Significance
This study unifies quantitative methods with dated molecular phylogenies of different lineages to identify biogeographical regions and understand the spatial and temporal evolution of the biota in one of the most biodiverse hotspots of the planet, the tropical Andes. We found complex distribution patterns reflected in a significantly higher number of bioregions than previous regionalization work has identified. In addition, this study found evidence that bioregions’ drivers were processes of Andean uplift and mountain dispersal facilitated by temperature oscillations during the Pleistocene. Therefore, Andean bioregions were formed from a combination of vicariance and dispersal events, which occurred in different time periods. Our results will help set conservation priorities that preserve the evolutionary patterns of biodiversity.
Keywords: vicariance, biogeography, biogeographic regions
Abstract
Understanding the spatial and temporal evolution of biota in the tropical Andes is a major challenge, given the region’s topographic complexity and high beta diversity. We used a network approach to find biogeographic regions (bioregions) based on high-resolution species distribution models for 151 endemic bird taxa. Then, we used dated molecular phylogenies of 14 genera to reconstruct the area history through a sequence of allopatric speciation processes. We identified 15 biogeographical regions and found 26 events of isolation and diversification within their boundaries that are independently confirmed with disjunct distributions of sister taxa. Furthermore, these events are spatially congruent with six geographical barriers related to warm and/or dry river valleys, discontinuities in elevation, and high peaks separating fauna from different range slopes. The most important barrier is the Marañon River Valley, which limits the boundaries of four bioregions and is congruent with eight phylogenetic distribution breaks, separating the Central and Northern Andes, where the most bioregions are found. We also show that many bioregions have diffuse and overlapping structures, with contact and transition zones that challenge previous conceptions of biogeographical regions as spatially simple in structure. This study found evidence that the drivers of our identified bioregions were processes of Andean uplift and mountain dispersal facilitated by temperature oscillations of the Pleistocene. Therefore, Andean bioregions were not formed from one simple biogeographical event in a certain time frame, but from a combination of vicariance and dispersal events, which occurred in different time periods.
The tropical Andes of South America is one of the most biodiverse regions on earth, exhibiting high levels of endemism and spatial turnover in the distribution of species (1–3). Understanding the complex mechanisms of isolation and diversification of the Andean biota has been one of the main challenges of biogeography since Humboldt (4). The first hypotheses of faunal evolution in the Andes were proposed by the American ornithologist Frank M. Chapman, based on several expeditions to Colombia and Ecuador that aimed to reveal the geographic origins of birds in South America (5, 6). At the core of Chapman’s ideas are speciation between elevational gradients and the formation of barriers, along with long-distance dispersal and colonization following the opening of new environments. These ideas have been supported by contemporary studies with birds and multiple lines of evidence from other biological groups (reviewed by ref. 7).
Recent research using dated molecular phylogenies suggests that the final uplift of the Andes during the Late Miocene and Pliocene (8) in conjunction with the climatic oscillation of the Pleistocene, had a strong effect on the diversification of Andean biota (9). On one hand, Andean uplift created isolated “sky islands” surrounded by drier valleys which act as barriers to the dispersal of species (10–12). On the other hand, the climate change of the Pleistocene caused repetitive range expansion and contraction of species distribution, allowing down-slope migration through lowland elevation barriers (valleys) (11, 13). Alternatively, Ramírez-Barahona and Eguiarte (14) hypothesized that the advance of ice sheets and páramo ecosystems during glacial maxima could have contracted humid montane species into refugia. These ideas notwithstanding, currently there is general agreement on the historical geography of the Andes and the origin of its biota, but it is the spatial and temporal evolution within the region that has remained less clear (12, 15). This task can be approached with the identification of areas that are characterized by distinct assemblages of living species, i.e., biogeographical regions (16).
Modern understanding conceptualizes biogeographical regions, or areas of endemism, as regional species pools shaped by stochastic, ecological, and evolutionary processes (17) that act as dynamic entities in space and time (18). However, species within a bioregion do not necessarily share the same history of speciation, because there are different events of isolation and diversification occurring at several time and elevational intervals generating different evolutionary trajectories (12). Nevertheless, it is expected that the combined ranges of species pools within a region should be more similar than the combined ranges of species in other regions, due to the presence of barriers that limit dispersal (19). The identification of these regions is a crucial step in addressing the spatial evolution of biodiversity (20) and delimiting important units of conservation. The identification of endemic bird areas of the world (21) was a major effort to set priority conservation areas and shows many areas with a high number of narrowly distributed species in the tropical Andes. Despite these advances, this study (21) cannot be considered as regionalization, because the authors used an arbitrary range size to select species with narrow distribution and it is well established that widely distributed species are also important for identifying biogeographic regions (22). The most recent study of Andean regionalization by Morrone (23) delimited five subregions and 15 provinces, with the goal of providing a standardized nomenclature and area diagnosis. However, in Morrone’s work there is no explicit or quantitative method used to conduct the delimitation of regions. It was made with a consensus of previous work, most of which also did not use any explicit method and some of which dates back to Sclater (24). This is questionable, given that the distribution of taxa was scarcely known in previous centuries compared with today’s occurrence records available as digitally accessible knowledge (25) and the rise of sophisticated methods for bioregion identification (26, 27). Furthermore, Morrone’s study does not evaluate how well supported these areas are in the light of independent evidence, despite recent calls to incorporate biogeography in a more robust hypothesis testing framework (28, 29). For instance, it has long been recognized that phylogenies of different lineages are key elements that aid the discovery of common patterns and temporal sequence of processes that influence the formation of biogeographic regions (30). The use of phylogenies is absent in this study. As a result of these drawbacks, many mountain regions that belong to the Andes were entirely excluded in that study, such as the Western and Central Cordilleras of Colombia, and the Mérida Cordillera of Venezuela, despite their biogeographic affinities with the rest of the Andes (2, 31–33). Therefore, the evolutionary history of the region and how it is represented by current distributional patterns has not been adequately assessed to date.
