Abstract
Electrospinning is a simple, low-cost and versatile approach to fabricate multifunctional materials useful in drug delivery and tissue engineering applications. Despite its emergence into other manufacturing sectors, electrospinning has not yet made a transformative impact in the clinic with a pharmaceutical product for use in humans. Why is this the current state of electrospun materials in biomedicine? Is it because electrospun materials are not yet capable of overcoming the biological safety and efficacy challenges needed in pharmaceutical products? Or, is it that technological advances in the electrospinning process are needed? This review investigates the current state of electrospun materials in medicine to identify both scientific and technological gaps that may limit clinical translation.
Keywords: : clinical applications, drug delivery, electrospinning, medicated nanofibers, scale-up fabrication
Electrospun fibers have a long history of research and development but there are currently no US FDA approved clinical products for biomedical use in humans incorporating materials made by electrospinning. However, since its first description over a century ago [1], electrospun materials have advanced into several other manufacturing sectors resulting in the development of commercial products such as filtration systems [2] catalysts [3], apparel [4] and electronics [5]. These products capitalize on the micro- and nano-scale features of electrospun fibers to achieve unique performance attributes that are difficult or impossible to realize from materials made by conventional processes. The interest in electrospun fibers has led to rapid innovations in production technologies that are trying to keep pace with the global market demand, which is estimated to be over US$2 billion by the end of the current decade [1]. Yet, despite an existing industry to make electrospun materials and a deep interest in their healthcare applications, the platform has not resulted in any FDA-approved pharmaceutical products and only a paucity of human clinical studies [6]. This review attempts to investigate both the scientific and technological gaps that may still persist in limiting the clinical translation of electrospun fibers. Our focus is to review the opportunities and challenges in developing functional electrospun materials for use in humans, but excludes any discussion of their development for clinical diagnostics.
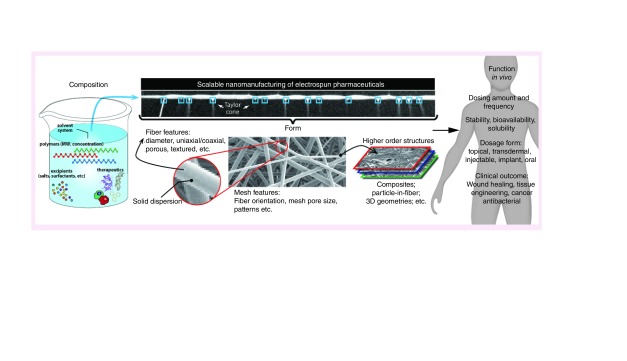
Many thousands of scientific manuscripts have been published on the topic of electrospinning since the seminal work of Reneke et al. who first detailed the fundamental principles of using electrostatic forces to emanate a charged fluid jet into continuous fibers with small diameter [2,3]. There are already several in-depth reviews on the biomedical applications of electrospun fibers [7–9], as well as reviews on technological advances that have enabled electrospinning at quantities needed to support large-scale applications [10,11]. In contrast to these separate reviews, here we investigate the convergence of topics related to the composition, form, function and manufacturing of electrospun materials for their clinical use in humans and emphasize work published since 2012 from these disparate fields (Figure 1). We first assess the state of biomedical electrospinning by describing the current clinical landscape for electrospun materials (Section ‘Electrospun materials in the clinic: commercial products and clinical activity’). From these examples, we attempt to identify common design criteria and development strategies for electrospun fibers in drug delivery (Section ‘Design of electrospun fibers for clinical applications in drug delivery’). The current literature specific to these applications is reviewed from the perspective where device design intersects with biological constraints needed to realize clinical function. Finally, these concepts are framed within the context of development considerations and scalability for pharmaceutical manufacturing (Section ‘Scalable nanomanufacturing of electrospinning for clinical development’).
Figure 1. . Convergence of composition, form, function and nanomanufacturing to enable clinical applications of electrospun materials.
The composition of the precursor solution must be selected for a scalable electrospinning process to realize the form needed for clinical function.
The overall purpose of this review is to identify opportunities that will advance the development of clinical products based on functional electrospun materials. We expect that the most rapid progress will occur by concurrently synergizing across multiple gaps in biology, medicine, engineering and scalable nanomanufacturing.
Clinical applications for electrospinning in drug delivery
Here, we summarize the current clinical landscape for electrospun materials by identifying existing and emerging commercial products. We use these products as a framework to then consider design of electrospun fibers for clinical applications specific to drug delivery. Throughout this section, we provide our perspective on the topics of composition, form and function of electrospun materials that most enable clinical translation.
Electrospun materials in the clinic: commercial products & clinical activity
Table 1 lists products incorporating electrospun fibers that have advanced into humans for biomedical use or testing. While none of these products are approved by the FDA, Nicast obtained Conformitè Europèenne (CE) certification in 2008 for its AVfloTM product [12] and Biotronik received a CE mark in 2013 for its PK Papyrus [12]. Out of these products, AVflo is comprised almost entirely of electrospun fibers whereas PK Papyrus only incorporates an electrospun coating of a coronary metal stent [12]. AVflo is a multilayered scaffold electrospun from polycarbonate-urethane and silicone copolymers intended to be used for vascular access in hemodialysis [13]. Its composition and form is hypothesized to be more biocompatible, which is expected to increase patency rates due to lower graft stenosis, and exhibit shorter times for cannulation due to better resealing properties [13]. However, a clinical study of AVflo showed that patency rates were similar to values reported for ePTFE grafts [14]. Future clinical studies will need to measure the advantage of shortened cannulation time for AVflow compared with existing grafts given that patency rates were not improved significantly.
Table 1. . Electrospun biomedical products for use in humans.
| Electrospun products | Electrospinning polymer | Therapeutic | Application |
|---|---|---|---|
| Commercial products | |||
| AVFlowTM (Nicast) [13,14] | Polyurethane | Composite vascular graft | Implant |
| PK Papyrus (Biotronik) [12] | Polyurethane | Coating metal stent | Implant |
| Products tested in humans | |||
| Surgiclot® [15,16] | Dextran | Single mesh for protein delivery | Wound dressing |
| Pathon [17] | Polyurethane | Composite for NO delivery | Wound dressing |
| TPP-fibers [20] | Polyurethane-polyethylene glycol | Single mesh for photosensitizer | Wound dressing |
There are also several emerging electrospun products not yet commercially available but have been or are currently in human clinical studies. For example, SURGICLOT® developed by St. Teresa Medical, Inc.® (NCT02509208) is being evaluated as a hemostatic dressing that uses electrospun dextran fibers to deliver thrombin and fibrinogen proteins to promote blood clotting at a surgical or wound site [15,16]. A transdermal patch comprised four layers of electrospun polyurethane fibers and designed to release nitric oxide (NO) delivered as bound nitrite on polysulfonate beads has been evaluated in two different Phase III, double-blind clinical trials [17–19]. In a study for treatment of cutaneous leishmaniasis ulcers (NCT00317629), topical daily application of the NO patch for 20 days did not have sufficient efficacy compared with a standard treatment and the study was terminated [19]. A separate study of the same materials for treating diabetic foot ulcers is still pending [17]. Finally, a clinical study of 162 patients investigated electrospun fibers of polyurethane and polyethylene glycol (PEG-PU) doped with 1 wt% tetraphenyporphyrin (TPP) for the treatment of chronic leg ulcers [20]. Participants who received TPP-doped fibers with 60 min of illumination showed a decrease in average ulcer area with 13% of patients experiencing complete healing.
Collectively, these examples of electrospun biomedical products in the clinic or in commercial development have several commonalities with respect to their composition, form and function. First, almost all of the products involve materials that have a history of safe use in medical applications. For example, polyurethane is represented in the majority of electrospun products on the market or in clinical testing (Table 1). PU and other materials used such as PTFE, dextran and hyaluronic acid are all generally regarded as safe (GRAS) and listed in the FDA inactive ingredient list [21]. Use of materials that are not GRAS increase the design space but could potentially lengthen a study. A second theme is that these products mostly employ random fibers constructed into single layer meshes or multilayered composites, whereas patterned fibers or 3D constructs are rare. In the cases where pharmaceutically active ingredients are used (blood-clotting proteins, TPP), they are included at the time of fiber formation rather than in a postprocessing step. A final shared feature is that the route of administration for these products is either as a surgical implant or topical wound dressing, which highlights opportunities as well as practical challenges for electrospun materials in different clinical applications and is the focus of our next section.
