Significance
Activated G protein-coupled receptors (GPCRs) internalize and can continue to signal from endosomes. The contribution of endosomal signaling to human disease is unknown. Proteases that are generated in the colon of patients with irritable bowel syndrome (IBS) can cleave protease-activated receptor-2 (PAR2) on nociceptors to cause pain. We evaluated whether PAR2 generates signals in endosomes of nociceptors that mediate persistent hyperexcitability and pain. Biopsies of colonic mucosa from IBS patients released proteases that induced PAR2 endocytosis, endosomal signaling, and persistent hyperexcitability of nociceptors. When conjugated to the transmembrane lipid cholestanol, PAR2 antagonists accumulated in endosomes and suppressed persistent hyperexcitability. The results reveal the therapeutic potential of endosomally targeted PAR2 antagonists for IBS pain, and expand the contribution of endosomal GPCR signaling to encompass processes that are relevant to disease.
Keywords: endosomes, receptors, pain, proteases
Abstract
Once activated at the surface of cells, G protein-coupled receptors (GPCRs) redistribute to endosomes, where they can continue to signal. Whether GPCRs in endosomes generate signals that contribute to human disease is unknown. We evaluated endosomal signaling of protease-activated receptor-2 (PAR2), which has been proposed to mediate pain in patients with irritable bowel syndrome (IBS). Trypsin, elastase, and cathepsin S, which are activated in the colonic mucosa of patients with IBS and in experimental animals with colitis, caused persistent PAR2-dependent hyperexcitability of nociceptors, sensitization of colonic afferent neurons to mechanical stimuli, and somatic mechanical allodynia. Inhibitors of clathrin- and dynamin-dependent endocytosis and of mitogen-activated protein kinase kinase-1 prevented trypsin-induced hyperexcitability, sensitization, and allodynia. However, they did not affect elastase- or cathepsin S-induced hyperexcitability, sensitization, or allodynia. Trypsin stimulated endocytosis of PAR2, which signaled from endosomes to activate extracellular signal-regulated kinase. Elastase and cathepsin S did not stimulate endocytosis of PAR2, which signaled from the plasma membrane to activate adenylyl cyclase. Biopsies of colonic mucosa from IBS patients released proteases that induced persistent PAR2-dependent hyperexcitability of nociceptors, and PAR2 association with β-arrestins, which mediate endocytosis. Conjugation to cholestanol promoted delivery and retention of antagonists in endosomes containing PAR2. A cholestanol-conjugated PAR2 antagonist prevented persistent trypsin- and IBS protease-induced hyperexcitability of nociceptors. The results reveal that PAR2 signaling from endosomes underlies the persistent hyperexcitability of nociceptors that mediates chronic pain of IBS. Endosomally targeted PAR2 antagonists are potential therapies for IBS pain. GPCRs in endosomes transmit signals that contribute to human diseases.
There is a growing realization that G protein-coupled receptors (GPCRs), which were formerly considered to function principally at the surface of cells, can continue to signal from endosomes by mechanisms that involve β-arrestins (βARRs) and G proteins (1). Although GPCR signaling begins at the plasma membrane, activated receptors associate with βARRs, which mediate receptor desensitization and endocytosis (2). These processes efficiently terminate GPCR signaling at the plasma membrane. The detection of GPCR signaling complexes in endosomes, and the finding that disruption of endocytosis can suppress signaling, both suggest that GPCRs signal from endosomes (3–12). GPCRs in endosomes can generate persistent signals in subcellular compartments that control gene transcription and neuronal excitation (8, 11, 12). Although endosomal signaling of GPCRs can regulate important physiological processes, including pain transmission (8, 12), the contribution of endosomal signaling to human disease is far from clear.
Protease-activated receptor-2 (PAR2) mediates the proinflammatory and pronociceptive actions of proteases (13). Given the irreversible mechanism of proteolytic activation, PAR2 may be capable of persistent signaling at the plasma membrane and in endosomes. Trypsin and mast cell tryptase activate PAR2 by canonical mechanisms that induce receptor association with βARRs, endocytosis, and endosomal signaling (5, 14). Neutrophil elastase (NE) and macrophage cathepsin S (CS) cleave PAR2 at different sites and activate PAR2 by biased mechanisms that do not induce receptor interactions with βARRs or endocytosis (15, 16). The contribution of PAR2 signaling at the plasma membrane and in endosomes to the disease-relevant actions of proteases is unknown.
Proteases and PAR2 have been implicated in the hypersensitivity of sensory nerves in the colon that may account for chronic pain in patients with irritable bowel syndrome (IBS) (17). Biopsies of colonic mucosa from IBS patients secrete proteases, including tryptase and trypsin-3, which induce PAR2-dependent hyperexcitability of nociceptors and colonic nociception in mice (18–21). PAR2 agonists induce a remarkably long-lasting hyperexcitability of neurons by unknown mechanisms (22, 23). Whether PAR2 at the plasma membrane or in endosomes is a target for the treatment of IBS pain remains to be determined.
We examined the hypothesis that two components of PAR2 signaling contribute to the persistent hyperexcitability of nociceptors in IBS: the irreversible mechanism of proteolytic activation and the capacity of PAR2 to generate sustained signals from endosomes.
Results
PAR2-Mediated Nociception.
Proteases may induce pain by activating PAR2 on nociceptors or other cell types. To determine the contribution of PAR2 on nociceptors, we bred mice expressing Par2 flanked by LoxP sites (Par2lox/lox) with mice expressing Cre recombinase targeted to nociceptors using the NaV1.8 promoter (Scn10a). Par2-NaV1.8 mice lacked immunoreactive PAR2 in NaV1.8+ neurons of the dorsal root ganglia (DRG) (Fig. 1A). Whereas 31% (20 of 65) of small-diameter (<25 µm) DRG neurons from WT mice responded to trypsin (100 nM) with increased [Ca2+]i, only 6% (3 of 51) of neurons from Par2-NaV1.8 mice responded (Fig. 1B and SI Appendix, Fig. S1 A and B). We assessed nociception by measuring withdrawal responses to stimulation of the plantar surface of the hindpaw with von Frey filaments (VFF). In WT mice, intraplantar injection (10 µL) of trypsin (80 nM), NE (3.9 µM), or CS (5 µM) induced mechanical allodynia within 30 min, which was maintained for 180 min (Fig. 1 C–E). In Par2-NaV1.8 mice, the initial responses were maintained, but responses after 120 min were diminished. At 180 min, when mechanical allodynia in WT mice was fully maintained, responses in Par2-NaV1.8 mice had returned to baseline (trypsin, NE) or were significantly attenuated (CS). In WT mice, intraplantar trypsin increased paw thickness—measured using calipers—which peaked at 1 h and was maintained for 4 h, and stimulated an influx of neutrophils after 4 h, consistent with inflammation (SI Appendix, Fig. S1 C and D). Trypsin-induced inflammation was markedly diminished in Par2-NaV1.8 mice.
Fig. 1.
Protease-induced mechanical nociception. (A) Localization of PAR2 and NaV1.8 immunoreactivity in DRG from WT or Par2-NaV1.8 mice. White arrowheads: neurons coexpressing PAR2 and NaV1.8 in WT mice. Yellow arrowheads: neurons expressing NaV1.8 but not PAR2 in Par2-NaV1.8 mice. (B) Total number and number of trypsin (100 nM)-responsive DRG neurons (<25 µm) from WT and Par2-NaV1.8 mice. (C–E) VFF withdrawal responses in WT and Par2-NaV1.8 mice after intraplantar injection of trypsin (C, Tryp), NE (D), or CS (E). (F–K) VFF withdrawal responses in WT mice after intraplantar injection of Dy4 or Dy4 inact (F–H, dynamin inhibitor), PS2 or PS2 inact (I–K, clathrin inhibitor), or vehicle (Veh), followed 30 min later by intraplantar trypsin (F and I), NE (G and J), or CS (H and K). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Numbers in parentheses denote mouse number.
