Significance
The primate brain is specialized for social interaction, and a complex network of brain regions supports this important function. Face perception is central to social development, and both humans and nonhuman primates exhibit a spontaneous viewing preference for faces. This shared involuntary response underscores the importance of faces in the earliest stages of cognitive development, yet its neural basis is not well understood. Here we report that bilateral amygdala lesions in rhesus monkeys eliminate the robust viewing preference for both real faces and illusory faces. This demonstrates a fundamental role for the amygdala in guiding eye movements toward face stimuli, a critical behavior for normal social development and social interaction.
Keywords: face perception, spontaneous behavior, neurocircuitry, social development, visual salience
Abstract
In free-viewing experiments, primates orient preferentially toward faces and face-like stimuli. To investigate the neural basis of this behavior, we measured the spontaneous viewing preferences of monkeys with selective bilateral amygdala lesions. The results revealed that when faces and nonface objects were presented simultaneously, monkeys with amygdala lesions had no viewing preference for either conspecific faces or illusory facial features in everyday objects. Instead of directing eye movements toward socially relevant features in natural images, we found that, after amygdala loss, monkeys are biased toward features with increased low-level salience. We conclude that the amygdala has a role in our earliest specialized response to faces, a behavior thought to be a precursor for efficient social communication and essential for the development of face-selective cortex.
When unconstrained by task, primates look longer at faces than at other complex visual stimuli (1–8). This advantage for faces is evident in human infants tested moments after birth (4, 6) or immediately following the removal of bilateral cataracts (9). Although this advantage for faces is thought to be essential for typical social development, and perhaps responsible for the formation of face-selective cortex (8, 10), the neural basis for this behavior is unknown.
In addition to the faces of conspecifics, primates preferentially orient toward schematic faces and other face-like stimuli (4, 6), including illusory facial features in everyday objects such as fruit (1, 11). This suggests that the mechanism driving orienting behavior is broadly tuned to a basic feature template or structural code of a face, rather than the specific visual attributes associated with the realistic facial features of conspecifics. The numerous observations that viewing preferences generalize to schematic and illusory facial features are consistent with the idea that the mechanism involved in rapidly directing eye movements to faces is tuned to coarse visual features diagnostic of face detection, rather than local details specific to a particular species or individual (1, 12, 13).
A popular theoretical account posits that the amygdala is part of a neural network that detects relevant stimuli in the visual environment (14–16). Tract-tracing anatomical studies in macaques have identified a pathway between the amygdala and the anterior-most region of the core face-selective network (17), which is located in the inferior temporal cortex, along the ventral visual stream (18, 19). This connection suggests that the amygdala may play a role in face perception. Consistent with this idea, neurons in the amygdala are active in relation to face identity and facial expressions of emotion (20, 21). In addition, selective amygdala lesions alter typical eye-movement patterns when faces are presented at fixation (22). However, there has not yet been a direct test of the hypothesis that the amygdala is causally implicated in viewing-preference behavior, which is a necessary step in understanding the role of this face-responsive structure in face perception. If the amygdala plays a role in the prioritization of faces in natural viewing behavior, we predict that amygdala insult would reduce the spontaneous advantage for conspecific and illusory faces over nonface objects in a free-viewing task.
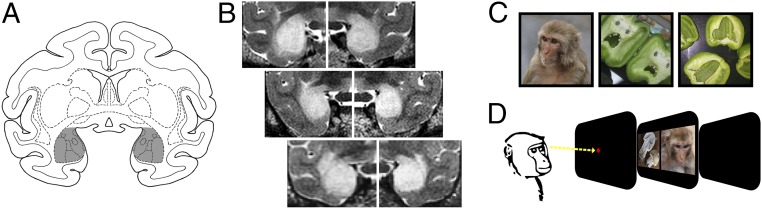
To directly assess the role of the amygdala in orienting toward faces, we presented rhesus monkeys that had received selective bilateral amygdala lesions (Fig. 1 A and B) with pairs of photographs from three categories—monkey faces, everyday objects containing illusory facial features, and similar nonface objects (Fig. 1C)—and measured the time they spent looking at each stimulus (Fig. 1D). It is well-established that primates (humans and macaques) look longer at faces than at other stimuli (1, 3–5, 23). Thus, if the amygdala has no role in guiding eye movements toward faces that are competing with other objects for attention, our subjects should spend longer looking at monkey faces than at nonface objects, as we observed when we tested intact controls in a previous study with the same stimuli (Fig. 2A) (1). Similarly, subjects should also look longer at illusory faces in inanimate objects than at similar objects without illusory face structure (Fig. 2A) (1). Additionally, we measured the location of fixations relative to stimulus features because fixation maps have been used previously to infer whether a monkey has detected facial structure (1, 24, 25).
Fig. 1.
Monkeys with selective bilateral amygdala lesions viewed pairs of stimuli. (A) A coronal section (17 mm anterior to the interaural plane) from a representative rhesus monkey brain showing the location and extent of the intended bilateral amygdala lesion in gray. (B) Postoperative T2-weighted MR scans from each of the three monkeys at levels matched to A. The white hypersignal indicates edema that is characteristic of cell death and confirms successful injections of ibotenic acid (also see SI Appendix, Fig. S1 and Table S1). (C) Example stimuli: (Left) monkey face; (Middle) illusory face; (Right) matched nonface object. Reprinted from ref. 1, Copyright (2017), with permission from Elsevier. (D) The trial procedure indicating the timing parameters. Monkeys initiated each trial by fixating a central fixation spot and were rewarded at the end of each trial with three drops of juice if the gaze remained within the bounds of the screen; viewing was otherwise unconstrained. Eye movements were recorded throughout the trial.