Birds have frequently been used as a typical model system in studies to address questions regarding biota diversification and distribution in the Neotropics (34–38). One of the most classical examples is how bird studies have contributed to establish some of the main hypotheses of the Amazon region. The “Pleistocene forest refuge hypothesis” (36) and the “rivers barrier or interfluve hypothesis” (35) were both proposed with birds and tried to explain the evolutionary biogeography of the Amazon. Thus, our study integrates a network approach for identifying biogeographical regions with molecular dated phylogenies of different lineages to elucidate the spatial and temporal evolution of the biota in the complex region of the tropical Andes. We had three major objectives that were assessed in a three-step workflow as follows: (i) to identify biogeographic regions (bioregions) within the tropical Andes using a network approach to detect clusters in the distribution of 151 bird taxa restricted to the mountains of the Andes; (ii) to reconstruct a sequence of hypothetical vicariance and dispersal events, inferred from the disjunct distributions of sister taxa using published dated molecular phylogenies of 14 genera of Andean birds; and (iii) to assess the spatial and temporal congruence of these events with known geographical barriers, climate events, and the boundaries of our identified bioregions.
Results
Biogeographic Regions in the Tropical Andes.
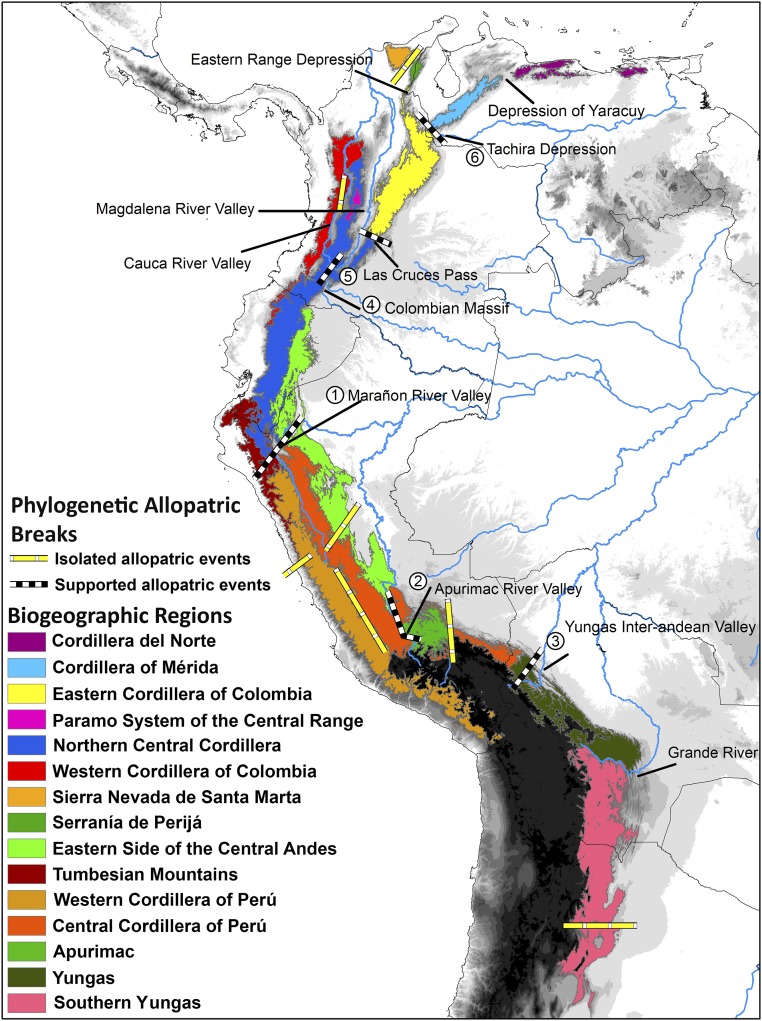
We found 15 bioregions in the tropical Andes (Fig. 1 and SI Appendix, Fig. S1). The highest number of bioregions was found north of the Colombian Massif where the Andes split into three ranges or cordilleras, each of them found to be a different biogeographic region: Northern Central Cordillera, and “Western” and “Eastern” Cordilleras of Colombia. There were also two isolated mountain systems identified as biogeographical regions to the north of South America: “Sierra Nevada de Santa Marta” and “Serranía de Perijá.” Within the Northern Central Cordillera region, we identified the smaller bioregion: the “Páramo System of the Central Range.” In addition, to the northeast in Venezuela we identified two other bioregions: “Cordillera del Norte” and “Cordillera of Mérida.” To the south of the Colombian Massif, the Western and Central Cordilleras extend into Ecuador, where a new bioregion arises on the east side of the Andes and goes to the south of Peru, “eastern side of the Central Andes.” In the southwest of Ecuador, there is an additional bioregion, the “Tumbesian Mountains,” which extends to the northern part of Peru. In Peru, three more biogeographic regions can be recognized: the Western and Central Cordilleras of Peru and Apurimac. Finally, the bioregions “Bolivian Yungas” and “Southern Andean Yungas” are on the eastern Andean side of Bolivia and northern Argentina.