Design of electrospun fibers for clinical applications in drug delivery
The design considerations in therapeutic drug delivery involves selecting the appropriate agent to be delivered at an effector site that will establish concentrations to provide an onset and duration of activity required for clinical efficacy. Drug delivery is one of the most promising clinical applications for electrospun fibers, and several comprehensive and recent reviews have been published on the topic [8,22–24]. The variety of materials that can be electrospun provides enormous flexibility to develop formulations for physicochemically diverse small molecule drugs, proteins and nucleic acids [23]. In addition, electrospinning has been shown to be an efficient process to achieve high loading of these therapeutic agents into fibers. The ability to fabricate electrospun materials into composites and 3D geometries, or use them as coating on existing biomedical devices, provides additional opportunities to customize their design to specific clinical applications in drug delivery. In this section, we consider this enormous platform versatility within the context of achieving specific fiber formulation attributes that will affect safety and bioavailability at target sites given different administration routes. In particular, we discuss key attributes of a formulation's composition, as well as macroscopic properties of the fiber and mesh structure, that are commonly manipulated to modulate drug release.
Effect of formulation composition on drug release from electrospun fibers
The composition of the fiber formulation has a significant impact on the electrospinning process as well as the final fiber solid-state properties, which will impact safety and efficacy. Table 2 lists the most commonly investigated carrier polymers used in electrospinning for drug-delivery applications [21]. Carrier polymers are the fiber-forming component of the formulation that constitutes a significant percentage of the solids in the finished materials. Therefore, carrier polymers play an integral role in the final performance attributes of the fiber dosage form. In general, polymers used for electrospinning have typically been categorized as being synthetic or natural and can be further differentiated based on their biodegradability and hydrophobicity/hydrophilicity. Hydrophobic polymers limit the rate of wetting and diffusion of water into the mesh, and typically result in slower drug release rates compared with hydrophilic polymers. However, rapid burst release from hydrophobic polymer fibers can also be observed if the drug partitions to the fiber surface. Polymer biodegradability can also significantly affect various attributes of a fiber-based drug delivery system. As biodegradable polymers break down, there is greater chain mobility and a larger free volume for diffusion. These attributes make biodegradable polymers attractive for drug-delivery applications requiring multiple or sustained dosing at sites that are difficult to access repeatedly.
Table 2. . Common attributes of electrospun materials used for biomedical applications in drug delivery and tissue engineering.
| Composition | Structure or form | Biomedical application | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Electrospinning polymer | *FDA inactives list (UNII) | ‡Solvent(s) | Therapeutic | Electrode configuration | §Fiber Diameter | Macroarchitecture (alignment, composites, etc) | In vivo study | Outcome measures | Ref.¶ |
| Synthetic polymers | |||||||||
| Poly(L-lactic acid) | F9S9FFU82N | CHCl3/C2H4Cl2/EtOAc | Small molecule drug (cyclosporin A) | Free surface | 10 nm- 10 μm | Single mesh | Mouse (topical, 0.1 mg/mouse, single dosage) | Expression of inflammatory genes | [54] |
| Poly(L-lactide-co-caprolactone) 70:30, poly(propylene glycol), sodium acetate | N/A, N/A, 4550K0SC9B | HFIP | N/A | Single nozzle | N/A | Single mesh, two layers (one random, one aligned) | Rats (subcutaneous, N/A, single dosage) | Electrophysiology amplitude recoverage, fibrous layer thickness, myelinated axon diameter | [151] |
| Poly(caprolactone), Poly(styrene) | N/A, N/A | CHCl3/DMF | Small molecule drug (apigenin) | Single nozzle | 200-1200 nm | Single mesh | Rat (transdermal, NDS, single dosage) | Wound healing | [39] |
| Poly(lactic-co-glycolic) acid | WE369×5600 | HFIP | Small molecule drug (vancomycin) | Single nozzle | 50-267 nm | Single mesh | Rat (implanted, NDS, single dosage) | Local antibiotic delivery (brain), histological evaluation | [45] |
| Poly(L-lactic acid), poly(ethylene oxide) | F9S9FFU82N, 11628IH70O | DMF | Peptide (nisin) | Single nozzle | 466 nm | Single mesh | Mouse (dermal, NDS, single dosage) | Wound healing, bacterial burden at wound site | [152] |
| Poly(lactic-co-glycolic) acid | WE369×5600 | THF/DMF | Small molecule drugs (fusidic acid, rifampicin) | Single nozzle | 285-656 nm | Single mesh | Rat (subcutaneous, NDS, single dosage) | Infection at implant site, histological evaluation | [44] |
| Poly(lactic-co-glycolic) acid | WE369×5600 | HFIP | Small molecule drug (bupivacaine HCL) | Single nozzle | N/A | Single mesh | Rat (suture, NDS, single dosage) | Local analgesia, wound healing | [153] |
| Poly (vinyl alcohol) | 532B59J990 | water | Small molecule drug (neostigmine) | Single nozzle | 500-1000 nm | Single mesh | Mouse (injection, 0.05 mg neostigmine, single dosage) | Tail-flick test, locomotor activity | [46] |
| Poly(lactic-co-glycolic) acid, poly(ester urethane urea) | WE369×5600, N/A | HFIP, HFIP | Small molecule drug (tetracycline hydrochloride) | Dual stream | 102-390 nm | Single mesh (PLGA + PEUU fibers) | Rat (implanted, NDS, single dosage) | Wound dehiscene, abcess formation | [37] |
| Poly(lactic-co-glycolic) acid | WE369×5600 | HFIP | Biologic (insulin) | Single nozzle | 1360 nm | Composite (PLGA fibers with attached hyaluronic acid microneedles) | Mouse (topical, 0.2 IU insulin, single dose) | Blood glucose level | [38] |
| Poly(caprolactone), poly(glycerol monostearate-co-caprolactone) | N/A, N/A | CHCl3/MeOH | Small molecule drug (SN-38) | Single nozzle | 1-5 μm | Layered mesh (hydrophobic shield layers around core layer) | Rat (subcutaneous, NDS, single dosage) | Tissue integration, foreign body response | [36] |
| Natural polymers | |||||||||
| Pullulan, dextran, fucoidan, gelatin, trisodium trimetaphosphate | N/A, K3R6ZDH4DU, N/A, 2G86QN327L, 3IH6169RL0 | water/DMF | Biologic (VEG-F) | Single nozzle | 500 nm | Single mesh | Mouse (subcutaneous, NDS, single dosage) | Migrated cells, formation of blood vessels | [154] |
| Hyaluronic acid | N/A | water | N/A | Single nozzle | 58-1016 nm | Single mesh | Pig (transdermal, N/A, single dosage) | Wound healing | [155] |
| Synthetic/natural polymers | |||||||||
| Gelatin, polyvinyl alcohol (PVA) | 2G86QN327L, 532B59J990 | AcOH/water, water | Small molecule drug (doxorubicin) | Coaxial nozzle | 211-339 nm | Single mesh | Mouse (implanted, 1mg/kg, single dosage) | Tumor volume, body weight, survival rate | [41] |
| Eudragit S 100, Zein | N/A | EtOH, MeOH | Small molecule drugs (aceclofenac, pantoprazole) | Single nozzle | 50-200 nm | Single mesh | Mouse (oral, 20 mg/kg, single dosage) | Stomach ulceration, infiltration of inflammatory cells into gut mucosa | [50] |
| Poly(caprolactone), poly(ethylene oxide), collagen I | N/A, 11628IH70O, N/A | HFIP | N/A | Single nozzle | N/A | Composite, with hydroxylapatite nanoparticles | Rat (bone implant, N/A, single dosage) | Endogenous cell infiltration | [156] |
| Gelatin, glyoxal, poly(caprolactone) | 2G86QN327L, N/A, N/A | AcOH/EtOAc/water | N/A | Single nozzle | 680 nm | Single mesh, gelatin/PCL/gelatin layers | Pig (peridontal, N/A, single dosage) | Epithelial wound closure | [157] |
| Poly(lactic-co-glycolic) acid, collagen | WE369×5600, 58×445TH30, 7SVE964630; N/A | HFIP | Small molecule drugs (vancomycin, gentamicin, lidocaine) | Single nozzle | 55-314 nm | Single mesh, three layers (PLGA/collagen, PLGA/drugs, PLGA collagen) | Rat (transdermal, NDS, singal dosage) | Wound healing, histological evaluation | [40] |
| Poly(lactic-co-glycolic) acid, poly (ethylene glycol), 2-hydroxypropyl-beta-cyclodextrin | WE369×5600, 58×445TH30, 7SVE964630; 30IQX730WE, 1I96OHX6EK | DCM (shell), DMSO (core) | Small molecule drug (hydroxycamptothecin) | Single nozzle, emulsion | 540 nm | Single mesh (core/shell) | Mouse (subcutaneous, 4 mg/kg, single dosage) | Tumor volume, body weight, survival rate | [42] |
| Poly(caprolactone), gelatin | N/A, 2G86QN327L | TFE | Small molecule drug (doxorubicin) | Single nozzle | N/A | Single mesh | Mouse (subcutaneous, NDS, single dosage) | Tumor suppression | [43] |
*Unique ingredient identifier (UNII) for specific material compositions and molecular weights as listed on the USFDA inactive ingredient database (www.accessdata.fda.gov/scripts/cder/iig/index.cfm); may not correspond to actual material used in reference.