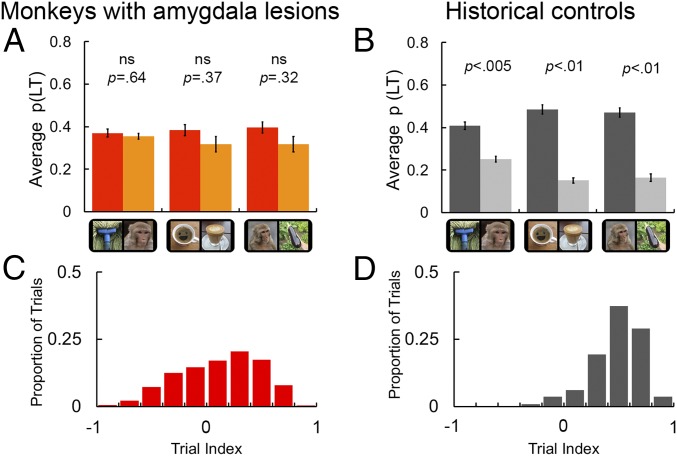
Fig. 2.
Results for viewing preference as a function of visual stimulus condition. (A) Looking-time data from five male intact historical control monkeys for comparison with B. Data are reproduced from ref. 1. Note that p(LT) was calculated as the proportion of time relative to the length of the trial. The three conditions of interest were illusory faces vs. monkey faces, illusory faces vs. objects, and monkey faces vs. objects. Error bars indicate ±SEM. ns, not significant. Reprinted from ref. 1, Copyright (2017), with permission from Elsevier. (B) The mean proportion of total looking time [p(LT)] for monkeys with amygdala lesions as a function of stimulus type for each of the three comparisons of interest. See SI Appendix, Fig. S2A for individual subject results (also see SI Appendix, Table S2 to compare subjects in both groups). Same conventions as in A. (C) A distribution of face-bias indices for trials completed by intact controls. The face-bias index is calculated as a weighting of which stimulus subjects looked at the most on each trial by the formula: face-bias index = [monkey face LT − nonface object LT]/[monkey face LT + nonface object LT]. Indices approaching −1 indicate a trial on which subjects favored the nonface object, and approaching +1 reflect trials on which subjects favored the face stimulus. An index of 0 indicates no bias in looking time toward either stimulus. Individual subject contributions can be viewed in SI Appendix, Fig. S2B. (D) The corresponding distribution of viewing preference for face vs. object trials for monkeys with amygdala lesions (n = 3).
Results
Changes in Viewing Preferences.
We recorded the eye movements of three rhesus monkeys (Macaca mulatta) with bilateral excitotoxic lesions of the amygdala while they were presented with pairs of stimuli on a color monitor screen (for details, see Experimental Procedures). The monkeys received a juice reward for maintaining their gaze within the screen region during each trial. Stimuli consisted of 15 monkey faces, 15 illusory faces, and 15 nonface objects. The paradigm was identical to that used in our previous study showing that intact rhesus monkeys (used here as historical controls) perceive illusory faces when they occur by chance in the natural environment (1). The inclusion of natural examples of illusory faces (i.e., examples of face pareidolia) here in addition to monkey faces has two key benefits. First, the illusory faces are matched visually and semantically to the control nonface objects, thus providing a valuable face–nonface comparison where features such as color are equally variable. Second, this stimulus set allows examination of the generalizability of any observed effect of amygdala lesions on spontaneous viewing behavior beyond the faces of conspecifics.
We presented all unique pairings of the 45 stimuli; thus, each monkey completed 1,980 trials in total. The behavioral measure, looking time (LT), was expressed as the proportion of time that the animals spent exploring each visual stimulus compared with total presentation time (4 s). Remarkably, we found no evidence that the amygdalectomized monkeys spent more time looking at monkey faces than at nonface objects (mean difference 0.08; t2 =1.3, P = 0.32, η2 = 0.46; Fig. 2B). These subjects also showed no looking preference for illusory faces compared with matched objects without illusory faces (mean difference 0.07; t2 = 1.9, P = 0.37, η2 = 0.39; Fig. 2B). It is important to note that from this result, we cannot determine whether monkeys with amygdala lesions perceive illusory faces or not because they exhibited no preference for conspecific faces. The inference of illusory face perception in intact monkeys is based on this preferential looking behavior toward real faces. The data also indicated that, on average, monkeys with amygdala lesions had no preference for an illusory or a monkey face when presented simultaneously (mean difference 0.01; t2 = 0.55, P = 0.64, η2 = 0.13; Fig. 2B). An independent analysis of the first fixation data yielded the same pattern of results: no advantage for faces, real or illusory, over nonface objects (SI Appendix, Fig. S2). The elimination of a strong bias toward the faces of conspecifics and illusory faces represents a striking departure from the behavior of intact controls (reproduced in Fig. 2A) and a number of previous studies that have established that primates reliably orient toward faces (1, 4, 5, 26) even in cluttered displays composed of multiple objects (20, 27). To date, the only exceptions have been human studies testing clinical populations of individuals living with social developmental disorders (28–31).
