Significance
Actin is required for many general and specialized cellular functions. Two isoforms, β-actin and γ-actin, are ubiquitously expressed and 99% identical in amino acid sequence, yet previous studies indicated only β-actin is indispensable for life. The nucleotide sequence of each gene also varies, providing additional regulation that may make β-actin indispensable. To separate the effects of protein and nucleotide sequences, the β-actin gene was edited to encode γ-actin protein, while retaining any regulation contained in the nucleotide sequence. The mice, which lack β-actin protein, are viable and appear to be normal. However, they develop progressive hearing loss as auditory sensory cells degenerate. Together, these results show that β-actin function depends on both its nucleotide and protein sequence.
Keywords: β-actin, γ-actin, gene edited, auditory hair cell, stereocilia
Abstract
The highly similar cytoplasmic β- and γ-actins differ by only four functionally similar amino acids, yet previous in vitro and in vivo data suggest that they support unique functions due to striking phenotypic differences between Actb and Actg1 null mouse and cell models. To determine whether the four amino acid variances were responsible for the functional differences between cytoplasmic actins, we gene edited the endogenous mouse Actb locus to translate γ-actin protein. The resulting mice and primary embryonic fibroblasts completely lacked β-actin protein, but were viable and did not present with the most overt and severe cell and organismal phenotypes observed with gene knockout. Nonetheless, the edited mice exhibited progressive high-frequency hearing loss and degeneration of actin-based stereocilia as previously reported for hair cell-specific Actb knockout mice. Thus, β-actin protein is not required for general cellular functions, but is necessary to maintain auditory stereocilia.
The actin cytoskeleton contributes to a wide range of cellular functions, such as muscle contraction, cytokinesis, migration, chromatin remodeling, transcriptional regulation, and regulating cell shape (1). Although often thought of as a single protein, there are actually six actin isoforms encoded by separate genes. Four actins are considered muscle specific while the cytoplasmic β- and γ-actin isoforms are ubiquitously expressed. The cytoplasmic actins are highly similar at the level of both gene and protein: the coding sequences of Actb and Actg1 are 89% identical and β- and γ-actin differ at just 4 out of 375 aa residues. Furthermore, human and mouse Actb coding sequences are 90% identical and β- and γ-actin primary sequences are identical across birds and mammals (2). The fact that selective pressure has maintained these seemingly slight differences for hundreds of millions of years has confounded cell biologists since the cytoplasmic actins were initially cloned four decades ago: Why do cells apparently require two such highly similar proteins?
Corresponding to the evolutionary conservation of β- and γ-actin, several lines of evidence demonstrate that both isoforms are required for normal cellular function. In humans, point mutations in ACTB and ACTG1 cause a spectrum of developmental defects as well as progressive hearing loss, demonstrating lack of compensation between the isoforms (3–5). In mice, genetic ablation of each isoform results in different phenotypes. Loss of Actb results in embryonic lethality and severe cellular proliferation and migration defects in null fibroblasts, while Actg1 knockouts survive, although with decreased fitness (6–8). Finally, ablation of each isoform in auditory sensory hair cells causes different patterns of progressive hearing loss and degeneration of actin-based protrusions (9).
The phenotypes arising from conventional gene knockout may result from loss of isoform specific transcript or protein. Most notably, the Actb transcript has a 3′-UTR “zipcode” sequence not found in the Actg1 transcript that targets the transcript to subcellular regions and regulates translation (10, 11). In addition, the transcripts differ in ribosome density and translation rate (12). On the protein level, purified β- and γ-actin have slightly different polymerization rates and differentially interact with a subset of actin binding proteins (13, 14). Considering that nucleotide and amino acid sequences are evolutionally maintained, both may contribute to critical cellular functions.
To separate the effects of noncoding regulation from differences in β- and γ-actin protein, we edited the Actb gene encoding β-actin to instead encode γ-actin while retaining all of the endogenous Actb regulatory elements. We found the resulting mice, despite lacking β-actin protein, were born in Mendelian ratios and that the mice and derived fibroblasts appeared phenotypically normal. These results, together with those of a recent study using a similar approach (12) demonstrate that the nucleotide sequence is a critical determinant of actin isoform-specific function. However, we also found that β-actin protein is required to maintain the shape and function of auditory hair cells, demonstrating that other functions of cytoplasmic actins are defined by their amino acid sequences.
Results
Actbc-g Mice Coding γ-Actin Instead of β-Actin Protein Are Grossly Normal.