Fig. 1.
Biogeographical regions identified in the tropical Andes using 151 endemic bird taxa with the network approach in Infomap Bioregions. The phylogenetic allopatric breaks of the 14 genera phylogenies were generated by the software Vicariance Inference Program (VIP).
The structure and boundaries of the biogeographic regions, as visualized with richness maps of indicative species reveal a set of overlapping bioregions and diffuse limits (SI Appendix, Fig. S2). The limits are more distinguishable in small bioregions where richness is concentrated (e.g., Sierra Nevada de Santa Marta, Serranía de Perijá, Cordillera of Mérida). Also, some bioregions have a strong concentration of species richness and present very diffuse limits spreading from this core, such as the eastern side of the Central Andes, where richness is accumulated in the Marañon River Valley and diffuses extensively to the north and south. Most notably, the boundaries of several bioregions diffuse to overlap with the core of indicative species richness of other contiguous bioregions: the Eastern Cordillera of Colombia overlaps with the core of the Cordillera of Mérida and Serranía Perijá; the Northern Central Cordillera with the core area of Western Cordillera of Colombia; the Central Cordillera of Peru with the core area of Apurimac; and the Southern Yungas with the core of the Bolivian Yungas. In addition, there was a complete biogeographic region inside another larger region, the Páramo Systems of the Central Range and Central Cordillera of Colombia, respectively. Finally, there were also some overlapping regions where the boundaries of each presented low indicative species richness: the Tumbesian Mountains and Western Cordillera of Peru and the Central and Western Cordilleras of Peru.
Geographic Barriers and Events of Isolation and Diversification.
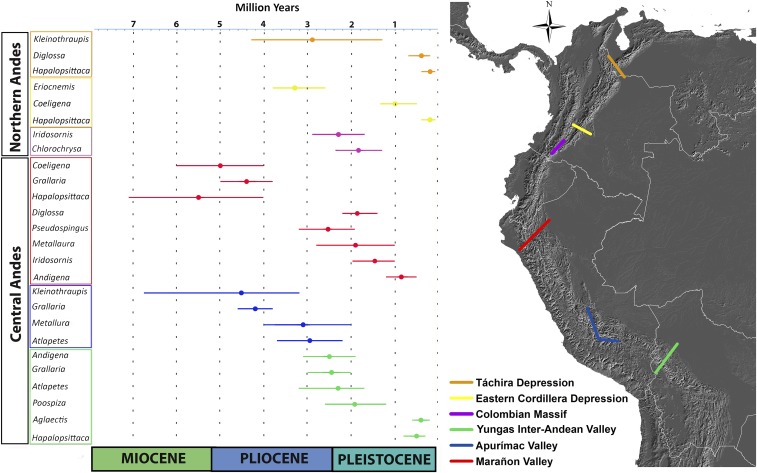
We found 33 traceable allopatry events formed by disjunct distributions in sister taxa (SI Appendix, Figs. S3–S8) based on published dated molecular phylogenies of 14 bird genera. Among these, 26 are supported allopatry events (SAEs) that are independently confirmed by more than one phylogeny, and are spatially congruent with six geographic barriers across the tropical Andes: the Marañon, Apurimac, and Yungas Inter-Andean Valleys; the Colombian Massif, Las Cruces Pass, and the Táchira Depression (Figs. 1 and 2). The SAEs occurred between the last Miocene to the Pleistocene (7–0.08 Ma, Fig. 2) and most of them were asynchronous even for the same barrier. Therefore, they are related to different isolation and diversification processes and not only to a single event.
Fig. 2.
Spatial and temporal analysis of the six congruent allopatry events supported by more than one phylogeny. Colors of the divergence interval time of each genus correspond to the colors of the barriers on the map to the Right. Numbers 1–6 represent the six congruent allopatry events.
When we compare the barriers related to these isolation and diversification events with the boundaries of the biogeographical regions identified, it is easy to realize that they present a strong spatial match. In fact, 10 of the 13 allopatric distribution breaks found (six SAEs and seven by only a single phylogeny) match with the boundaries of the bioregions. In addition, the six SAEs (see SI Appendix, Fig. S2B for bioregion 4 and the Colombian Massif barrier) are also spatially consistent with the boundaries of the bioregions. Moreover, there are some SAEs, with barriers that are congruent with the boundaries of not only one, but several bioregions (e.g., the Marañon River Valley).
