Significance
Retrosplenial cortex (RSC) is a major relay of hippocampal formation output to other neocortical areas and is critical for spatial and some other forms of learning. We show here that the sparse, orthogonal, “place cell” sequence activity in RSC develops gradually over several days and is severely attenuated by hippocampal damage. These data support the theory that hippocampus endows RSC (and possibly other cortical areas) with an index-like, continuous representation of the context in which events occur, that could support coordinated retrieval of recent memory.
Keywords: retrosplenial cortex, hippocampus, spatial sequence coding, spatial learning, hippocampal indexing theory
Abstract
Retrosplenial cortex (RSC) is involved in visuospatial integration and spatial learning, and RSC neurons exhibit discrete, place cell-like sequential activity that resembles the population code of space in hippocampus. To investigate the origins and population dynamics of this activity, we combined longitudinal cellular calcium imaging of dysgranular RSC neurons in mice with excitotoxic hippocampal lesions. We tracked the emergence and stability of RSC spatial activity over consecutive imaging sessions. Overall, spatial activity in RSC was experience-dependent, emerging gradually over time, but, as seen in the hippocampus, the spatial code changed dynamically across days. Bilateral but not unilateral hippocampal lesions impeded the development of spatial activity in RSC. Thus, the emergence of spatial activity in RSC, a major recipient of hippocampal information, depends critically on an intact hippocampus; the indirect connections between the dysgranular RSC and the hippocampus further indicate that hippocampus may exert such influences polysynaptically within neocortex.
The retrosplenial cortex (RSC) is a midline association region that integrates thalamic, (para)hippocampal, and neocortical information (1–5). Similar to the hippocampus (6), RSC is also essential for spatial learning and memory (7–9). Consistent with its proposed role in translating between world-centered and body-centered views (10), RSC neurons carry various navigation-related signals such as head direction, positional, and conjunctive allocentric and egocentric information (11–17). RSC neurons also show spatial activity resembling the activity of hippocampal CA1 place cells (15). The sources of spatial signals in RSC are unknown; however, hippocampus is an obvious possibility, and hippocampal lesions or inactivation impair immediate early gene expression in RSC (18, 19). On the other hand, both rodent and human studies have suggested the opposite direction of information flow, that RSC may send sensory and contextual information to the hippocampus (20, 21), possibly through RSC projections to the medial entorhinal cortex (22). Here we studied the emergence of spatial activity in RSC upon repeated exploration of the same environment and tested the impact of the hippocampus on this activity.
Results
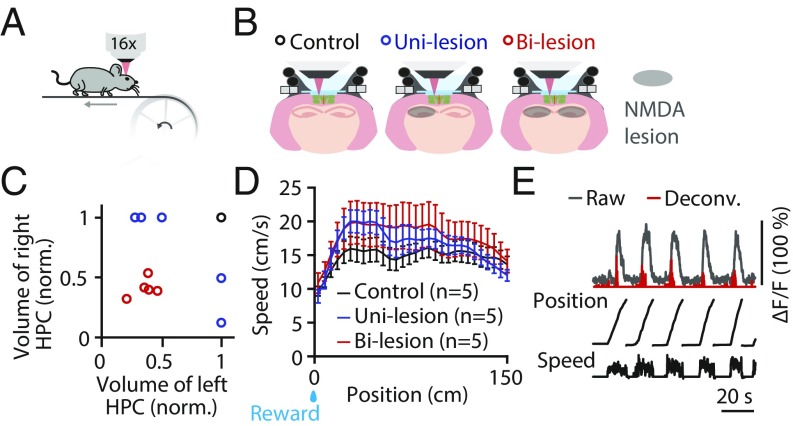
We investigated RSC neuronal activity in mice in a head-fixed, treadmill assay (15, 23) (Fig. 1A). Fifteen adult transgenic mice specifically expressing calcium indicator GCaMP6 in excitatory neurons (24, 25) were divided into three experimental groups: control, unilateral hippocampal lesion, and bilateral hippocampal lesions (n = 5 in each group; NMDA lesion) (Fig. 1B). Mice in the lesion groups sustained extensive neuron/tissue loss in the dorsal hippocampal formation (Fig. 1C and SI Appendix, Fig. S1). Movement trajectories were similar across groups (average speed between 30- and 120-cm position: control, 15.4 ± 1.7; unilesion, 17.7 ± 1.8; bilesion, 18.9 ± 3.5 cm/s; all mean ± SEM, n = 5 in each group; P = 0.61, one-way ANOVA) (Fig. 1D). We measured cellular activity in the superficial layers (100 μm to 200 µm deep) of dysgranular RSC using two-photon calcium imaging (26) (SI Appendix, Fig. S2). We inferred activity from raw calcium fluorescence signals using deconvolution (SI Appendix, Fig. S2) (27). We studied the degree to which RSC neurons encode the animal’s position on the treadmill. Consistent with our previous work (15), a substantial fraction of RSC neurons showed repeated activation at specific positions on the treadmill (Fig. 1E and SI Appendix, Fig. S1D), similar to the activity of hippocampal place cells.