We designed transcription activator-like effector nucleases (TALENs) and a single-stranded oligo (ssOligo) donor template to edit the endogenous Actb locus within the mouse genome (SI Appendix, Fig. S1A). A single founder was edited so the Actb exon 2 encoded E2–4 and I10 instead of D2–4 and V10, thus producing γ-actin protein instead of β-actin (SI Appendix, Fig. S1 B and C). We named the edited allele as Actbc-g for Actb coding gamma.
Whereas conventional cre-mediated knockout of Actb results in embryonic lethality (7), homozygous Actbc-g mice were born in Mendelian ratios (27% Actb+/+, 50% Actbc-g/+, 23% Actbc-g/c-g; χ2 = 0.80) and in normal litter sizes (average of 7 pups per litter; 17 litters) (15). In addition, homozygous Actbc-g mice were visibly indistinguishable from Actb+/+ littermates, grew at the same rate as their littermates, and exhibited no perinatal or early onset death out to 6 mo of age (n = 30).
Cre-mediated knockout of Actb in skeletal muscle or the central nervous system resulted in a mild myopathy or hyperactivity, respectively (16, 17). These phenotypes were not detected in homozygous Actbc-g mice. Actbc-g homozygous mice displayed no evidence of histopathology in quadriceps muscles at 6 mo of age (SI Appendix, Fig. S2 A–C), had no defects in muscle contractile function (SI Appendix, Fig. S2D and Table S1), and behaved normally in an open field assay (SI Appendix, Fig. S2 E and F). Overall, these results suggest that β-actin protein is dispensable for mouse viability if γ-actin protein is expressed from the endogenous Actb locus.
Actg1/γ-Actin Responds to Loss of β-Actin Protein in Actbc-g Mice.
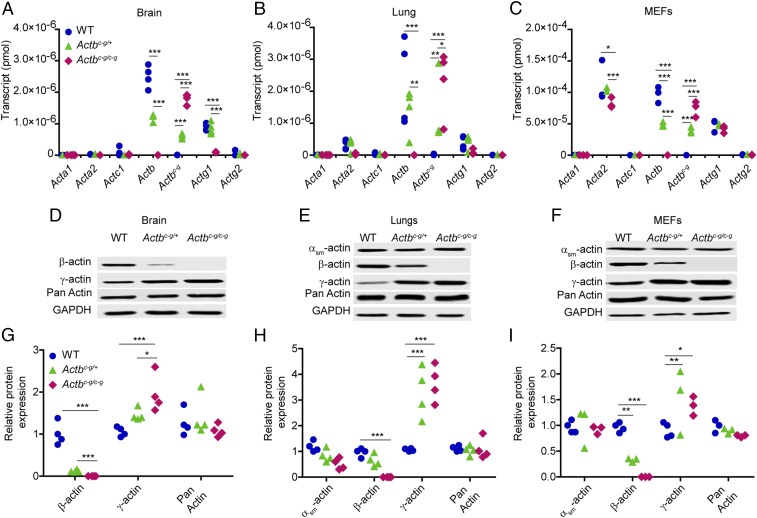
We next measured transcript and protein levels for each actin isoform in tissue and cells from Actbc-g mice to determine how the actin isoform composition changed without β-actin expression. Actb transcript and β-actin protein in brain, lung, and primary mouse embryonic fibroblasts (MEFs) were decreased in Actbc-g heterozygotes and absent in homozygous mice. Correspondingly, the edited Actbc-g transcript increased in parallel (Fig. 1 A–C), which correlated with increased γ-actin protein expression (Fig. 1 D–I). Transcripts and protein levels for muscle-specific actin isoforms were largely unchanged (Fig. 1). Acta2 transcript, encoding αsm-actin, was decreased in Actbc-g/c-g MEFs, which contrasts with Acta2 up-regulation previously observed following cre-mediated knockout of Actb (6, 7, 18). Interestingly, WT Actg1 transcripts were significantly decreased in Actbc-g homozygous brain (Fig. 1A), suggesting cells can distinguish β- and γ-actin proteins, and down-regulate endogenous Actg1 transcription to compensate for increased expression of γ-actin protein from the edited Actbc-g locus (Fig. 1 D and G).
Fig. 1.
Actbc-g transcript is synthesized from the edited Actb locus and correlates with a twofold increase in γ-actin protein expression. (A–C) Calculated quantity of isoactin transcript via qRT-PCRs in brain (n = 4 mice), lung tissues (n = 4 mice), and MEFs (n = 3 embryos). The x axis denotes actin isoform; the y axis denotes transcript in pmol. (D–F) Representative Western blots in brain, lung, and MEFs. (G–I) Calculated relative isoactin protein expression in WT, heterozygous, and homozygous Actbc-g mice brain (n = 4 mice), lung (n = 4 mice), and MEFs (n ≥ 3 embryos). The x axis denotes actin isoform; the y axis denotes relative protein expression which was normalized to GAPDH and relative to a WT sample. Two-way ANOVA with Bonferroni posttest was performed. *P < 0.05, **P < 0.01, ***P < 0.001.