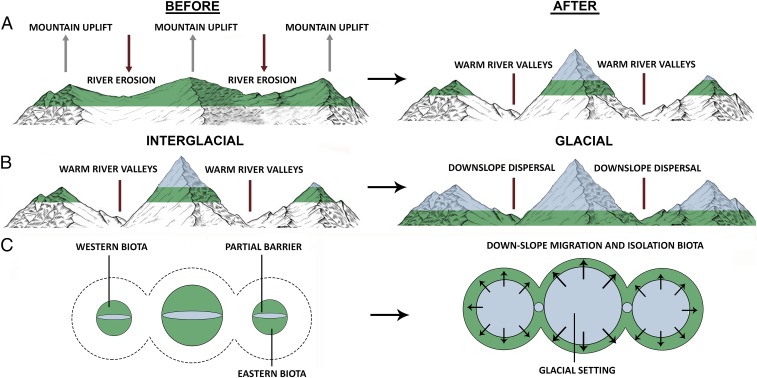
We conclude that these geographic barriers and their associated historical isolation and diversification events may have been some of the main drivers for these biogeographic regions, and are consistent with three models of speciation, two of vicariance, and one of dispersal and subsequently isolation (Fig. 3): (i) A vicariance model of Andean uplift, which together with the incision of the deep canyons (river valleys) caused by erosion has passively isolated populations associated with high mountain humid ecosystems, (ii) a dispersal model facilitated by Pleistocene temperature oscillations, where highland Andean biota spread out with increased connections to lower ecosystems with temperature decrease, and subsequent isolation by the increase of temperature during interglacial cycles, (iii) a vicariance model where the distribution of sister populations from midelevations occurring in different slopes of the same range become isolated because the high peaks that partially separated them became a stronger barrier during glacial cycles. A complete account of how these three models of speciation are related to each of the individual barriers is available in SI Appendix.
Fig. 3.
Three biogeographical models of speciation found in the tropical Andes. (A) Vicariance model of Andean uplift, together with the incision of the deep canyons (river valleys). (B) Dispersal model facilitated by Pleistocene temperature oscillations, where highland Andean biota spread out with increased connections to lower elevations with temperature decrease. (C) Vicariance model (aerial view from above) where the distribution of sister populations from midelevations occurring in different slopes (eastern and western) of the same range become isolated because the high peaks that partially separated them became a stronger barrier during glacial intervals. Green areas represent the biota restricted to mountain ecosystems. Blue light represents the covering of the glaciers.
Discussion
The present work identified 15 biogeographic regions in the tropical Andes and found that the boundaries of these bioregions can be related to conspicuous topographical barriers such as warm and/or dry valleys, discontinuities in elevation and high peaks separating fauna from different slopes. Furthermore, these boundaries also show a strong spatial congruence with events of isolation and diversification that are independently confirmed with disjunct distributions of sister taxa, and we find evidence that the drivers of these events were vicariance processes of Andean uplift and dispersal facilitated by temperature oscillation of the Pleistocene. Together, these processes produced three models of speciation that interacted through different time scales to generate current distributional patterns and therefore high endemism and diversity turnover in small spatial scales (2, 32). Thus, Andean bioregion formations are not related to just one simple biogeographical event framed to a single period of time, but it was a combination of vicariance and dispersal events, which occurred in different time periods.
The geography of speciation in the Andes is exemplified in the eastern ranges found in the Northern Andes. Here, two barriers coincide with elevation discontinuities for three of our identified bioregions: Las Cruces Pass between the Northern Central Andes and the Eastern Cordillera of Colombia regions, and the Táchira Depression in the Mérida Cordillera region. In these areas sister groups from two independent phylogenies (Eriocnemis and Kleinothraupis) were isolated and diverged during the Pliocene (4.2–2.8 Ma). This is congruent with the final and fast phase of the uplift of these Cordilleras (8, 39) that was accentuated with the appearance of warm valleys and sharp elevation discontinuities. A second period of isolation and divergence happened in the Early to Middle Pleistocene (1.5–0.05 Ma), and could be associated with dispersal events, because it occurred when these Cordilleras were already uplifted (8, 39, 40). Well-documented paleoecological studies in the Eastern Cordillera of Colombia and Cordillera of Mérida confirm that ecosystems above the forest tree line (páramos) underwent strong vertical shifts in vegetation belts around 2.7–0.02 Ma, causing a range expansion and contractions of the flora and fauna (12, 15, 40, 41). In fact, present day Páramo of Colombia is only 5% of its former area (15) and there are records of this ecosystem reaching at elevations as low as 1900 m (40). Thus, highland mountain ecosystems were connected during glacial periods, and subsequently during interglacial periods, warm climatic conditions fragmented these into isolated islands, separating the biota specific from them.
