Significance
Pore-forming toxins (PFTs) are the largest class of bacterial exotoxins mediating virulence. Soluble toxin monomers oligomerize upon binding to cellular membrane and convert to stable membrane-integrated pores, causing cell death. This conversion to an active form occurs in absence of extrinsic factors and is governed solely by molecular determinants in the protein and target membrane. Here we demonstrate the existence of cholesterol-binding motifs in ClyA, which stabilize structural intermediates in the assembly pathway in presence of cholesterol. Our finding elucidates the basis for selective targeting of the toxin to eukaryotic membranes. Molecular engineering of these signatures could advance application of PFTs in cytolytic therapy.
Keywords: pore-forming toxin, membrane, cholesterol, single-molecule imaging, molecular dynamics
Abstract
Pore-forming toxins (PFTs) form nanoscale pores across target membranes causing cell death. Cytolysin A (ClyA) from Escherichia coli is a prototypical α-helical toxin that contributes to cytolytic phenotype of several pathogenic strains. It is produced as a monomer and, upon membrane exposure, undergoes conformational changes and finally oligomerizes to form a dodecameric pore, thereby causing ion imbalance and finally cell death. However, our current understanding of this assembly process is limited to studies in detergents, which do not capture the physicochemical properties of biological membranes. Here, using single-molecule imaging and molecular dynamics simulations, we study the ClyA assembly pathway on phospholipid bilayers. We report that cholesterol stimulates pore formation, not by enhancing initial ClyA binding to the membrane but by selectively stabilizing a protomer-like conformation. This was mediated by specific interactions by cholesterol-interacting residues in the N-terminal helix. Additionally, cholesterol stabilized the oligomeric structure using bridging interactions in the protomer–protomer interfaces, thereby resulting in enhanced ClyA oligomerization. This dual stabilization of distinct intermediates by cholesterol suggests a possible molecular mechanism by which ClyA achieves selective membrane rupture of eukaryotic cell membranes. Topological similarity to eukaryotic membrane proteins suggests evolution of a bacterial α-toxin to adopt eukaryotic motifs for its activation. Broad mechanistic correspondence between pore-forming toxins hints at a wider prevalence of similar protein membrane insertion mechanisms.
Pore-forming toxins (PFTs) are cell membrane-rupturing proteins and form the largest class of toxins that mediate bacterial virulence (1–3). PFTs are secreted as water-soluble monomers that bind strongly to the lipid membrane of eukaryotic cells by adopting structures that traverse the membrane via helices (α-PFT) or sheets (β-PFT). This allows the passage of molecules from within the cell to the exterior, resulting in host cell lysis. The conformational transition of a PFT from a water-soluble structure to a distinct membrane-associated protomer form is not understood in mechanistic detail. For example, do components in the eukaryotic cell membrane drive the conformational transitions that result in an assembly competent state? Does the membrane play an active role in stabilization of intermediates that allow membrane insertion and pore formation? Membrane components that are essential in the β-PFT assembly pathway have been well characterized and include protein receptors, carbohydrates, or eukaryotic lipids such as cholesterol and sphingomyelin (1). Determinants of membrane selectivity are poorly understood in the case of α-PFTs with the exception of some reports of sphingomyelin as a cofactor for toxin function for certain actinoporins (4, 5). Another crucial but less probed aspect of the assembly pathway is the role of the toxin’s lateral motion on membranes. Toxin self-assembly is contingent on establishing interprotomer contacts, which is influenced by lateral motion of the individual protein molecules on the membrane surface. However, little is known about toxin dynamics on the lipid membrane and its implication on the formation of higher-order structures.
Here we address these questions using a prototypical α-PFT, Cytolysin A, which is produced by strains of Escherichia coli, Shigella, and Salmonella (6). The water-soluble ClyA monomer produced by these bacteria is composed of a five–α-helix bundle and a hydrophobic β-hairpin (β-tongue) (7) (SI Appendix, Fig. S1). The protein assembles into a dodecameric cation-selective pore complex upon membrane binding. The structure of the ClyA pore (assembled in detergents) displayed large structural alterations in protomers of the pore, compared with the monomeric water-soluble form (8). The β-tongue in the monomer transforms into a helix–loop–helix, and reorganization of α-helices causes the N-terminal helix to switch orientation by ∼180°, to form the inner lumen of the pore.
Detergent-induced ClyA oligomerization experiments have indicated that conformational transition in ClyA is the rate-limiting step in the assembly pathway (9, 10). However, ClyA assembly driven by surfactants results in significantly slower kinetics compared with membrane rupture and leakage (11). Apart from kinetic modeling studies (12), little is understood about the kinetics of this process in phospholipid bilayers. More importantly, it is not clear how pore-like ClyA complexes as observed on bacterial outer membranes vesicles (OMVs) can form without altering bacterial viability, even upon overexpression, whereas similar oligomeric complexes are cytotoxic in eukaryotic membranes (13, 14).
Here we characterize the initial steps of interaction of ClyA with model membranes and events that ultimately lead to the formation of a pore. Using single-molecule fluorescence-based particle tracking and photobleaching analysis on supported lipid bilayers (SLBs), we identify distinct diffusive states that represent altered conformations of the toxin. The distribution of sampled states is sensitive to cholesterol content, and specific cholesterol-interacting residues selectively promote membrane insertion of the transmembrane segment of ClyA in presence of cholesterol. Furthermore, all-atom molecular dynamics simulations reveal specific cholesterol–protein interactions that mediate membrane binding and oligomerization. Together, these cholesterol-stabilized ClyA intermediates bias the assembly pathway towards pore formation.
Results
Cholesterol Enhances Membrane Rupture by ClyA.
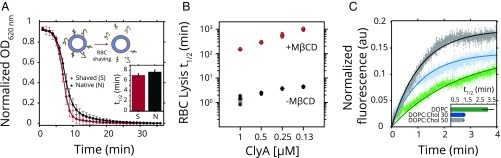
ClyA interacts directly with the lipid membranes (6), but it is not clear whether any additional receptors are required for efficient ClyA pore formation on cellular membranes. We therefore monitored rabbit erythrocyte lysis following proteolytic cleavage of exposed cell surface proteins (Fig. 1A and SI Appendix, Fig. S2A) and continued to observe efficient and equivalent lysis. In contrast, erythrocytes pretreated with methyl β-cyclodextrin (MβCD), which selectively reduces the cholesterol content of the plasma membrane (15, 16), displayed a drop in cell lysis by at least two orders in magnitude over a wide range of toxin concentrations (Fig. 1B). Cholesterol-regulated ClyA activity was also observed in vesicle dye leakage assays (6). For example, incorporation of cholesterol in DOPC (1:1 mol %) vesicles resulted in a threefold enhancement in ClyA lytic activity (Fig. 1C and SI Appendix, Fig. S2B).
Fig. 1.