Actbc-g MEFs Proliferate and Migrate Normally.
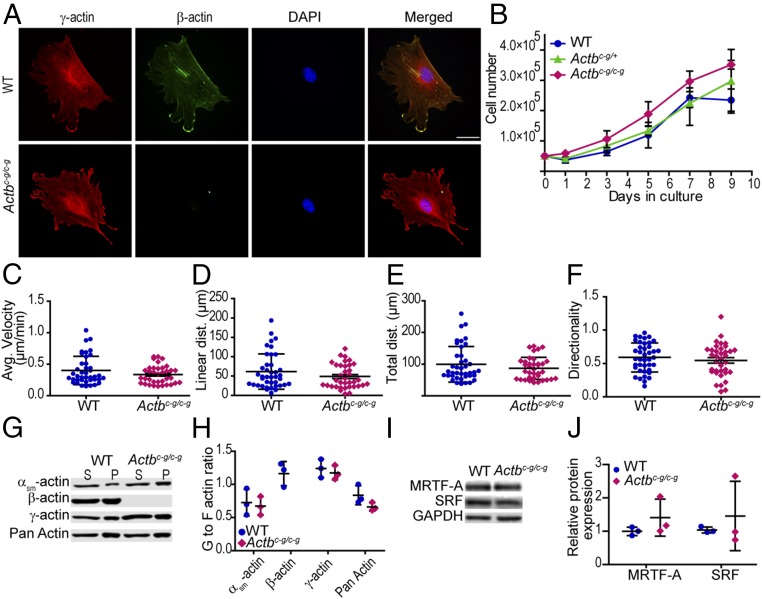
We previously demonstrated that MEFs with cre-mediated knockout of Actb failed to proliferate and migrated less efficiently than control cells (7). Actbc-g/c-g MEFs, as gauged by immunostaining with actin isoform-specific antibodies, localize γ-actin to stress fibers and the periphery of lamellipodial protrusions, and do not express β-actin (Fig. 2A). Despite lacking β-actin, heterozygous and homozygous Actbc-g MEFs proliferated similarly to control cells in culture (Fig. 2B). Similarly, Actbc-g homozygous MEFs migrated normally, displaying average velocity, linear distance, total distance, and directionality persistence comparable to WT MEFs (Fig. 2 C–F). Thus, β-actin protein is not required for cell proliferation or migration.
Fig. 2.
Actbc-g MEFs display cell proliferation and random migration rates not different from WT. (A) Representative immunofluorescence images of WT MEFs and Actbc-g MEFs. γ-Actin is labeled in red, β-actin is labeled in green, nucleus is labeled in blue, and the merged image is on the Right. (Scale bar: 50 μm.) (B) MEF growth curve analysis, cultured from 0 to 9 d. n = 3 embryos. (C–F) Calculated average velocity, linear distance, total distance, and directionality from random migration assay. n ≥ 4 embryos, n ≥ 40 cells per genotype. (G and H) G-to F-actin ratio in WT and Actbc-g MEFs. The x axis denotes actin isoform; the y axis denotes G- to-F-actin ratio. n = 3 embryos. (I and J) Representative and calculated relative isoactin protein expression in WT, homozygous Actbc-g MEFs for SRF and MRTF-A. n = 3 embryos. The x axis denotes actin isoform; the y axis denotes relative protein expression which was normalized to GAPDH and relative to a WT sample. Error bars are SD.
Finally, actin exists both as a monomer (G-actin) and as filaments (F-actin); and the G-to F-actin dynamic plays a key role in the serum response factor (SRF)/myocardin-related transcription factor (MRTF) signaling pathway (19). Cre-mediated β-actin knockout in primary MEFs caused a significant decrease in G-actin which correlated with altered gene expression (8). In contrast, we found αsm-, γ-, and total actin G- to F-actin ratios were not altered in Actbc-g homozygous MEFs (Fig. 2 G and H). Expression of SRF and MRTF-A proteins were also not altered compared with WT MEFs (Fig. 2 I and J). Overall, these results suggest that γ-actin and β-actin are functionally redundant for G-actin–mediated regulation of gene expression.
Progressive Hearing Loss and Stereocilia Degeneration in Actbc-g Mice.
