involved the Erk signaling pathway. Although there are three putative Ets binding sites, our results suggested that the EtsB site is a functional site that mediates cyclin D1 regulation by FAK. Although the JNK signaling pathway has also been suggested to mediate FAK regulation of cell cycle progression, we did not find a role for the AP-1 or Cre sites, which are both targets of JNK. Interestingly, a recent study by D'Amico et al. (2000) identified the Cre site of the cyclin D1 promoter as a mediator of cyclin D1 regulation by ILK in a PI 3-kinase dependent manner. Because both FAK and ILK are major mediators of integrin signaling, these results suggested the interesting possibility of combinatory regulation of cyclin D1 promoter by two distinct cytoplasmic to nuclear signaling pathways (ILK-PI3K-Cre and FAK-Erk-EtsB) emanating from the integrins. It is possible that one of these two pathways may play a more dominant role in different cell types (e.g., fibroblasts vs. epithelial cells). It will be interesting to examine the relative roles of these pathways in cells where both pathways are active.
ACKNOWLEDGMENTS
We are grateful to Dr. T. Hunter of Salk Institute, CA for pRK5-D1 construct, Drs. O. Tetsu and F. McCormack of UCSF, CA, for the cyclin D1 promoter mutant reporter constructs, Dr. Y. Xiong of UNC, NC, for antibodies against p21 and cyclin D1, Dr. Roy Levine of Cornell University for the EF1α cDNA, and Dr. C. Deng of NIH for the p21−/− and control fibroblasts. We thank Dong Cho Han, Xu Peng, Tanglong Shen, Luis Rodriguez, Tamas Nagy, Boyi Gan, Dan Rhoads, Xiaoyang Wu, Smita Abbi, and Jie W. Wu for their critical reading of the manuscript and helpful comments. This research was supported by NIH grant GM52890 to J.-L. Guan. J.-L. Guan is an Established Investigator of American Heart Association.
Abbreviations used:
- FAK
focal adhesion kinase
- ECM
extracellular matrix
- Erk
extracellular signal-related kinase
- JNK
c-Jun N-terminal kinase
- PI3K
phosphatidylinositol 3 kinase
- FN
fibronectin
- PLL
poly-l-lysine
- BrdU
5-bromodeoxyuridine
- Ets
Epstein-Barr virus transforming substrate
- AP-1
activator protein 1
- CREB
cAMP-responsive element binding protein
- LEF-1
lymphoid enhancer factor
- EMSA
electrophoretic mobility shift assay
- cdk
cyclin-dependent kinase
- ILK
integrin linked kinase
REFERENCES
- Aktas H, Cai H, Cooper GM. Ras links growth factor signaling to the cell cycle machinery via regulation of cyclin D1 and the Cdk inhibitor p27KIP1. Mol Cell Biol. 1997;17:3850–3857. doi: 10.1128/mcb.17.7.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell RG. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- Arber N, Hibshoosh H, Moss SF, Sutter T, Zhang Y, Begg M, Wang S, Weinstein IB, Holt PR. Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis. Gastroenterology. 1996;110:669–674. doi: 10.1053/gast.1996.v110.pm8608874. [DOI] [PubMed] [Google Scholar]
- Assoian RK. Anchorage-dependent cell cycle progression. J Cell Biol. 1997;136:1–4. doi: 10.1083/jcb.136.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian RK, Schwartz MA. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev. 2001;11:48–53. doi: 10.1016/s0959-437x(00)00155-6. [DOI] [PubMed] [Google Scholar]
- Barberis L, Wary KK, Fiucci G, Liu F, Hirsch E, Brancaccio M, Altruda F, Tarone G, Giancotti FG. Distinct roles of the adaptor protein Shc and focal adhesion kinase in integrin signaling to ERK. J Biol Chem. 2000;275:36532–36540. doi: 10.1074/jbc.M002487200. [DOI] [PubMed] [Google Scholar]
- Chen HC, Appeddu PA, Parsons JT, Hildebrand JD, Schaller MD, Guan JL. Interaction of focal adhesion kinase with cytoskeletal protein talin. J Biol Chem. 1995;270:16995–16999. doi: 10.1074/jbc.270.28.16995. [DOI] [PubMed] [Google Scholar]
- Chen HC, Guan JL. Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1994;91:10148–10152. doi: 10.1073/pnas.91.21.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YR, Tan TH. Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene. 1998;17:173–178. doi: 10.1038/sj.onc.1201941. [DOI] [PubMed] [Google Scholar]
- Chen YR, Zhou G, Tan TH. c-Jun N-terminal kinase mediates apoptotic signaling induced by N-(4-hydroxyphenyl)retinamide. Mol Pharmacol. 1999;56:1271–1279. doi: 10.1124/mol.56.6.1271. [DOI] [PubMed] [Google Scholar]