Cholesterol stimulates the pore-forming activity of ClyA. (A) Turbidity assay to determine activity of ClyA is performed by measuring the optical density of rabbit erythrocytes (black) with time after addition of ClyA. Proteolytic shaving of erythrocyte membranes (shaved RBC; red) did not change the t1/2 of the lysis (Inset) or the extent of lysis. Solid lines represent Boltzmann sigmoid fits to the data. (B) Partial removal of cholesterol from erythrocytes by treatment with MβCD (red) increases the t1/2 for lysis in turbidity assays by more than 100-fold compared with untreated erythrocytes (black). (C) Vesicle dye leakage kinetics of ClyA for DOPC (green) and DOPC with 30% cholesterol (blue) and 50% cholesterol (gray) concentrations are shown. Solid lines represent single exponential fits to the leakage data. The dye leakage t1/2 in presence of cholesterol is reduced by approximately threefold for 30–50 mol % cholesterol (Inset). Error bars represent SD of at least three experiments.
ClyA Binding to Artificial Bilayer Membranes.
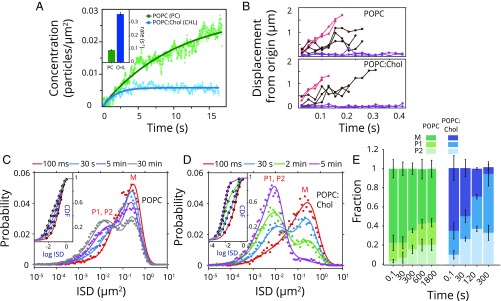
We first asked if the stimulation in activity in the presence of cholesterol was merely a consequence of enhanced binding. We examined ClyA interaction with lipid membranes by single-molecule particle tracking of fluorescently labeled ClyA on phospholipid bilayers using total internal reflection fluorescence (TIRF) microscopy (17, 18). We established a single ClyA imaging assay on supported lipid bilayers using a functionally active, singly labeled (∼60% labeling efficiency) ClyA (Q56C) mutant (10). ClyA appeared to be immobile on conventional membrane platforms (SI Appendix, Figs. S3 and S4A), possibly because of strong surface effects of the underlying substrate (19, 20). Hence, polymer-supported bilayers (PEG-SLBs) were used in all subsequent experiments (SI Appendix, SI Results and Fig. S4 B and C). Toxin binding to the membrane was assessed by monitoring the appearance of fluorescent spots on the membrane surface as ClyA (100 pM) was introduced into a microchannel containing PEG-SLBs. Unimodal intensity distribution and single-step photobleaching suggested monomeric ClyA as the dominant membrane population (SI Appendix, Fig. S5). The binding of ClyA reached equilibrium within tens of seconds for POPC PEG-SLBs and those containing 27.5% cholesterol (Fig. 2A). We limited our experiments to these two membrane compositions (referred as POPC and POPC:Chol) because reports exist of membrane phase separation with higher concentrations of cholesterol either in POPC or in ternary lipid mixtures (21) that might complicate data interpretation.
Fig. 2.
Single-ClyA molecule dynamics on supported bilayers. (A) Binding kinetics of ClyA on POPC (green) and POPC:Chol (blue) SLB as determined by single exponential fit (line) to the increase in the number of ClyA particles observed on the membrane. (B) Representative single-particle trajectories (displacements from the origin) for ClyA molecules displaying low mobility (purple), high mobility (red), and heterogeneous behavior (black) on POPC (Top) and POPC:Chol (Bottom) membranes are shown. (C) Time series of ISD distribution for ClyA (n = 1,000–5,000 for each set) on POPC membranes is shown (ntotal = 37,000). Solid lines represent fits to a Gaussian mixture model (GMM) with three species. (D) Corresponding time series of ISD distribution (n = 800–1,200 for each set) on POPC:Chol bilayers are shown (ntotal = 18,000). The CDF of ISD is fit to three diffusive species for all time points (C and D, Insets). (E) Fraction of the ClyA diffusive species for both POPC (shades of green) and POPC:Chol (shades of blue) membranes is displayed for slow (P2, light), intermediate (P1, dark), and fast (M, darkest shade) populations at different time points after ClyA binding. Error bars represent SD.
Binding of ClyA reached equilibrium faster by a factor of ∼3 (0.36 ± 0.015 s−1 in POPC:Chol vs. 0.095 ± 0.004 s−1 in POPC) on cholesterol-containing membranes. However, contrary to cholesterol stimulation of ClyA activity, approximately fivefold less ClyA was bound to cholesterol-containing membranes at equilibrium even though the initial rates of ClyA binding were similar (∼0.003 particles per μm2 per s), suggesting (approximately fivefold) higher rates of unbinding from cholesterol-containing membranes. However, the rapid binding of ClyA to lipid membranes (seconds) contrasted with the much longer time scales (t1/2 ∼ 2–7 min) for detecting cell lysis or vesicle leakage, suggesting that ClyA binding to membranes was not the rate-limiting step in pore assembly.
Diffusional Dynamics of ClyA on the Membrane.
We asked if changes in the lateral mobility of the membrane-bound toxin explain the observed stimulation of ClyA activity. Therefore, we examined the diffusional properties of single ClyA at low densities (25–100 pM) on PEG-SLBs. We observed a large heterogeneity in the lateral displacements for ClyA particles on POPC and POPC:Chol membranes (Fig. 2B). On POPC PEG-SLBs, ClyA molecules predominantly exhibited a single distribution of instantaneous squared displacements (ISDs) with high mobility at early time points (∼100 ms) (Fig. 2C). A linear correlation between MSD and the lag times indicated that the motion was Brownian for the initial fast species (SI Appendix, Fig. S6A). However, the fast diffusing population gradually diminished with a concomitant increase in a broad population of lower-mobility states (Fig. 2C and SI Appendix, Fig. S6B). Global fitting of the cumulative distribution function (CDF) of instantaneous squared displacements yielded the presence of at least three diffusive states: a fast (M; DM = 1.43 ± 0.07 μm2/s), an intermediate (P1; DP1 = 0.24 ± 0.05 μm2/s), and a slow mobility state (P2; DP2 = 0.040 ± 0.006 μm2/s) (Fig. 2 C, Inset and 2E and SI Appendix, SI Results). The lower-mobility population did not demonstrate an increase in single-particle intensity compared with the initial fast ClyA species, hence arguing against oligomerization causing the reduction in mobility (SI Appendix, Fig. S6 B and C). The ISD distribution reached equilibrium within 10 min, but ∼55% of the molecules remained in the fast mobility state (M) (Fig. 2 C and E).
On POPC:Chol bilayers, the protein initially displayed rapid diffusion (Fig. 2D), with diffusion coefficients comparable to those determined in POPC membranes (SI Appendix, Fig. S7A and Table S1). However, ClyA quickly converted to the lower-mobility form with near-complete disappearance of the fast species (∼5 min), without any discernible increase in single-particle intensity (SI Appendix, Fig. S7B). The slow moving P2 fraction was similar in both POPC and POPC:Chol membranes, indicating that major changes occurred between the M and P1 states (Fig. 2E). This was further validated by quantifying the rates of conversion between the diffusive states for individual ClyA molecules.
Protein Conformational Transitions Underlie the Switch in Mobility States.