To assess the contribution of endocytosis to protease-induced nociception, Dyngo4a (Dy4, dynamin inhibitor) (24), PitStop2 (PS2, clathrin inhibitor) (25), inactive (inact) analogs (50 µM), or vehicle (0.2% DMSO, 0.9% NaCl) (10 µL) was administered by intraplantar injection to mice. After 30 min, trypsin (10 nM), NE (1.2 µM), or CS (2.5 µM) (10 µL) was injected into the same paw. In controls (vehicle or inactive analogs), trypsin, NE, and CS induced mechanical allodynia that was fully maintained for 4 h (Fig. 1 F–K). Dy4 and PS2 inhibited trypsin-induced allodynia at 1 and 2 h (Fig. 1 F and I), whereas NE- (Fig. 1 G and J) and CS- (Fig. 1 H and K) induced allodynia was unchanged. Endocytic inhibitors or proteases did not influence withdrawal responses of the noninjected contralateral paw (SI Appendix, Fig. S2 A and B). Trypsin, NE, and CS increased paw thickness, consistent with edema (SI Appendix, Fig. S2 C–H). Dynamin and clathrin inhibitors did not affect edema.
The results suggest that proteases induce persistent nociception and neurogenic inflammation in large part by activating PAR2 on NaV-1.8+ neurons. PAR2 endocytosis is necessary for the nociceptive actions of trypsin, but not NE or CS.
PAR2-Mediated Hyperexcitability of Nociceptors.
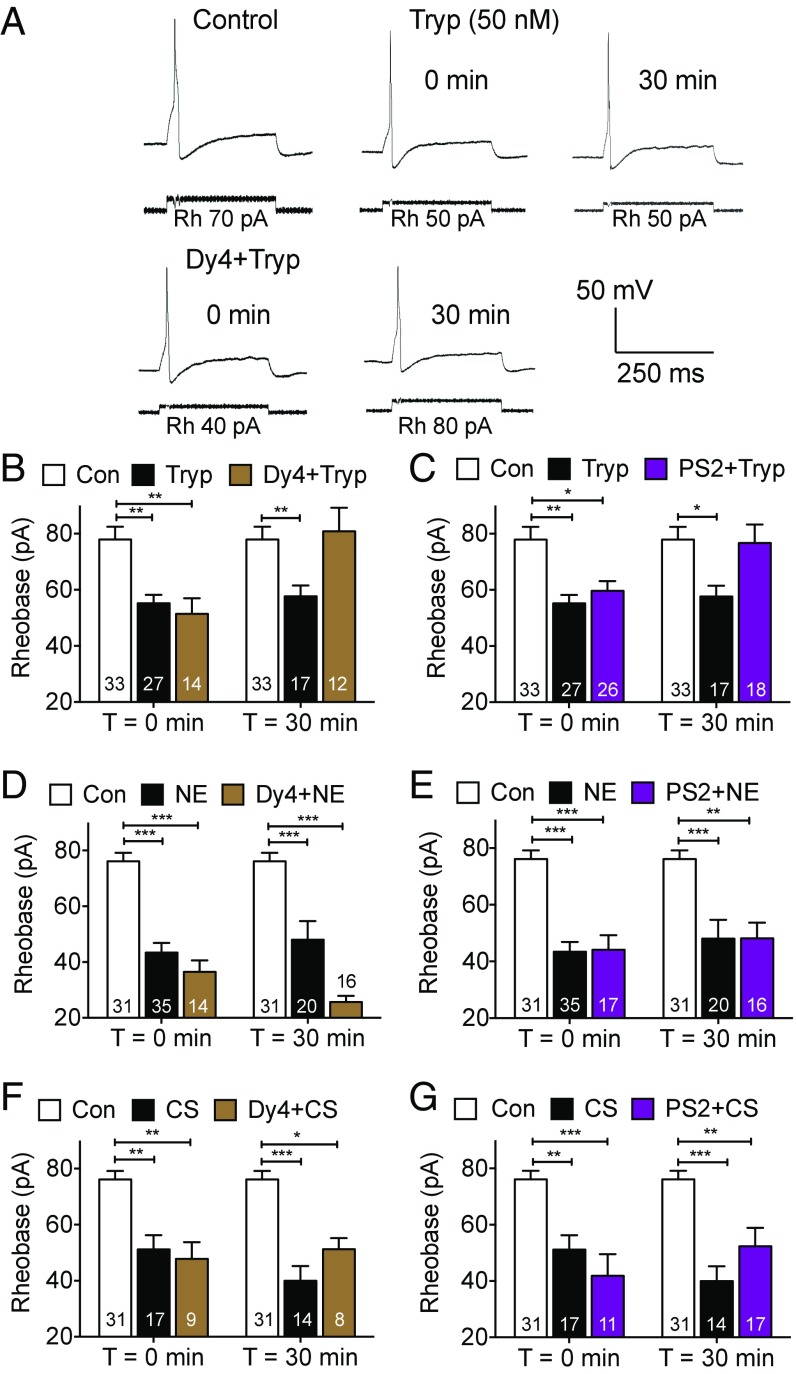
To evaluate the contribution of endocytosis to protease-induced hyperexcitability of nociceptors, the rheobase (the minimal input current required to fire one action potential) of small diameter neurons of mouse DRG was measured by patch-clamp recording. Neurons were preincubated with trypsin (50 nM, 10 min), NE (390 nM, 30 min), CS (500 nM, 60 min) (conditions selected to cause robust hyperexcitability), or vehicle, and washed. Rheobase was measured 0 or 30 min after washing. The mean rheobase of protease- or vehicle-treated neurons was calculated. Trypsin, NE, and CS decreased rheobase at 0 and 30 min, indicating an initial hyperexcitability that is maintained for at least 30 min (Fig. 2). Dy4 (30 µM) or PS2 (15 µM) did not affect the capacity of trypsin, NE, or CS to cause initial hyperexcitability (0 min). Dy4 and PS2 abolished the persistent effects of trypsin (Fig. 2 A–C), but not of NE (Fig. 2 D and E) or CS (Fig. 2 F and G) (30 min). Dy4, PS2, or vehicle (0.3% DMSO) did not affect the basal excitability of DRG neurons (SI Appendix, Fig. S3).
Fig. 2.
Protease-induced hyperexcitability of nociceptors. Rheobase of mouse DRG neurons preincubated with Dy4 (A, B, D, and F, dynamin inhibitor), PS2 (C, E, and G, clathrin inhibitor), or vehicle control (Con). Neurons were challenged with trypsin (A–C), NE (D and E), or CS (F and G), washed, and rheobase was measured 0 or 30 min later. (A) Representative traces. Rh, rheobase. (B–G) Mean responses. *P < 0.05, **P < 0.01, ***P < 0.001. Numbers in bars denote neuron numbers.
I-343 is an imidazopyridazine derivative that has been described as a potent PAR2 antagonist in the patent literature (26) (SI Appendix, Fig. S4A). I-343 belongs to the same family of PAR2 antagonists as I-191, a full antagonist of PAR2 that inhibits multiple components of PAR2 signaling, including those that may mediate protease-induced pain (27). We investigated whether I-343 inhibits PAR2 signaling in HT-29 and HEK293 cells, which express endogenous PAR2, and in KNRK cells transfected with human (h) PAR2. Accumulation of inositol phosphate-1 (IP1) was measured in response to trypsin or the PAR2-selective agonist 2-Furoyl-LIGRLO-NH2 (2F), an analog of the trypsin-exposed tethered ligand. I-343 inhibited 2F (300 nM)-induced IP1 in HT-29 cells (pIC50 8.93 ± 0.11, IC50 1.1 nM) and 2F (100 nM)-induced IP1 in KNRK-hPAR2 cells (pIC50 6.18 ± 0.11, IC50 666 nM) (SI Appendix, Fig. S4 B–D). I-343 inhibited trypsin (30 nM)-induced IP1 in HEK293 cells (pIC50 9.36 ± 0.20, IC50 0.4 nM) and in KNRK-hPAR2 cells (pIC50 5.13 ± 0.14, IC50 7507 nM). I-343 did not affect ATP (10 µM)-stimulated IP1 in KNRK cells (SI Appendix, Fig. S4E).