‡Reported solvents that have been used to electrospin polymer class. Solvents: CHCl3 = chloroform; C2H4Cl2 = 1,2 dichloroethane; AcOH = acetic acid; DMF = dimethylformamide; DMSO = dimethylsulfoxide; EtOH = ethanol; EtOAc = ethyl acetate; DCM = methylene chloride; HFIP = hexafluoro-2-propanol; MeOH = methanol; THF = tetrahydrofuran; TFE = tetrafluoroethylene
§Reported fiber diameters resulting from electrospinning specified polymer class.
¶Reference is to specific biomedical application described.
N/A: Not applicable or not found; NDS: No dose specified.
Careful consideration of the thermal and solid-state properties of polymers used in the formulation is also critically important for modulating drug release from electrospun fibers. Polymer crystallinity defines the ratio of ordered, aligned crystalline segments of polymer chains relative to the random, disorganized amorphous regions. The degree of crystallinity influences mechanical properties such as hardness, modulus, tensile strength and melting temperature but will also impact drug release kinetics. Ball et al. found that the ratio of semicrystalline poly-L-lactic acid (PLLA) to amorphous poly-D,L-lactic acid (PDLLA) could be used to modulate the release of a water-soluble antiretroviral drug [25]. Although the polymers were chemically identical, incorporating more amorphous PDLLA likely enhanced the rate of water penetration into the polymer network and led to faster drug release from the fibers. The melt temperature (Tm) is used to measure the degree of crystallinity of both semi-crystalline polymers and crystalline drugs. Differential scanning calorimetry enables observation of melt peaks for both polymers and drugs, and can be used to characterize crystalline content in the solid dispersion. The absence of a polymer of drug melt peak can be an indicator of an amorphous dispersion and shifts in polymer Tm may be evidence of drug–polymer interactions [26]. As was observed by Verreck et al. upon reaching 40% loading of itraconazole and ketanserin in polyurethane fibers, high drug loading can result in crystalline drug formation [27]. Polymer glass transition temperature (Tg), which is the temperature at which the polymer transitions from a hard, rigid ‘glassy’ state to a more compliant, pliable ‘rubbery’ state due to increased chain mobility, is also an important factor influencing drug release from electrospun fibers. Peng et al. observed that incorporating a small molecule drug into PDLLA and poly(ethylene glycol-co-D,L-lactic acid) (PELA) electrospun fibers reduced the polymer glass transition, likely due to the drug allowing for more facile movement of polymer chains [28]. The Tg of electrospun fibers was also decreased by higher percentage of PELA relative to PDLLA, and correlated with faster drug release. Drastic alterations in polymer Tg can directly influence the clinical relevance of electrospun materials. For example, if the Tg is below body temperature, fibers may transition into an undesired amorphous state and cause rapid drug release. Polymer crystallinity, Tg and Tm are not necessarily independent properties, but allow for strategic design of formulations with desired clinical performance.
Solid-state properties of the fiber-formulated drug or biologic also affects its solubility, bioavailability and stability. Amorphous solid dispersions (ASDs) occur when both the carrier polymer and drug co-exist within the fiber matrix in a molecularly dispersed state. ASDs are a useful category of drug-delivery systems as they are able to achieve transient drug concentrations significantly higher than the equilibrium solubility of the drug due to the absence of crystalline lattice energy in amorphous solids. Depending on polymer–drug compatibility, the high surface to volume ratio of electrospun fibers promotes rapid solvent evaporation and minimization of lattice energy between the polymer and drug. This may be particularly useful for pharmaceutical actives that are difficult to formulate or have poor solid-state properties alone [29]. For example, electrospinning poorly water-soluble drugs in carrier polymers as ASDs can enhance drug dissolution compared with the crystalline form, which may be more hydrophobic as well as needing to overcome the crystalline lattice energy [30] For example, Verreck et al. showed the release rate of two poorly water-soluble drugs was tunable by altering the drug-to-polymer ratio in hydrophobic polyurethane fibers [27]. Because both drugs were distributed in an amorphous form within the carrier polymer, the rate-limiting solubilization step was avoided and a supersaturated system was generated to provide the chemical potential for drug release. ASDs of biologic payloads in a carrier polymer can also enable long-term stability of proteins as well as preserve bioactivity. Kim et al. showed that lysozyme formulated in fiber blends of PCL and PEO maintained 90% of its catalytic activity from the dried mesh [31]. In summary, the mild electrospinning process and resulting ASDs enable the delivery and stability of challenging pharmaceutics, including poorly water-soluble drugs and fragile biologics.
Effect of macroscopic properties on drug-release from electrospun fibers
Fiber size and architecture as well as macroscopic mesh properties have also been investigated for their effects on drug release. Manipulating the surface tension or viscosity of the precursor solution is a common strategy to alter fiber size [32]. However, varying fiber diameter in the nanometer to micrometer range typically does not significantly affect drug release [33]. On the other hand, coaxial or emulsion electrospinning to fabricate fibers with distinct core and shell geometry has been shown to effectively modulate the magnitude and shape of drug-release profiles [34]. In these cases, the drug can be isolated to the core or shell region and result in drastically different release kinetics [35]. On a macroscopic scale, composite electrospun meshes in which drug-rich hyodrophilic polymer fibers are sandwiched between hydrophobic layers that act as a diffusive barrier to water has been shown as an effective approach to sustain drug release [36]. These types of layered electrospun composites can be fabricated by dual-stream electrospinning, which enables concurrent but independent manipulation of the individual mesh properties by manipulating the ratio of the two fiber populations [37].
These examples highlight the importance of thoughtful fiber formulation and fabrication for clinical translation. First, the simple selection of compatible polymers, with appropriate crystallinity and solid-state properties can have a profound impact on the ability to deliver a variety of therapeutics at clinically relevant doses and drug release profiles. Electrospinning is a relatively mild process that allows for the distribution of compatible drugs and polymers into ASDs, which may enable superior tuning of drug release as well as long-term stability. Second, variations in fiber- and mat-level architecture may also be utilized to achieve precise control over drug-release rates, but may present more of a challenge for the scale-up production needed for translation to the clinic.
Case studies of electrospun fibers for drug-delivery applications
Here we look at specific case studies to identify emerging opportunities for drug-eluting fibers for specific routes of administration and clinical applications, including topical and transdermal delivery, implants and injectables, oral delivery and tissue engineering.
Case studies of drug-eluting fibers for topical & transdermal delivery
Electrospun fibers are well suited in applications for topical or transdermal delivery due to their inherent high surface to volume ratio and relatively high drug loading compared with other delivery platforms [23]. The pliable and elastic properties of electrospun fiber meshes allow them to conform easily to these sites of administration. As an example, Yang et al. capitalized on the pliable and versatile nature of electrospun PLGA fibers for transdermal delivery of insulin using an array of dissolving microneedles compared with a rigid patch, they found that the elasticity of the fiber mesh improved insertion of the microneedles and faster delivery of insulin in vivo [38]. In addition to drug delivery, the mesh structure itself has also been implicated to function in wound healing. Moteallah et al. showed that the porous and structured nature of the electrospun PCL/polystyrene fibers delivering apigenin, a natural flavone derived from chamomile, promoted formation of epithelial tissue but minimal collagen accumulation resulting in 99% healed wounds after 14 days of treatment [39]. Fibers for topical and transdermal delivery often employ composite structures that tailor each layer for specific uses. For example, Chen et al. treated burn wounds using a sandwich-structured mesh comprising a surface layer of PLGA and collagen on a separate PLGA layer loaded with vancomycin, gentamicin and lidocaine [40]. The sandwich structure promoted early stage wound healing and delivered bioactive drug for more than 3 weeks and stimulated wound closure. The noninvasive nature of dosing and elimination at topical and transdermal also expands the material compositions that can be used (e.g., nonbiodegradable polymers). However, drug penetration of epithelial barriers to access nonsuperficial sites of action can be a significant challenge, but one that is not unique to fiber-based delivery system.