Fig. 1.
Experimental design and retrosplenial place cell activity. (A) Treadmill locomotion assay. Two-photon calcium imaging was performed in head-fixed mice running on a treadmill belt endowed with tactile cues. (B) Cellular imaging of neuronal activity through a glass window in RSC in both hemispheres in mice with intact hippocampus (control), unilateral hippocampal lesion (unilesion), and bilateral hippocampal lesions (bilesion). (C) Scatter plot of the remaining volume (normalized) of the hippocampus in the left and right hemispheres. Colors correspond to animal groups shown in B. (D) Mean movement speed profiles as a function of position for the three experimental groups. Error bars are SEM over animals. (E) Calcium time courses (raw and deconvolved) of an example RSC place cell. Position and speed traces are shown below. deconv, deconvolved; norm, normalized.
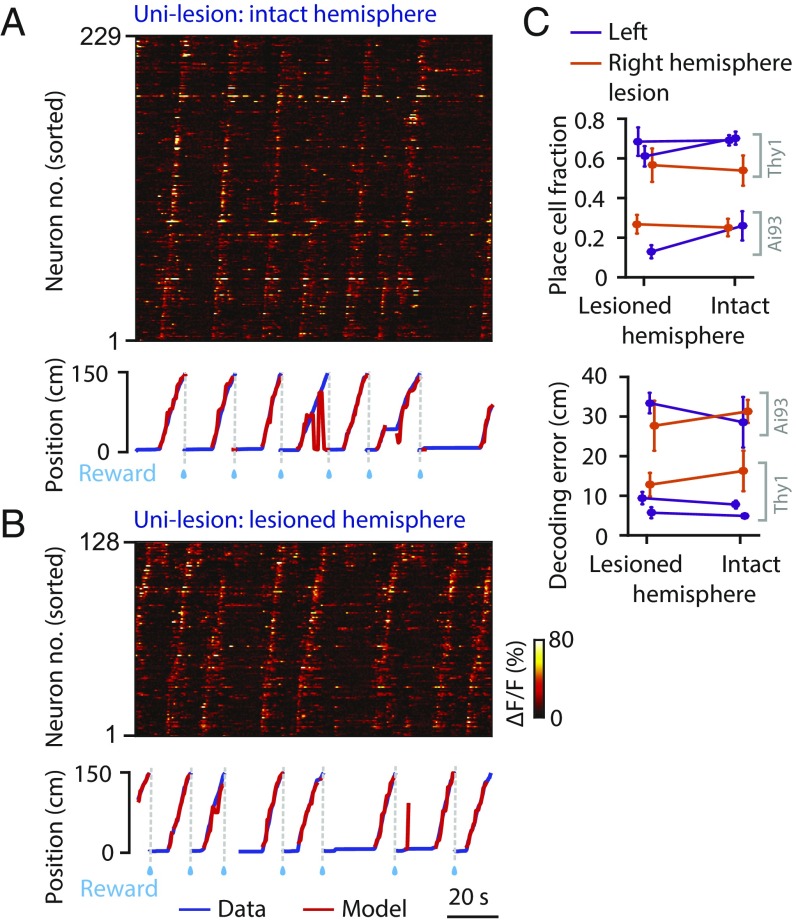
Hippocampal projections to RSC are mostly ipsilateral (2); we therefore tested whether a unilateral hippocampal lesion disrupts “place” cell activity in ipsilateral RSC. We compared activity in the lesioned and intact hemispheres measured from the same animals. Unilateral hippocampal lesion had no discernible impact on RSC spatial activity (Fig. 2). RSC neuronal ensembles showed sequential activation that was locked to position during movement in both the intact and lesioned hemispheres (Fig. 2 A and B). To quantify the encoding of spatial information by RSC neuronal population, we built a Bayesian decoding model (SI Appendix, Methods and Fig. S3A) to predict the animal’s position from all imaged neurons using separate sets of trials for training and testing (Fig. 2 A and B) (28). We observed no significant difference in the place cell fraction (P = 0.30, paired t test) or the position decoding error (P = 0.98, paired t test) between the lesioned and intact hemispheres (Fig. 2C).