Therefore, discontinuities in the elevation and warm river valleys with the interaction of climate change in the Pleistocene were significant factors that affected the distribution patterns of Andean species that are restricted to the highlands ecosystems (cloud forests and páramo). Indeed, dry and warm river valleys constitute four of our six identified geographic barriers. The most notable example is the Marañon River Valley, which separates and limits the boundaries of four biogeographical regions separating the Central and Northern Andes. Our study was not only able to detect bioregions separated by notorious and well-documented barriers like the Marañon, but the boundaries of our bioregions are congruent with the boundaries of areas of high endemism of plant and animal taxa in the Eastern Andean slope of Peru and Bolivia (42, 43) that have no obvious limits. In addition, many of the biogeographic regions identified in this work are also congruent with areas with high richness of narrowly distributed bird species (21). Thus, we recover previous indications of these regions as biogeographic units, although the evolutionary history of these areas still needs to be further explored.
We found that the complex structures and transition zones of the biogeographical regions revealed by the richness maps of indicative species support new ideas of recent studies that contradict previous conceptions of biogeographical regions as nonoverlapping and spatially simple in scale (44). In addition, it was possible to detect contact areas between bioregions, which correspond to either depauperate or species-rich transition zones (45), where the overlap area presents a progressive loss or gain of taxa, respectively. These biogeographical areas indicate that the distribution patterns of Andean biota are significantly more complex than the most recent regionalization (23), especially to the north of the Andes where they reach their maximum topographic complexity.
In conclusion, our study found complex patterns of biota distribution reflected in the high number of bioregions at small spatial scales, each of them with a unique combination of species, and therefore they are essential units of conservation. Furthermore, the integration of multiple phylogenies of different lineages allowed us to identify that one of the primary drivers of these regions was the formation of sizeable warm river valleys during periods of high Andean uplift, which isolated species restricted to highland ecosystems. Then, these ecosystems underwent strong vertical shifts in vegetation belts toward these valleys during glacial periods of the Pleistocene, allowing species community dispersal. Thus, Andean bioregion drivers were a combination of vicariance and dispersal events, which occurred in different time periods. We expanded beyond decades-old interpretations that remained fixed on minor improvements for delimiting biogeographic regions in this complex area. We also successfully incorporated phylogenies and robust methods of bioregion identification with high-resolution species distribution models to identify distribution patterns and their drivers in one of the most endemic and biodiverse places on Earth. We recommend that future studies of regionalization of the Andes include additional biological groups of plants and animals and use a hypothesis-driven framework to assess the contribution of different ecological and evolutionary processes.
Methods
Study Area and Taxa Selection.
Our study area encompasses the mountains of the tropical Andes (>500 m; 9° 27′ N; 15° 74′ S; 80° 41′; 69° 26′ W) excluding the Puna. We chose avian genera that have overlapping distributions of monophyletic lineages across the entire region (e.g., Andigena, Diglossa, and Metallura) to help us unmask biogeographic patterns at a higher level and to make use of published dated molecular phylogenies. We selected all taxa within a genus when possible, but excluded those with only a handful of records and/or present in few localities. Our final selection included 151 taxa from 28 genera, 142 species, and 9 subspecies with a balance between different life histories and ecology. We followed the taxonomy of the South American Classification Committee (46), with subspecies treatment by Handbook of the Birds of the World Alive (47) and accepted the proposed split of Ocreatus by Schuchmann et al. (48) (SI Appendix, Table S1).
Distribution Mapping.
We sought to generate high-resolution species maps that minimize distributional gaps and that capture the complexity and small scale turnover in the tropical Andes. We made use of the exponential growth of occurrence records for bird species available as digital accessible knowledge (25) to estimate potential distribution maps with Maxent (49). We gathered occurrences of our selected species from eBird (eBird 2017) and the Global Biodiversity Information Facility (GBIF; https://www.gbif.org). To produce a final map, we used expert criteria to refine the output models by generating a polygon encompassing the filtered occurrence points. We did this to avoid overestimation of the distribution range in areas of known absence and to account for dispersal limitations (50). Please refer to SI Appendix, Supporting Methods for further details.
Biogeographic Region Identification.
We used a recently developed network approach to identify biogeographic regions, integrated in the Infomap Bioregions web application (51). Here, the distribution of all species is organized with an adaptive resolution method into spatially explicit grid cells to reflect differences on the data density. Then, a bipartite network is generated between species and the grid cells, and bioregions are produced by clustering the network with the widely used algorithm known as Infomap. This procedure using network theory has been shown to outperform the classic turnover measures based on beta diversity that are frequently used in biogeography (26, 52). Because the results are more scale independent, the method allows endemic taxa to contribute more strongly to the identification of bioregions, and is less impacted by sampling biases (53).