To determine whether the viewing preferences for monkeys with amygdala lesions were significantly reduced relative to those of intact monkeys, we calculated the mean difference in p(LT) between the two stimuli in each of the three conditions of interest [(i) monkey faces vs. nonface objects; (ii) illusory faces vs. nonface objects; and (iii) illusory faces vs. monkey faces] for monkeys in both groups. These data were analyzed using a mixed ANOVA with condition (three levels) as the repeat factor and group (two levels) as the between-subjects factor. This analysis yielded a significant effect of group (F1,6 = 16.17, P = 0.007, η2p = 0.73), indicating that viewing preferences were significantly stronger in intact monkeys relative to those with amygdala lesions. In addition, there was a main effect of condition (F2,12 = 19.53, P < 0.001, η2p = 0.76), indicating that viewing patterns differed across the three conditions. Notably, we also found a significant interaction between condition and group (F2,12 = 4.99, P = 0.03, η2p = 0.45) that was followed up with a series of pairwise comparisons to test for group differences within each condition (two-tailed independent-sample t tests, equal variance assumed, Bonferroni correction applied to adjust for multiple comparisons). Monkeys with amygdala lesions had reduced viewing preferences relative to historical controls in each of the three conditions (monkey faces vs. nonface objects: mean difference between groups 0.23, t6 = 3.4, P = 0.015, ηp2 = 0.66; illusory faces vs. nonface objects: mean difference between groups 0.27, t6 = 4.41, P = 0.005, ηp2 = 0.76; illusory faces vs. monkey faces: mean difference between groups 0.14, t6 = 3.31, P = 0.016, ηp2 = 0.65).
To more closely examine the impact of amygdala loss on the critical condition (i.e., trials contrasting monkey faces vs. nonface objects, for which a strong face-viewing preference would be predicted), we aimed to determine whether the significant decrease in viewing preferences was the consequence of a genuine lack of viewing preference for faces or, alternatively, whether there exists an equal number of trials with opposing preferences, which would manifest as an apparent lack of preference when only the mean data are considered (i.e., if only a subset of trials elicited a strong preference for faces, and another subset elicited a strong opposing preference for objects). To achieve this, we created an index of viewing bias (“face-bias index”) to characterize which stimulus was favored on each individual completed trial and examined the corresponding distribution. The face-bias index was calculated from the monkey face vs. nonface object trials only (450 trials per monkey) using the following formula: face-bias index = [monkey face LT − nonface object LT]/[monkey face LT + nonface object LT]. In Fig. 2 C and D, we show the distribution of face-bias indices as a proportion of the total number of trials completed for controls (median face-bias index = 0.51, minimum = −0.57, maximum = 0.87) and monkeys with amygdala lesions (median face-bias index = 0.17, minimum = −0.90, maximum = 0.85). A permutation test on the median scores revealed a statistically significant difference between the median face-bias index for controls compared with that for monkeys with lesions (shuffling the group labels over 1,000 permutations, P < 0.001).
For the controls, over 95% of trials had an index greater than 0, a much greater percentage than we observed for the monkeys with amygdala lesions (63%). For the intact monkeys, half of the trials elicited a very clear (almost fivefold) preference for faces over objects, reflected in the strongly peaked distribution in Fig. 2D. In comparison, the distribution of viewing bias is much flatter for the monkeys with amygdala lesions in Fig. 2C, indicative of a lack of a clear preference for faces over other stimuli. Collectively, these results reveal that amygdala loss alters spontaneous viewing behavior in monkeys, markedly reducing the advantage for faces.
Altered Fixation Patterns.
Primates reliably fixate on certain facial features such as eyes and mouth. To further evaluate the amygdalectomized monkeys’ spontaneous response to face stimuli, we examined their fixation patterns for the three classes of stimuli. We divided each stimulus into 121 equally sized, square bins (1° in height and width) and tallied the distribution of fixations directed to each of the 45 stimuli across all trials. For each subject, we created a two-dimensional plot normalized to the maximum number of fixations. In Fig. 3A, we present the normalized fixation density data for the three monkeys with amygdala lesions, averaged across subjects (also see SI Appendix, Fig. S3). At first glance, these average fixation maps appear to be highly disorganized, irrespective of stimulus category (i.e., faces or nonfaces). However, when we compared these fixation maps with those of the intact controls, we found evidence that amygdala lesions selectively decreased the consistency with which subjects view face stimuli.
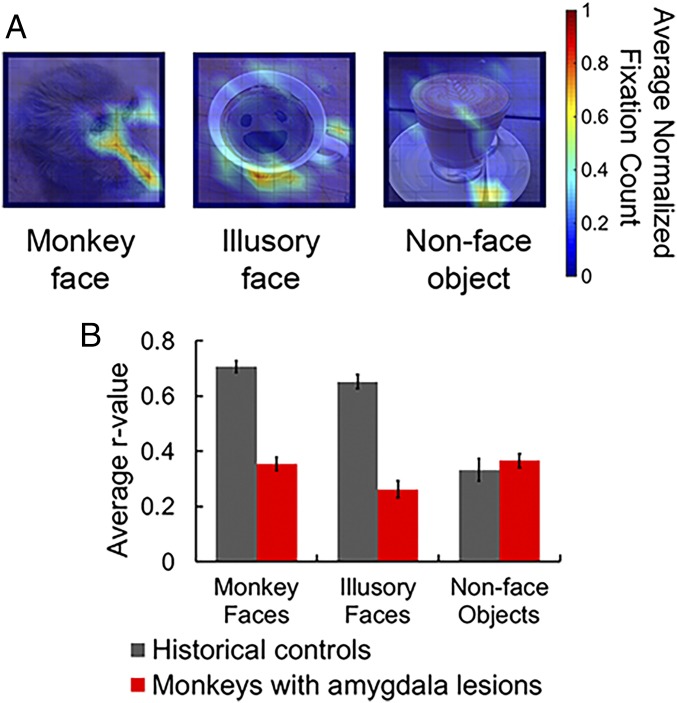
Fig. 3.
Fixation density plots for three example stimuli and the correlation across all maps as a function of stimulus category and presence/absence of an amygdala; see also SI Appendix, Fig. S3. (A) Example fixation maps show the average number of fixations (≥150 ms) as two-dimensional density plots in degrees of visual angle [an example from each stimulus type: (Left) monkey face; (Middle) illusory face; (Right) nonface object] in monkeys with amygdala lesions. Data were normalized to each monkey’s maximum fixation count and then averaged across all three subjects before being smoothed for illustration purposes using MATLAB’s surf function with interpolated shading. The unsmoothed data for every experimental stimulus are available in SI Appendix, Fig. S3. (B) The average r values for the experimental stimuli as a function of both stimulus type (monkey faces, illusory faces, and nonface objects) and subject group (monkeys with amygdala lesions and intact controls from ref. 1 are plotted for comparison). Error bars indicate ±SEM. Reprinted from ref. 1, Copyright (2017), with permission from Elsevier.