Case studies of drug-eluting fibers for implants & injectables
Although a more invasive route for administration, electrospun fibers have been used successfully as implants and injectables in applications for tumor suppression, implant infection prevention and epidural pain relief in vivo. Innovative designs capitalizing on the versatility of electrospun materials have been implemented in several preclinical studies for cancer treatment. For example, Yang et al. fabricated a composite of micelles electrospun in coaxial fibers of a PVA-core surrounded by a cross-linked gelatin shell. These materials were used to deliver doxorubicin directly to a solid tumor site, and resulted in high local concentrations of drug that minimized dosing frequency [41]. Luo et al. used emulsion electrospinning to fabricate core-sheath fibers for sustained delivery of an anti-cancer drug, hydroxycamptothecin (HCPT) in a poly(D,L-lactic acid)-PEG (PELA) copolymer. The core-shell structure allowed for encapsulation of HCPT, which is insoluble in water, and led to decreased tumor size and higher survival rate in vivo compared with free HCPT [42]. Chen et al. combined chemotherapy and photothermal therapy in electrospun fiber composites using nanoparticles with a doxorubicin core and Cu9S5@SiO2 shell loaded into PCL/gelatin blend nanofibers. When implanted at the tumor site, they found that photothermal treatment combined with chemotherapy could successfully decrease tumor size [43].
Electrospun materials have also been used as coating on implants to prevent infection. For example, Gilchrist et al. coated titanium implants with electrospun PLGA fibers eluting fusidic acid and rifampicin [44]. When used in rats, the coated implants were found to significantly decrease adherence of methicillin-resistant Staphylococcus aureus (MRSA) to the implants by greater than 99.9%. Tseng et al. showed that mice administered vancomycin fibers postoperatively had high and sustained levels of the antibiotic for up to 8 weeks without evidence of an inflammatory reaction [45]. Electrospun fibers have also been used effectively as injectables. Yosefifard et al. evaluated micronized PVA fibers loaded with neostigmine for use as an epidural in mice to help reduce pain and found that the materials achieved sustained release of neostigmine in vivo [46]. As implantables and injectables, electrospun fibers can deliver therapeutics locally over the appropriate timescales, eliminating the need to systemic administration and are then resorbed by the body.
Case studies of drug-eluting fibers for oral delivery
Drug-eluting fibers are also useful for oral delivery as they can be formulated using hydrophilic polymers for quick dissolution, pH-dependent polymers for dissolution in specific gastrointestinal locations and polymer blends for sustained release of physicochemically diverse drugs. Illangakoon et al. used polyvinylpyrrolidone (PVP), a hydrophilic polymer, to load paracetamol and caffeine, a pain reliever and stimulant for oral delivery. Using a high-speed camera, they found that their fibers quickly dissolved (<0.5 s) in 15 ml of saliva stimulant and caused rapid drug release [47]. For populations with difficulties swallowing medication, Noolkar et al., developed quick dissolving PVP fibers that deliver the nonsteroidal anti-inflammatory (NSAID) compound meloxicam. The investigators showed that the drug-loaded fibers rapidly disintegrated in an ex vivo tissue model saturated with saliva, but complete dissolution of meloxicam took approximately 2 h [48]. Although PVP supports the encapsulation of physicochemically diverse drugs such as caffeine and meloxicam, the drastically different rate of dissolution of the drugs due to variance in crystallinity.
Stimuli responsive polymers that show pH-dependent solubility have also been used for oral administration to target dissolution in the GI tract. Commercially available Eudragit® copolymers comprised methacrylic acid and methyl methacrylate in ratios that allow tuning of pH-dependent ionization have been widely investigated for this function. For example, Shen et al. formulated diclofenac sodium in Eudragit L100–55 fibers and showed minimal release at acidic pH of the stomach (pH < 2) but sustained release at pH 7, making the formulation effective for colon-targeted drug delivery [49]. Karthikeyan et al. showed that a zein/Eudragit S100 carrier system could deliver aceclofenac (in zein) and pantoprazole (in Eudragit S100) [50]. Pantoprazole, which is used to treat gastroesophageal reflux, was delivered in combination with aceclofenac, an NSAID, to prevent gastrointestinal ulcer, using Eudragit S100 to prevent degradation. This formulation was shown to reduce gastrointestinal toxicity induced in vivo by NSAIDs. The clinical efficacy of drug-eluting electrospun fibers relies on carrier polymers with the appropriate dissolution rate and solid-state properties to deliver therapeutics to the target region.
Case studies of drug-eluting fibers for tissue engineering
The field of tissue engineering aims to use biological substitutes for the regeneration, maintenance or improvement of tissue [9]One of the most successful approaches toward this goal has been the use of polymeric scaffolds, with specific mechanical and biological properties similar to that of native extracellular matrix (ECM), which is composed of proteins and glycosaminoglycans arranged in nanoscale features. The spatial and temporal ECM provides cues for cells to dynamically influence cellular migration, proliferation, differentiation, gene expression and signaling. Electrospinning offers a simple and cost-effective way to fabricate tissue engineering scaffolds in the submicron range. Furthermore, a nano- or microfibrous material is suited for tissue engineering applications because of the ability within the manufacturing process to adjust for pore size and create random or aligned fibers to help promote cell adhesion and remodeling of the ECM [51]. Natural and synthetic polymer choices expand the design space to achieve required mechanical strength of the material as well as cellular growth [52].
Encapsulating signaling molecules directly into polymeric fibers can enhance the biological activity and potential for tissue growth of electrospun fibrous tissue engineering scaffolds. Holan et al. used PLA as a scaffold and carrier polymer to deliver Cyclosporine A (CsA), a T-cell and calcineurin inhibitor to serve as scaffolds for cell therapies to increase cellular proliferation without eliciting an immune response [53]. Jiang et al. electrospun cross-linked zein nanofibers, using citric acid, to improve water stability of fibers and promote cellular adhesion. They observed a threefold increase of cell attachment using cross-linked zein nanofibers containing citric acid compared with zein films [54]. Loh et al. electrospun an FDA-approved triblock hydrogel PEG-PPG-PEG in a 1:1 ratio with PCL loaded with bovine serum albumin (BSA) to enhance cell adhesion and proliferation. The triblock hydrogel is a known thermoresponsive polymer that can increase the release of BSA when electrospun with PCL compared with PCL alone. Their findings suggest increased cell attachment in the hydrogel nanofiber with BSA compared with PCL alone because of the enhanced hydrolytic degradation of the hydrogel nanofibers resulting in a more biomimetic matrix [55]. Li et al. incorporated bone morphogenetic protein 2 (BMP-2) and hydroxyapatite nanoparticles (nHAP) into an electrospun silk fibroin scaffold for use in bone tissue engineering [56]. They found that the coexistence of BMP-2 and nHAP resulted in the highest calcium deposition and upregulation of BMP-2 transcript levels. These approaches show that incorporating signaling molecules into drug-eluting electrospun fibers are a promising strategy for the design of tissue engineering scaffolds for clinical translation.
Outlook on the design & development of drug-eluting fibers
Drug-eluting electrospun fibers have been formulated for use in a wide variety of applications, including topical, transdermal, implantable, injectable and oral delivery. A number of trends are apparent as far as the functionality and clinical efficacy of these delivery platforms. First, for any delivery route, it is important to formulate the fibers with compatible drugs and carrier polymers. Dispersing the drug in an amorphous form throughout the carrier polymer allows the polymer to act with the intended release properties. Furthermore, composites of electrospun fibers, whether layers of different polymers to promote tissue integration or loading the fibers with particles to achieve a desired release rate, exploits the malleable nature of fibers and allows for the desired release profile, in addition to addressing challenges associated with each route of administration. Finally, the combination of electrospun fibers with other drug-delivery systems, such as dissolving microneedles or as coatings on implants, shows the breadth but also limitations of fiber function.