To run the analyses on Infomap Bioregions, we decided to use a grid size with cell ranges between 0.125° and 0.25°, given that our species records were extracted in a resolution lower than 0.15°. We used the default settings with maximum cell capacity = 100 and minimum cell capacity = 10, which sets the limits for the adaptive resolution algorithm to operate. Also, we used number of trials = 1, and number of cluster cost = 1.0, which are recommended. The application gives an output of the most common species for each bioregion and the most indicative species, the latter defined as the ratio between the frequency of the species in a bioregion against all bioregions, thus reflecting its degree of endemicity (51) (SI Appendix, Table S2). We created richness maps of the indicative species that have at least 75% of their distribution inside a bioregion to visualize the structure and boundaries of the bioregions (42, 53) (SI Appendix, Fig. S2). We adjusted the scale resolution of the bioregions at 30 arcseconds based on the raw output map of Infomap Bioregions (SI Appendix, Fig. S1), the richness map of the indicative species, and an elevation model. We adjust the scale resolution of the bioregions at 30 arcseconds.
Spatial and Temporal Analysis of Allopatry.
To identify spatial and temporal events of isolation and diversification, we applied an allopatry analysis using Hovenkamp’s protocol (54, 55) with modifications. The protocol aims to reconstruct the history of a geographic area through a sequence of vicariance events inferred from the allopatric distributions of sister taxa. This allows for reticulate and divergent patterns without assuming hierarchical relationship of areas, avoiding unrealistic assumptions prevalent in traditional cladistic biogeographical methods (56). The focus of the protocol is on detecting barriers that separate biotas, not on the relationship between predefined areas of endemism, and it is therefore suited for disentangling the origins of current distributional patterns of taxa (57) and the boundaries of our bioregions.
Our analysis was conducted following four steps using previously chosen dated molecular phylogenies of 14 genera of birds (SI Appendix, Table S3). First, we searched for allopatric distributions in each node of the phylogenies, called traceable allopatric events (TAEs). We used the software Vicariance Inference Program (VIP; www.zmuc.dk/public/phylogeny/vip) (57) to search for allopatric distributions using a grid of 0.1° and the von Neumann neighborhood options and left the maximum fill option unchecked. We allowed 20% of maximum overlap in the distribution between sister groups, and we disallowed the use of the partial removal option, which sets a cost to the partial distribution removal for terminals. The search was conducted with 1,000 iterations, keeping 20 reconstructions per iteration.
In the second step, we extracted the temporal sequence information of the allopatric events in each phylogeny. In the third step, we searched for allopatric events that were confirmed by more than a single sister-group relationship, or SAEs. We achieved this by visualizing the spatial congruence of allopatric distribution in independent nodes or phylogenies (SI Appendix, Figs. S3–S8). In the fourth step, we ordered the supported allopatric events into a temporal sequence based on the temporal information extracted from the second step (for details see ref. 44). We made use of divergence times as a source of temporal information, modifying steps 2 and 4 because dated phylogenies were scarce when the original method was proposed.
Supplementary Material
Acknowledgments
We thank Rebecca Clement and Robert J. Kallal for English editing; Carlos Jaramillo for providing us valuable literature about Andes uplift and climate change in the Pleistocene; Soroush Parsa for comments throughout the development of the study; Iván Moreno for help with the design and elaboration of Fig. 3; and three anonymous reviewers and the editor for helpful suggestions to improve the manuscript. N.A.H. thanks Gustavo Hormiga, Robert J. Kallal, and Thiago da Silva Moreira for comments and discussion during the first phases of the study.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1803908115/-/DCSupplemental.
References
- 1.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 2.Kattan GH, Franco P, Rojas V, Morales G. Biological diversification in a complex region: A spatial analysis of faunistic diversity and biogeography of the Andes of Colombia. J Biogeogr. 2004;31:1829–1839. [Google Scholar]
- 3.Sklenář P, Hedberg I, Cleef AM. Island biogeography of tropical alpine floras. J Biogeogr. 2014;41:287–297. [Google Scholar]
- 4.von Humboldt A, Bonpland A. Essai sur la géographie des plantes. Chez Levrault, Schoell et compagnie, libraires; Paris: 1805. [Google Scholar]
- 5.Chapman FM. The distribution of bird-life in Colombia : A contribution to a biological survey of South America. Bull Am Mus Nat Hist. 1917;36:1–729. [Google Scholar]
- 6.Chapman FM. The distribution of bird-life in in Ecuador: A contribution to a study of the origin of Andean bird-life. Bull Am Mus Nat Hist. 1926;55:1–784. [Google Scholar]
- 7.Kattan GH, Tello SA, Giraldo M, Cadena CD. Neotropical bird evolution and 100 years of the enduring ideas of Frank M. Chapman. Biol J Linn Soc Lond. 2016;117:407–413. [Google Scholar]
- 8.Hoorn C, et al. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science. 2010;330:927–931. doi: 10.1126/science.1194585. [DOI] [PubMed] [Google Scholar]
- 9.Luebert F, Weigend M. Phylogenetic insights into Andean plant diversification. Front Ecol Evol. 2014;2:27. [Google Scholar]
- 10.Sánchez-González LA, Navarro-Sigüenza AG, Krabbe NK, Fjeldså J, García-Moreno J. Diversification in the Andes: The Atlapetes brush-finches. Zool Scr. 2015;44:135–152. [Google Scholar]
- 11.Winger BM, et al. Inferring speciation history in the Andes with reduced-representation sequence data: An example in the bay-backed antpittas (Aves; Grallariidae; Grallaria hypoleuca s. l.) Mol Ecol. 2015;24:6256–6277. doi: 10.1111/mec.13477. [DOI] [PubMed] [Google Scholar]
- 12.Flantua SGA, Hooghiemstra H. Historical Connectivity and Mountain Biodiversity. Wiley-Blackwell; Chichester, UK: 2018. pp. 171–185. [Google Scholar]
- 13.Quintero E, Ribas CC, Cracraft J. The Andean Hapalopsittaca parrots (Psittacidae, Aves): An example of montane-tropical lowland vicariance: Biogeography of Hapalopsittaca parrots. Zool Scr. 2013;42:28–43. [Google Scholar]
- 14.Ramírez-Barahona S, Eguiarte LE. The role of glacial cycles in promoting genetic diversity in the Neotropics: The case of cloud forests during the last glacial maximum. Ecol Evol. 2013;3:725–738. doi: 10.1002/ece3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzette F, et al. 2014. Connectivity dynamics since the Last Glacial Maximum in the northern Andes; a pollen-driven framework to assess potential migration. Paleobotany and Biogeography: A Festschrift for Alan Graham in His 80th Year, Monographs in systematic botany from the Missouri Botanical Garden, eds Graham A, Stevens WD, Raven PH (Missouri Botanical Garden, St. Louis), pp 98–123.