To quantify this effect, we vectorized the subjects’ normalized fixation density plots and cross-correlated across subjects to compute an average r value for each stimulus. These average r values could then be compared directly with the r values that were generated previously (1), when intact monkeys performed the same task (independent-sample t tests, two-tailed; Fig. 3B). These comparisons indicated that monkeys with amygdala lesions fixated images containing faces with significantly less consistency than intact monkeys (monkey faces: t28 = 10.96, P < 0.001, η2 = 0.98; illusory faces: t28 = 9.87, P < 0.001, η2 = 0.98). At the same time, there was no evidence of a difference between groups when the subjects viewed nonface objects (t28 = 0.7, P = 0.49, η2 = 0.2). In the study of intact monkeys, the consistent pattern of fixations associated with the two face categories (real and illusory) was argued to be a marker of face perception (1) because behavioral studies have reliably shown that humans and monkeys frequently fixate the internal facial features, particularly the eyes (1, 5, 22, 32). Indeed, deviation from this typical fixation map has been viewed as pathological (30, 33). The absence of a reliable fixation pattern in monkeys with amygdala lesions, together with the lack of a viewing preference for faces, is evidence that amygdala loss selectively reduces the inherent salience of facial features, and fits with previous reports linking amygdala activity with fixations on the eyes of a face (20, 22, 25).
Comparison with Maps of Saliency.
Studies of human viewing behavior have reasoned that individuals who have difficulty directing visual attention toward socially relevant stimuli in free-viewing tasks might orient instead to objects that have higher salience, as defined by low-level visual properties (28–30). Since the monkeys with amygdala lesions exhibit decreased orienting toward faces, a socially relevant category of stimuli, we tested whether their fixations instead corresponded to image regions with relatively high visual salience. To test this hypothesis, we first mapped the low-level visual salience in our stimuli using two different algorithms loosely inspired by the tuning properties of neurons in the primary visual cortex in response to static natural scenes: (i) the Itti–Koch algorithm (34) and (ii) the graph-based visual saliency algorithm [GBVS (35)], both implemented in MATLAB (version R2016a; MathWorks). To facilitate comparison with gaze preferences, the output from each saliency algorithm for each stimulus was binned and normalized using the same process as used for the fixation data for individual subjects (all maps are available in SI Appendix, Figs. S4–S6).
For each stimulus, we then cross-correlated the average fixation data from monkeys with amygdala lesions with three different predictive maps: (i) the average fixation data collected from intact controls, used as a proxy for social salience; (ii) the Itti–Koch visual saliency map; and (iii) the GBVS map (Fig. 4A and SI Appendix, Figs. S4–S6). The bar graphs in Fig. 4B plot the average correlation between the fixation density maps elicited from monkeys with amygdala lesions and these three predictive maps as a function of stimulus category. We analyzed these data using a repeated-measures ANOVA applied to the data for each stimulus image. The analysis included two factors: stimulus category (monkey faces, illusory faces, nonface objects) and predictive map (intact behavior, Itti–Koch map, GBVS map). The results revealed the expected effect of predictive map (F2,28 = 30.32, P < 0.001, η2p = 0.68) in addition to a main effect of stimulus category (F2,28 = 5.93, P = 0.007, η2p = 0.30) and an interaction between both factors (F4,56 = 5.73, P = 0.001, η2p = 0.29).
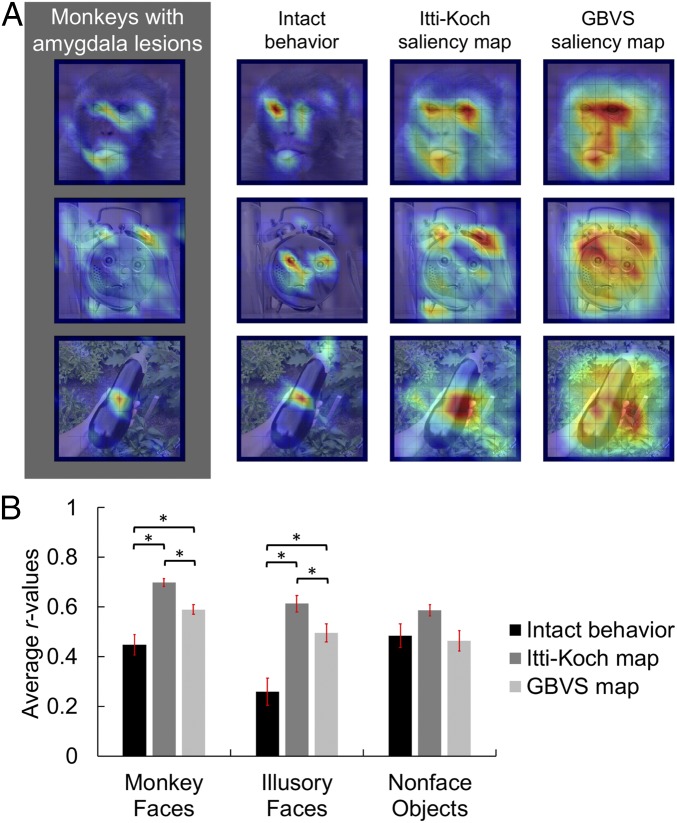
Fig. 4.