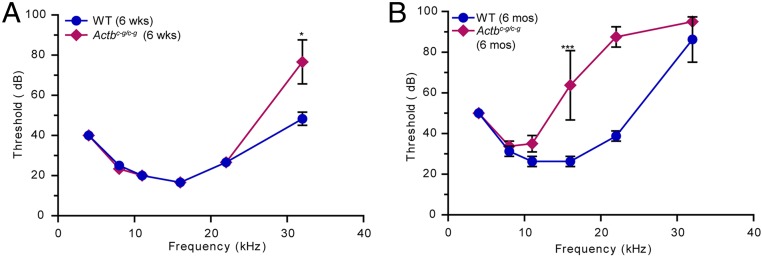
We previously demonstrated that cre-mediated knockout of Actb resulted in progressive, high-frequency hearing loss corresponding to inappropriate shortening of stereocilia, which are actin-based protrusions on auditory hair cells that detect sound (9, 20). We therefore assessed hearing in Actbc-g mice by measuring auditory brainstem responses (ABRs) to a range of pure frequency tones. At 6 wk of age, control and Actbc-g mice had comparable thresholds, except at 32 kHz, the highest frequency tested, where Actbc-g mice had some hearing loss as demonstrated by their higher ABR threshold (Fig. 3A). This high-frequency hearing loss was more advanced by 6 mo of age, with significant elevation of ABR thresholds at and above 16 kHz in Actbc-g mice compared with control mice (Fig. 3B).
Fig. 3.
Actbc-g mice suffer from progressive hearing loss. (A) ABR of 6-wk-old WT and homozygous Actbc-g mice. n = 3 mice. (B) ABR of 6-mo-old WT and homozygous Actbc-g mice. n = 4 mice. The x axis denotes defined frequency in kilohertz; the y axis denotes threshold (in decibels) sound that elicits an ABR. Student t test. *P < 0.05, ***P < 0.001. Error bars are SD.
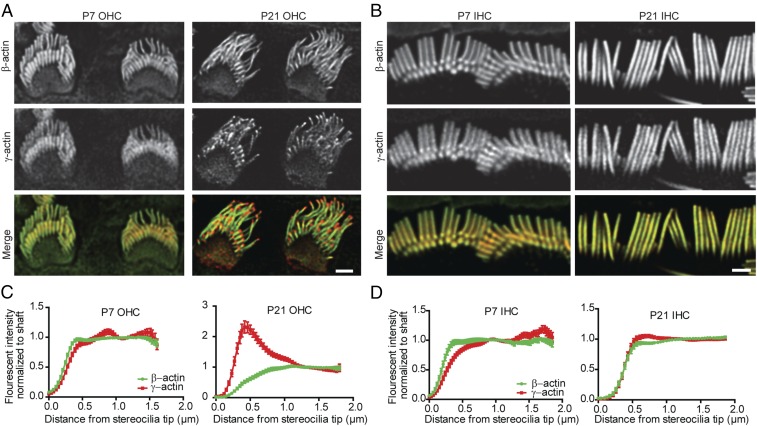
We immunostained cochlear tissue to assess the distribution of β- and γ-actin in stereocilia on inner hair cells (IHCs) and outer hair cells (OHCs), which both detect sound but have different stereocilia morphologies. As expected, β-actin was not detected in Actbc-g stereocilia (SI Appendix, Fig. S3). In wild-type hair cells at P7, when bundles are developing, β-actin and γ-actin are colocalized to the OHC stereocilia. However, β-actin was reduced and γ-actin markedly enriched at the tips of OHC stereocilia in mature P21–P40 mice, where stereocilia lengths are unchanging (Fig. 4 A and C). IHC stereocilia showed a similar trend, with a higher proportion of β-actin at the tips of developing stereocilia (Fig. 4 B and D). Thus, increased levels of β-actin at stereocilia tips correlates with growth, suggesting that β-actin protein drives stereocilia growth.
Fig. 4.
β-Actin and γ-actin colocalized in developing OHC stereocilia. (A) Representative immunofluorescent images of the OHC in P7 and P21 C57BL/6 mice. β-Actin is labeled in green, γ-actin is labeled in red. (B) Representative immunofluorescent images of the IHC in P7 and P21 C57BL/6 mice. β-Actin is labeled in green, γ-actin is labeled in red. (C) Calculated fluorescent intensity of β- and γ-actin normalized to the shaft in the OHC. n = 3 mice, n ≥ 40 stereocilia. (D) Calculated fluorescent intensity of β- and γ-actin normalized to the shaft in the IHC. (Scale bar: 1 µm.) n = 3 mice, n ≥ 40 stereocilia. Error bars are SEM.