- 16.Wallace AR. The Geographical Distribution of Animals; with a Study of the Relations of Living and Extinct Faunas as Elucidating the Past Changes of the Earth’s Surface. Vol 1 Macmillan & Co; London: 1876. [Google Scholar]
- 17.Ricklefs RE. Disintegration of the ecological community. Am Nat. 2008;172:741–750. doi: 10.1086/593002. [DOI] [PubMed] [Google Scholar]
- 18.Noguera-urbano EA. Areas of endemism: Travelling through space and the unexplored dimension. Syst Biodivers. 2016;14:131–139. [Google Scholar]
- 19.Hausdorf B. Units in biogeography. Syst Biol. 2002;51:648–652. doi: 10.1080/10635150290102320. [DOI] [PubMed] [Google Scholar]
- 20.Ficetola GF, Mazel F, Thuiller W. Global determinants of zoogeographical boundaries. Nat Ecol Evol. 2017;1:89. doi: 10.1038/s41559-017-0089. [DOI] [PubMed] [Google Scholar]
- 21.Stattersfield AJ, Crosby MJ, Long AJ, Wege DC. Endemic Bird Areas of the World: Priorities for Biodiversity Conservation. BirdLife International; Cambridge, UK: 1998. [Google Scholar]
- 22.Vilhena DA, Antonelli A. Beyond similarity : A network approach for identifying and delimiting biomes. Nat Commun. 2014;6:1–9. doi: 10.1038/ncomms7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrone JJ. Biogeographical regionalisation of the Andean region. Zootaxa. 2015;3936:207–236. doi: 10.11646/zootaxa.3936.2.3. [DOI] [PubMed] [Google Scholar]
- 24.Sclater PL. On the general geographical distribution of the members of the class aves. J Proc Linn Soc London Zool. 1858;2:130–136. [Google Scholar]
- 25.Sousa-Baena MS, Garcia LC, Peterson AT. Completeness of digital accessible knowledge of the plants of Brazil and priorities for survey and inventory. Divers Distrib. 2014;20:369–381. [Google Scholar]
- 26.Kreft H, Jetz W. A framework for delineating biogeographical regions based on species distributions: Global quantitative biogeographical regionalizations. J Biogeogr. 2010;37:2029–2053. [Google Scholar]
- 27.Holt BG, et al. An updated of Wallace’s zoogeographic regions of the World. Science. 2013;339:74–79. doi: 10.1126/science.1228282. [DOI] [PubMed] [Google Scholar]
- 28.Crisp MD, Trewick SA, Cook LG. Hypothesis testing in biogeography. Trends Ecol Evol. 2011;26:66–72. doi: 10.1016/j.tree.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Warren DL, Cardillo M, Rosauer DF, Bolnick DI. Mistaking geography for biology: Inferring processes from species distributions. Trends Ecol Evol. 2014;29:572–580. doi: 10.1016/j.tree.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Wiley EO. Vicariance biogeography. Annu Rev Ecol Syst. 1988;19:513–542. [Google Scholar]
- 31.Halffter G, editor. La Diversidad Biologica de Iberoamerica. 1st Ed. CYTED-D, Programa Iberoamericano de Ciencia y Tecnologia para el Desarollo, Instituto de Ecologia, A.C., Secretaria de Desarrollo Social; Xalapa, México: 1992. Centros de Endemismo en Colombia; pp. 175–190. [Google Scholar]
- 32.Graham CH, Silva N, Velásquez-Tibatá J. Evaluating the potential causes of range limits of birds of the Colombian Andes: Range limits of birds of the Colombian Andes. J Biogeogr. 2010;37:1863–1875. [Google Scholar]
- 33.Gutiérrez EE, et al. The taxonomic status of Mazama bricenii and the significance of the Táchira depression for mammalian endemism in the Cordillera de Mérida, Venezuela. PLoS One. 2015;10:e0129113. doi: 10.1371/journal.pone.0129113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith BT, et al. The drivers of tropical speciation. Nature. 2014;515:406–409. doi: 10.1038/nature13687. [DOI] [PubMed] [Google Scholar]
- 35.Cracraft J. Historical biogeography and patterns of differentiation within the South American avifauna: Areas of endemism. Ornithol Monogr. 1985;36:49–84. [Google Scholar]
- 36.Haffer J. Speciation in amazonian forest birds. Science. 1969;165:131–137. doi: 10.1126/science.165.3889.131. [DOI] [PubMed] [Google Scholar]