Correlation between fixation patterns and saliency. (A) Three illustrative mean fixation density maps, one example from each stimulus category [(Top) monkey face; (Middle) illusory face; (Bottom) nonface object]. From Left to Right: observed viewing pattern, a social saliency map from intact monkeys, the Itti–Koch visual saliency map, and the GBVS map. Maps for all stimuli are available in SI Appendix, Figs. S4–S6. Reprinted from ref. 1, Copyright (2017), with permission from Elsevier. (B) Mean (±SEM) correlation between the fixation maps elicited from monkeys with amygdala lesions and each of the predictive maps as a function of stimulus category. *P < 0.01.
Follow-up contrasts for the effect of predictive map indicated that the average correlation between the behavior of the monkeys with amygdala lesions and both the Itti–Koch and GBVS maps was higher than with the maps from the intact monkeys (Itti–Koch vs. intact behavior: t14 = 6.39, P < 0.001, η2 = 0.74; GBVS vs. intact behavior: t14 = 3.52, P = 0.003, η2 = 0.47). There was also evidence that the Itti–Koch algorithm was superior to the GBVS algorithm in terms of correspondence with the current data, when averaging across stimulus type (t14 = 7.5, P < 0.001, η2 = 0.8). All observed P values were adjusted for multiple comparisons using the conservative Bonferroni correction. These results support the hypothesis that low-level visual salience is a better predictor of where the monkeys with amygdala lesions fixated on each stimulus than the fixation patterns elicited from intact monkeys.
We investigated the source of the interaction between stimulus type and predictive map (Fig. 4B) using a set of nine a priori pairwise contrasts (applying the Bonferroni correction for multiple comparisons; Fig. 4B). The fixations of monkeys with amygdala lesions while viewing conspecific faces correlated better with the Itti–Koch maps than with the GBVS maps (t14 = 6.72, P = 0.004, η2 = 0.76) or with intact monkey behavior (t14 = 5.77, P = 0.004, η2 = 0.7). Similarly, there was a greater correspondence between the fixations of monkeys with amygdala lesions and the GBVS maps than with the intact monkey behavior (t14 = 3.86, P = 0.02, η2 = 0.51). Collectively, these comparisons further indicate that the behavior of the monkeys with amygdala lesions is better captured by the maps of low-level salience than by the average fixation maps generated from historical control monkeys (1).
The same pattern of results held for illusory face stimuli, with a greater correspondence between the fixation behavior of the monkeys with amygdala lesions and the saliency maps than with the fixation patterns of the control monkeys (Itti–Koch vs. intact behavior: t14 = 6.18, P < 0.001, η2 = 0.73; GBVS vs. intact behavior: t14 = 5.07, P < 0.001, η2 = 0.65; Itti–Koch vs. GBVS: t14 = 3.49, P = 0.04, η2 = 0.46). However, for nonface objects, there was no evidence that the relationship between the behavior of monkeys with amygdala lesions and any of the three predictive maps differed (Itti–Koch vs. intact behavior: t14 = 1.72, P > 0.9, η2 = 0.17; GBVS vs. intact behavior: t14 = 0.31, P > 0.9, η2 < 0.01; Itti–Koch vs. GBVS: t14 = 3.15, P = 0.06, η2 = 0.41). Thus, when analyzing the response to nonface objects, there was no evidence that low-level visual salience was a better predictor of the current data than the behavior of intact monkeys. Importantly, this result shows that low-level visual saliency retains predictive power for both groups of subjects when they are shown nonface stimuli. The difference in fixation patterns between intact monkeys and those with amygdala lesions appears to be specific to faces and face-like stimuli.
Discussion
Here we report that adult monkeys with selective bilateral amygdala lesions fail to spontaneously orient toward faces. In a free-viewing visual preference paradigm, intact monkeys were found to have a strong viewing preference for both faces (3, 5, 8, 20) and illusory faces (1). By contrast, we found that monkeys with amygdala lesions looked equally often at photographs of monkey faces, illusory faces, and everyday nonface objects. Further, amygdalectomized monkeys looked more often at stimulus regions with greater low-level visual salience than regions with greater social meaning, such as facial features. Although prior studies have demonstrated a causal role for the amygdala in preferential viewing of certain facial features (e.g., eyes, mouth) when faces are presented in the central visual field, the present results reveal that, in addition, the amygdala is essential for guiding eye movements toward faces in visual scenes with competing visual stimuli. Thus, the amygdala has a causal role in the well-established visual preference for faces spontaneously exhibited by both nonhuman primates and humans.
A Causal Link Between the Amygdala and a Spontaneous Behavioral Response Toward Faces.
It is generally assumed that most aspects of face perception, including detection and discrimination, are mediated by mechanisms located within face-selective areas of the inferior temporal cortex (12, 36–38). In contrast, the role of the amygdala in face perception has largely been framed in terms of relevance—with the amygdala responding more to visual stimuli with greater valence, such as faces expressing fear (39–41). However, most studies investigating the neural mechanisms underlying face perception have examined fixations toward faces presented in the central visual field (12, 19, 37, 39, 42, 43). Comparatively little is known about how we detect and perceive faces in the periphery and then orient toward them. Our data clearly implicate the amygdala in directing eye movements toward faces when they occur with another nonface object in the visual environment.
Moreover, we report dramatic changes in the spatial location of fixations within each stimulus made by monkeys with amygdala lesions. This suggests that, in addition to an absence of a general face-viewing preference, amygdala loss in adult monkeys results in changed face-viewing behavior, with no advantage for the eyes and internal facial features over other parts of the face. Previous studies of single-cell responses have concluded that the amygdala may act as an eye detector in natural scenes (25). Furthermore, amygdalectomized monkeys have previously been shown to fixate less frequently on the eyes of monkey faces presented centrally (22). Here we demonstrate that this holds for both monkey faces and illusory faces in objects when these compete with other stimuli for attention in a preferential looking paradigm. Our data are consistent with the observation of human patient SM with bilateral amygdala damage (44), who exhibited a profound deficit in recognizing fear from photographs of facial expressions and did not spontaneously fixate the eye region when freely viewing faces. Intriguingly, when SM was explicitly directed to fixate the eyes, her performance in recognizing fear improved. Our demonstration of atypical eye-fixation patterns on faces following amygdala loss further suggests that it plays a critical role in directing eye movements to faces in a stereotypical manner (20, 25).