Fig. 2.
Unilateral hippocampal lesion does not impair ipsilateral RSC place cell activity. (A) (Top) Raw calcium time courses of 229 simultaneously imaged RSC neurons in the intact hemisphere of an example unilesion mouse. Neurons were sorted by the positions that elicited their maximum responses. (Bottom) Real (blue) and Bayesian decoded (red) position traces. (B) The same as A but for the lesioned hemisphere RSC of the same mouse. (C) Mean place cell fractions and position decoding errors in unilesion mice. Note that two mouse lines were used here (Thy1 and Ai93). Error bars are SEM over sessions. For unknown reasons, there were intrinsic differences between these lines in the observed place cell fractions.
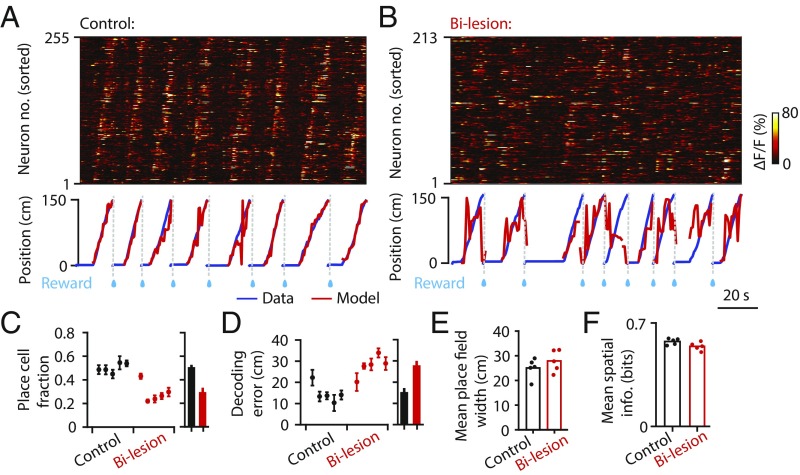
While unilateral hippocampal lesions did not disrupt ipsilateral RSC spatial activity, RSC could also receive spatial information from the intact, contralateral hemisphere (2). We compared RSC place cell activity between intact animals and animals with bilateral hippocampal lesions. Indeed, bilateral hippocampal lesions significantly impaired place cell activity in RSC (Fig. 3). RSC spatial sequence activity was severely disrupted by bilateral hippocampal lesions (Fig. 3 A and B). The fraction of RSC neurons showing stable place fields dropped dramatically in animals with bilateral lesions (place cell fraction: control, 0.50 ± 0.02; bilesion, 0.29 ± 0.04; mean ± SEM, n = 5 each group; P = 0.0005, one-tailed t test) (Fig. 3C). An alternative, spatial information-based criterion of place cell selection yielded similar results (SI Appendix, Fig. S3 B–G). Position estimates inferred from Bayesian decoding of population activity (using all cells) were also severely disrupted in bilaterally lesioned animals (decoding error: control, 14.60 ± 1.98 cm; bilesion, 27.68 ± 2.20 cm; mean ± SEM; P = 0.0011, one-tailed t test) (Fig. 3D). These effects were not explained by other variables such as neuron or trial counts (SI Appendix, Fig. S3 H and I). Notably, while the proportion of place cells was reduced in animals with bilateral hippocampal lesions, the properties of neurons with place cell activity were similar (place field width: P = 0.28; spatial information: P = 0.21, two-tailed t test) (Fig. 3 E and F and SI Appendix, Figs. S3J and S4). The extent of hippocampal lesions may explain the residual place cell activity observed in bilaterally lesioned animals, since the magnitude of the effect was proportional to the proportion of hippocampal damage (SI Appendix, Fig. S3J). These results indicate that the hippocampus is necessary for the expression of place cell activity in RSC.