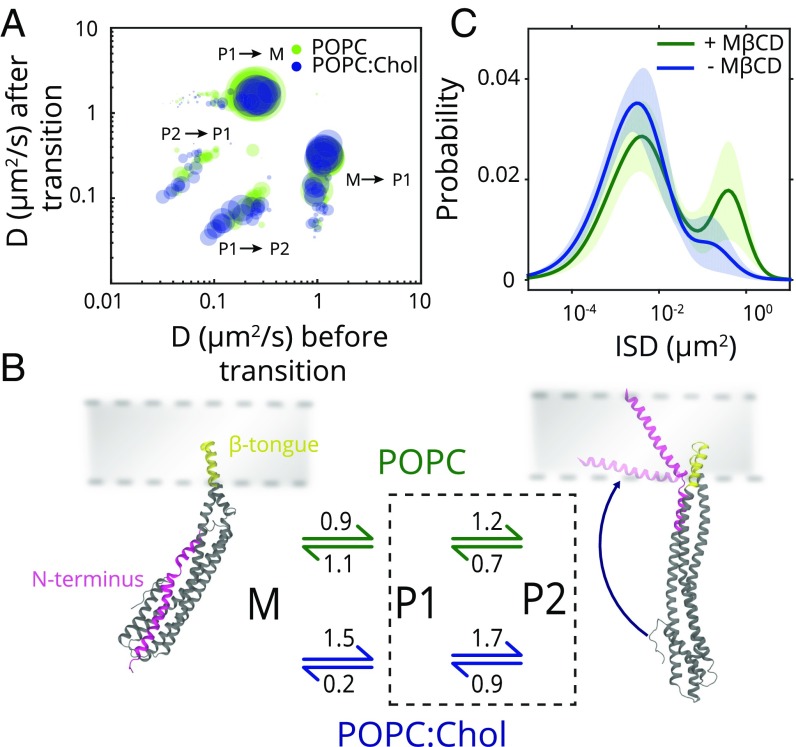
To determine the kinetics of transition between the ClyA mobility states, we employed hidden Markov model (HMM) analysis of the particle trajectories and assigned three diffusive states (22). We pooled all transitions across different time points for the two membrane compositions to generate a transition probability matrix (Fig. 3A). The cluster of transitions between the mobility states largely overlap between POPC and POPC:Chol membranes, indicating that ClyA sampled identical diffusive states in both POPC and POPC:Chol membranes. As observed in the CDF analysis (Fig. 2E), the majority of the transitions observed were between the M to P1 mobility states. Similar but less prominent transitions were observed between the P1 and P2 states, with a large distribution of diffusion coefficients. We estimated the mean diffusion coefficients of the three diffusive states to be 1.2–1.3 μm2/s, 0.06–0.12 μm2/s, and 0.02–0.03 μm2/s by pooling all of the particle trajectories for HMM analysis (SI Appendix, Table S1). Comparable values obtained for the diffusion coefficients for each of the mobility states with cumulative ISD fitting as well as HMM analysis support the existence of these distinct diffusive states. The HMM analysis indicated that ClyA could reversibly interconvert between these states.
Fig. 3.
Kinetics of diffusion state transitions and correspondence to structural states. (A) Transition probability matrix plotted from HMM-based assignments of transitions between different diffusive states for all time points for both POPC and POPC:Chol clusters is depicted (n = 37,000 for POPC and n = 18,000 for POPC:Chol). The major clusters representing transitions from monomer, M, to intramembrane, P1, and between P1 and P2 states and vice versa are marked. (B) Kinetic scheme of postulated ClyA conformations on the membrane with transition rates (s−1) is depicted. The two membrane domains in the protein (dark gray), namely, N-terminal helix (magenta) and β-tongue (yellow), and their locations with respect to the membrane (light gray) are highlighted. (C) ClyA ISD distribution before (blue) and after (green) MβCD treatment is shown with solid lines representing the GMM fits (shaded region indicates SD).
The average forward and reverse rates from the HMM analysis were computed (SI Appendix, Table S2). In POPC membranes, the kinetic rates for transition between monomer, M, and P1 states (kM → P1, POPC = 0.86 ± 3.3 × 10−6 s−1 and kP1 → M, POPC = 1.08 ± 1.3 × 10−5 s−1) and interconversion between the P1 and P2 states were fast (kP1 → P2, POPC = 1.2 ± 1.5 × 10−5 s−1 and kP2 → P1, POPC = 0.7 ± 1.0 × 10−5 s−1). However, low rates of direct conversion between M and P2 implied that M ↔ P1 ↔ P2 is the dominant pathway (Fig. 3 A and B and SI Appendix, Table S2). Therefore, the M ↔ P2 transitions were ignored in further analysis. In the presence of cholesterol, the rate for P1 → M conversion decreased approximately sixfold (kP1 → M, Chol = 0.18 ± 2 × 10−6 s−1) without significant changes in the rates of conversion between the other states. The cumulative and most significant effect of these changes in rates is a net increase (∼10-fold) in the formation of the P1 state in the presence of cholesterol. Thus, cholesterol affects the kinetics of transitions between the ClyA states by stabilizing the first intermediate (P1), postmembrane binding.
At the low concentrations used, ClyA did not oligomerize, nor were there changes in membrane fluidity or the presence of membrane domains in the two membrane systems used (SI Appendix, Fig. S8), ruling them out as factors inducing changes in ClyA mobility states. We therefore surmised that the lowered mobility state (P1) was a consequence of specific protein–lipid interactions. Based on order of appearance of the diffusive states upon membrane binding and consistent with the structural data (8), we assigned the diffusive states M and P1 as arising from two distinct conformations of ClyA, i.e., a β-tongue only and a β-tongue with N-terminal helix membrane-inserted forms (Fig. 3B). M and P1 states structurally resembled peripheral and transmembrane protein conformations, and their mobilities compared well with those reported for peripheral membrane (0.8–2 μm2/s) and transmembrane proteins (0.02–0.2 μm2/s) (23–27). Indeed, frictional effects of a single transmembrane helix have been reported to result in >55% reduction in diffusivity of a peripheral membrane protein (23). This is comparable to the decrease in diffusivity between the M and P1 states in ClyA, attributed to the insertion of the N-terminal helix. In addition, the M and P1 structures were the two prominent membrane-interacting conformations observed by Giri Rao et al. (28), where structure-based models captured the conformational transitions of ClyA from the monomer to the membrane-inserted protomer. In the same study, the order of the appearance of M and P1 states corresponded with those observed in our experiments. Although the identity of the P2 state is unclear at this stage, it could arise from the residual pinning of the transmembrane helix to the underlying glass surface or another structural state.
The significantly enhanced rate of switching of M to P1 state in cholesterol-containing membranes suggested that the distinct conformation of the P1 state is stabilized by the interaction of cholesterol with ClyA. To test this, we incubated POPC:Chol membranes with ClyA till diffusive states reached equilibrium and then treated these membranes with MβCD. We observed the reappearance of the fast-moving M species to comparable levels as observed in cholesterol-free membranes confirming that the direct interaction of cholesterol with ClyA was responsible for mediating the changes in toxin mobility (Fig. 3C).
N-Terminal Helix Interacts with Membrane Cholesterol.