Scalable nanomanufacturing of electrospinning for clinical development
The incredible variety in possible clinical applications inspires innovation in electrospinning technology. Figure 2 illustrates the hierarchical complexity that can be achieved with electrospinning by simply altering the precursor formulation and specific electrospinning process configurations. At the fiber-level, several approaches have been used to control diameter and fiber structure (core-shell, surface features, porosity, etc.). At the mesh-level, aligning or complex patterning of fibers as well as controlling mesh pore size has been investigated for various materials and applications. For these reasons, electrospinning has emerged as a popular technology because it is a simple, low-cost approach to nanomaterial fabrication that has proven to be extremely versatile. Understanding what elements in the large electrospinning design space to prioritize is important for rapid and efficient clinical development. In addition, unnecessary complexity in pharmaceutical development can incur high costs and may be difficult – or impossible – to realize in a scalable manufacturing process.
Figure 2. . Design of submicron and macroscopic attributes of electrospun fibers enhances their versatility and function.
Fiber-level design: (A) Small and (B) large fiber diameter; (C) core-shell fiber, reprinted with permission from [57] © Elsevier; (D); porous, reprinted with permission from [58] © Elsevier; and (E) spongy, reprinted with permission from [59] © Elsevier, fiber morphology. Mesh-level design: (F) Random and (G) aligned fiber orientation; (H) patterned grid reprinted with permission from [60] © Elsevier; (I) composite nanoparticle (arrow) – nanofiber, reprinted with permission from [61] © Elsevier; and (J) nanosphere-immobilized nano-fibrous scaffolds, reprinted with permission from [62] © Elsevier.
Scale bars: 2 μm (A, B); 200 nm (C, D); 5 μm (E); 10 μm (F, G, I) and 200 μm (H, J).
In this section, we focus on advances in electrospinning technology that enable the compositions and form identified for the biomedical applications discussed at the beginning of this article (Figure 1). The simple electrospinning instrumentation can be modified in many different methods, which are organized here by efforts specifically focused on the precursor solution, the electrospinning set-up design and post-processing techniques. We also describe the current industrial landscape of electrospinning; these advances are framed within the context of scalability for clinical development.
Effect of precursor solution
Much of the versatility in the electrospinning process deals specifically with the composition of the precursor solution. As discussed previously, many different polymers can be electrospun with requirements for different solvents, solution concentrations and electrospinning process conditions to be successful (Table 2). All of these factors contribute to the design of nanofiber mesh characteristics. Fiber morphology is often a reported process output or response where inputs are manipulated to control fiber diameter and diameter distribution, fiber matrix pore size and pore size distribution, prevalence of ‘beads on string’ phenomenon [63] and percent of nonfiber regions that may emerge in the finished materials. Other process responses, such as throughput or material yield are also important to consider when evaluating precursor materials. These responses will collectively have varying importance in the design and function of electrospun biomedical materials.
Because fiber responses are affected by both the materials and solution properties, a considerable number of possibilities emerge given the many polymers (molecular weight, crystallinity, hydrophobicity/hydrophilicity) that can be used alone or as blends [6. Solution properties such as surface tension, viscosity and conductivity have a dynamic effect on fiber outcomes, which can be tuned by manipulating the solvent system (using solvent ratios to precisely tune surface tension), polymer concentration and including additives such as salts [64]. For example, the presence of an active ingredient such as a drug or other therapeutic in the precursor solution will naturally affect solution properties and the electrospinning process, and must be considered in the clinical development process [65]. Here, we discuss innovative precursor manipulations that have emerged to meet challenges of clinical applications and consider constraints for their implementation.
Electrospinning solid suspensions
Precursor solutions of solid suspensions are commonly used in drug delivery applications. The simplest example is electrospinning of drugs that are insoluble in the electrospinning solvent [66] In the case where insoluble drugs are simply added into the polymer solution, drug release profiles can be further tuned by material selection (polymer Tg, crystallinity, wetting) and understanding of nanofiber erosion or degradation. For example, electrospinning allows for the fabrication of amorphous solid dispersions of poorly water-soluble drugs, such as ibuprofen in polyvinylpyrrolidone, wherein the electrospun fiber formulations dissolved greater than tenfold more quickly than the physical mixture of polymer and drug [67]. Engineered drug-delivery vehicles can also be electrospun as solid suspensions [68–74]. Solid suspensions containing drug-loaded silica mesoporous nanoparticles can also achieve sustained drug release with distinct release profiles depending on drug loading and drug type [71]. Incorporating drugs into particles can also help preserve activity of the drug or be used for cell targeting [68]. Some efforts have involved fabrication of electrospun cell bearing matrices, which has largely been evaluated in a review by Jayasinghe [75]. Although key challenge is ensuring cells survive the electrospinning process, bacteria have successfully been electrospun [76] and cell-bearing fiber matrices have successfully been tested in mouse models [77].
Electrospinning of solid suspensions has several challenges when considering scalability of processing these materials. Most of this work is conducted on the simple needle electrospinning apparatus, and needle clogging issues observed in continuous production from these instruments could be exacerbated by the presence of solids. A common preparation strategy is to suspend the solutions by sonication [68] or homogenization immediately before electrospinning. While this is an effective short-term solution for conducting experiments, continued processing requires a stabilized suspension to prevent temporal evolution of solids loading in the product. Finally, the question of uniformity of solid distribution within fiber mats remains uninvestigated. To address some of these questions, free surface electrospinning of solid suspensions from a rotating wire electrode has been shown [66]. Within this context, modeling efforts have been used to inform the theoretical maximum particle diameter that should be electrospinnable as a function of polymer/solvent selection and solution properties [78].
Emulsion electrospinning
Emulsion electrospinning has emerged as a convenient method to fabricate fibers with interesting morphologies (Figure 3C & D). In emulsion electrospinning, the precursor solution mixed in a precise oil to water phase ratio to achieve the desired fiber characteristics. One favorable advantage of emulsion electrospinning for drug delivery applications is the ability to solubilize drugs and polymer in separate solvent systems for drug and polymer combinations with different hydrophillicity [79]. Another application of emulsion electrospinning is in fabricating core-shell nanofibers from W/O or O/W emulsions, which have been used for delivery of bioactive proteins, sustained release of small molecules drugs and tissue engineering scaffolds [80–85]. The ratio of W/O solvent in these systems can be manipulated to span the fiber morphology design space, with an optimum W/O ratio resulting in nanofibers with a distinct core-shell morphology [86]. The effect of various surfactants (type and amount) has also been commonly investigated to stabilize emulsions and understand their effect on fiber diameter, mechanical strength and activity of proteins or other bioactives [80,81,83,84].
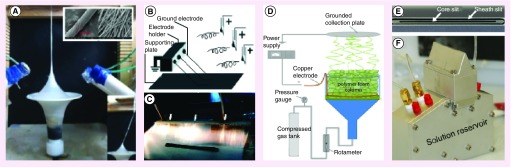
Figure 3. . Free surface electrospinning electrode configurations.
Examples of electrode configurations used in free-surface electrospinning. (A) Rotating cylinder, (B) rotating wire and (C) static wire electrode (top, showing entrained precursor solution and emanating charged fluid jets) and oscillating carriage module (bottom).
Although most emulsion electrospinning studies are conducted with a simple needle electrospinning set up, translation of emulsion electrospinning to two different scalable free-surface designs has been shown [87,88]. One design uses a rotating wire module where solution entrainment occurs by passing a wire electrode through an emulsion bath [87]. This study uses a simple mass transport model to design solutions with compatible properties, including evaporation rate, viscosity and conductivity, to optimize the jetting instability wavelength parameter for each phase (core-shell) in order to produce uniform core-shell fibers [87]. Another design attempts to overcome the requirement of precisely compatible solutions by using a dual-wire spinneret that draws droplet bridges into the jetting zone by capillary force [88]. Although emulsion electrospinning shows promise as a scalable technique, current limitations still preclude the ability to translate nonspecific material formulations to the scalable free surface designs. Further, precise and independent control of core and shell diameters is not possible in these designs due to limited degrees of freedom in parameter selection. Core-shell fibers can also be fabricated with a coaxial design, which is further discussed in Section 2.2.2. We refer the reader to Yarin et al, who present a detailed review of core-shell nanofiber production through coaxial and emulsion electrospinning conducted prior to 2011 [89].