I-343 (10 µM) prevented the decrease in rheobase 30 min after trypsin and CS, but not NE (Fig. 3 A–C). However, I-343 prevented the decrease in rheobase 0 min after NE (Fig. 3D). I-343 (100 nM, 300 nM) also prevented the decrease in rheobase 0 min after trypsin (SI Appendix, Fig. S5A). When neurons were incubated with thrombin (50 nM, 20 min) and washed, there was an immediate decrease in rheobase that was prevented by preincubation with the PAR1 antagonist SCH79797 (1 µM, 10 min) (28); SCH79797 alone had no effect (SI Appendix, Fig. S5B) and SCH79797 did not affect the response to trypsin (SI Appendix, Fig. S5C). Thus, PAR2 mediates the immediate and persistent actions of trypsin, the persistent actions of CS, and the initial effects of NE; NE causes persistent hyperexcitability by a different mechanism. PAR1 does not mediate the initial actions of trypsin. Another PAR2 antagonist, GB88, also prevents trypsin, NE, and CS activation of nociceptors (29). Trypsin-activated PAR2 signals from endosomes by βARR- and Raf-1–dependent processes, which activate ERK (5). PD98059 (50 µM), which inhibits activation of mitogen-activated protein kinase kinase-1 (MEK1) (30), did not affect initial trypsin-induced hyperexcitability, but prevented persistent trypsin-induced hyperexcitability (Fig. 3E). In contrast, GF109203X (Bis-1, 10 µM), which inhibits PKCα and other kinases (30), prevented the initial but not the persistent effects of trypsin (Fig. 3F).
Fig. 3.
Mechanisms of protease-induced hyperexcitability of nociceptors. Rheobase of mouse DRG neurons preincubated with I-343 (A–D, PAR2 antagonist), PD98059 (E, MEK1 inhibitor), or GF109203X (F, GFX, PKC inhibitor). Neurons were challenged with trypsin (A, E, and F, Tryp), NE (B and D), or CS (C), washed, and rheobase was measured 0 or 30 min later. *P < 0.05, **P < 0.01, ***P < 0.0001. Numbers in bars denote neuron numbers.
The results suggest that trypsin induces initial hyperexcitability of nociceptors by PAR2/PKC signaling from the plasma membrane, and persistent hyperexcitability by PAR2/ERK signaling from endosomes. Adenylyl cyclase and PKA mediate NE- and CS-induced hyperexcitability of nociceptors (15, 16), which was not further studied.
PAR2 Endocytosis and Compartmentalized Signaling in Nociceptors.
To assess endocytosis of PAR2 in nociceptors, we transfected mouse (m) PAR2-GFP into mouse DRG neurons. In vehicle-treated neurons, mPAR2-GFP was detected at the plasma membrane and in intracellular compartments that may correspond to the prominent stores of PAR2 in the Golgi apparatus (Fig. 4A) (31). Trypsin, but not NE or CS (100 nM, 30 min), induced removal of mPAR2-GFP from the plasma membrane and accumulation in endosomes (Fig. 4 A and B). Dy4, but not Dy4 inact, inhibited trypsin-induced endocytosis of mPAR2-GFP (Fig. 4C). To determine whether PAR2 recruits βARRs, which mediate endocytosis of PAR2 (14), we expressed bioluminescence resonance energy transfer (BRET) sensors for PAR2-RLuc8 (donor) and βARR2-YFP (acceptor) in mouse DRG neurons. Trypsin, but not NE or CS, stimulated PAR2-RLuc8/βARR2-YFP BRET over 25 min (Fig. 4D).
Fig. 4.
PAR2 endocytosis, βARR2 recruitment, and compartmentalized signaling in nociceptors. (A–C) PAR2 endocytosis. (A) Representative images (of three experiments) of effects of trypsin (Tryp) on the distribution of mPAR2-GFP in mouse DRG neurons. Arrowheads (A, Left) show PAR2-GFP at the plasma membrane. Arrows (A, Right) show PAR2-GFP in endosomes. (B and C) Cytosol/plasma membrane ratio of mPAR2-GFP in mouse DRG neurons after 30-min incubation with trypsin, NE, or CS (B), or after preincubated with Dy4 or Dy4 inact and then trypsin (C). (D) PAR2-RLuc8/βARR2-YFP BRET in mouse DRG neurons exposed to trypsin, NE or CS. AUC, area under curve (25 min) *P < 0.05 to vehicle. n, experimental replicates, triplicate observations. (E–J) Compartmentalized signaling. Effects of trypsin on PKC activity at the plasma membrane (E and F) and in the cytosol (G), and on ERK activity in the cytosol (H and I), and nucleus (J) of rat DRG neurons. Numbers in bars denote neuron numbers. *P < 0.05, **P < 0.01 to vehicle.
To determine whether trypsin causes PAR2-dependent activation of PKC and ERK, which respectively mediate the initial and persistent phases of trypsin-induced hyperexcitability of nociceptors, we expressed genetically encoded FRET biosensors in neurons. The biosensors are targeted to subcellular compartments and are reversibly modified by kinases and phosphatases. They are suitable for analysis of signaling in subcellular compartments with high spatial and temporal resolution (8, 12). Biosensors for plasma membrane PKC (pmCKAR), cytosolic PKC (CytoCKAR), cytosolic ERK (CytoEKAR), and nuclear ERK (NucEKAR) were expressed in DRG neurons from rat, because pilot studies revealed more robust and consistent PAR2 responses than in mouse neurons. Trypsin (100 nM) activated PKC at the plasma membrane but not in the cytosol (Fig. 4 E–G), and activated ERK in the cytosol and nucleus (Fig. 4 H–J). The PAR2 antagonist I-343 (10 µM) inhibited trypsin-induced activation of PKC and ERK, whereas the PAR1 antagonist SCH530348 (100 nM) had no effect (Fig. 4 F and I). At the end of experiments, neurons were challenged with the positive controls phorbol 12,13-dibutyrate (PDBu) for EKAR biosensors or PDBu plus phosphatase inhibitor mixture-2 for CKAR biosensors, to ensure that the response of the biosensor was not saturated.
The results suggest that trypsin, but not NE or CS, stimulates βARR2 recruitment and dynamin-dependent endocytosis of PAR2 in nociceptors. Trypsin causes PAR2-dependent activation of PKC at the plasma membrane and ERK in the cytosol and nucleus.
Mechanisms of PAR2 Endocytosis and Endosomal Signaling.
We examined the mechanism of PAR2 endocytosis and endosomal signaling in HEK293 cells. To quantify the removal of PAR2 from the plasma membrane and its accumulation in early endosomes, we measured bystander BRET between PAR2 and proteins that are resident at the plasma membrane (Ras-like protein expressed in many tissues, or RIT) and early endosomes (Ras-related protein Rab5a) (8, 12). This application of BRET takes advantage of nonspecific protein–protein interactions to track movement of membrane proteins through different compartments (32). Trypsin induced a decrease in PAR2-RLuc8/RIT-Venus BRET (EC50 2.9 nM), and an increase in PAR2-RLuc8/Rab5a-Venus BRET (EC50 2.7 nM) (Fig. 5 A and B and SI Appendix, Fig. S6 A–D). Neither NE nor CS (100 nM) affected PAR2-RLuc8/RIT-Venus or Rab5a-Venus BRET (Fig. 5 A and B). PS2, but not PS2 inact, suppressed the trypsin-induced decrease in PAR2-RLuc8/RIT-Venus BRET and increase in PAR2-RLuc8/Rab5a-Venus BRET (Fig. 5 C and D and SI Appendix, Fig. S6 E and F). Dominant-negative dynaminK44E (DynK44E), which is deficient in GTP binding (33), inhibited the increase in PAR2-RLuc8/Rab5a-Venus BRET, but did not affect PAR2-RLuc8/RIT-Venus BRET (Fig. 5 C and D and SI Appendix, Fig. S6 G and H). WT dynamin (DynWT) had minimal effects. Because GTP binding is required for scission of budding vesicles from the plasma membrane, DynK44E presumably traps PAR2 in membrane vesicles, which would impede interaction with Rab5a but not RIT. Thus, trypsin, but not CS or NE, induces clathrin- and dynamin-dependent endocytosis of PAR2.
Fig. 5.
PAR2 endocytosis and compartmentalized ERK signaling in HEK293 cells. (A–D) BRET assays of endocytosis. PAR2-RLuc8/RIT-Venus BRET (A and C) and PAR2-RLuc8/Rab5a-Venus BRET (B and D). (E–K) FRET assays of cytosolic (E, G, H, and J) and nuclear (F, G, I, and K) ERK activity. AUC, area under curve. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with trypsin alone. n, experimental replicates in triplicate.