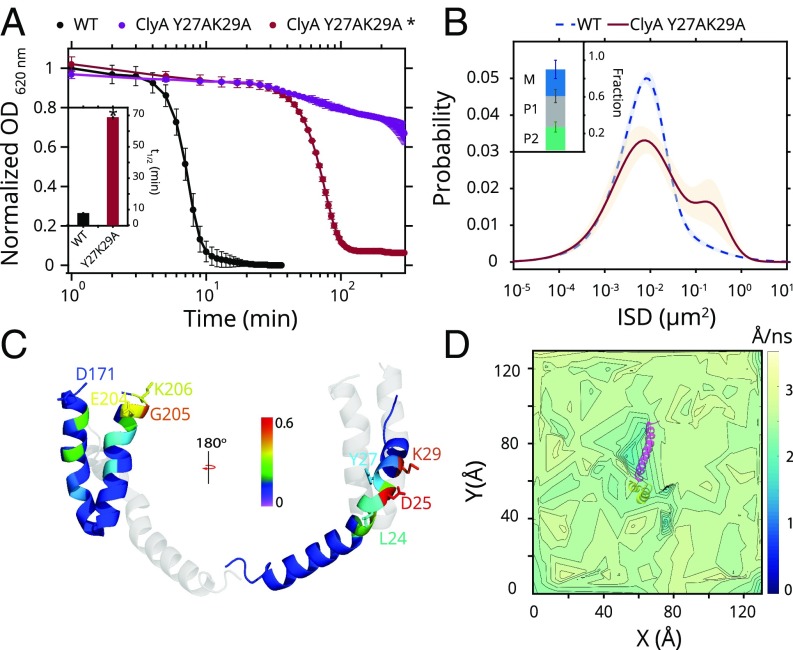
Attenuation in the rate of transition from the intramembrane state P1 to the M state in the absence of cholesterol suggested that the P1 state was able to sample cholesterol in the membrane. Thus, we hypothesized that the membrane-interacting segments (β-tongue and N-terminal helix) of ClyA may harbor cholesterol interacting motif(s). Upon inspection of the transmembrane segment of the N-terminal helix in the protomer form, we identified residues that bore strong resemblance to a previously characterized cholesterol interaction motif [cholesterol recognition and consensus motif (CRAC)] (29, 30) (SI Appendix, Fig. S9 A and B). However, the loose definition of this consensus sequence appears to result in a large repertoire of proteins comprising these residues, many of which may not participate in specific interactions with cholesterol. Therefore, site-directed mutagenesis was performed to mutate two residues that are reported as critical to cholesterol interaction in the CRAC motif. ClyA Y27AK29A was severely compromised in cell lysis activity and vesicle leakage (Fig. 4A and SI Appendix, Fig. S9C). The distribution of ISDs of ClyA Y27AK29A on POPC:Chol membranes displayed a significant fraction of fast moving molecules resembling the profile observed with wild-type ClyA in membranes devoid of cholesterol (Fig. 4B). Lysis experiments with single mutations of ClyAK29A, Y27A, and Y27F established that central Tyr-27 was the key determinant for cholesterol interaction (SI Appendix, Fig. S9D). Therefore, the N-terminal helix interacted with cholesterol via specific residues, which stabilized the P1 state, and P1 and M mobility species were indeed two distinct conformations, with or without a membrane-inserted N-terminal helix, respectively (Fig. 3B). Because the conformational transition of ClyA has been demonstrated to be the rate-limiting step in the assembly pathway even at much higher concentrations of the protein (10, 28), cholesterol-induced structural stabilization will operate at physiological concentrations of ClyA and promote lytic activity.
Fig. 4.
Cholesterol interactions with the amino terminus of ClyA. (A) Erythrocyte lysis activity (OD620) for ClyA (500 nM, black) and ClyA Y27AK29A (500 nM, purple, and 10 μM, red and *) with Boltzmann sigmoid fits (lines) is shown. Cell lysis t1/2 for ClyA (500 nM) and ClyA Y27AK29A* (10 μM) is shown in Inset. Error bars represent SD. (B) GMM fit to ISD distribution (at 5 min) for ClyA Y27AK29A mutant (solid brown line) and ClyA (dashed blue line) in POPC:Chol (shaded region represents SD). (C) Fractional cholesterol occupancy values from MD simulations of the ClyA protomer are shown for the transmembrane N-terminal helix (Right) as well as the β-tongue region (Left). (D) Cholesterol lateral mobility (from MD) calculated is plotted near the ClyA protomer (top view). Only the membrane-residing αA1 N-terminal helix (magenta) and β-tongue region (yellow) segments are shown for clarity.
To further ascertain the nature and extent of interaction between ClyA and cholesterol, we conducted all-atom, molecular dynamics (MD) simulations of the single ClyA protomer modeled from the full pore in 70% DOPC and 30% cholesterol bilayers with explicit water for a duration of 0.9 μs (SI Appendix, Fig. S10A). Cholesterol molecules showed a distinctly high occupancy around the CRAC motif residues in contrast to the rest of the membrane-inserted α-helix (Fig. 4C and SI Appendix, Fig. S10B and Table S3). A preferred propensity was observed at residues D25 and K29 highlighted by cholesterol hydroxyl group interactions with the carboxylate oxygen of D25 and ε-amino group of K29 side chains (Fig. 4C). In-plane mobility maps based on the displacement of cholesterol molecules revealed a significantly reduced mobility of the cholesterol molecules in the vicinity of the N-terminal helix further supporting direct cholesterol interaction (Fig. 4D). High cholesterol occupancy and reduced mobility of cholesterol were also observed near the residues (E204–K206) that lie close to the lipid–water interface in the β-tongue, indicating the possibility of distinct secondary site for cholesterol interaction (Fig. 4 C and D and SI Appendix, Fig. S10C).
Assembly of the ClyA Pore Is Enhanced by Cholesterol.
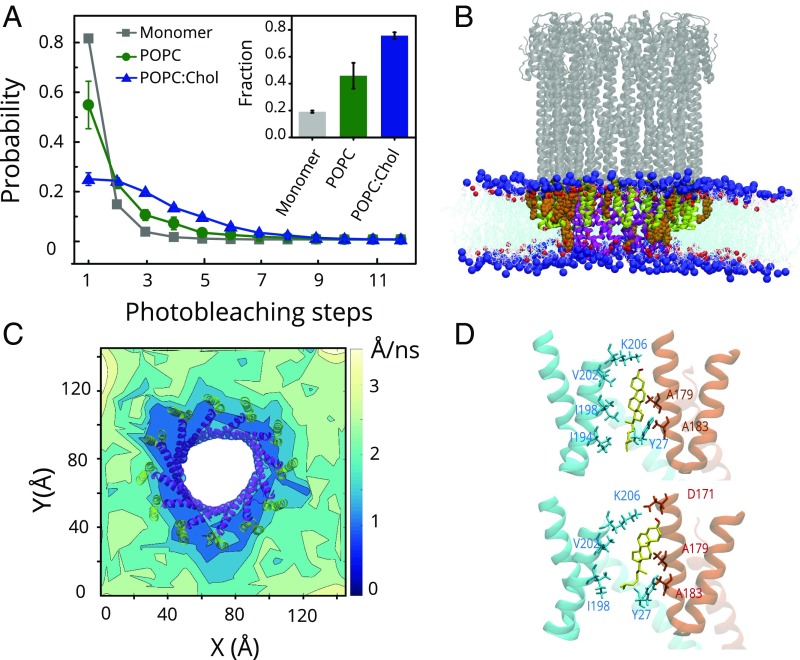
Because the dodecameric ClyA pores can be assembled in detergents and E. coli (14, 31), it is apparent that cholesterol is not essential for the ClyA pore assembly. Compared with kinetic analysis in detergents (9), our results indicate an enhanced rate of pore formation in membranes, which is further stimulated by cholesterol (Fig. 1 B and C). Although the presence of membrane cholesterol is essential for stabilization of the transmembrane helix, it may also affect oligomerization. We examined this by inducing oligomerization of ClyA on the bilayers by employing concentrations of the toxin (10 nM) comparable to those used for erythrocyte lysis. ClyA molecules initially bound homogeneously to the supported bilayer and then reorganized to form brighter spots and large punctate features (SI Appendix, Fig. S11A). Diffraction-limited ClyA complexes were quantified for fluorophore tags present per spot, by estimating the number of photobleaching steps (32–34). Compared with a single photobleaching step observed for the ClyA monomer, the ClyA puncta consistently displayed a higher number of photobleaching steps that increased with time, indicating formation of ClyA oligomers. At equilibrium (∼45 min, beyond which no significant changes were detected), the fraction of particles with more than one photobleaching step was significantly higher in the presence of cholesterol (Fig. 5A and SI Appendix, Fig. S11B). This showed that cholesterol played a role in promoting formation of higher-order ClyA oligomers.