In situ cross-linking
Cross-linking can lead to favorable outcomes in polymer nanofiber networks, such as enhanced mechanical properties and controllable degradation. For example, Jiang et al. show that using citric acid to cross-link electrospun zein fibers allowed the fibers to retain their ultrafine nanfibrous structure for up to 15 days, as compared with less than 2 days for zein alone, which promoted cellular adhesion and viability [54]. By cross-linking during the electrospinning process, greater control over the cross-linking reaction can be maintained and the need for post-processing is eliminated. A common strategy is adding cross-linking agents into the precursor solution immediately before electrospinning to ensure that the reaction progresses with an appropriate timescale by controlling reactant concentrations [90–93]. The reaction kinetics dictate transient properties in the electrospinning solution, such as a dramatic increase in solution viscosity as the reaction progresses, which can result in a change over time of nanofiber properties such as fiber diameter and degradation timescale [90,92]. Cross-linking reactions which require a catalyst can be further controlled by catalyst concentration and time of catalyst addition [92,93]. Materials developed with these methods will have a spatial dependence on material properties orthogonal to the collecting substrate. Although a gradient of cross-linking density from the top to bottom layer of the fiber mat may be preferred for a desired degradation profile, a consistent cross-linking density can be achieved by using a double syringe pump with mixing head [94]. Another example of cross-linking during the electrospinning process is using photochemical cross-linking and positioning a UV lamp between the needle tip and collector so that the polymer cross-links while in flight to the collector [95].
Although these in situ cross-linking strategies seem inherently scalable, the techniques have yet to be demonstrated in a scaled instrument and several challenges would need to be overcome. For example, cross-linking reaction residence times would have to be translated and maintained in a scale-up design. Also, stability of cross-linked nanofiber products needs to be investigated further. Any residual cross linking agents in unpurified nanofiber fabrics could continue to slowly progress the cross-linking reaction after electrospinning and also result in impurities within the biomedical materials.
Melt electrospinning
Melt electrospinning is a process in which fiber formation arises from a polymer-based melt without requirement for a solvent. Many interesting products intended for clinical applications have been developed with this technology, including quick dissolving drug delivery systems and tissue engineering scaffolds [96–98]. High-throughput production of a melt set-up has also been shown using a rotary metal disc free surface electrospinning instrument, and the effect of electrode distance and electric field magnitude on melt electrospun fiber morphology has been investigated [99]. Although melt-electrospinning technology has possibilities for scaled production, the applications are more limited due to the requirement of polymers with low melting points.
Effect of electrospinning set-up design
Many innovative electrospinning configurations have been demonstrated to fulfill specific demands for various clinical applications. This section summarizes electrospinning configurations most interesting for clinical applications from a scalability perspective. New approaches are continually being evaluated to make electrospinning more versatile in fabricating more complex nanofiber products of interest in the application space. As electrospinning processes become more focused on scalability, current designs will continue to be improved with more focus on throughput and compatibility with process control. Topics discussed subsequently include free surface electrospinning, coaxial electrospinning, strategies to control fiber orientation, electrospinning of nanofiber yarns and bundles, direct electrospinning of 3D structures and foam (Figure 4) electrospinning.
Figure 4. . Emerging scalable electrospinning technologies.
(A) Nanofiber yarn production with vertical airflow [109] © John Wiley & Sons. (B, C) Aligned nanofibers with single jet eject method [110] © ACS Publications, (D) foam electrospinning, reprinted with permission from [111] © John Wiley & Sons, and (E, F) slit electrospinning [112].
Free surface electrospinning
Free surface (or nozzle-less) electrospinning initiates charged fluid jets from a free surface entrained with a thin film of solution rather than from a solution pumped through a needle tip [100]. Free surface electrospinning is of particular interest for this review due to the high-throughput achievable for these designs. Advantages of free surface technology emerge from avoiding needle clogging issues and avoiding nozzle–nozzle interactions since jets emanating from a free surface will achieve self-optimize spacing based on applied voltage and solution properties. Free surface designs may also allow for scale-up of nanofibers containing particles or fiber mats fabricated with distinct microarchitectures for drug-delivery applications [101,102]. Although both free surface and multi-nozzle designs have their advantages, it seems the industrial landscape has become more focused on free surface designs because of the increased compatibility with manufacturing. Many different free surface electrospinning designs have been developed, which can be evaluated by throughput capabilities for comparable electrode dimensions, compatibility with technologies discussed previously, and tunability of mass deposition profile (Table 3). Free surface designs widely used in academia include the rotating wire design [78,87,103,104], rotating coil design [105], oscillating carriage design [106], rotary cone design [107] and rotating disc/cylinder [99] (Figure 3). The rotating cylinder (Figure 3A), disc or wire (Figure 3B) designs are popular due to the simplicity of set-up and the detailed modeling efforts the Rutledge group has conducted focused on this design specifically [78,87,103,104]. However, these electrode configurations have fewer design parameters available to tune mass deposition of nanofibers. Further, the open solution bath may cause issues with solution aging in continuous processing. Although other free surface designs are not as widely studied, other configurations with more factors to control mass deposition include the rotating coil design [108] (spiral distance, maximum coil diameter, relative coil diameters and wire diameter) and oscillating carriage design [106] (carriage speed and orifice size) (Figure 3C). Although free surface configurations are evolving to meet challenges in clinical applications, more research and development is required in order to accommodate emerging designs and translation of multiphase precursors to free surface instrumentations.
Table 3. . Electrospinning platforms for scalable manufacturing.
| Production-scale electrospinning instrumentation | Configurable attributes for biomedical applications | |||||||
|---|---|---|---|---|---|---|---|---|
| Electrode Configuration [Ref.] | *Max. production rate, basis weight | Instrument manufacturers | Commercialized products | Pros/Cons | Fiber dimater | Core-shell fibers? | Fiber alignment? | Other |
| Multinozzle [10,111,136–138] | ≤0.2g/h per nozzle | Linari Engineering, E-Spin Nanotech, Holmarc, NaBond, Inovenson (Nanospinner), Donaldson, Yflow, Kato-Tech, IME technologies | Filtration products (HVAC, HEPA, etc.), textiles/apparel, acoustics | Pros: Most versatile, compatibile with coaxial electrospinning Cons: Lowest throughput, nozzle-nozzle interactions, needle clogging, waste from non-optimal flow rate |
≥50 nm | Yes | Yes | 3D volumes |
| Free Surface [67,88,102,105,139–141] | ≤60g/(h•m) for wire electrodes; 0.1-10 gsm | Elmarco, Revolution Fibers, Nfiber | Filtration products (HVAC, HEPA, etc.); skin care/cosmetics; composite reinforcement, acoustics | Pros: Higher throughput, self-optimized jet spacing and throughput, simple designs, many design options/versatile. Cons: Coaxial electrospinning challenging |
≥50 nm | Emulsion; parallel slits | Yes | 3D volumes |
| Melt [10,136,142,143] | NR | NaBond, Electrospunra | NR | Pros: No solvent required Cons: Limited polymers (only low melting point), products not thermally stable, no ultrathin fibers, not compatible with making composites or interesting architectures |
Typically ≥0.5 μm | NR | Yes | NR |
| Centrifugal [10,136,144–148] | NR | Dienes | NR | Pros: Highest throughput, aligned fibers. Cons: Highest capital costs, least versatile with interesting architectures/composite materials |
≥50 nm | NR | Yes | NR |
*Values are highly dependent on materials and electrospinning conditions.
NR: Not reported.