We investigated the contribution of endocytosis to trypsin-induced signaling in HEK293 cells expressing Flag-PAR2-HA11 and FRET biosensors for cytosolic and nuclear ERK (CytoEKAR, NucEKAR), plasma membrane and cytosolic PKC (pmCKAR, CytoCKAR), and plasma membrane and cytosolic cAMP (pmEpac, CytoEpac). Trypsin (10 nM), but not NE or CS (100 nM), stimulated a rapid and persistent activation of ERK in the cytosol and nucleus (EC50, 5 nM) (Fig. 5 E and F and SI Appendix, Fig. S7 A–F). I-343 (10 µM) but not the PAR1 antagonist SCH530348 (100 nM) inhibited trypsin activation of cytosolic and nuclear ERK (Fig. 5G). PS2 and DynK44E inhibited trypsin-stimulated activation of cytosolic and nuclear ERK compared with PS2 inact and DynWT controls (Fig. 5 H and I and SI Appendix, Fig. S7 G–J). AG1478 (1 µM), an inhibitor of EGF receptor tyrosine kinase (34), UBO-QIC (100 nM), which inhibits Gαq and certain Gβγ signals (35), and Gö6983 (1 µM), which inhibits all isoforms of PKC (36), suppressed trypsin-stimulated activation of cytosolic ERK (Fig. 5J and SI Appendix, Fig. S7K). UBO-QIC and Gö6983 also inhibited activation of nuclear ERK (Fig. 5K and SI Appendix, Fig. S7L). The results suggest that PAR2 signals from endosomes by Gαq-dependent mechanisms to activate ERK in the cytosol and nucleus.
To determine whether trypsin induces translocation of βARR and Gαq to endosomes, we measured BRET between βARR1-RLuc8 or Gαq-RLuc8 and Rab5a-Venus in HEK293 cells. Trypsin (100 nM) stimulated an increase in βARR1-RLuc8/Rab5a-Venus BRET and in Gαq-RLuc8/Rab5a-Venus BRET (SI Appendix, Fig. S8 A and B). We used immunofluorescence and structured illumination microscopy to localize PAR2-HA, Gαq and early endosomal antigen-1 (EEA1) in HEK-293 cells. In unstimulated cells, PAR2 was confined to the plasma membrane, although Gαq was detected in early endosomes (SI Appendix, Fig. S8C). Trypsin (10 nM, 30 min) induced translocation of PAR2 to early endosomes containing Gαq. The results support the hypothesis that trypsin causes assembly of a PAR2/βARR/Gαq signalosome in early endosomes.
Trypsin (10 nM) caused a rapid and sustained activation of PKC and generation of cAMP at the plasma membrane and in the cytosol of HEK293 cells (SI Appendix, Fig. S9 A–H). DynK44E strongly inhibited these signals, but DynWT had no effect. I-343, but not SCH530348, inhibited trypsin stimulation of PKC and cAMP, which thus depend on PAR2 (SI Appendix, Fig. S9 G and H). These results suggest that endocytosis is necessary for multiple components of PAR2 signaling. cAMP signaling at the plasma membrane is usually desensitized by βARR delivery of phosphodiesterases, which degrade cAMP (37). The sustained plasma membrane cAMP response to trypsin support the existence of mechanisms that allow persistent PAR2 signaling, which warrant further investigation. Stimulation of cells with the positive controls PDBu (EKAR), PDBu + phosphatase inhibitor mixture-2 (CKAR), or forskolin + 3-isobutyl-1-methylxanthine (Epac) revealed that responses to proteases did not saturate the FRET biosensors (Fig. 5 E and F and SI Appendix, Fig. S9 A–D).
IBS-Induced Hyperexcitability of Nociceptors.
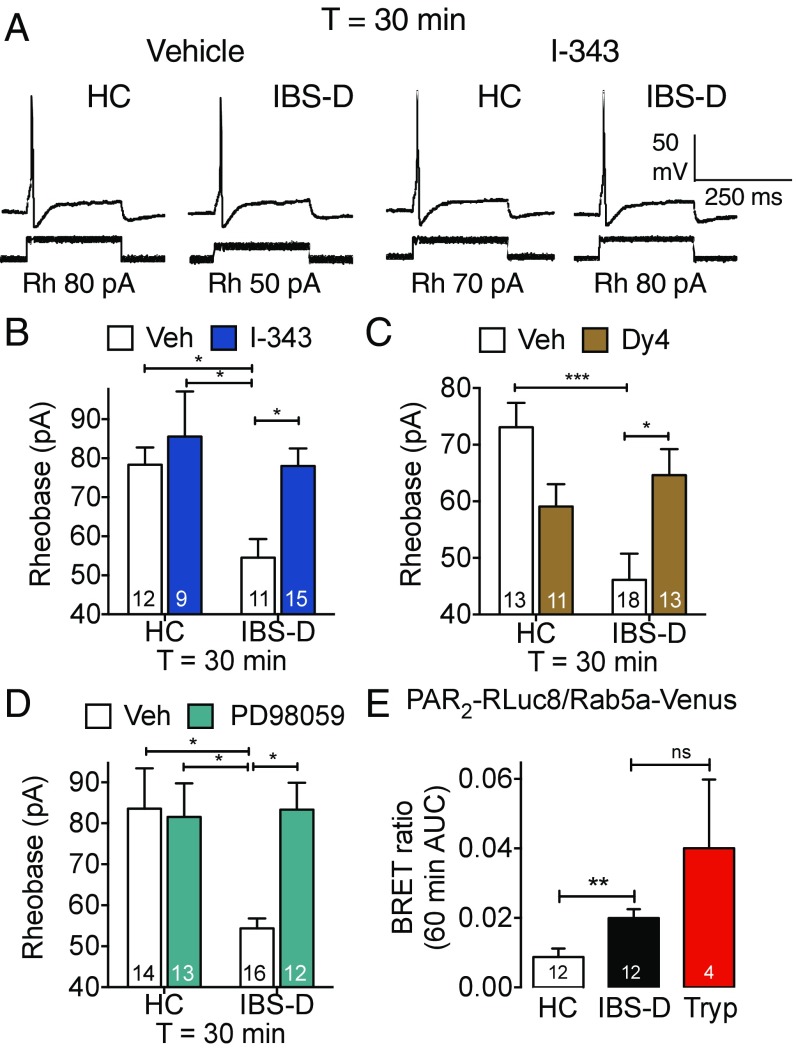
We investigated whether proteases from mucosal biopsies of IBS patients cause a persistent hyperexcitability of nociceptors by a mechanism that entails endosomal signaling of PAR2. Biopsies of colonic mucosa from patients with diarrhea-predominant IBS (IBS-D) or healthy control (HC) subjects were placed in culture medium (24 h, 37 °C). Mouse DRG neurons were then exposed to biopsy supernatants (30 min, 37 °C) and washed. Rheobase was measured 30 min after washing to assess persistent hyperexcitability. Supernatants of biopsies from IBS-D patients caused a persistent decrease in rheobase, consistent with hyperexcitability, compared with supernatants from HC subjects (rheobase at 30 min: HC, 78.33 ± 4.41 pA, 12 neurons, supernatant from four HC; IBS-D, 54.55 ± 4.74 pA, 11 neurons, supernatant from four IBS-D; P < 0.05; ANOVA, Tukey’s multiple comparisons test) (Fig. 6 A and B). I-343 (PAR2 antagonist, 10 µM), Dy4 (dynamin inhibitor, 30 µM), and PD98059 (MEK1 inhibitor, 50 µM) abolished IBS-D–induced hyperexcitability of nociceptors (Fig. 6 A–D). Dy4 caused a nonsignificant decrease in rheobase of neurons exposed to HC supernatant, but I-343 and PD98059 had no effect.
Fig. 6.
IBS-D–induced hyperexcitability of nociceptors. (A–D) Rheobase of mouse nociceptors 30 min after exposure to supernatant from biopsies of colonic mucosa from HC and IBS-D subjects. (A) Representative traces of vehicle- or I-343–treated neurons. Rh, rheobase. (B–D) Mean responses of neurons preincubated with I-343 (B, PAR2 antagonist), Dy4 (C, dynamin inhibitor), or PD98059 (D, MEK1 inhibitor). (E) PAR2-RLuc8/Rab5a-Venus BRET in HEK293 cells measured after 60-min incubation with HC or IBS-D biopsy supernatant, or trypsin. *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant. Numbers in bars denote neuron numbers.