Fig. 5.
Role of cholesterol in ClyA oligomerization on bilayers. (A) Distribution of the photobleaching steps for ClyA monomer (gray, n = 5,000) and assembled ClyA particles on POPC (green, n = 16,000) and POPC:Chol (blue, n = 15,250) is plotted. The fraction of multiple (>1) photobleaching steps is shown in Inset (same colors). Error bars represent SD. (B) Side view of the pore in membrane. Blue and red spheres represent phosphocholine and cholesterol head groups, respectively. Selected cholesterol molecules (with atoms within 0.5 nm of β-tongue) in upper leaflet are represented with an orange space-filling model (colors are the same as in Fig. 4D). (C) Cholesterol mobility map of the full ClyA pore in DOPC with 30% cholesterol is superimposed with membrane segments of the ClyA (colors are the same as in Fig. 4D). (D) Snapshots of the cholesterol moiety (yellow, hydroxyl head group in red) in the protomer–protomer interface formed between the β-tongues (two subunits colored in orange and blue) in the two major conformations (Top, at 100 ns, and Bottom, 300 ns of simulation time) for the ClyA dimer are shown. The residues in cholesterol pocket defined by charged side chains (K206 and D171) at the top, a hydrophobic interior (V202, A179, and A183), and isoleucine-rich tail (I194 and I198) region as well as residue Y27 from the N-terminal helix are highlighted.
To examine the molecular interactions of ClyA with cholesterol that manifests in enhanced assembly, we conducted MD simulations (0.6–0.8 μs) of the dodecameric pore (Fig. 5B and SI Appendix, Fig. S12) and a ClyA dimer (SI Appendix, Fig. S13A) in the protomer conformation in cholesterol membranes. In-plane mobility maps revealed a dramatically reduced mobility (and hence stronger binding) for cholesterol in the immediate vicinity of the pore complex (Fig. 5C). This was surprising because the cholesterol-interacting residues in the N-terminal helix now formed part of the pore lumen and were inaccessible to cholesterol. This behavior was also observed for the ClyA dimer, suggesting a crucial role of cholesterol in stabilizing the ClyA oligomers (SI Appendix, Fig. S13B and Table S4). Cholesterol was now observed to interact with the pocket flanked by adjacent residues of the neighboring β-tongues (Fig. 5 B and D). The cholesterol occupancy plots with the β-tongues revealed the presence of a dominant binding site at the dimer interface that harbors K206, D171, and K175 at the top of the pocket, a highly hydrophobic cavity (lined with I198, I194, and L192 at the bottom and G201, V202, A179, G180, and A183 in the middle) with Y178 providing stacking interactions. The cholesterol moiety spent ∼97% of the simulation time in the dimer pocket and sampled two dominant orientations with shared interactions between the two adjacent β-tongues (Fig. 5D and SI Appendix, Fig. S13C). Both the membrane-inserted protomer and dimer were found to form a distinct transmembrane water channel solvating the hydrophilic residues of the N terminus (SI Appendix, Fig. S14), signifying the onset of pore formation.
Discussion
PFT assembly has been the subject of growing investigation to understand protein conversion from soluble forms to stable membrane-integrated structures, especially considering the resemblance of their mechanism of action to amyloid proteins (35). Target membranes do not serve merely as passive scaffolds for adsorption of PFTs from solution but also contain determinants that are essential for their function. Investigating PFT assembly in detergent solution, although useful for determining oligomerized structures, fails to assess both the modulatory role played by lipids and the influence of protein dynamics on the membrane surface that impinge on the assembly and kinetic pathways. Here we elucidate the mechanistic basis for stimulation of toxin activity by membrane cholesterol.
Membrane specificity in pore-forming activity can be achieved by molecular determinants, interactions with heterogeneous membrane domains, or global physicochemical properties of the host membrane (1). Cholesterol, a major component in the plasma membranes of mammalian cells (36), plays complex roles that includes stabilizing the plasma membrane and enhancing several membrane–protein interactions both as an individual cofactor and as part of selectively enriched microdomains (37, 38). Pathogens can employ interaction with eukaryotic membrane components like cholesterol in various ways for recognition. For example, in the case of cholesterol-dependent cytolysin family of β-PFTs, binding to a membrane cholesterol is accomplished by a conserved tryptophan-rich loop motif in the D4 domain which allosterically activates distal domains essential for toxin assembly (39, 40). Our findings reveal the role of cholesterol in modulating the diffusional dynamics, structural states, and assembly for the amphipathic and lesser understood α-PFTs and thereby enhancing PFT activity.
Analysis of single-molecule diffusion kinetics on supported lipid bilayers allowed us to discern conformational heterogeneity of an α-PFT, Cytolysin A, that arises due to large structural rearrangements on the membrane. Post-membrane binding (using the β-tongue), membrane insertion of the N-terminal helix reduces the lateral mobility of the ClyA protein. We propose that this heterogeneous diffusion may be a signature feature in α-PFTs, due to the obligate requirement for conformational change before pore formation which has been previously reported for equinatoxin II (41). Furthermore, presence of cholesterol led to conformational selection of the membrane-inserted protomer-like form, a key structural intermediate in the assembly pathway. MD simulations demonstrated that this was mediated by interactions with a cholesterol recognition motif in the N-terminal helix of ClyA. This bore strong similarities to CRAC motifs that have been widely observed and characterized in many eukaryotic transmembrane proteins (42–46). A similar role of specific lipids in membrane partitioning of the N-terminal transmembrane helix for actinoporins, equinatoxin II, and fragaceatoxin C (FraC) has been demonstrated (47, 48), suggesting conservation of membrane insertion mechanisms among the α-PFT family of proteins.
In addition to stabilizing the N-terminal inserted state, MD simulations revealed that cholesterol played an important role in the ensuing stages of oligomerization by preferentially binding to a previously uncharacterized pocket formed between two adjacent β-tongues. Therefore, cholesterol plays a dual role: first in conformational selection of the N-terminal helix-inserted form and second in stabilization of the assembled oligomers. Bridging lipids were also observed to stabilize the pore structure of FraC (5), hinting at conserved modes of dual stabilization of intermediates among members of the α-PFT class.