Our results reveal a causal link between amygdala activity and the preference toward faces during a free-viewing task. Exactly how this behavioral effect is manifest, however, is unclear. One possibility is that the amygdala loss has an effect on visual processing in the temporal cortex and it is this disruption that eliminates viewing preferences for faces. This idea is consistent with an earlier finding that selective amygdala lesions disrupt the modulation of activity in the monkey inferior temporal cortex (39). The idea is further supported by a previous structural MRI study that reported a significant reduction in gray matter in the visual areas of the temporal cortex in human subjects with bilateral amygdala lesions (45). Importantly, our data indicate that amygdala activity is necessary for the prioritization of faces over objects in free viewing. Moreover, in the context of the present study, the analysis of the spatial location of fixations within images suggests that the behavioral consequences of amygdala loss are limited to stimuli with social content (i.e., real and illusory faces).
The Role of the Amygdala in the Typical Development of Face-Selective Cortex.
Preferential looking behavior has been suggested to be crucial for the development of category selectivity in the inferior temporal cortex, including face selectivity (8, 10). Monkeys reared without exposure to faces do not develop normal face patches, although they develop cortical domains for other categories, such as scenes and body parts, for which they had visual experience (8). Given that these findings indicate the amygdala plays a causal role in the prioritization of face stimuli over other objects in natural viewing, it is possible that the amygdala also plays an essential role during the early stages of development for the formation of face-selective cortex in primates.
Coincidently, we report two observations that create an interesting parallel between amygdala loss in monkeys and autism spectrum disorders (ASDs) in humans. First, the monkeys with amygdala lesions looked longer at visually salient features of images, not socially salient features (30). Individuals with ASDs similarly make reduced fixations on facial features, such as the eyes and mouth, when viewing photographs of faces (46). Second, we found no evidence that monkeys with amygdala lesions experienced the illusion of face pareidolia, namely perceiving faces within inanimate objects. Similarly, unlike typically developing children, children with ASDs show a reduced looking preference for face-like objects in the upright orientation (31, 47). As we note above, however, it is difficult to infer whether monkeys with amygdala lesions perceive illusory facial features from these data because the scan paths for conspecific faces were also irregular. Nonetheless, the observation of abnormal scan paths for socially relevant stimuli in amygdalectomized monkeys here converges with earlier arguments (15), suggesting that the rhesus monkey could serve as a model for the development of the social brain.
Understanding Object Representations Outside of Controlled Fixation.
The selection and prioritization of behaviorally relevant objects of interest via eye movements are critical steps in how the brain achieves coherent natural viewing by reducing the incoming computational load. In the laboratory, face processing has mainly been studied in experimental paradigms in which faces are presented centrally at fixation. A more recent complementary approach aims to understand face processing under more natural viewing conditions (25, 48). A complete understanding of visual function in the primate brain requires understanding the complex mechanisms that govern involuntary behaviors, such as our orienting response to faces and stereotypical scan paths. The demonstration of a causal role for the amygdala in spontaneous viewing preferences is a step toward revealing how the primate visual system has evolved to cope with complex and continuous input from the entire field of view.
Experimental Procedures
Subjects.
Three adult male rhesus macaques (monkeys E, B, and C; M. mulatta, weighing between 6.6 and 9 kg at the time of testing) participated in this experiment. Subjects were 4.9, 5.1, and 6.1 y old at first surgery, and 6, 7, and 18 y old at the start of testing, respectively. They were originally acquired from a breeding facility in the United States, where they were housed in large groups until their transfer to the National Institute of Mental Health at the age of ∼5 y. After that, they were housed in a large colony room on a 12-h light/dark cycle, with auditory and visual contact with at least 20 other conspecifics. Food was available ad libitum and water was controlled as needed to maintain testing motivation, with their weight remaining above 85% of baseline. All procedures were in accordance with the Guide for the Care and Use of Laboratory Animals (49) and were approved by the National Institute of Mental Health Animal Care and Use Committee.
Procedures.
Surgical procedure.
We used standard methods for making bilateral excitotoxic amygdala lesions, as described in detail elsewhere (50, 51). In our laboratory, these methods produce a median of 82% amygdala damage with 2% or less damage to surrounding structures (51). Briefly, we acquired a structural MR scan of each monkey, plotted a series of 16 to 19 injection sites along 8 or 9 vertical tracks, tailored to the individual size and shape of the amygdala in each hemisphere, and separated in all planes by ∼2 mm. During aseptic surgery, we opened a bilateral bone flap over the injection sites, lowered a 30-gauge Hamilton syringe to the ventral-most site in a track, injected 0.6 to 1.2 µL of ibotenic acid (10 mg/mL; Sigma) at a rate of 0.2 µL/min, waited 2 min to allow diffusion, raised the needle of the syringe to the next injection site, and repeated for each site along the track, waiting 3 min before removing the needle from the brain. This method was repeated for each additional track. For health reasons, we performed the operation in two stages, allowing 2 wk of recovery after each hemisphere. Three to 7 days after each surgery, we acquired T2-weighted MR scans to visualize edema and confirm successful injections. Amygdala damage can be partially predicted in vivo by MRI, with complete coverage by hypersignal indicating that a majority of the amygdala is damaged and extraamygdala damage generally being less than indicated by hypersignal (51). In all three subjects, the edema covered the extent of the intended amygdala lesion, indicating that the majority of the amygdala was damaged, and extended only minimally to surrounding tissue, indicating little extraamygdala damage (SI Appendix, Table S1).