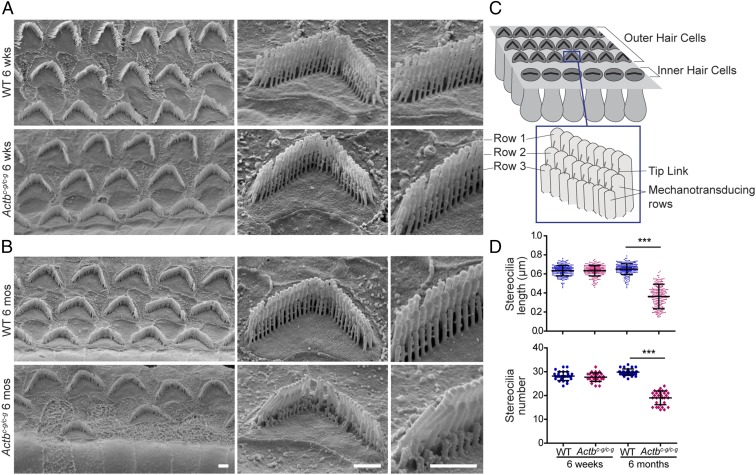
The morphology of stereocilia bundles is closely associated with their mechanotransductive function (Fig. 5C). We used scanning electron microscopy (SEM) to examine OHC stereocilia from the middle turn of the cochlea, which corresponds to the frequency range where ABR thresholds in Actbc-g mice are normal at 6 wk of age, but elevated at 6 mo of age. Six-week-old control and Actbc-g mice had OHC bundles that appeared normal, featuring similar numbers of stereocilia that were of similar height (Fig. 5 A and D). In contrast, at 6 mo of age, the mechanotransducing stereocilia in Actbc-g mice had started to degenerate and were shorter and fewer in number than in control OHCs. In addition, some OHC stereocilia bundles were missing in Actbc-g mice (Fig. 5 B and D). Toward the base of the cochlea where higher pitch sounds are detected, a similar phenotype was observed in Actbc-g OHC stereocilia at 6 wk of age (SI Appendix, Fig. S4). Together, these data suggest that Actbc-g mechanotransducing OHC stereocilia selectively and progressively degenerate, leading to hearing loss. Notably, this pattern of hearing loss and stereocilia degeneration is similar to that previously observed in hair cell-specific Actb knockout mice (9, 20), demonstrating that this protein isoform is required for stereocilia length maintenance.
Fig. 5.
Actbc-g mice show evidence of stereocilia degeneration. Representative scanning electron microcopy images of (A) 6-wk-old and (B) 6-mo-old OHC stereocilia from the middle turn of the cochlea. (Scale bar: 1 µm.) (C) Cartoon image of the stereocilia. (D) Length (in micrometers) of stereocilia in row 3 of OHC bundles in 6-wk-old and 6-mo-old WT and homozygous Actbc-g mice. n = 4 mice, n ≥ 200 stereocilia. Number of stereocilia in row 3 of OHC bundles in 6-wk-old and 6-mo-old WT and homozygous Actbc-g mice. n = 4 mice, n ≥ 28 cells. One-way ANOVA with Tukey’s posttest was performed. ***P < 0.001. Error bars are SD.
Discussion
To distinguish the relative contributions of nucleotide and amino acid sequences to the unique functions of closely related cytoplasmic actins, we used TALENs to engineer the endogenous Actb gene to express γ-actin instead of β-actin. This approach edited only the nucleotides required to substitute the β-actin N-terminal amino acids D2–4 and V10 for γ-actin residues E2–4 and I10. The resulting mice and cells, which produce no β-actin protein, lacked the most severe and overt phenotypes of previously characterized Actb knockouts. These findings demonstrate that many functions of the highly conserved and expressed Actb gene reside in the nucleotide sequence and do not depend on the protein sequence. However, homozygous Actbc-g mice did develop progressive hearing loss due to degeneration of actin-rich protrusions on sensory cells. Therefore, β-actin protein has functions in some specialized cell types that cannot be compensated for by γ-actin.
A recent study using clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 methodology also edited the endogenous Actb locus to express γ-actin protein (12), and the resulting mouse line is nearly identical to the one we independently generated and characterized here. Several of the same phenotypes were assessed, and very similar results were obtained. In particular, Actbc-g mice are viable and appear to develop normally without evidence of premature mortality (12). In addition, Actbc-g MEFs also have proliferation and migration rates similar to WT (Fig. 2 and ref. 12). We further show that Actbc-g muscle viability and physiology, as well as the activity level of the mice, was comparable to wild type. Overall, these two independent studies are highly consistent and support the major finding that β-actin protein is dispensable for most cellular functions.