The specific interactions described above allow us to reconcile the observation of pore-like structures of ClyA on E. coli OMVs. The absence of cholesterol in bacterial membranes limits the insertion of N-terminal helices, thereby resulting in complexes that do not puncture the membrane. This would explain the nontoxicity of the oligomeric structures toward E.coli (14). However, OMVs containing oligomeric ClyA have been shown to be highly toxic to eukaryotic cells (14), perhaps due to interaction with cholesterol in target cells upon fusing to host membrane.
Until now, the cholesterol binding motifs have been primarily found to be associated with transmembrane helices of mammalian integral membrane proteins. Presence of specific cholesterol interaction sites in a bacterial toxin reinforces our argument that the motif could have evolved as a mechanism for selective targeting of eukaryotic membranes. Mechanistic similarities among PFTs as well as other membrane proteins, such as viral envelope proteins and amyloid proteins (1, 35, 49), suggests that similar lipid-specific protein interactions, which stabilize transmembrane protein insertion and oligomerization, might serve as a broad strategy for achieving cell selectivity. In addition, a detailed understanding of the pore formation process at the spatiotemporal scales of protein assembly and intermolecular interactions could potentially provide a path for developing novel drug targets that compromise pore formation on target membranes and a potential route to mitigating rising bacterial virulence.
Materials and Methods
Erythrocyte Lysis Assay.
For kinetic measurements of erythrocyte lysis, ClyA variants were incubated with 200 µL of 1% suspension (vol/vol) of rabbit erythrocytes in phosphate buffered saline (PBS) at indicated concentrations. Turbidity was monitored by measuring optical density at 620 nm using a microplate reader (Tecan) with time, with intermittent orbital shaking at 37 °C. Lysis data obtained were fit to a Boltzmann sigmoid function to extract half-life (t1/2).
Erythrocyte shaving of membrane surface exposed protein fragments was achieved by incubation with 0.6 units of Proteinase K at 37 °C for 1 h. Reaction was terminated by addition of PMSF to a final concentration of 2 mM, and the erythrocytes were washed with PBS and diluted to a final 1% (vol/vol) suspension for the hemolysis assay. Cholesterol depletion in rabbit erythrocytes was carried out by incubation of 1% (vol/vol) suspension of RBCs with 1 mM methyl-β-cyclodextrin for 20 min at 25 °C. Cells were then washed with PBS and taken for lysis assay. All animal experiments were approved by the Institutional Animal Ethics Committee of the Indian Institute of Science.
PEG-Supported Lipid Bilayer.
PEG-SLBs were synthesized as described earlier (50) with some modifications. PEG-SUVs (SI Appendix, SI Methods) were diluted 1:1 in PBS (containing 3 mM CaCl2). Twenty μL of this vesicle solution was introduced into the chamber using a micropipette. The channel assembly was incubated at 37 °C for 1 h in a humidifying chamber. The channels were washed with PBS to remove unfused vesicles. Bilayer coverage and fluidity of PEG-SLBs were assessed by confocal imaging and fluorescence recovery after photobleaching (SI Appendix).
Fluorescence Microscope Setup.
The single-particle experiments were performed on an inverted microscope (Olympus IX81). A 532-nm laser (Sapphire; Coherent) was used to excite the Cy3-labeled ClyA Q56C molecules on a custom-built objective-type total internal reflection microscope. A combination of 25.4- and 300-mm biconvex lenses (Thor Laboratories) were used to expand the laser beam before a lens of focal length 150 mm was used to focus the (16-mW) laser beam on its back focal plane (BFP) of the objective (UAPON 100× OTIRF; Olympus). The laser spot at the BFP was translated away from the optical axis to achieve total internal reflection. Fluorescence emission from 80 μm × 40 μm area was collected by the objective and passed through a dichroic mirror (FF545/650-Di01-25 × 36; Semrock) and a long-pass filter (BLP02-561R-23.3-D; Semrock) before detection on an electron multiplying charge-coupled device (Andor ixon Ultra 897). Shutter (LS6; Vincent Associates) was used to control the laser illumination time.
ClyA Interaction with SLB Membrane.
For real-time binding to SLBs, ClyA was diluted to the working concentration in PBS containing 0.1 mg/mL BSA and was introduced into the channel (containing the SLB) using a syringe pump (NewEra) at a flow rate of 50 μL/min. Image sequences (500–2,000 frames) were acquired with a 25-ms exposure time immediately after flow into the microchannel and subsequently at different times over a time period of 30 min. All experiments were conducted at 25 °C. For experiments involving the depletion of cholesterol, ClyA was first incubated for a period of 5 min (to reach equilibrium) with POPC:Cholesterol SLBs. Subsequently, the bilayer was incubated with 10 mM methyl-β-cylclodextrin at 25 °C for 40 min to deplete cholesterol followed by washes with PBS before imaging and analyzing single-particle tracks.
Particle Tracking and Analysis of Squared Displacements.
Particle detection and tracking was performed using u-track MATLAB package on the image sequences acquired (51). Briefly, a 2D Gaussian fit with an SD of one pixel was performed on the diffraction-limited particles to estimate the subpixel location and intensity (after background correction). A gap length of zero frames was specified for tracking, and particles having a lifetime of fewer than three frames were discarded from analysis.
For calculating the rate of binding of ClyA to the SLBs, number of particles in each frame as detected by u-track was normalized for a unit μm2 area. This was fit to a single exponential distribution for estimating the apparent rate of interaction of ClyA with the membrane. The initial rate (slope at t = 0) was used to calculate the rate of ClyA binding.
Fraction and mobility of discrete diffusive states that are sampled by single molecules were obtained by analysis of their squared displacements (52). Instantaneous squared displacements (ISD) were calculated as
where τ is the lag time interval.
The probability density distribution of squared displacements (r2) for time interval, τ, for a Brownian particle motion with diffusion coefficient, D, is given by
where .
Integration of the equation above yields the cumulative distribution function
For a system with three distinct diffusive populations, the equation can be rewritten as
where α, β, and [1 − (α + β)] are the fractions of diffusive species with , , and as their mean squared displacements at time lag, τ, respectively. This expression was used to fit an empirical cumulative distribution function of the squared displacements to obtain the mean squared displacements and relative fractions of the three underlying components. All of the analyses were performed using custom scripts in MATLAB (Mathworks). Gaussian mixture modeling of the log transformed squared displacements was carried out using custom scripts in MATLAB. A total of 1,000 optimization iterations were carried out, and full covariance matrices were specified for the modeling. In addition, regularization was performed to prevent ill-conditioned covariance estimates.
Molecular Dynamics Simulations.
Single-protomer and dimer structures were extracted from the dodecameric structure of Cytolysin A pore (Protein Data Bank id: 2WCD) (8), and the complete dodecamer was used as is, for carrying out MD simulations. Initial structures were generated by placing the proteins (protomer, dimer, and pore) in a DOPC/Cholesterol (70:30) lipid bilayer using the Chemistry at Harvard Macromolecular Mechanics-Graphical User Interface (CHARMM-GUI) membrane builder (53). The system was solvated using TIP3P water, and sodium and chloride ions were added for electroneutrality. The full pore simulation was carried out at 0.15 M salt concentration. AMBER99SB-ILDN force field (54) with φ corrections (55) was used to capture the dynamics of ClyA with the Slipids force fields for the DOPC and cholesterol (56–58). Complete details of the simulations and parameters can be found in SI Appendix.