To examine whether proteases in IBS-D supernatants can stimulate endocytosis of PAR2, BRET was used to assess the proximity between PAR2-RLuc8 and Rab5a-Venus expressed in HEK293 cells. IBS-D supernatant increased PAR2-RLuc8/Rab5a-Venus BRET after 60 min compared with HC supernatant (Fig. 6E). Trypsin (10 nM, positive control) also increased PAR2-RLuc8/Rab5a-Venus BRET.
These results suggest that proteases that are released from biopsies of colonic mucosa from patients with IBS-D cause long-lasting hyperexcitability of nociceptors by a mechanism that requires dynamin-dependent endocytosis of PAR2 and PAR2 ERK signaling from endosomes.
Antagonist Delivery to PAR2 in Endosomes.
If endosomal signaling of PAR2 sustains the hyperexcitability of nociceptors exposed to supernatants from IBS-D patients, do PAR2 antagonists that are targeted to endosomal receptors effectively reverse this process? Conjugation to the transmembrane lipid cholestanol facilitates endosomal delivery of antagonists of the neurokinin 1 receptor (NK1R) and calcitonin receptor-like receptor (CLR), which provide more efficacious and long-lasting antinociception than conventional antagonists that do not target receptors in endosomes (8, 12). To evaluate whether PAR2 in endosomes is a therapeutic target, tripartite probes were synthesized comprising: cholestanol to anchor probes to membranes or ethyl ester that does not incorporate into membranes; a polyethylene glycol (PEG) 12 linker to facilitate presentation in an aqueous environment; and a cargo of cyanine 5 (Cy5) for localization or PAR2 antagonist I-343 (SI Appendix, Fig. S10 A and B). To determine whether tripartite probes accumulate in endosomes containing PAR2, mouse DRG neurons expressing mPAR2-GFP were incubated with Cy5-PEG-cholestanol (Cy5-Chol) or Cy5-PEG-Ethyl ester (Cy5-Ethyl ester) (200 nM, 60 min, 37 °C). Neurons were washed and imaged (37 °C). Cy5-Ethyl ester was not taken up by neurons, whereas Cy5-Chol inserted into the plasma membrane and then accumulated in endosomes of the soma and neurites over 3 h (Fig. 7). Trypsin induced endocytosis of PAR2-GFP into endosomes in close proximity to vesicles containing Cy5-Chol. Video-imaging revealed frequent association of endosomes containing PAR2-GFP and Cy5-Chol (Movie S1). I-343–PEG-cholestanol (MIPS15479) (SI Appendix, Fig. S10A) antagonized 2F-stimulated IP1 accumulation in HT-29 cells (pIC50 6.18 ± 0.07; IC50, 670 nM), albeit with reduced potency compared with the parent compound I-343 (pIC50 8.96 ± 0.10; IC50 1.1 nM) (SI Appendix, Fig. S10C).
Fig. 7.
Targeting PAR2 in endosomes of nociceptors. Representative images (of three experiments) of trafficking of Cy5 tripartite probes and mPAR2-GFP to the soma (A) and neurites (B) of mouse DRG neurons. The scale bar (5 µm) in the bright-field image applies to all panels in the same row, except for Inset, which is a magnification of the dashed box in the merged panels. Arrows show proximity of vesicles containing mPAR2-GFP and Cy5-Chol.
Antagonism of Endosomal PAR2 and Hyperexcitability of Nociceptors.
To evaluate the capacity of an endosomally targeted PAR2 antagonist to inhibit protease-induced hyperexcitability of nociceptors, mouse DRG neurons were preincubated with MIPS15479 (30 µM) or vehicle (60 min, 37 °C), washed, and recovered in antagonist-free medium for 180 min to allow accumulation of antagonist in endosomes (Fig. 8A). Transient incubation with trypsin decreased rheobase of vehicle-treated neurons at 0 and 30 min (Fig. 8B). MIPS15479 did not affect the initial excitability at 0 min, but prevented the persistent response at 30 min. MIPS15479 had no effect on baseline rheobase at either time point. Similarly, transient incubation with IBS-D supernatant decreased rheobase at 30 min compared with HC supernatant (Fig. 8C). MIPS15479 completely prevented the persistent actions of IBS-D supernatant on nociceptor excitability (rheobase at 30 min: vehicle IBS-D, 40 ± 3.89 pA, 12 neurons, supernatant from four patients; MIPS15479 IBS-D, 64.7 ± 3.84 pA, 17 neurons, supernatant from four patients; P < 0.05) (Fig. 8C). MIPS15479 did not affect excitability of neurons treated with HC supernatant. These results support the hypothesis that PAR2 in endosomes generates signals that underlie the persistent hyperexcitability of nociceptors, and is a potential therapeutic target for IBS pain.
Fig. 8.
Antagonism of endosomal PAR2 and hyperexcitability of nociceptors. (A and B) Trypsin-induced hyperexcitability of mouse DRG neurons. Neurons were preincubated with MIPS15479 or vehicle (control, con) for 60 min, washed, and recovered for 170 or 140 min. Neurons were then exposed to trypsin (10 min). Rheobase was measured 0 or 30 min after trypsin and 180 min post-MIPS15479. (C) IBS-induced hyperexcitability of mouse DRG neurons. Neurons were preincubated with MIPS15479 or vehicle (control, con) for 60 min, washed, and recovered for 60 min. Neurons were then exposed to HC or IBS-D supernatant for 30 min, washed, and rheobase was measured 30 min later (T 30 min), 120 min post-MIPS15479. *P < 0.05, **P < 0.01. Numbers in bars denote neuron numbers.
PAR2 Endosomal Signaling Mediates Trypsin-Induced Sensitization of Colonic Afferent Neurons and Colonic Nociception.
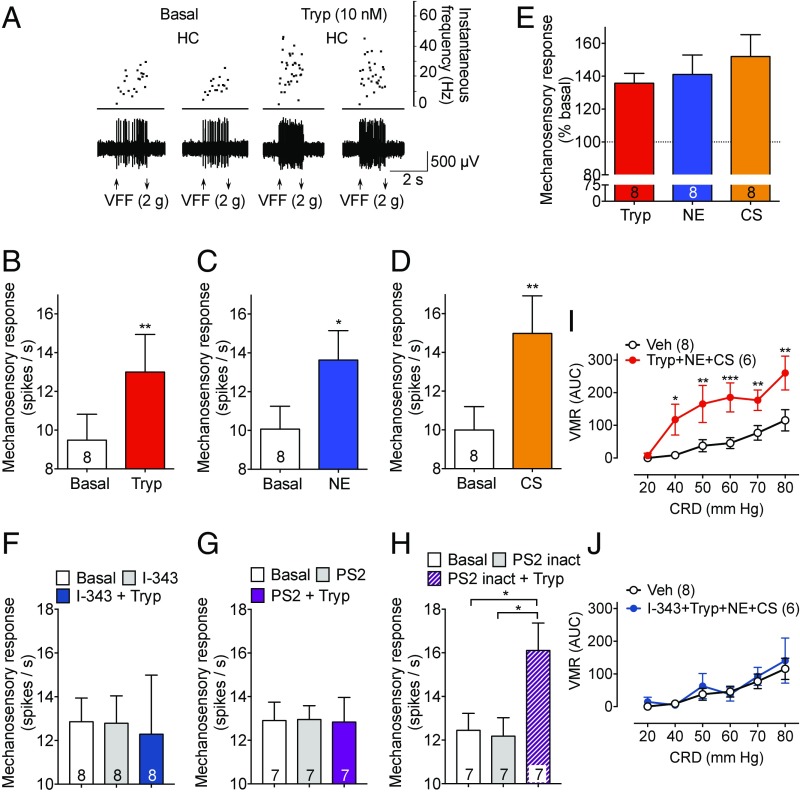
The sensitization of colonic afferent neurons to mechanical stimuli is a leading hypothesis for IBS pain (17). To examine whether proteases cleave PAR2 on the peripheral terminals of colonic nociceptors to induce mechanical hypersensitivity, we made single-unit recordings from afferent neurons innervating the mouse colon. Receptive fields were identified by mechanical stimulation of the mucosal surface with VFF, proteases were applied to the mucosal receptive fields, and mechanical responses were reevaluated to assess sensitization. Under basal conditions, repeated mechanical stimulation (2-g filament) induced reproducible firing (Fig. 9A). Exposure to trypsin (10 nM, 10 min) amplified the frequency of firing to mechanical stimulation by 35.8 ± 5.9%, to NE (100 nM, 10 min) by 41.0 ± 11.8%, and to CS (100 nM, 10 min) by 52.0 ± 13.2% (Fig. 9 B–E).