The Actb gene-edited mice (herein, ref. 12) contrast with conventional cre-mediated knockout of a floxed Actb allele, where exons 2 and 3 were deleted, and which caused embryonic lethality in mice (8). Tissue-specific knockout of the same allele also resulted in a variety of phenotypes with muscle showing progressive myofiber degeneration/regeneration, muscle weakness, impaired mitochondrial fission (17, 21), and the CNS showing tissue morphology defects that correlated with hyperactivity and cognitive behavior impairments (16). Additionally, isolated MEFs failed to divide or migrate, suggesting β-actin was required for fundamental cellular processes (6, 8). None of these phenotypes were observed in Actbc-g mice. It is possible that some of the phenotypes observed following cre-mediated deletion of Actb exons 2 and 3 are independent of normal Actb function. For example, there may be an essential element in the intervening intron. It is also possible that recombination produces toxic transcript and/or truncated protein. However, differences between cre-mediated knockouts and Actbc-g mice most likely suggest that regulatory information embedded in the nucleotide sequence is critical, as this would be altered in the Actb floxed knockout but not in the Actbc-g mice.
Singer and coworkers (22) first discovered and established the Actb zipcode as a key difference between Actb and Actg1 gene and transcript, which is conserved from birds to mammals (23). ZBP1 binds to this sequence to regulate both transcript localization and translation of β-actin (11). Consistent with well-regulated temporal and spatial β-actin protein translation, Actb transcript is more abundant than Actg1 transcript in primary MEFs even when β- and γ-actin protein levels are similarly expressed (6). Based on ref. 12 and our findings that the four amino acid variances between β- and γ-actin protein are not critical to most cellular functions, it seems likely that transcript targeting and regulated translation via the zipcode is the essential feature of Actb. A similar gene-editing approach can potentially address the importance of the Actb zipcode in vivo.
While β-actin protein is not essential to the function of many cell types, some tissues nonetheless seem to specifically detect and regulate the levels of β- and γ-actin. As Actbc-g transcript increased in heterozygous and homozygous brain tissue, the level of γ-actin protein increased as it was produced both from the engineered Actb locus and the endogenous Actg1 locus (Fig. 1 A–C). Interestingly, as γ-actin protein concentration increased, the amount of transcript from the endogenous Actg1 alleles decreased. In Actbc-g cells, as well as in most cells and tissues where Actb or Actg1 were knocked out, total actin protein levels remained constant across all genotypes even as transcript levels varied (7, 8). Thus, most cell and tissue types appear to maintain actin homeostasis via a protein-based feedback mechanism.
Clinically, spontaneous missense mutations in both ACTB and ACTG1 have been associated with Baraitser–Winter syndrome, a rare disease with complex phenotypes, including facial dysmorphism, learning disabilities, and deafness (24). Some of the mutations affect the polymerization properties of actin or interaction with actin binding proteins to cause a gain-of-function or a dominant-negative effect (25–27). Interestingly, the more severe forms of Baraitser–Winter syndrome are associated with mutations in ACTB over ACTG1 (4). Loss-of-function mutations in human ACTB also cause developmental delays, intellectual disability, internal organ malformations, hearing loss, and facial malformations (3). Together, these studies suggest that loss or mutation of cytoplasmic actins, although not necessarily lethal, perturbs a variety of specialized cells or cellular processes and decreases organismal fitness.
Stereocilia maintenance is one such specialized process that requires β-actin protein, which likely relates to deafness observed in humans with mutations in ACTB and ACTG1 (27). Actbc-g hair cells, like cre-mediated Actb knockout hair cells characterized previously, have a particular pattern of degeneration where the shorter rows of the bundle shorten irregularly as mice age, but the tallest row of the bundle is largely unaffected (9). In contrast, Actg1 knockout hair cells have a different phenotype where individual stereocilia are lost from bundles (9, 28). Together, these data point to a particular requirement for β-actin in maintaining stereocilia length. As with Actb knockout, blocking mechanotransduction also results in degeneration of the shorter rows of stereocilia, which can subsequently regrow when mechanotransduction is restored (29). Considering that the mechanotransductive apparatus is under constant mechanical stress, one possibility is that damage and repair during aging results in cycles of stereocilia shortening and regrowth (30). Efficient regrowth likely requires β-actin. Indeed, preferential localization of β-actin to the growing tips of developing stereocilia further supports a role for β-actin over γ-actin elongating stereocilia.