Supplementary Material
Acknowledgments
We thank Benjamin Schuler for sharing the ClyA 56C plasmid and Sunaina Banerjee, Subbarao Kanchi, Sreenath Balakrishnan, Ayush Agrawal, Rajat Desikan, and Aravind Penmatsa for reagents, technical assistance, and discussions. This work was supported by a Department of Science and Technology-Intensification of Research in High Priority Area (DST-IRHPA) grant (to K.G.A. and S.S.V.), Department of Biotechnology-Innovative Young Biotechnologist Award (DBT-IYBA) and DST grants (to R.R.), and computational facilities [Supercomputer Education and Research Centre (SERC)] at Indian Institute of Science (IISc). P.S., S.M., and A.B. are supported by fellowships from IISc.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721228115/-/DCSupplemental.
References
- 1.Dal Peraro M, van der Goot FG. Pore-forming toxins: Ancient, but never really out of fashion. Nat Rev Microbial. 2016;14:77–92. doi: 10.1038/nrmicro.2015.3. [DOI] [PubMed] [Google Scholar]
- 2.Parker MW, Feil SC. Pore-forming protein toxins: From structure to function. Prog Biophys Mol Biol. 2005;88:91–142. doi: 10.1016/j.pbiomolbio.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Tilley SJ, Saibil HR. The mechanism of pore formation by bacterial toxins. Curr Opin Struct Biol. 2006;16:230–236. doi: 10.1016/j.sbi.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Bakrač B, et al. Molecular determinants of sphingomyelin specificity of a eukaryotic pore-forming toxin. J Biol Chem. 2008;283:18665–18677. doi: 10.1074/jbc.M708747200. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka K, Caaveiro JMM, Morante K, González-Mañas JM, Tsumoto K. Structural basis for self-assembly of a cytolytic pore lined by protein and lipid. Nat Commun. 2015;6:6337. doi: 10.1038/ncomms7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oscarsson J, et al. Molecular analysis of the cytolytic protein ClyA (SheA) from Escherichia coli. Mol Microbial. 1999;32:1226–1238. doi: 10.1046/j.1365-2958.1999.01435.x. [DOI] [PubMed] [Google Scholar]
- 7.Atkins A, et al. Structure-function relationships of a novel bacterial toxin, hemolysin E. The role of α G. J Biol Chem. 2000;275:41150–41155. doi: 10.1074/jbc.M005420200. [DOI] [PubMed] [Google Scholar]
- 8.Mueller M, Grauschopf U, Maier T, Glockshuber R, Ban N. The structure of a cytolytic alpha-helical toxin pore reveals its assembly mechanism. Nature. 2009;459:726–730. doi: 10.1038/nature08026. [DOI] [PubMed] [Google Scholar]
- 9.Eifler N, et al. Cytotoxin ClyA from Escherichia coli assembles to a 13-meric pore independent of its redox-state. EMBO J. 2006;25:2652–2661. doi: 10.1038/sj.emboj.7601130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benke S, et al. The assembly dynamics of the cytolytic pore toxin ClyA. Nat Commun. 2015;6:6198. doi: 10.1038/ncomms7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sathyanarayana P, Desikan R, Ayappa KG, Visweswariah SS. The solvent-exposed C-terminus of the cytolysin A pore-forming toxin directs pore formation and channel function in membranes. Biochemistry. 2016;55:5952–5961. doi: 10.1021/acs.biochem.6b00593. [DOI] [PubMed] [Google Scholar]
- 12.Vaidyanathan MS, Sathyanarayana P, Maiti PK, Visweswariah SS, Ayappa KG. Lysis dynamics and membrane oligomerization pathways for Cytolysin A (ClyA) pore-forming toxin. RSC Adv. 2014;4:4930–4942. [Google Scholar]
- 13.Wallace AJ, et al. E. coli hemolysin E (HlyE, ClyA, SheA): X-ray crystal structure of the toxin and observation of membrane pores by electron microscopy. Cell. 2000;100:265–276. doi: 10.1016/s0092-8674(00)81564-0. [DOI] [PubMed] [Google Scholar]
- 14.Wai SN, et al. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell. 2003;115:25–35. doi: 10.1016/s0092-8674(03)00754-2. [DOI] [PubMed] [Google Scholar]
- 15.Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007;1768:1311–1324. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giddings KS, Johnson AE, Tweten RK. Redefining cholesterol’s role in the mechanism of the cholesterol-dependent cytolysins. Proc Natl Acad Sci USA. 2003;100:11315–11320. doi: 10.1073/pnas.2033520100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sako Y, Minoghchi S, Yanagida T. Single-molecule imaging of EGFR signalling on the surface of living cells. Nat Cell Biol. 2000;2:168–172. doi: 10.1038/35004044. [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara T, Ritchie K, Murakoshi H, Jacobson K, Kusumi A. Phospholipids undergo hop diffusion in compartmentalized cell membrane. J Cell Biol. 2002;157:1071–1081. doi: 10.1083/jcb.200202050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka M, Sackmann E. Polymer-supported membranes as models of the cell surface. Nature. 2005;437:656–663. doi: 10.1038/nature04164. [DOI] [PubMed] [Google Scholar]
- 20.Renner L, Pompe T, Lemaitre R, Drechsel D, Werner C. Controlled enhancement of transmembrane enzyme activity in polymer cushioned supported bilayer membranes. Soft Matter. 2010;6:5382–5389. [Google Scholar]
- 21.Veatch SL, Keller SL. Seeing spots: Complex phase behavior in simple membranes. Biochim Biophys Acta. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Persson F, Lindén M, Unoson C, Elf J. Extracting intracellular diffusive states and transition rates from single-molecule tracking data. Nat Methods. 2013;10:265–269. doi: 10.1038/nmeth.2367. [DOI] [PubMed] [Google Scholar]
- 23.Gambin Y, et al. Lateral mobility of proteins in liquid membranes revisited. Proc Natl Acad Sci USA. 2006;103:2098–2102. doi: 10.1073/pnas.0511026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knight JD, Lerner MG, Marcano-Velázquez JG, Pastor RW, Falke JJ. Single molecule diffusion of membrane-bound proteins: Window into lipid contacts and bilayer dynamics. Biophys J. 2010;99:2879–2887. doi: 10.1016/j.bpj.2010.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasquez JK, Chantranuvatana K, Giardina DT, Coffman MD, Knight JD. Lateral diffusion of proteins on supported lipid bilayers: Additive friction of synaptotagmin 7 C2A-C2B tandem domains. Biochemistry. 2014;53:7904–7913. doi: 10.1021/bi5012223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziemba BP, Falke JJ. Lateral diffusion of peripheral membrane proteins on supported lipid bilayers is controlled by the additive frictional drags of (1) bound lipids and (2) protein domains penetrating into the bilayer hydrocarbon core. Chem Phys Lipids. 2013;172-173:67–77. doi: 10.1016/j.chemphyslip.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saxton MJ, Jacobson K. Single-particle tracking: Applications to membrane dynamics. Annu Rev Biophys Biomol Struct. 1997;26:373–399. doi: 10.1146/annurev.biophys.26.1.373. [DOI] [PubMed] [Google Scholar]
- 28.Giri Rao VVH, Desikan R, Ayappa KG, Gosavi S. Capturing the membrane-triggered conformational transition of an α-helical pore-forming toxin. J Phys Chem B. 2016;120:12064–12078. doi: 10.1021/acs.jpcb.6b09400. [DOI] [PubMed] [Google Scholar]