Experimental procedure.
Stimuli were identical to those in our previous study of intact monkeys (1). Face stimuli comprised 15 color photographs of female rhesus monkeys that varied in pose, head position, size, color, gaze direction, and lighting condition. Illusory faces and content-matched objects comprised 15 examples of face pareidolia and 15 matched nonface objects. Subjects initiated a trial by fixating a central spot for 500 ms before two stimuli were presented side by side for 4 s. Each stimulus was 10.2° of visual angle in height and width. All 45 stimuli appeared once with every other stimulus, equally often on the left and the right (image center was horizontally displaced by ±8° of visual angle from the screen center). Therefore, each subject completed 1,980 trials in a unique order. Once a trial had been initiated, the monkeys were required to look anywhere on the screen for the full 4-s period to receive a subsequent liquid reward. The monkeys were free to look anywhere within the screen; however, if a monkey looked away from the screen or closed its eyes for a period longer than 300 ms, the trial was aborted and repeated at a later time. There was a timeout (an extended intertrial interval) following an aborted trial of 5 s; otherwise, the intertrial interval was 1 s.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Mental Health Intramural Research Program (Grant ZIAMH002918-09).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1807245115/-/DCSupplemental.
References
- 1.Taubert J, Wardle SG, Flessert M, Leopold DA, Ungerleider LG. Face pareidolia in the rhesus monkey. Curr Biol. 2017;27:2505–2509.e2. doi: 10.1016/j.cub.2017.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahl CD, Logothetis NK, Hoffman KL. Individuation and holistic processing of faces in rhesus monkeys. Proc Biol Sci. 2007;274:2069–2076. doi: 10.1098/rspb.2007.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugita Y. Face perception in monkeys reared with no exposure to faces. Proc Natl Acad Sci USA. 2008;105:394–398. doi: 10.1073/pnas.0706079105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goren CC, Sarty M, Wu PY. Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics. 1975;56:544–549. [PubMed] [Google Scholar]
- 5.Gothard KM, Erickson CA, Amaral DG. How do rhesus monkeys (Macaca mulatta) scan faces in a visual paired comparison task? Anim Cogn. 2004;7:25–36. doi: 10.1007/s10071-003-0179-6. [DOI] [PubMed] [Google Scholar]
- 6.Farroni T, et al. Newborns’ preference for face-relevant stimuli: Effects of contrast polarity. Proc Natl Acad Sci USA. 2005;102:17245–17250. doi: 10.1073/pnas.0502205102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosa Salva O, Farroni T, Regolin L, Vallortigara G, Johnson MH. The evolution of social orienting: Evidence from chicks (Gallus gallus) and human newborns. PLoS One. 2011;6:e18802. doi: 10.1371/journal.pone.0018802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arcaro MJ, Schade PF, Vincent JL, Ponce CR, Livingstone MS. Seeing faces is necessary for face-domain formation. Nat Neurosci. 2017;20:1404–1412. doi: 10.1038/nn.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mondloch CJ, Lewis TL, Levin AV, Maurer D. Infant face preferences after binocular visual deprivation. Int J Behav Dev. 2013;37:148–153. [Google Scholar]
- 10.Livingstone MS, et al. Development of the macaque face-patch system. Nat Commun. 2017;8:14897. doi: 10.1038/ncomms14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wardle SG, Seymour K, Taubert J. 2017. Characterizing the response to face pareidolia in human category-selective visual cortex. bioRxiv:10.1101/233387. Preprint, posted December 13, 2017.
- 12.Tsao DY, Livingstone MS. Mechanisms of face perception. Annu Rev Neurosci. 2008;31:411–437. doi: 10.1146/annurev.neuro.30.051606.094238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morton J, Johnson MH. CONSPEC and CONLERN: A two-process theory of infant face recognition. Psychol Rev. 1991;98:164–181. doi: 10.1037/0033-295x.98.2.164. [DOI] [PubMed] [Google Scholar]
- 14.Bachevalier J, Málková L, Mishkin M. Effects of selective neonatal temporal lobe lesions on socioemotional behavior in infant rhesus monkeys (Macaca mulatta) Behav Neurosci. 2001;115:545–559. doi: 10.1037//0735-7044.115.3.545. [DOI] [PubMed] [Google Scholar]
- 15.Bachevalier J. The orbitofrontal-amygdala system in nonhuman primates: Function, development, and early insult. In: Bauman ML, Kemper TL, editors. The Neurobiology of Autism. 2nd Ed. Johns Hopkins Univ Press; Baltimore: 2005. pp. 177–189. [Google Scholar]
- 16.Sander D, Grafman J, Zalla T. The human amygdala: An evolved system for relevance detection. Rev Neurosci. 2003;14:303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- 17.Grimaldi P, Saleem KS, Tsao D. Anatomical connections of the functionally defined “face patches” in the macaque monkey. Neuron. 2016;90:1325–1342. doi: 10.1016/j.neuron.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Logothetis NK, Guggenberger H, Peled S, Pauls J. Functional imaging of the monkey brain. Nat Neurosci. 1999;2:555–562. doi: 10.1038/9210. [DOI] [PubMed] [Google Scholar]
- 19.Taubert J, Van Belle G, Vanduffel W, Rossion B, Vogels R. The effect of face inversion for neurons inside and outside fMRI-defined face-selective cortical regions. J Neurophysiol. 2015;113:1644–1655. doi: 10.1152/jn.00700.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minxha J, et al. Fixations gate species-specific responses to free viewing of faces in the human and macaque amygdala. Cell Rep. 2017;18:878–891. doi: 10.1016/j.celrep.2016.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG. Neural responses to facial expression and face identity in the monkey amygdala. J Neurophysiol. 2007;97:1671–1683. doi: 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- 22.Dal Monte O, Costa VD, Noble PL, Murray EA, Averbeck BB. Amygdala lesions in rhesus macaques decrease attention to threat. Nat Commun. 2015;6:10161. doi: 10.1038/ncomms10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gothard KM, Brooks KN, Peterson MA. Multiple perceptual strategies used by macaque monkeys for face recognition. Anim Cogn. 2009;12:155–167. doi: 10.1007/s10071-008-0179-7. [DOI] [PubMed] [Google Scholar]