The ability of β-actin to lengthen stereocilia may be because it has a faster polymerization rate than γ-actin (13) under calcium bound conditions, which may reasonably occur in mechanotransducing stereocilia. Besides polymerization kinetics, multiple actin binding proteins may also differentially interact with β- and γ-actin. For example, nonmuscle myosinIIa and myosinVIIa both exhibit higher ATPase activity with γ-actin filaments (31). MyosinVIIa is a particularly interesting candidate for mediating actin isoform-specific effects since it is also localized to stereocilia. Finally, the actin filament severing protein cofilin regulates β- and γ-actin differently (14), which is intriguing since mice lacking the severing protein actin depolymerizing factor (ADF) phenocopy the pattern of stereocilia degeneration seen in Actbc-g mice (30).
Whether through polymerization kinetics or differential binding partners, differences in β- and γ-actin function in stereocilia depend on the four amino acid differences in the N terminus. One substitution is a valine for an isoleucine, while the other three substitutions are in an acidic block (DDD in β- and EEE in γ-actin). Auditory function in mice expressing β-actin from the Actg1 locus seem likely to phenocopy Actg1 nulls, while swapping the EEE acidic block or the isoleucine from Actg1 into the Actb locus would help resolve the relative contributions of each feature to β-actin protein function.
Regardless of the mechanism by which β-actin protein contributes to stereocilia maintenance, this isoform has a cellular function that is not met by γ-actin protein. Therefore, it is likely that other cell types also depend on β-actin protein to form or maintain specialized features. However, this scenario is considerably less common than previously appreciated and, in agreement with Vedula et al. (12), we conclude that the nucleotide sequence rather than amino acid sequence determines the majority of the divergent phenotypes previously associated with Actb gene disruption.
Methods
Animal Care.
Animals were housed and treated in accordance with the standards set by the University of Minnesota Institutional Animal Care and Use Committee.
Statistics.
All statistical analysis was analyzed using the GraphPad Prism software.
Detailed descriptions of materials and methods, including TALEN construction, cell culture, the open field activity assay, muscle histology and physiology, immunofluorescence microscopy of MEFs and cochlea, scanning electron microscopy, auditory brainstem response, live cell imaging, qRT-PCR, Western blotting, and the G-to F-actin ratio assay are given in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Kevin Sonnemann for his advice on the manuscript. This work was supported by NIH Grant AR049899 (to J.M.E.) and NIH Grant DC015495 (to B.J.P.).
Footnotes
Conflict of interest statement: D.F.V. is a named inventor on several patents involving the use of transcription activator-like effector nucleases (TALENs) to create targeted genome modifications.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1807895115/-/DCSupplemental.
References
- 1.Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perrin BJ, Ervasti JM. The actin gene family: Function follows isoform. Cytoskeleton (Hoboken) 2010;67:630–634. doi: 10.1002/cm.20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuvertino S, et al. ACTB loss-of-function mutations result in a pleiotropic developmental disorder. Am J Hum Genet. 2017;101:1021–1033. doi: 10.1016/j.ajhg.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Donato N, et al. Severe forms of Baraitser-Winter syndrome are caused by ACTB mutations rather than ACTG1 mutations. Eur J Hum Genet. 2014;22:179–183. doi: 10.1038/ejhg.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivière J, et al. De novo mutations in the actin genes ACTB and ACTG1 cause Baraitser-Winter syndrome. Nat Genet. 2012;44:440–444, S1–S2. doi: 10.1038/ng.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patrinostro X, et al. Relative importance of βcyto- and γcyto-actin in primary mouse embryonic fibroblasts. Mol Biol Cell. 2017;28:771–782. doi: 10.1091/mbc.E16-07-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunnell TM, Burbach BJ, Shimizu Y, Ervasti JM. β-Actin specifically controls cell growth, migration, and the G-actin pool. Mol Biol Cell. 2011;22:4047–4058. doi: 10.1091/mbc.E11-06-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunnell TM, Ervasti JM. Delayed embryonic development and impaired cell growth and survival in Actg1 null mice. Cytoskeleton (Hoboken) 2010;67:564–572. doi: 10.1002/cm.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perrin BJ, Sonnemann KJ, Ervasti JM. β-actin and γ-actin are each dispensable for auditory hair cell development but required for Stereocilia maintenance. PLoS Genet. 2010;6:e1001158. doi: 10.1371/journal.pgen.1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang F, Saha S, Shabalina SA, Kashina A. Differential arginylation of actin isoforms is regulated by coding sequence-dependent degradation. Science. 2010;329:1534–1537. doi: 10.1126/science.1191701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross AF, Oleynikov Y, Kislauskis EH, Taneja KL, Singer RH. Characterization of a beta-actin mRNA zipcode-binding protein. Mol Cell Biol. 1997;17:2158–2165. doi: 10.1128/mcb.17.4.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vedula P, et al. Diverse functions of homologous actin isoforms are defined by their nucleotide, rather than their amino acid sequence. eLife. 2017;6:e31661. doi: 10.7554/eLife.31661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergeron SE, Zhu M, Thiem SM, Friderici KH, Rubenstein PA. Ion-dependent polymerization differences between mammalian beta- and gamma-nonmuscle actin isoforms. J Biol Chem. 2010;285:16087–16095. doi: 10.1074/jbc.M110.110130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapustina M, Read TA, Vitriol EA. Simultaneous quantification of actin monomer and filament dynamics with modeling-assisted analysis of photoactivation. J Cell Sci. 2016;129:4633–4643. doi: 10.1242/jcs.194670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silver LM. 2001 Reproductive characteristics of some important inbred strains. Mouse Genome Informatics, Jackson Lab. Available at www.informatics.jax.org/silver/tables/table4-1.shtml. Accessed January 22, 2018.