Fig. 9.
Sensitization of colonic afferents and colonic nociception. (A–H) Mechanosensory responses in heathy control mice to stimulation of the colonic mucosa with a 2 g VFF under basal conditions and after exposure of receptive fields to trypsin (A, B, and E–H, Tryp), NE (C and E), or CS (D and E). (A) Representative results. (B–D and F–H) Mean responses. (E) Responses as percent basal. Numbers in bars denote afferent numbers. (I and J) VMR to CRD in awake normal mice. Numbers in parentheses denote mouse number. *P < 0.05, **P < 0.01, ***P < 0.001.
Colitis in mice induces hypersensitivity of colonic afferent neurons that persists even after inflammation is resolved (17). This chronic hypersensitivity resembles postinfectious/inflammatory IBS. To determine whether proteases can further amplify chronic hypersensitivity, mice were treated with trinitrobenzene sulphonic acid (TNBS enema) to induce colitis. At 28 d post-TNBS, when inflammation was resolved, mechanical stimulation of the colon induced a larger firing rate than in healthy control mice, consistent with chronic hyperexcitability (SI Appendix, Fig. S11 A–D). Compared with basal responses, trypsin further amplified responses by 16.4 ± 7.9%, NE by 30.6 ± 9.0%, and CS by 29.6 ± 9.2%. Thus, proteases can still amplify the excitability of colonic nociceptors even when they are already sensitized as a result of prior inflammation.
To determine the contribution of endosomal PAR2 signaling to trypsin-induced sensitization of colonic afferent neurons in normal healthy mice, I-343 (10 µM), PS2, or PS2 inact (50 µM) was applied to the receptive fields before exposure to trypsin. I-343 and PS2 did not affect basal mechanical sensitivity, but abolished trypsin-induced sensitization of mechanical responses (Fig. 9 F and G). PS2 inact did not affect basal responses or trypsin-induced sensitization (Fig. 9H).
Noxious colorectal distension (CRD) triggers the visceromotor response (VMR), a nociceptive brainstem reflex consisting of contraction of abdominal muscles, which can be monitored by electromyography. This approach allows assessment of visceral sensitivity in awake mice (38). To examine protease-induced hypersensitivity, a protease mixture (10 nM trypsin + 100 nM NE + 100 nM CS) or vehicle (saline) (100 µL) was instilled into the colon (enema) of healthy mice. After 15 min, the VMR was measured in response to graded CRD (20–80 mm Hg) with a barostat balloon. In vehicle-treated mice, CRD induced a graded VMR (Fig. 9I). The protease mixture amplified VMR at all pressures from 40 to 80 mm Hg. Administration of I-343 (30 mg/kg) into the colon (100 µL enema) 30 min before the protease mixture, abolished the response (Fig. 9J). Because alterations in the compliance of the colon can alter VMR to CRD, the pressure/volume relationship was measured at all distending pressures. Compliance of the colon was unaffected by the protease mixture or I-343 (SI Appendix, Fig. S11 E and F).
The results support the hypothesis that PAR2 endocytosis is required for trypsin-induced sensitization of colonic afferent neurons and colonic nociception.
Discussion
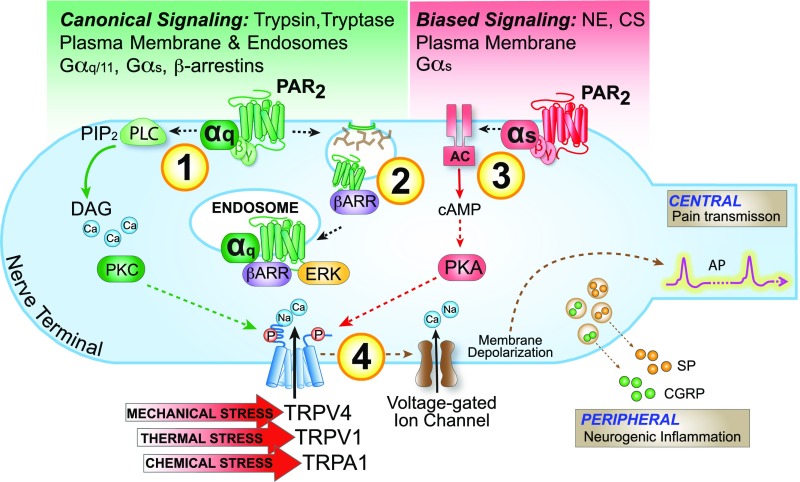
We propose that two components of PAR2 signaling contribute to persistent hyperexcitability on nociceptors: irreversible proteolytic activation and sustained signaling from endosomes or the plasma membrane, depending on the mechanism of activation (Fig. 10 and Movie S2). In the case of trypsin and proteases from the mucosa of patients with IBS-D, PAR2 endocytosis and endosomal ERK signaling mediate persistent hyperexcitability. In the case of NE and CS, which do not cause PAR2 endocytosis, plasma membrane signaling underlies persistent hyperexcitability. The observation that an endosomally targeted PAR2 antagonist blocks the persistent actions of trypsin and IBS proteases identifies PAR2 in endosomes as a therapeutic target. The combined results reveal that endosomal GPCR signaling can contribute to processes that are relevant to human disease, and support the proposal that GPCRs in endosomes are a target for therapy (8, 12).
Fig. 10.
Mechanisms of protease- and PAR2-induced hyperexcitability of nociceptors. After activation by canonical mechanisms, PAR2 signals at the plasma membrane to activate PKC, which mediates initial hyperexcitability (1). PAR2 then undergoes clathrin-, dynamin-, and βARR-dependent endocytosis (2). PAR2 continues to signal from endosomes by βARR- and Gαq-mediated mechanisms to activate ERK, which mediates persistent hyperexcitability. After activation by biased mechanisms, PAR2 signals from the plasma membrane to activate adenylyl cyclase (AC) and PKA, which mediate the initial and persistent hyperexcitability (3). Kinases may regulate the activity of TRP channels and voltage-gated ion channels, to control nociceptor hyperexcitability (4).
Mechanisms of Persistent Hyperexcitability of Nociceptors and Nociception.
The contribution of plasma membrane and endosomal signaling of PAR2 to protease-induced hyperexcitability of nociceptors depends on the protease and the timing of the response. Trypsin, but not NE or CS, caused dynamin- and clathrin-dependent endocytosis of PAR2 in nociceptors. Because inhibitors of endocytosis did not affect the initial effects of trypsin, NE, or CS on excitability, hyperexcitability initially involves PAR2 signaling at the plasma membrane. Clathrin and dynamin inhibitors prevented persistent trypsin-induced hyperexcitability (at a time when activated PAR2 was in endosomes), and also suppressed trypsin-induced sensitization of colonic afferent nociceptors and somatic mechanical allodynia. Thus, PAR2 endocytosis and continued endosomal signaling are necessary for persistent trypsin-induced hyperexcitability of nociceptors and nociception. Inhibitors of endocytosis did not affect NE- or CS-induced neuronal hyperexcitability or mechanical allodynia, which is consistent with the inability of these proteases to induce PAR2 endocytosis.
PAR2/PKC signaling at the plasma membrane mediates initial trypsin-induced hyperexcitability of nociceptors, because trypsin induced PAR2-dependent PKC activation at the plasma membrane, and a PKC inhibitor blocked the initial hyperexcitability. Several observations indicate that PAR2/ERK signaling from endosomes mediates persistent trypsin-induced hyperexcitability: trypsin stimulated PAR2-dependent ERK activity in the cytosol and nucleus, clathrin and dynamin inhibitors suppressed ERK activation, and a MEK1 inhibitor prevented the persistent actions of trypsin. The finding that inhibitors of Gαq, PKC, and the EGF receptor block trypsin activation of ERK, suggest that several pathways mediate PAR2 signaling in endosomes. A PAR2/βARR/Raf-1/MEK signaling complex in endosomes mediates activation of ERK in the cytosol (5), and Gαq and Gαs transduce signals from other GPCRs in endosomes (7, 8, 12). Gαq was detected in early endosomes of HEK293 cells under basal conditions and after exposure to trypsin by immunofluorescence and superresolution microscopy. In agreement with previous studies (5, 14), trypsin induced translocation of PAR2 and βARR to early endosomes, detected by BRET and microscopy. Together, these findings support involvement of Gαq and βARR in endosomal signaling of PAR2. NE and CS induce PAR2-dependent stimulation of adenylyl cyclase and protein kinase A-mediated activation of transient receptor potential vanilloid 4 in nociceptors, which likely underlie hyperexcitability (15, 16). The inability to recruit βARRs and internalize may contribute to the sustained signaling of NE- and CS-activated PAR2 at the plasma membrane.