- 24.Leonard TK, Blumenthal G, Gothard KM, Hoffman KL. How macaques view familiarity and gaze in conspecific faces. Behav Neurosci. 2012;126:781–791. doi: 10.1037/a0030348. [DOI] [PubMed] [Google Scholar]
- 25.Mosher CP, Zimmerman PE, Gothard KM. Neurons in the monkey amygdala detect eye contact during naturalistic social interactions. Curr Biol. 2014;24:2459–2464. doi: 10.1016/j.cub.2014.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- 27.Di Giorgio E, Turati C, Altoè G, Simion F. Face detection in complex visual displays: An eye-tracking study with 3- and 6-month-old infants and adults. J Exp Child Psychol. 2012;113:66–77. doi: 10.1016/j.jecp.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, et al. Atypical visual saliency in autism spectrum disorder quantified through model-based eye tracking. Neuron. 2015;88:604–616. doi: 10.1016/j.neuron.2015.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459:257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Constantino JN, et al. Infant viewing of social scenes is under genetic control and is atypical in autism. Nature. 2017;547:340–344. doi: 10.1038/nature22999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guillon Q, et al. Intact perception but abnormal orientation towards face-like objects in young children with ASD. Sci Rep. 2016;6:22119. doi: 10.1038/srep22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker-Smith GJ, Gale AG, Findlay JM. Eye movement strategies involved in face perception. Perception. 1977;6:313–326. doi: 10.1068/p060313. [DOI] [PubMed] [Google Scholar]
- 33.Chawarska K, Shic F. Looking but not seeing: Atypical visual scanning and recognition of faces in 2 and 4-year-old children with autism spectrum disorder. J Autism Dev Disord. 2009;39:1663–1672. doi: 10.1007/s10803-009-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itti L, Koch C, Niebur E. A model of saliency-based visual attention for rapid scene analysis. IEEE Trans Pattern Anal Mach Intell. 1998;20:1254–1259. [Google Scholar]
- 35.Harel J, Koch C, Perona P. Graph-based visual saliency. In: Scholkoph B, Platt JC, Hofmann T, editors. Proceedings of the 19th International Conference on Neural Information Processing Systems. MIT Press; Cambridge, MA: 2006. pp. 545–552. [Google Scholar]
- 36.Afraz A, Boyden ES, DiCarlo JJ. Optogenetic and pharmacological suppression of spatial clusters of face neurons reveal their causal role in face gender discrimination. Proc Natl Acad Sci USA. 2015;112:6730–6735. doi: 10.1073/pnas.1423328112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Afraz SR, Kiani R, Esteky H. Microstimulation of inferotemporal cortex influences face categorization. Nature. 2006;442:692–695. doi: 10.1038/nature04982. [DOI] [PubMed] [Google Scholar]
- 38.Sadagopan S, Zarco W, Freiwald WA. A causal relationship between face-patch activity and face-detection behavior. eLife. 2017;6:e18558. doi: 10.7554/eLife.18558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadj-Bouziane F, et al. Amygdala lesions disrupt modulation of functional MRI activity evoked by facial expression in the monkey inferior temporal cortex. Proc Natl Acad Sci USA. 2012;109:E3640–E3648. doi: 10.1073/pnas.1218406109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams RB, Jr, Gordon HL, Baird AA, Ambady N, Kleck RE. Effects of gaze on amygdala sensitivity to anger and fear faces. Science. 2003;300:1536. doi: 10.1126/science.1082244. [DOI] [PubMed] [Google Scholar]
- 41.Adolphs R, et al. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37:1111–1117. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 42.Taubert J, Van Belle G, Vanduffel W, Rossion B, Vogels R. Neural correlate of the Thatcher face illusion in a monkey face-selective patch. J Neurosci. 2015;35:9872–9878. doi: 10.1523/JNEUROSCI.0446-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freiwald WA, Tsao DY, Livingstone MS. A face feature space in the macaque temporal lobe. Nat Neurosci. 2009;12:1187–1196. doi: 10.1038/nn.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adolphs R, et al. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- 45.Boes AD, et al. Changes in cortical morphology resulting from long-term amygdala damage. Soc Cogn Affect Neurosci. 2012;7:588–595. doi: 10.1093/scan/nsr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pelphrey KA, et al. Visual scanning of faces in autism. J Autism Dev Disord. 2002;32:249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- 47.Pavlova MA, et al. Social cognition in autism: Face tuning. Sci Rep. 2017;7:2734. doi: 10.1038/s41598-017-02790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russ BE, Leopold DA. Functional MRI mapping of dynamic visual features during natural viewing in the macaque. Neuroimage. 2015;109:84–94. doi: 10.1016/j.neuroimage.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed Natl Acad Press; Washington, DC: 2011. [Google Scholar]
- 50.Murray EA, Mishkin M. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J Neurosci. 1998;18:6568–6582. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basile BM, Karaskiewicz CL, Fiuzat EC, Malkova L, Murray EA. MRI overestimates excitotoxic amygdala lesion damage in rhesus monkeys. Front Integr Neurosci. 2017;11:12. doi: 10.3389/fnint.2017.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.