- Cheng M, Sexl V, Sherr CJ, Roussel MF. Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1) Proc Natl Acad Sci USA. 1998;95:1091–1096. doi: 10.1073/pnas.95.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico M, Hulit J, Amanatullah DF, Zafonte BT, Albanese C, Bouzahzah B, Fu M, Augenlicht LH, Donehower LA, Takemaru K, Moon RT, Davis R, Lisanti MP, Shtutman M, Zhurinsky J, Ben-Ze'ev A, Troussard AA, Dedhar S, Pestell RG. The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3beta and cAMP-responsive element-binding protein-dependent pathways. J Biol Chem. 2000;275:32649–32657. doi: 10.1074/jbc.M000643200. [DOI] [PubMed] [Google Scholar]
- Day ML, Foster RG, Day KC, Zhao X, Humphrey P, Swanson P, Postigo AA, Zhang SH, Dean DC. Cell anchorage regulates apoptosis through the retinoblastoma tumor suppressor/E2F pathway. J Biol Chem. 1997;272:8125–8128. doi: 10.1074/jbc.272.13.8125. [DOI] [PubMed] [Google Scholar]
- Diehl JA, Zindy F, Sherr CJ. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 1997;11:957–972. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- Draetta GF. Mammalian G1 cyclins. Curr Opin Cell Biol. 1994;6:842–846. doi: 10.1016/0955-0674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Guan JL, Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992;358:690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- Huang S, Chen CS, Ingber DE. Control of cyclin D1, p27(Kip1), and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol Biol Cell. 1998;9:3179–3193. doi: 10.1091/mbc.9.11.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce D, Bouzahzah B, Fu M, Albanese C, D'Amico M, Steer J, Klein JU, Lee RJ, Segall JE, Westwick JK, Der CJ, Pestell RG. Integration of Rac-dependent regulation of cyclin D1 transcription through a nuclear factor-kappaB-dependent pathway. J Biol Chem. 1999;274:25245–25249. doi: 10.1074/jbc.274.36.25245. [DOI] [PubMed] [Google Scholar]
- Kerkhoff E, Rapp UR. Induction of cell proliferation in quiescent NIH 3T3 cells by oncogenic c-Raf-1. Mol Cell Biol. 1997;17:2576–2586. doi: 10.1128/mcb.17.5.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie JN, L'Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Albanese C, Stenger RJ, Watanabe G, Inghirami G, Haines GK, 3rd, Webster M, Muller WJ, Brugge JS, Davis RJ, Pestell RG. 60(v-src) induction of cyclin D1 requires collaborative interactions between the extracellular signal-regulated kinase, p38, and Jun kinase pathways. A role for cAMP response element-binding protein and activating transcription factor-2 in pp60(v-src) signaling in breast cancer cells. J Biol Chem. 1999;274:7341–7350. doi: 10.1074/jbc.274.11.7341. [DOI] [PubMed] [Google Scholar]
- Motokura T, Arnold A. PRAD1/cyclin D1 proto-oncogene: genomic organization, 5′ DNA sequence, and sequence of a tumor-specific rearrangement breakpoint. Genes Chromosomes Cancer. 1993;7:89–95. doi: 10.1002/gcc.2870070205. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Wang TC, Zukerberg L, Odze R, Togawa K, May GH, Wilson J, Rustgi AK. The targeting of the cyclin D1 oncogene by an Epstein-Barr virus promoter in transgenic mice causes dysplasia in the tongue, esophagus and forestomach. Oncogene. 1997;14:1185–1190. doi: 10.1038/sj.onc.1200937. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M, Roberts JM. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science. 1993;259:1908–1912. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
- Oktay M, Wary KK, Dans M, Birge RB, Giancotti FG. Integrin-mediated activation of focal adhesion kinase is required for signaling to Jun NH2-terminal kinase and progression through the G1 phase of the cell cycle. J Cell Biol. 1999;145:1461–1469. doi: 10.1083/jcb.145.7.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestell RG, Albanese C, Reutens AT, Segall JE, Lee RJ, Arnold A. The cyclins and cyclin-dependent kinase inhibitors in hormonal regulation of proliferation and differentiation. Endocr Rev. 1999;20:501–534. doi: 10.1210/edrv.20.4.0373. [DOI] [PubMed] [Google Scholar]
- Quelle DE, Ashmun RA, Shurtleff SA, Kato JY, Bar-Sagi D, Roussel MF, Sherr CJ. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- Reiske HR, Zhao J, Han DC, Cooper LA, Guan JL. Analysis of FAK-associated signaling pathways in the regulation of cell cycle progression. FEBS Lett. 2000;486:275–280. doi: 10.1016/s0014-5793(00)02295-x. [DOI] [PubMed] [Google Scholar]