The finding that a cholestanol-conjugated PAR2 antagonist inhibited the ability of trypsin and IBS-D proteases to cause persistent hyperexcitability of nociceptors reinforces the importance of endosomal PAR2 signaling. After transient incubation and recovery, cholestanol-conjugated tripartite probes were removed from the plasma membrane of nociceptors and accumulated in endosomes containing PAR2, which demonstrates effective targeting. The observation that I-343–PEG-cholestanol and inhibitors of clathrin and dynamin inhibited the persistent, but not initial, actions of trypsin and IBS-proteases on nociceptor excitability suggests selective targeting of PAR2 in endosomes represents a viable therapeutic strategy. Becaause I-343–PEG-cholestanol abolished persistent hyperexcitability despite reduced potency, endosomal targeting, retention, and local concentration are probably important determinants of efficacy.
There are several limitations of this investigation. Further studies are required to characterize the composition of PAR2 signaling complexes in different subcellular domains, and to define the mechanisms by which PAR2/PKC signaling at the plasma membrane and PAR2/ERK signaling in endosomes amplify the sensitivity of nociceptors. Involvement of PKC and ERK are consistent with reports that painful stimulants activate PKCε and ERK1/2 in primary sensory neurons, and that inhibitors of PKCε and MEK attenuate nociception (39, 40). PKC and ERK can activate channels that control the sensitivity of nociceptors (41). GPCRs in endosomes can also regulate transcription, which may contribute to chronic hyperexcitability of nociceptors (8, 11). Additional studies are required to define the specificity of I-343 and I-343–PEG-cholestanol (MIPS15479) for PAR2 over other GPCRs. Although I-343 shows remarkable potency for PAR2, high concentrations of I-343 can inhibit thrombin signaling, suggesting antagonism of PAR1 (26). However, I-343, at concentrations that do not affect thrombin signaling, prevented trypsin-induced hyperexcitability of nociceptors, whereas a PAR1-selective antagonist had no effect. Thus, PAR2, not PAR1, mediates these actions of trypsin.
A limitation to the use of inhibitors of endocytosis is that clathrin and dynamin control the trafficking of many receptors and channels that control neuronal activity and nociception. Although Dy4 may have off-target effects (42), we obtained similar results by overexpressing dominant-negative dynamin or inhibiting clathrin. Dy4 and PS2 did not affect basal excitability of nociceptors or affect hyperexcitability or allodynia to NE or CS, which do not cause PAR2 endocytosis. These findings argue against nonselective effects of inhibitors on the function of nociceptors. The finding that an endosomally targeted PAR2 antagonist (I-343–PEG-cholestanol) replicated the inhibitory actions of Dy4 and PS2 on trypsin-induced hyperexcitability, supports the involvement of endosomal PAR2 signaling.
PAR2 Endosomal Signaling and IBS Pain.
PAR2 endosomal signaling may underlie persistent hyperexcitability of nociceptors in patients with IBS-D, because an endosomally targeted PAR2 antagonist and inhibitors of dynamin and MEK1 prevented the persistent effects of IBS-D proteases on the hyperexcitability of nociceptors. Although we did not identify the proteases that are released from colonic biopsies that mediate these effects, tryptase and trypsin-3 are likely candidates; both proteases are activated in the colonic mucosa of IBS patients, and tryptase and trypsin inhibition or PAR2 deletion prevent IBS-dependent hyperexcitability of nociceptors (18–21). The finding that a dynamin inhibitor and an endosomally targeted PAR2 antagonist prevented hypersensitivity supports involvement of proteases that activate PAR2 by canonical mechanisms and induce receptor endocytosis, such as tryptase and trypsin-3 (13).
Hypersensitivity to colorectal distension is a hallmark of IBS (17). Our results show that proteases that activate PAR2 by canonical (trypsin) and biased (NE, CS) mechanisms sensitize colonic nociceptors to mechanical stimuli in basal and postinflammatory states. Trypsin-evoked sensitization requires PAR2 endocytosis and endosomal signaling. The administration of a mixture of proteases (trypsin, NE, CS) into the colonic lumen of mice amplified VMR to CRD, which is consistent with mechanical hyperalgesia in the colon. I-343 abolished these effects, which are thus dependent on PAR2. We did not determine whether inhibitors of endocytosis or endosomally targeted PAR2 antagonists suppress trypsin-evoked colonic nociception due to the impracticality of systemic administration of broadly acting and lipophilic drugs. Such studies will require pharmacokinetic studies of endocytic inhibitors and I-343–PEG-cholestanol to determine whether these drugs are capable of targeting pain-sensing neurons in the colonic wall. However, when administered by local injection into the paw, inhibitors of dynamin and clathrin prevented the ability of trypsin to cause mechanical allodynia. These results support a role for endosomal signaling in mechanical nociception.
Our results show that PAR2 expressed by NaV1.8+ neurons mediates the long-lasting pronociceptive actions of trypsin, NE, and CS, because the sustained actions of these proteases were absent or diminished in Par2-NaV1.8 mice. Because global deletion of PAR2 attenuates the algesic actions of these proteases (15, 16, 43), the initial effects may involve activation of PAR2 on other cell types involved in nociception, including keratinocytes and colonocytes that highly express PAR2 (13). The findings that deletion of PAR2 in NaV1.8+ neurons or treatment with a PAR2 antagonist suppressed trypsin signaling in DRG cultures support the suggestion that proteases can directly activate nociceptors by cleaving PAR2. However, we cannot exclude the possibility that proteases activate PAR2 on nonneuronal cells that control nociceptor function, or that proteases may cause pain by activating other receptors, such as PAR1. PAR2 in NaV1.8+ neurons markedly inhibited trypsin-induced edema and neutrophil infiltration, which supports the proposal that trypsin causes inflammation by a neurogenic mechanism (44).
Our results have implications for the treatment of IBS pain. GPCRs are the target of over one-third of therapeutic drugs, most of which are designed to target cell surface receptors. The realization that GPCRs can continue to signal from endosomes to control important pathophysiological processes, has led to the proposal that receptors in endosomes are a target for therapy (1, 8, 12). The capacity of endosomally targeted PAR2 antagonists to abolish IBS-dependent persistent hyperexcitability of nociceptors highlights the importance of endosomal signaling of GPCRs for human disease, and reveals endosomal PAR2 as a therapeutic target for IBS pain.
Materials and Methods
Human Subjects.
The Queen’s University Human Ethics Committee approved human studies. All subjects gave informed consent.
Animal Subjects.
Institutional Animal Care and Use Committees of Queen’s University, Monash University, Flinders University, New York University, and the South Australian Health and Medical Research Institute approved studies in mice and rats.
Nociception, Inflammation, Nociceptor Hyperexcitability.
The analysis of somatic nociception and inflammation (8, 12), nociceptor hyperexcitability (21), sensitization of colonic afferent neurons, and colonic nociception (38) have been described.
BRET, FRET Assays.
Endocytosis was studied by BRET and compartmentalized signaling was analyzed by FRET, as described previously (8, 12).
Statistics.
Results are mean ± SEM. Differences were assessed using Student’s t test (two comparisons) or one- or two-way ANOVA (multiple comparisons).
Supplementary Material
Acknowledgments
This work was supported by NIH Grant NS102722 (to N.W.B.); National Health Medical and Research Council (NHMRC) Grants 63303, 1049682, and 1031886 (to N.W.B.); Australian Research Council Centre of Excellence in Convergent Bio-Nano Science and Technology (N.W.B.); Takeda Pharmaceuticals, Inc. (N.W.B.); NIH Grant DE026806 (to N.W.B. and B.L.S.); NIH Grant DE025393 (to B.L.S.); and the Canadian Institute of Health (S.J.V.). M.L.H. is an NHMRC RD Wright Career Development Fellow (1061687); S.M.B. is an NHMRC RD Wright Biomedical Research Fellow (1126378); and M.C. is a Monash Fellow.