- 37.Fjeldså J, Bowie RCK, Rahbek C. The role of mountain ranges in the diversification of birds. Annu Rev Ecol Evol Syst. 2012;43:249–265. [Google Scholar]
- 38.Quintero I, Jetz W. Global elevational diversity and diversification of birds. Nature. 2018;555:246–250. doi: 10.1038/nature25794. [DOI] [PubMed] [Google Scholar]
- 39.Gregory-Wodzicki KM. Uplift history of the Central and Northern Andes: A review. Geol Soc Am Bull. 2000;112:1091–1105. [Google Scholar]
- 40.Hooghiemstra H, Wijninga VM, Cleef AM. The paleobotanical record of Colombia: Implications for biogeography and biodiversity 1. Ann Mo Bot Gard. 2006;93:297–325. [Google Scholar]
- 41.Torres V, Hooghiemstra H, Lourens L, Tzedakis PC. Astronomical tuning of long pollen records reveals the dynamic history of montane biomes and lake levels in the tropical high Andes during the quaternary. Quat Sci Rev. 2013;63:59–72. [Google Scholar]
- 42.Swenson JJ, et al. Plant and animal endemism in the eastern Andean slope: Challenges to conservation. BMC Ecol. 2012;12:1. doi: 10.1186/1472-6785-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young BE, et al. Using spatial models to predict areas of endemism and gaps in the protection of Andean slope birds. Auk. 2009;126:554–565. [Google Scholar]
- 44.Bertelli S, et al. Mexican land birds reveal complexity in fine-scale patterns of endemism. J Biogeogr. 2017;44:1836–1846. [Google Scholar]
- 45.Ferro I, Morrone JJ. Biogeographical transition zones: A search for conceptual synthesis. Biol J Linn Soc Lond. 2014;113:1–12. [Google Scholar]
- 46.Remsen JV, et al. 2017 A classification of the bird species of South America. Version 22 April 2017. Available at www.museum.lsu.edu/∼Remsen/SACCBaseline.htm. Accessed December 18, 2017.
- 47. del Hoyo J, Elliot A, Sargatal J, Christie DA, de Juana E, eds (2017) Handbook of the Birds of the World Alive. Available at www.hbw.com/. Accessed January 5, 2018.
- 48.Schuchmann K-L, Weller A-A, Jürgens D. Biogeography and taxonomy of racket-tail hummingbirds (Aves: Trochilidae: Ocreatus): Evidence for species delimitation from morphology and display behavior. Zootaxa. 2016;4200:83. doi: 10.11646/zootaxa.4200.1.3. [DOI] [PubMed] [Google Scholar]
- 49.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Modell. 2006;190:231–259. [Google Scholar]
- 50.Barve N, et al. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol Modell. 2011;222:1810–1819. [Google Scholar]
- 51.Edler D, Guedes T, Zizka A, Rosvall M, Antonelli A. Infomap bioregions: Interactive mapping of biogeographical regions from species distributions. Syst Biol. 2016;66:197–204. doi: 10.1093/sysbio/syw087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bloomfield NJ, Knerr N, Encinas-Viso F. A comparison of network and clustering methods to detect biogeographical regions. Ecography. 2018;41:1–10. [Google Scholar]
- 53.Vilhena DA, Antonelli A. A network approach for identifying and delimiting biogeographical regions. Nat Commun. 2015;6:6848. doi: 10.1038/ncomms7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hovenkamp P. Vicariance events, not areas, should be used in biogeographical analysis. Cladistics. 1997;13:67–79. doi: 10.1111/j.1096-0031.1997.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 55.Hovenkamp P. A direct method for the analysis of vicariance patterns. Cladistics. 2001;17:260–265. doi: 10.1111/j.1096-0031.2001.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 56.Fattorini S. Hovenkamp’s ostracized vicariance analysis: Testing new methods of historical biogeography. Cladistics. 2008;24:611–622. doi: 10.1111/j.1096-0031.2007.00193.x. [DOI] [PubMed] [Google Scholar]
- 57.Arias JS, Szumik CA, Goloboff PA. Spatial analysis of vicariance: A method for using direct geographical information in historical biogeography. Cladistics. 2011;27:617–628. doi: 10.1111/j.1096-0031.2011.00353.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.