- 29.Jamin N, et al. Characterization of the cholesterol recognition amino acid consensus sequence of the peripheral-type benzodiazepine receptor. Mol Endocrinol. 2005;19:588–594. doi: 10.1210/me.2004-0308. [DOI] [PubMed] [Google Scholar]
- 30.Fantini J, Barrantes FJ. How cholesterol interacts with membrane proteins: An exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front Physiol. 2013;4:31. doi: 10.3389/fphys.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J-Y, et al. Engineered bacterial outer membrane vesicles with enhanced functionality. J Mol Biol. 2008;380:51–66. doi: 10.1016/j.jmb.2008.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGuire H, Aurousseau MRP, Bowie D, Blunck R. Automating single subunit counting of membrane proteins in mammalian cells. J Biol Chem. 2012;287:35912–35921. doi: 10.1074/jbc.M112.402057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson JR, Cronin B, Bayley H, Wallace MI. Rapid assembly of a multimeric membrane protein pore. Biophys J. 2011;101:2679–2683. doi: 10.1016/j.bpj.2011.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulbrich MH, Isacoff EY. Subunit counting in membrane-bound proteins. Nat Methods. 2007;4:319–321. doi: 10.1038/NMETH1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lashuel HA, Lansbury PT., Jr Are amyloid diseases caused by protein aggregates that mimic bacterial pore-forming toxins? Q Rev Biophys. 2006;39:167–201. doi: 10.1017/S0033583506004422. [DOI] [PubMed] [Google Scholar]
- 36.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: Where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hulce JJ, Cognetta AB, Niphakis MJ, Tully SE, Cravatt BF. Proteome-wide mapping of cholesterol-interacting proteins in mammalian cells. Nat Methods. 2013;10:259–264. doi: 10.1038/nmeth.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 39.Soltani CE, Hotze EM, Johnson AE, Tweten RK. Structural elements of the cholesterol-dependent cytolysins that are responsible for their cholesterol-sensitive membrane interactions. Proc Natl Acad Sci USA. 2007;104:20226–20231. doi: 10.1073/pnas.0708104105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dowd KJ, Farrand AJ, Tweten RK. The cholesterol-dependent cytolysin signature motif: A critical element in the allosteric pathway that couples membrane binding to pore assembly. PLoS Pathog. 2012;8:e1002787, and correction (2012) 8, 10.1371/annotation/750e7055-3a67-44ac-88e1-673d017a15c7. doi: 10.1371/journal.ppat.1002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subburaj Y, et al. Bax monomers form dimer units in the membrane that further self-assemble into multiple oligomeric species. Nat Commun. 2015;6:8042. doi: 10.1038/ncomms9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Yao Z, Degenhardt B, Teper G, Papadopoulos V. Cholesterol binding at the cholesterol recognition/interaction amino acid consensus (CRAC) of the peripheral-type benzodiazepine receptor and inhibition of steroidogenesis by an HIV TAT-CRAC peptide. Proc Natl Acad Sci USA. 2001;98:1267–1272. doi: 10.1073/pnas.031461598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh AK, et al. Multiple cholesterol recognition/interaction amino acid consensus (CRAC) motifs in cytosolic C tail of Slo1 subunit determine cholesterol sensitivity of Ca2+- and voltage-gated K+ (BK) channels. J Biol Chem. 2012;287:20509–20521. doi: 10.1074/jbc.M112.356261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sengupta D, Chattopadhyay A. Identification of cholesterol binding sites in the serotonin1A receptor. J Phys Chem B. 2012;116:12991–12996. doi: 10.1021/jp309888u. [DOI] [PubMed] [Google Scholar]
- 45.Vincent N, Genin C, Malvoisin E. Identification of a conserved domain of the HIV-1 transmembrane protein gp41 which interacts with cholesteryl groups. Biochim Biophys Acta. 2002;1567:157–164. doi: 10.1016/s0005-2736(02)00611-9. [DOI] [PubMed] [Google Scholar]
- 46.Brown AC, Koufos E, Balashova NV, Boesze-Battaglia K, Lally ET. Inhibition of LtxA toxicity by blocking cholesterol binding with peptides. Mol Oral Microbial. 2016;31:94–105. doi: 10.1111/omi.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rojko N, et al. Membrane damage by an α-helical pore-forming protein, equinatoxin II, proceeds through a succession of ordered steps. J Biol Chem. 2013;288:23704–23715. doi: 10.1074/jbc.M113.481572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morante K, Caaveiro JMM, Tanaka K, González-Mañas JM, Tsumoto K. A pore-forming toxin requires a specific residue for its activity in membranes with particular physicochemical properties. J Biol Chem. 2015;290:10850–10861. doi: 10.1074/jbc.M114.615211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schroeder C. Cholesterol-binding viral proteins in virus entry and morphogenesis. Subcell Biochem. 2010;51:77–108. doi: 10.1007/978-90-481-8622-8_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yee CK, Amweg ML, Parikh AN. Direct photochemical patterning and refunctionalization of supported phospholipid bilayers. J Am Chem Soc. 2004;126:13962–13972. doi: 10.1021/ja047714k. [DOI] [PubMed] [Google Scholar]
- 51.Jaqaman K, et al. Robust single-particle tracking in live-cell time-lapse sequences. Nat Methods. 2008;5:695–702. doi: 10.1038/nmeth.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schütz GJ, Schindler H, Schmidt T. Single-molecule microscopy on model membranes reveals anomalous diffusion. Biophys J. 1997;73:1073–1080. doi: 10.1016/S0006-3495(97)78139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu EL, et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J Comput Chem. 2014;35:1997–2004. doi: 10.1002/jcc.23702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindorff-Larsen K, et al. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins. 2010;78:1950–1958. doi: 10.1002/prot.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nerenberg PS, Head-Gordon T. Optimizing protein-solvent force fields to reproduce intrinsic conformational preferences of model peptides. J Chem Theory Comput. 2011;7:1220–1230. doi: 10.1021/ct2000183. [DOI] [PubMed] [Google Scholar]
- 56.Jämbeck JPM, Lyubartsev AP. An extension and further validation of an all-atomistic force field for biological membranes. J Chem Theory Comput. 2012;8:2938–2948. doi: 10.1021/ct300342n. [DOI] [PubMed] [Google Scholar]
- 57.Jämbeck JPM, Lyubartsev AP. Derivation and systematic validation of a refined all-atom force field for phosphatidylcholine lipids. J Phys Chem B. 2012;116:3164–3179. doi: 10.1021/jp212503e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jämbeck JPM, Lyubartsev AP. Another piece of the membrane puzzle: Extending slipids further. J Chem Theory Comput. 2013;9:774–784. doi: 10.1021/ct300777p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.