- Resnitzky D. Ectopic expression of cyclin D1 but not cyclin E induces anchorage-independent cell cycle progression. Mol Cell Biol. 1997;17:5640–5647. doi: 10.1128/mcb.17.9.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnitzky D, Gossen M, Bujard H, Reed SI. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles AI, Rodriguez-Puebla ML, Glick AB, Trempus C, Hansen L, Sicinski P, Tennant RW, Weinberg RA, Yuspa SH, Conti CJ. Reduced skin tumor development in cyclin D1-deficient mice highlights the oncogenic ras pathway in vivo. Genes Dev. 1998;12:2469–2474. doi: 10.1101/gad.12.16.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roovers K, Davey G, Zhu X, Bottazzi ME, Assoian RK. Alpha5beta1 integrin controls cyclin D1 expression by sustaining mitogen-activated protein kinase activity in growth factor-treated cells. Mol Biol Cell. 1999;10:3197–3204. doi: 10.1091/mbc.10.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- Schulze A, Zerfass-Thome K, Berges J, Middendorp S, Jansen-Durr P, Henglein B. Anchorage-dependent transcription of the cyclin A gene. Mol Cell Biol. 1996;16:4632–4638. doi: 10.1128/mcb.16.9.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ. D-type cyclins. Trends Biochem Sci. 1995;20:187–190. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- Shtutman M, Zhurisky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Wang D, Grammer JR, Cobbs CS, Stewart JE, Liu Z, Rhoden R, Hecker TP, Ding Q, Gladson CL. p125 focal adhesion kinase promotes malignant astrocytoma cell proliferation in vivo. J Cell Sci. 2000;113 Pt 23:4221–4230. doi: 10.1242/jcs.113.23.4221. [DOI] [PubMed] [Google Scholar]
- Wang LD, Shi ST, Zhou Q, Goldstein S, Hong JY, Shao P, Qiu SL, Yang CS. Changes in p53 and cyclin D1 protein levels and cell proliferation in different stages of human esophageal and gastric-cardia carcinogenesis. Int J Cancer. 1994a;59:514–519. doi: 10.1002/ijc.2910590414. [DOI] [PubMed] [Google Scholar]
- Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994b;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- Wasylyk B, Hagman J, Gutierrez-Hartmann A. Ets transcription factors: nuclear effectors of the Ras-MAP-kinase signaling pathway. Trends Biochem Sci. 1998;23:213–216. doi: 10.1016/s0968-0004(98)01211-0. [DOI] [PubMed] [Google Scholar]
- Watanabe G, Lee RJ, Albanese C, Rainey WE, Batlle D, Pestell RG. Angiotensin II activation of cyclin D1-dependent kinase activity. J Biol Chem. 1996;271:22570–22577. doi: 10.1074/jbc.271.37.22570. [DOI] [PubMed] [Google Scholar]
- Weber JD, Raben DM, Phillips PJ, Baldassare JJ. Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem J. 1997;326:61–68. doi: 10.1042/bj3260061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwick JK, Lambert QT, Clark GJ, Symons M, Van Aelst L, Pestell RG, Der CJ. Rac regulation of transformation, gene expression, and actin organization by multiple, PAK-independent pathways. Mol Cell Biol. 1997;17:1324–1335. doi: 10.1128/mcb.17.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwick JK, Lee RJ, Lambert QT, Symons M, Pestell RG, Der CJ, Whitehead IP. Transforming potential of Dbl family proteins correlates with transcription from the cyclin D1 promoter but not with activation of Jun NH2-terminal kinase, p38/Mpk2, serum response factor, or c-Jun. J Biol Chem. 1998;273:16739–16747. doi: 10.1074/jbc.273.27.16739. [DOI] [PubMed] [Google Scholar]
- Wu RC, Schonthal AH. Activation of p53–p21waf1 pathway in response to disruption of cell- matrix interactions. J Biol Chem. 1997;272:29091–29098. doi: 10.1074/jbc.272.46.29091. [DOI] [PubMed] [Google Scholar]
- Xing Z, Chen HC, Nowlen JK, Taylor SJ, Shalloway D, Guan JL. Direct interaction of v-Src with the focal adhesion kinase mediated by the Src SH2 domain. Mol Biol Cell. 1994;5:413–421. doi: 10.1091/mbc.5.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Nanney LB, Luongo C, Lamps L, Heppner KJ, DuBois RN, Beauchamp RD. Concurrent overexpression of cyclin D1 and cyclin-dependent kinase 4 (Cdk4) in intestinal adenomas from multiple intestinal neoplasia (Min) mice and human familial adenomatous polyposis patients. Cancer Res. 1997;57:169–175. [PubMed] [Google Scholar]
- Zhao J, Zheng C, Guan J. Pyk2 and FAK differentially regulate progression of the cell cycle. J Cell Sci. 2000;113:3063–3072. doi: 10.1242/jcs.113.17.3063. [DOI] [PubMed] [Google Scholar]
- Zhao JH, Inoue T, Shoji W, Nemoto Y, Obinata M. Direct association of YY-1 with c-Myc and the E-box binding protein in regulation of glycophorin gene expression. Oncogene. 1998a;17:1009–1017. doi: 10.1038/sj.onc.1202026. [DOI] [PubMed] [Google Scholar]
- Zhao JH, Reiske H, Guan JL. Regulation of the cell cycle by focal adhesion kinase. J Cell Biol. 1998b;143:1997–2008. doi: 10.1083/jcb.143.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Ohtsubo M, Bohmer RM, Roberts JM, Assoian RK. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J Cell Biol. 1996;133:391–403. doi: 10.1083/jcb.133.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]