Fig. 3.
Bilateral hippocampal lesions severely impair place cell activity in RSC. (A) (Top) Raw calcium time courses of 255 simultaneously imaged RSC neurons from an example control mouse. Neurons were sorted by the positions that elicited their maximum responses. (Bottom) Real (blue) and Bayesian decoded (red) position traces. (B) The same as A but from an example bilateral lesion mouse. (C) (Left) Mean place cell fractions of individual mice in the control and bilesion groups (all Thy1 mice). Error bars are SEM over sessions. (Right) Bar plots of the average place cell fractions for the two groups. Error bars are SEM over animals. (D) The same as C but for decoding errors. (E) Bar plot of the mean place field width. Colored dots correspond to the mean place field width of individual mice in the control and bilesion groups. (F) The same as E but for the mean spatial information of identified place cells in each animal.
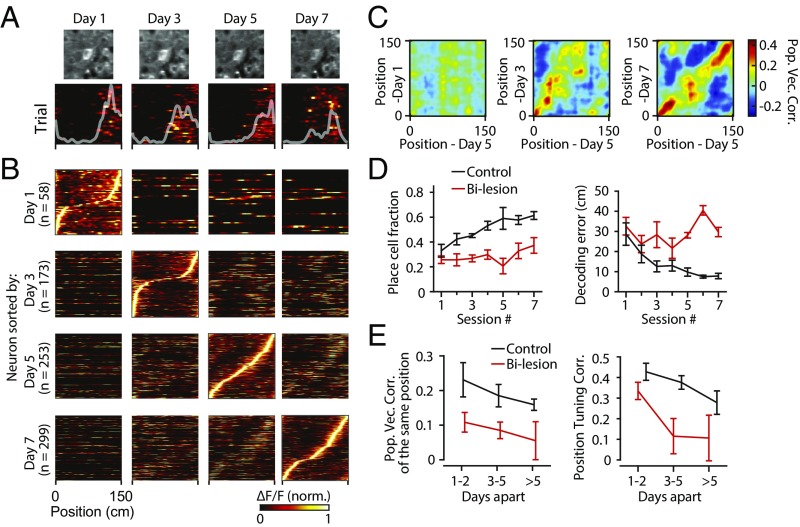
We next investigated how RSC place cell activity emerges over repeated exploration of the same environment and what the role of the hippocampus is in this process. We monitored the activity of the same RSC neuronal population over a period of 2 wk to 3 wk in daily imaging sessions (control, n = 4; bilesion, n = 4). Activity from the same neuronal cell bodies could be imaged across days (Fig. 4A and SI Appendix, Fig. S5 A and B). Place cell activity increased gradually with experience in the control group but not in mice with bilateral hippocampal lesions (place cell fraction change: control, r = 0.96, P = 0.0006; bilesion, r = 0.61, P = 0.17) (Fig. 4D). Similar to what is observed in the hippocampus (29), RSC population representations of position on the track changed dynamically across days (Fig. 4B), and more adjacent days had more highly correlated population activity as experience increased (Fig. 4 B and C). The control group showed more repeatable population representations across days than the bilateral lesion group (population vector correlations: control, 0.19 ± 0.02; bilesion, 0.09 ± 0.02, P = 0.02, two-tailed t test) (Fig. 4E and SI Appendix, Fig. S5C). Position tuning curves (occupancy-normalized neuronal responses as a function of position) of individual neurons were also more correlated between days in control mice than in bilateral lesion mice (P = 0.02, two-tailed t test) (Fig. 4E). These effects were not explained by the number of place cells included in the calculation (SI Appendix, Fig. S5F). We did not observe a difference in spatial activity correlations (population vector correlations or position tuning correlations of individual neurons) between the two hemispheres in mice with unilateral hippocampal lesion (SI Appendix, Fig. S5 D and E). These results indicate that RSC population representation of spatial context improves progressively over time and that the hippocampus is necessary for the emergence of spatial sequence coding in RSC.