- 16.Cheever TR, Li B, Ervasti JM. Restricted morphological and behavioral abnormalities following ablation of β-actin in the brain. PLoS One. 2012;7:e32970. doi: 10.1371/journal.pone.0032970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prins KW, Call JA, Lowe DA, Ervasti JM. Quadriceps myopathy caused by skeletal muscle-specific ablation of β(cyto)-actin. J Cell Sci. 2011;124:951–957. doi: 10.1242/jcs.079848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tondeleir D, et al. Cells lacking β-actin are genetically reprogrammed and maintain conditional migratory capacity. Mol Cell Proteomics. 2012;11:255–271. doi: 10.1074/mcp.M111.015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11:353–365. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrin BJ, et al. β-actin and fascin-2 cooperate to maintain stereocilia length. J Neurosci. 2013;33:8114–8121. doi: 10.1523/JNEUROSCI.0238-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Rourke AR, et al. Impaired muscle relaxation and mitochondrial fission associated with genetic ablation of cytoplasmic actin isoforms. FEBS J. 2018;285:481–500. doi: 10.1111/febs.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kislauskis EH, Zhu X, Singer RH. Sequences responsible for intracellular localization of beta-actin messenger RNA also affect cell phenotype. J Cell Biol. 1994;127:441–451. doi: 10.1083/jcb.127.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Artman L, Dormoy-Raclet V, von Roretz C, Gallouzi IE. Planning your every move: The role of β-actin and its post-transcriptional regulation in cell motility. Semin Cell Dev Biol. 2014;34:33–43. doi: 10.1016/j.semcdb.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Ampe C, Van Troys M. Mammalian actins: Isoform-specific functions and diseases. Handb Exp Pharmacol. 2016;235:1–37. doi: 10.1007/164_2016_43. [DOI] [PubMed] [Google Scholar]
- 25.Jepsen L, Kruth KA, Rubenstein PA, Sept D. Two deafness-causing actin mutations (DFNA20/26) have allosteric effects on the actin structure. Biophys J. 2016;111:323–332. doi: 10.1016/j.bpj.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston JJ, et al. NIH Intramural Sequencing Center Functional analysis of a de novo ACTB mutation in a patient with atypical Baraitser-Winter syndrome. Hum Mutat. 2013;34:1242–1249. doi: 10.1002/humu.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubenstein PA, Wen KK. Insights into the effects of disease-causing mutations in human actins. Cytoskeleton (Hoboken) 2014;71:211–229. doi: 10.1002/cm.21169. [DOI] [PubMed] [Google Scholar]
- 28.Belyantseva IA, et al. Gamma-actin is required for cytoskeletal maintenance but not development. Proc Natl Acad Sci USA. 2009;106:9703–9708. doi: 10.1073/pnas.0900221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vélez-Ortega AC, Freeman MJ, Indzhykulian AA, Grossheim JM, Frolenkov GI. Mechanotransduction current is essential for stability of the transducing stereocilia in mammalian auditory hair cells. eLife. 2017;6:e24661. doi: 10.7554/eLife.24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narayanan P, et al. Length regulation of mechanosensitive stereocilia depends on very slow actin dynamics and filament-severing proteins. Nat Commun. 2015;6:6855. doi: 10.1038/ncomms7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller M, et al. Distinct functional interactions between actin isoforms and nonsarcomeric myosins. PLoS One. 2013;8:e70636. doi: 10.1371/journal.pone.0070636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.