Coaxial electrospinning
Coaxial electrospinning is a technique that has been used for about a decade to produce high-quality core shell nanofibers [113], which are particularly useful for controlled drug release applications. A recent review on coaxial electrospinning and how structure and composition can tune drug release is presented by Jiang et al. [114]. Many studies investigate the effect of shell thickness on drug release [115] by controlling the inner and outer diameter of core shell nanofibers as a function of relative core and shell flow rates and needle tip design in addition to other electrospinning parameters. Interestingly, coaxial set-ups have also been used with a nonspinning solution in the shell component resulting in typical single-layer nanofibers with round morphology and narrower fiber diameter distribution [116]. Recently, coaxial has been expanded to triaxial nozzles to achieve for even more complex and interesting fiber morphology possibilities [117]. Zero-order drug release can be obtained with a triaxial set up by using the same polymer solution for each of the three components while manipulating the drug concentration in each feed to achieve linear release profiles [118]. In addition, Yang et al. used triaxial pH-sensitive fibers comprising Eudragit S100 and an unspinnable lecithin-diclogenac sodium solution to promote dissolution and oral colon-targeted drug delivery [119]. Another study combines emulsion and coaxial technologies by using an emulsion in the core of a coaxial set-up to achieve nanofibers with three partitions, resulting in sustained drug release out to 20 days [120]. While both coaxial and emulsion electrospinning can lead to fabrication of core-shell nanofibers, there are specific advantages to each technique. For example, coaxial electrospinning can produce more consistent core shell fibers with tunable relative diameters. In contrast, relative diameter control for emulsion systems is difficult to achieve because the emulsion composition is constrained to ensure stable core-shell structure formation. However, emulsion technology can be more easily translated between electrospinning designs and is compatible with free surface scalable technology despite current material limitations in free surface emulsion electrospinning. Although coaxial and triaxial electrospinning work has been almost entirely confined to single needle systems, a recent study describes ‘slit electrospinning’, which is an innovative high-throughput and scalable example of coaxial electrospinning [112] (Figure 4 E & F).
Electrospinning aligned fibers, nanofiber yarns & 3D volumes
Although traditional electrospinning yields nonwoven mats of randomly oriented nanofibers, aligned nanofiber matrices are often desired for tissue engineering scaffolds and materials with high unidirectional tensile strength. Common strategies to produce aligned nanofibers include electric field manipulation with parallel plate collecting electrode [121], rotating mandrel collecting electrode [122], high frequency electric relay [123] and centrifugal electrospinning [124] (Figure 4B & C). An exception is the high frequency electric relay concept, where the grounded electrode is switched between two locations with a high frequency electric relay that causes fibers to be aligned across the two grounded electrodes [123]. Although this design seems promising, it has not been investigated in detail, likely due to the relative complexity of the equipment required to set up a high frequency relay system capable of over 20 kV. To date, the only design that has successfully been proven with throughput higher than a single needle system is a multi-jet and collector jet ejection design [110,125]. Another strategy to improve mechanical strength of nanofiber fabrics is by bundling aligned nanofibers into nanofiber yarns (Figure 4A). Many different designs have been implemented to fabricate nanofiber yarn, which can be categorized into devices that use mechanical twisting [126–129] and devices that use rotational air flow [109,130,131]. Niu et al. demonstrate nanofiber yarn production from a free surface electrode with rotating wire collector to bundle the yarn [127], and He et al. present a design using airflow to bundle fibers capable of production of over 6 g/h of nanofiber yarn [109]. Finally, electrospinning can also be used to directly spin 3D volumes. One electrospinning design to promote 3D volume product form is using a grounded spherical dish with an array of needle like probes as the collecting electrode [132]. However, 3D nanofiber structures have also been spun directly onto flat Al foil substrates [133] and have achieved throughput of over 14 g/h when produced on a simple rotating mandrel from a free surface spinning electrode [134] Tunability of the 3D fiber formation mechanism remains a barrier in manufacturing high-quality 3D fiber products.
Perspectives on scalable electrospinning for pharmaceutical manufacturing
As shown in Table 3, there are four classes of electrospinning equipment that are commercially available, and of these the multi-nozzle and free-surface classes are the most mature. Through understanding of the current industrial landscape as well as the research literature, we feel that the free surface class of equipment is best suited for production of pharmaceutical products and tissue engineering scaffolds through electrospinning. A major consideration is process throughput; the multi-nozzle configuration is far less productive than mature fiber and film production technologies making economics unfavorable. Further, free-surface electrospinning is well poised to confront the remaining scalability challenges in production of electrospun medical fabrics. The self-optimized jet spacing and throughput and elimination of nozzle–nozzle interactions and jet clogging makes the free surface technologies more compatible with development of process and quality control mechanisms. Further, the relative simplicity of the design and minimal contact components could also be beneficial for process approval as well as routine maintenance and cleaning requirements for pharmaceutical production. Finally, in lieu of coaxial spinning capabilities emulsion electrospinning from free surface electrodes is an emerging technique capable of producing the core-shell fiber architectures popular, especially in controlled drug release applications. Despite the recent progress, there are still some key gaps for free surface technologies as discussed [10,66,87,101,104,110,135–147].
Industry scale electrospinning
Although clinical applications of electrospun fibers have not yet made it to market, electrospun manufacturing exists in other sectors. Persano et al. presents a comprehensive summary of the currently available products and companies involved in both the production of electrospun nanofiber products and electrospinning equipment [11] For example, Bioweb™ is an electrospun PTFE graft developed by Zeus® for applications as tissue scaffolds and coatings for stents and implantable devices [7]. Nicast, who also developed AVfloTM, uses the same polycarbonate-urethane polymers to electrospin a mesh (NovaMeshTM) for intra-abdominal treatment of ventral hernias [148]. NanoNerve, Inc. has developed a patterned electrospun nanofiber mesh based on synthetic bioresorbable polymers as a dural substitute for use in neurosurgical procedures [149].
There are also several companies that provide electrospinning services to support development and manufacturing of electrospun materials for biomedical applications. For example, the Electrospinning Company based in the UK offers services to support academics and businesses develop and manufacture electrospun materials for clinical application in tissue regeneration [8]. Biosurfaces, Inc. based in the US is an electrospinning service company as well as developer of electrospun products such as wound dressings, artificial small diameter arteries and coatings for biomedical devices [9]. Arsenal Medical uses its AxiCore™ slit-surface technology to scale up traditional co-axial fiber electrospinning [10] (Figure 4E & F). Finally, SNS Nano Fiber Technology, LLC markets ISO certified facilities to produce custom electrospun products at industrial scale using a continuous process.
Conclusion & future perspective
With our understanding of the state-of-the-art electrospinning technology, we can now evaluate the specific characteristics limiting the progression of clinical electrospun nanofiber products into the market. As evidenced in the first part of this article, electrospun fabrics have shown enormous potential to address therapeutic challenges in both drug delivery and tissue engineering. The platform is amenable to various compositions of polymers, therapeutics and excipients as well as process controls that can achieve both fiber- and mat-level attributes best suited for in vivo function. It is also clear that existing and emerging scalable electrospinning instrumentation can be used to realize the form and structure of these multifunctional electrospun materials. However, economic and manufacturing challenges still need to be addressed to fully enable electrospinning for clinical applications. From an economic perspective, compared with other nonwoven production technologies, electrospinning still has too low of throughput for demand, and electrospinning products require highly skilled and multidisciplinary employees to produce and develop [11]. From a business model perspective, Hayes writes that most researchers interested in electrospinning have no business model or plan and fail to communicate with industry early enough in product development [150]. These economic issues are exacerbated for clinical applications, where the economic burden of bringing a product through clinical trial and approval are added to the burden of implementing an emerging technology. With respect to manufacturability, Persano et al. suggests one of the greatest limitations in current electrospinning technology is the lack of robust process control and product quality control structures [11]. In this regard, although process throughput is commonly studied, other manufacturing challenges specific to electrospinning of nanofibers for clinical applications are also important. For example, spatial mass accumulation profile during electrospinning is completely uninvestigated. Electrospinning of products with uniform basis weight is required for clinical applications, especially for pharmaceuticals with specified active ingredient dosage. Therefore, understanding tunability of the mass accumulation profile is an important topic for clinical electrospinning. Further, temporal instability during longer electrospinning runs is another topic largely unexplored. Temporal variation of throughput, fiber diameter, mass deposition and yield can arise in electrospinning due to charge accumulation, solution aging or polymer accumulation on spinning electrode. For high-quality clinical nanofiber products, advances will be required in understanding these phenomena behind temporal evolution of process outputs. Addressing the simultaneous gaps that persist at the interface of designing and manufacturing of multifunctional electrospun materials is likely to lead to the most rapid advances toward the clinic.
Executive summary.
The overall purpose of this review is to identify opportunities that will advance the development of clinical products based on functional electrospun materials.