Fig. 4.
Hippocampal destruction disrupts experience-dependent emergence of spatial coding in RSC. (A) Average fluorescence images (centered on the target neuron) and position activity maps of the target neuron imaged on days 1, 3, 5, and 7. Position tuning curves (white traces) are overlaid on the position activity maps. (B) Sorted, trial-averaged position activity maps for all RSC place cells from an example control mouse on days 1, 3, 5, and 7. Same neuronal population was imaged across days. Neurons were selected and sorted by corresponding days. (C) Population vector correlation matrices between days for data shown in B. (D) Mean place cell fractions and decoding errors as a function of imaging session for the control and bilateral lesion animals. Error bars are SEM over animals. (E) Mean population vector correlations of the same position (distance < 15 cm, dashed area in SI Appendix, Fig. S5C) and mean position tuning (white traces in A) correlations for all place cells as a function of different imaging intervals. Error bars are SEM over animals. norm, normalized; Pop Vec Corr, population vector correlation.
Discussion
Our data indicate that the spatial context coding in RSC improves with experience, and this process relies on instructive signals from the hippocampus. This may reflect a direct impact of the hippocampus or an indirect effect through intermediate regions after lesioning the hippocampus. Indeed, the specific RSC subregion we studied, the dysgranular RSC, receives weak direct hippocampal input (1, 3, 4). Our results demonstrate the importance of the hippocampus in shaping neocortical activity. The pronounced experience-dependent spatial activity observed may reflect a general principle of the influences of hippocampal outflow on the association neocortex in terms of spatiotemporal contextual processing. Sequential activation of large groups of neurons has been observed in several cortical regions, including the posterior parietal and prefrontal cortices (30, 31). These sequences reflect information processing along the spatial and/or temporal dimensions, with concurrent sensory experience and events superimposed. Being uniquely situated at the intermediate layer within the default mode network (DMN) (32), RSC may be critical for episodic memory processes by mediating functional interactions between the cortical and subcortical DMN subsystems (33, 34). The RSC may play a critical role in the transfer of hippocampal place/memory sequence codes to other regions of the neocortex, to associate information across different cortical modalities (35–37) and to guide complex behaviors (38).
Methods
All animal procedures were performed in compliance with protocols approved by the ethical research committee of the University of Lethbridge. Fifteen adult male and female transgenic GCaMP6 mice [20 g to 25 g, 2 mo to 4 mo old at the time of surgery, including 13 Thy1 GCaMP6s GP4.3 mice and 2 Ai93 (TITL-GCaMP6f) || CaMK2a-tTA || Rasgrf2-2A-dCre mice] were used in this study. Mice were divided into three experimental groups: sham lesion (control, n = 5 Thy1 mice), unilateral hippocampal lesion (n = 3 Thy1 mice and n = 2 Ai93 mice), and bilateral hippocampal lesions (n = 5 Thy1 mice). Mice were habituated and trained to run on a linear treadmill track with head fixed.
Full methods can be found in SI Appendix, Methods.