Footnotes
Conflict of interest statement: P.M. and G.A.H. work for Takeda Pharmaceuticals, Inc. Research in N.W.B.’s laboratory is supported in part by Takeda Pharmaceuticals, Inc. N.W.B. and G.A.H. are founding scientists of Endosome Therapeutics, Inc.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721891115/-/DCSupplemental.
References
- 1.Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW. Endosomes: A legitimate platform for the signaling train. Proc Natl Acad Sci USA. 2009;106:17615–17622. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 3.Cheloha RW, Gellman SH, Vilardaga J-P, Gardella TJ. PTH receptor-1 signalling-mechanistic insights and therapeutic prospects. Nat Rev Endocrinol. 2015;11:712–724. doi: 10.1038/nrendo.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeFea KA, et al. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta-arrestin-dependent scaffolding complex. Proc Natl Acad Sci USA. 2000;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeFea KA, et al. Beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eichel K, Jullié D, von Zastrow M. β-Arrestin drives MAP kinase signalling from clathrin-coated structures after GPCR dissociation. Nat Cell Biol. 2016;18:303–310. doi: 10.1038/ncb3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irannejad R, et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–538. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen DD, et al. Neurokinin 1 receptor signaling in endosomes mediates sustained nociception and is a viable therapeutic target for prolonged pain relief. Sci Transl Med. 2017;9:eaal3447. doi: 10.1126/scitranslmed.aal3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May V, Parsons RL. G protein-coupled receptor endosomal signaling and regulation of neuronal excitability and stress responses: Signaling options and lessons from the PAC1 receptor. J Cell Physiol. 2017;232:698–706. doi: 10.1002/jcp.25615. [DOI] [PubMed] [Google Scholar]
- 10.Thomsen ARB, et al. GPCR-G protein-β-arrestin super-complex mediates sustained G protein signaling. Cell. 2016;166:907–919. doi: 10.1016/j.cell.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsvetanova NG, von Zastrow M. Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nat Chem Biol. 2014;10:1061–1065. doi: 10.1038/nchembio.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yarwood RE, et al. Endosomal signaling of the receptor for calcitonin gene-related peptide mediates pain transmission. Proc Natl Acad Sci USA. 2017;114:12309–12314. doi: 10.1073/pnas.1706656114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ossovskaya VS, Bunnett NW. Protease-activated receptors: Contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 14.Déry O, Thoma MS, Wong H, Grady EF, Bunnett NW. Trafficking of proteinase-activated receptor-2 and beta-arrestin-1 tagged with green fluorescent protein. beta-arrestin-dependent endocytosis of a proteinase receptor. J Biol Chem. 1999;274:18524–18535. doi: 10.1074/jbc.274.26.18524. [DOI] [PubMed] [Google Scholar]
- 15.Zhao P, et al. Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4. J Biol Chem. 2014;289:27215–27234. doi: 10.1074/jbc.M114.599712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao P, et al. Neutrophil elastase activates PAR2 and TRPV4 to cause inflammation and pain. J Biol Chem. 2015;290:13875–13887. doi: 10.1074/jbc.M115.642736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azpiroz F, et al. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil. 2007;19(Suppl 1):62–88. doi: 10.1111/j.1365-2982.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- 18.Barbara G, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 19.Cenac N, et al. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636–647. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolland-Fourcade C, et al. Epithelial expression and function of trypsin-3 in irritable bowel syndrome. Gut. 2017;66:1767–1778. doi: 10.1136/gutjnl-2016-312094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valdez-Morales EE, et al. Sensitization of peripheral sensory nerves by mediators from colonic biopsies of diarrhea-predominant irritable bowel syndrome patients: A role for PAR2. Am J Gastroenterol. 2013;108:1634–1643. doi: 10.1038/ajg.2013.241. [DOI] [PubMed] [Google Scholar]
- 22.Kayssi A, Amadesi S, Bautista F, Bunnett NW, Vanner S. Mechanisms of protease-activated receptor 2-evoked hyperexcitability of nociceptive neurons innervating the mouse colon. J Physiol. 2007;580:977–991. doi: 10.1113/jphysiol.2006.126599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reed DE, et al. Mast cell tryptase and proteinase-activated receptor 2 induce hyperexcitability of guinea-pig submucosal neurons. J Physiol. 2003;547:531–542. doi: 10.1113/jphysiol.2002.032011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson MJ, Deane FM, Robinson PJ, McCluskey A. Synthesis of Dynole 34-2, Dynole 2-24 and Dyngo 4a for investigating dynamin GTPase. Nat Protoc. 2014;9:851–870. doi: 10.1038/nprot.2014.046. [DOI] [PubMed] [Google Scholar]
- 25.Robertson MJ, et al. Synthesis of the Pitstop family of clathrin inhibitors. Nat Protoc. 2014;9:1592–1606. doi: 10.1038/nprot.2014.106. [DOI] [PubMed] [Google Scholar]
- 26.Farmer LJ. 2013. Imidazopyridazines useful as inhibitors of the PAR-2 signaling pathway. US Patent Appl WO 2015/048245.
- 27.Jiang Y, et al. A potent antagonist of protease-activated receptor 2 that inhibits multiple signaling functions in human Cancer cells. J Pharmacol Exp Ther. 2018;364:246–257. doi: 10.1124/jpet.117.245027. [DOI] [PubMed] [Google Scholar]
- 28.Ahn HS, et al. Inhibition of cellular action of thrombin by N3-cyclopropyl-7-[[4-(1-methylethyl)phenyl]methyl]-7H-pyrrolo[3, 2-f]quinazoline-1,3-diamine (SCH 79797), a nonpeptide thrombin receptor antagonist. Biochem Pharmacol. 2000;60:1425–1434. doi: 10.1016/s0006-2952(00)00460-3. [DOI] [PubMed] [Google Scholar]
- 29.Lieu T, et al. Antagonism of the proinflammatory and pronociceptive actions of canonical and biased agonists of protease-activated receptor-2. Br J Pharmacol. 2016;173:2752–2765. doi: 10.1111/bph.13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen DD, et al. Protein kinase D and Gβγ subunits mediate agonist-evoked translocation of protease-activated receptor-2 from the Golgi apparatus to the plasma membrane. J Biol Chem. 2016;291:11285–11299. doi: 10.1074/jbc.M115.710681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lan TH, Liu Q, Li C, Wu G, Lambert NA. Sensitive and high resolution localization and tracking of membrane proteins in live cells with BRET. Traffic. 2012;13:1450–1456. doi: 10.1111/j.1600-0854.2012.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herskovits JS, Burgess CC, Obar RA, Vallee RB. Effects of mutant rat dynamin on endocytosis. J Cell Biol. 1993;122:565–578. doi: 10.1083/jcb.122.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levitzki A, Gazit A. Tyrosine kinase inhibition: An approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 35.Gao ZG, Jacobson KA. On the selectivity of the Gαq inhibitor UBO-QIC: A comparison with the Gαi inhibitor pertussis toxin. Biochem Pharmacol. 2016;107:59–66. doi: 10.1016/j.bcp.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gschwendt M, et al. Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase c isoenzymes. FEBS Lett. 1996;392:77–80. doi: 10.1016/0014-5793(96)00785-5. [DOI] [PubMed] [Google Scholar]
- 37.Perry SJ, et al. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science. 2002;298:834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- 38.Castro J, et al. Cyclic analogues of alpha-conotoxin Vc1.1 inhibit colonic nociceptors and provide analgesia in a mouse model of chronic abdominal pain. Br J Pharmacol. 2017;175:2384–2398. doi: 10.1111/bph.14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aley KO, et al. Nociceptor sensitization by extracellular signal-regulated kinases. J Neurosci. 2001;21:6933–6939. doi: 10.1523/JNEUROSCI.21-17-06933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khasar SG, et al. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24:253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PubMed] [Google Scholar]
- 41.Cheng JK, Ji RR. Intracellular signaling in primary sensory neurons and persistent pain. Neurochem Res. 2008;33:1970–1978. doi: 10.1007/s11064-008-9711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park RJ, et al. Dynamin triple knockout cells reveal off target effects of commonly used dynamin inhibitors. J Cell Sci. 2013;126:5305–5312. doi: 10.1242/jcs.138578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vergnolle N, et al. Proteinase-activated receptor-2 and hyperalgesia: A novel pain pathway. Nat Med. 2001;7:821–826. doi: 10.1038/89945. [DOI] [PubMed] [Google Scholar]
- 44.Steinhoff M, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.