Supplementary Material
Acknowledgments
We thank V. Lapointe and A. Demchuk for help with histology, and F. Battaglia for comments on the manuscript. This research was supported through the Alberta Innovates–Health Solutions Polaris award (to B.L.M.) and a graduate studentship (to D.M.), Natural Sciences and Engineering Research Council of Canada Discovery Grant 40352 (to M.H.M.) and RGPIN-2017-03857 (to B.L.M.), Research Foundation–Flanders (Fonds voor Wetenschappelijk Onderzoek - Vlaanderen) Grant G0D0516N (to V.B.), KU Leuven Research Council Grant C14/16/048 (to V.B.), National Science Foundation Grant 1631465 (to B.L.M.), Canada Foundation for Innovation Grant 33598 (to M.H.M. and B.L.M.), and Defense Advanced Research Projects Agency Grant HR0011-18-2-0021 (to B.L.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data related to this work has been deposited on Gin and is available at https://web.gin.g-node.org/dunmao/RSC_HPC.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1803224115/-/DCSupplemental.
References
- 1.van Groen T, Wyss JM. Connections of the retrosplenial dysgranular cortex in the rat. J Comp Neurol. 1992;315:200–216. doi: 10.1002/cne.903150207. [DOI] [PubMed] [Google Scholar]
- 2.Wyss JM, Van Groen T. Connections between the retrosplenial cortex and the hippocampal formation in the rat: A review. Hippocampus. 1992;2:1–11. doi: 10.1002/hipo.450020102. [DOI] [PubMed] [Google Scholar]
- 3.Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Brain Res Rev. 2007;56:1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugar J, Witter MP, van Strien NM, Cappaert NL. The retrosplenial cortex: Intrinsic connectivity and connections with the (para)hippocampal region in the rat. An interactive connectome. Front Neuroinform. 2011;5:7. doi: 10.3389/fninf.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Groen T, Wyss JM. Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol. 2003;463:249–263. doi: 10.1002/cne.10757. [DOI] [PubMed] [Google Scholar]
- 6.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland RJ, Whishaw IQ, Kolb B. Contributions of cingulate cortex to two forms of spatial learning and memory. J Neurosci. 1988;8:1863–1872. doi: 10.1523/JNEUROSCI.08-06-01863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czajkowski R, et al. Encoding and storage of spatial information in the retrosplenial cortex. Proc Natl Acad Sci USA. 2014;111:8661–8666. doi: 10.1073/pnas.1313222111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vann SD, Aggleton JP. Selective dysgranular retrosplenial cortex lesions in rats disrupt allocentric performance of the radial-arm maze task. Behav Neurosci. 2005;119:1682–1686. doi: 10.1037/0735-7044.119.6.1682. [DOI] [PubMed] [Google Scholar]
- 10.Byrne P, Becker S, Burgess N. Remembering the past and imagining the future: A neural model of spatial memory and imagery. Psychol Rev. 2007;114:340–375. doi: 10.1037/0033-295X.114.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen LL, Lin L-H, Green EJ, Barnes CA, McNaughton BL. Head-direction cells in the rat posterior cortex. I. Anatomical distribution and behavioral modulation. Exp Brain Res. 1994;101:8–23. doi: 10.1007/BF00243212. [DOI] [PubMed] [Google Scholar]
- 12.Cho J, Sharp PE. Head direction, place, and movement correlates for cells in the rat retrosplenial cortex. Behav Neurosci. 2001;115:3–25. doi: 10.1037/0735-7044.115.1.3. [DOI] [PubMed] [Google Scholar]
- 13.Alexander AS, Nitz DA. Retrosplenial cortex maps the conjunction of internal and external spaces. Nat Neurosci. 2015;18:1143–1151. doi: 10.1038/nn.4058. [DOI] [PubMed] [Google Scholar]
- 14.Smith DM, Barredo J, Mizumori SJ. Complimentary roles of the hippocampus and retrosplenial cortex in behavioral context discrimination. Hippocampus. 2012;22:1121–1133. doi: 10.1002/hipo.20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao D, Kandler S, McNaughton BL, Bonin V. Sparse orthogonal population representation of spatial context in the retrosplenial cortex. Nat Commun. 2017;8:243. doi: 10.1038/s41467-017-00180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander AS, Nitz DA. Spatially periodic activation patterns of retrosplenial cortex encode route sub-spaces and distance traveled. Curr Biol. 2017;27:1551–1560.e4. doi: 10.1016/j.cub.2017.04.036. [DOI] [PubMed] [Google Scholar]
- 17.Vedder LC, Miller AMP, Harrison MB, Smith DM. Retrosplenial cortical neurons encode navigational cues, trajectories and reward locations during goal directed navigation. Cereb Cortex. 2017;27:3713–3723. doi: 10.1093/cercor/bhw192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albasser MM, Poirier GL, Warburton EC, Aggleton JP. Hippocampal lesions halve immediate-early gene protein counts in retrosplenial cortex: Distal dysfunctions in a spatial memory system. Eur J Neurosci. 2007;26:1254–1266. doi: 10.1111/j.1460-9568.2007.05753.x. [DOI] [PubMed] [Google Scholar]
- 19.Kubik S, Miyashita T, Kubik-Zahorodna A, Guzowski JF. Loss of activity-dependent Arc gene expression in the retrosplenial cortex after hippocampal inactivation: Interaction in a higher-order memory circuit. Neurobiol Learn Mem. 2012;97:124–131. doi: 10.1016/j.nlm.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Cooper BG, Mizumori SJ. Temporary inactivation of the retrosplenial cortex causes a transient reorganization of spatial coding in the hippocampus. J Neurosci. 2001;21:3986–4001. doi: 10.1523/JNEUROSCI.21-11-03986.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auger SD, Zeidman P, Maguire EA. A central role for the retrosplenial cortex in de novo environmental learning. eLife. 2015;4:e09031. doi: 10.7554/eLife.09031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czajkowski R, et al. Superficially projecting principal neurons in layer V of medial entorhinal cortex in the rat receive excitatory retrosplenial input. J Neurosci. 2013;33:15779–15792. doi: 10.1523/JNEUROSCI.2646-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Royer S, et al. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat Neurosci. 2012;15:769–775. doi: 10.1038/nn.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dana H, et al. Thy1-GCaMP6 transgenic mice for neuronal population imaging in vivo. PLoS One. 2014;9:e108697. doi: 10.1371/journal.pone.0108697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madisen L, et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron. 2015;85:942–958. doi: 10.1016/j.neuron.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldey GJ, et al. Removable cranial windows for long-term imaging in awake mice. Nat Protoc. 2014;9:2515–2538. doi: 10.1038/nprot.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pnevmatikakis EA, et al. Simultaneous denoising, deconvolution, and demixing of calcium imaging data. Neuron. 2016;89:285–299. doi: 10.1016/j.neuron.2015.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davidson TJ, Kloosterman F, Wilson MA. Hippocampal replay of extended experience. Neuron. 2009;63:497–507. doi: 10.1016/j.neuron.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziv Y, et al. Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci. 2013;16:264–266. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harvey CD, Coen P, Tank DW. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature. 2012;484:62–68. doi: 10.1038/nature10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujisawa S, Amarasingham A, Harrison MT, Buzsáki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nat Neurosci. 2008;11:823–833. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 33.Kaboodvand N, Bäckman L, Nyberg L, Salami A. The retrosplenial cortex: A memory gateway between the cortical default mode network and the medial temporal lobe. Hum Brain Mapp. 2018;39:2020–2034. doi: 10.1002/hbm.23983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- 35.Teyler TJ, DiScenna P. The hippocampal memory indexing theory. Behav Neurosci. 1986;100:147–154. doi: 10.1037//0735-7044.100.2.147. [DOI] [PubMed] [Google Scholar]
- 36.McNaughton BL. Cortical hierarchies, sleep, and the extraction of knowledge from memory. Artif Intell. 2010;174:205–214. [Google Scholar]
- 37.Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- 38.Yamawaki N, Radulovic J, Shepherd GM. A corticocortical circuit directly links retrosplenial cortex to M2 in the mouse. J Neurosci. 2016;36:9365–9374. doi: 10.1523/JNEUROSCI.1099-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.