Electrospun materials in the clinic: commercial products & clinical activity
Almost all of the clinical products involve materials that have a history of safe use in medical applications and employ random fibers constructed into single-layer meshes or multilayered composites, whereas patterned fibers or 3D constructs are rare.
Design of electrospun fibers for clinical applications in drug delivery
Selection of compatible polymers, with appropriate crystallinity and solid-state properties can have a profound impact on the ability to deliver a variety of therapeutics and variations in fiber- and mat-level architecture may also be utilized to achieve precise control over drug-release rates.
Case studies of electrospun fibers for drug delivery applications
Formulation of fibers with compatible drugs and carrier polymers is imperative, and the use of electrospun fiber composites and combinations with other drug-delivery systems exploits the malleable nature of fibers and allows for complete control of drug-release profile.
Effect of precursor solution
Solution properties such as surface tension, viscosity and conductivity have a dynamic effect on electrospun fiber outcomes, which can be tuned by manipulating the solvent, polymer concentration and including additives.
Effect of electrospinning set-up design
New approaches are continually being evaluated to make electrospinning more versatile in fabricating more complex nanofiber products and as electrospinning processes become more focused on scalability, current designs will continue to be improved with respect to throughput and process control.
Industry-scale electrospinning
Although clinical applications of electrospun fibers have not yet made it to market, there are commercially available electrospinning instruments and several companies that provide electrospinning services.
Conclusion & future perspective
The electrospun fiber platform is amenable to various compositions of polymers, therapeutics and excipients that can achieve both fiber- and mat-level attributes best suited for in vivo function, however, economic and manufacturing challenges still need to be addressed to fully enable electrospinning for clinical applications.
Footnotes
Financial & competing interests disclosure
The work is supported by NIH/NIAID grant AI112002 and funding from the Bill and Melinda Gates Foundation (OPP1067729, OPP1110945). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Greiner A, Wendorff JH. Electrospinning: a fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007;46(30):5670–5703. doi: 10.1002/anie.200604646. [DOI] [PubMed] [Google Scholar]
- 2.Gopal R, Kaur S, Ma Z, Chan C, Ramakrishna S, Matsuura T. Electrospun nanofibrous filtration membrane. J. Membr. Sci. 2006;281(1):581–586. [Google Scholar]
- 3.Tong Y, Lu X, Sun W, Nie G, Yang L, Wang C. Electrospun polyacrylonitrile nanofibers supported Ag/Pd nanoparticles for hydrogen generation from the hydrolysis of ammonia borane. J. Power Sources. 2014;261:221–226. [Google Scholar]
- 4.Khatri Z, Mayakrishnan G, Hirata Y, Wei K, Kim IS. Cationic-cellulose nanofibers: preparation and dyeability with anionic reactive dyes for apparel application. Carbohydr. Polym. 2013;91:434–443. doi: 10.1016/j.carbpol.2012.08.046. [DOI] [PubMed] [Google Scholar]
- 5.Persano L, Dagdeviren C, Su Y, et al. High performance piezoelectric devices based on aligned arrays of nanofibers of poly (vinylidenefluoride-co-trifluoroethylene) Nature Communications. 2013;4:1633. doi: 10.1038/ncomms2639. [DOI] [PubMed] [Google Scholar]
- 6.Meinel AJ, Germershaus O, Luhmann T, Merkle HP, Meinel L. Electrospun matrices for localized drug delivery: current technologies and selected biomedical applications. Eur. J. Pharmaceutics Biopharm. 2012;81(1):1–13. doi: 10.1016/j.ejpb.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal S, Wendorff JH, Greiner A. Use of electrospinning technique for biomedical applications. Polymer. 2008;49:5603–5621. [Google Scholar]; • Provides a comprehensive outlook on electrospun fibers for drug delivery and tissue engineering applications.
- 8.Liang D, Hsiao BS, Chu B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Del. Rev. 2007;59(14):1392–1412. doi: 10.1016/j.addr.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12(5):1197–1211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 10.Luo CJ, Stoyanov SD, Stride E, Pelan E, Edirisinghe M. Electrospinning versus fibre production methods: from specifics to technological convergence. Chem. Soc. Rev. 2012;41(13):4708–4735. doi: 10.1039/c2cs35083a. [DOI] [PubMed] [Google Scholar]
- 11.Persano L, Camposeo A, Tekmen C, Pisignano D. Industrial upscaling of electrospinning and applications of polymer nanofibers: a review. Macromol. Mat. Engineering. 2013;298(5):504–520. [Google Scholar]; •• Discusses the scale-up of electrospun fibers beyond the context of biomedical engineering applications.
- 12.Ryan CNM, Fuller KP, Larrañaga A, et al. An academic, clinical and industrial update on electrospun, additive manufactured and imprinted medical devices. Expert Rev. Med. Devices. 2015:1–12. doi: 10.1586/17434440.2015.1062364. Epub ahead of print. [DOI] [PubMed] [Google Scholar]; •• Complements the translational aspects of electrospun fibers discussed in this review.
- 13.Ferraresso M, Bertoli S, Nobili P, Bortolani EM. Early experience with a newly developed electrospun polycarbonate-urethane vascular graft for hemodialysis access. J. Vasc. Access. 2013;14(3):252–256. doi: 10.5301/jva.5000128. [DOI] [PubMed] [Google Scholar]
- 14.Wijeyaratne SM, Kannangara L. Safety and efficacy of electrospun polycarbonate-urethane vascular graft for early hemodialysis access: first clinical results in man. J. Vasc. Access. 2011;12(1):28–35. doi: 10.5301/jva.2011.6278. [DOI] [PubMed] [Google Scholar]
- 15.Floyd CT, Rothwell SW, Risdahl J, Martin R, Olson C, Rose N. Salmon thrombin-fibrinogen dressing allows greater survival and preserves distal blood flow compared with standard kaolin gauze in coagulopathic swine with a standardized lethal femoral artery injury. J. Spec. Oper. Med. 2012;12(12):16–26. doi: 10.55460/3ZJN-MK5S. [DOI] [PubMed] [Google Scholar]
- 16.Rothwell SW, Reid TJ, Dorsey J, et al. A salmon thrombin-fibrin bandage controls arterial bleeding in a swine aortotomy model. J. Trauma Acute Care Surg. 2005;59(1):143. doi: 10.1097/01.ta.0000171528.43746.53. [DOI] [PubMed] [Google Scholar]
- 17.Silva SY, Rueda LC, Márquez GA, et al. Double blind, randomized, placebo controlled clinical trial for the treatment of diabetic foot ulcers, using a nitric oxide releasing patch: PATHON. Trials. 2007;8:26. doi: 10.1186/1745-6215-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva SY, Rueda LC, López M, et al. Double blind, randomized controlled trial, to evaluate the effectiveness of a controlled nitric oxide releasing patch versus meglumine antimoniate in the treatment of cutaneous leishmaniasis [ NCT00317629] Trials. 2006;7(1):14. doi: 10.1186/1745-6215-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.López-Jaramillo P, Rincón MY, García RG, et al. A controlled, randomized-blinded clinical trial to assess the efficacy of a nitric oxide releasing patch in the treatment of cutaneous leishmaniasis by Leishmania (V.) panamensis. Am. J. Trop. Med. Hyg. 2010;83(1):97–101. doi: 10.4269/ajtmh.2010.09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arenbergerova M, Arenberger P, Bednar M, Kubat P, Mosinger J. Light-activated nanofibre textiles exert antibacterial effects in the setting of chronic wound healing. Exp. Dermatol. 2012;21:619–624. doi: 10.1111/j.1600-0625.2012.01536.x. [DOI] [PubMed] [Google Scholar]
- 21.Research CFDEA. Inactive Ingredient Database. 2015.
- 22.Hu X, Liu S, Zhou G, Huang Y, Xie Z, Jing X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Rel. 2014;185:12–21. doi: 10.1016/j.jconrel.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Sill TJ, Von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29(13):1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Huang Z-M, Zhang YZ, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites Sci. Technol. 2003;63(15):2223–2253. [Google Scholar]
- 25.Ball C, Krogstad E, Chaowanachan T, Woodrow K. Drug-eluting fibers for HIV-1 inhibition and contraception. PLoS One. 2012;7(11):e49792. doi: 10.1371/journal.pone.0049792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian F, Huang J, Hussain MA. Drug–polymer solubility and miscibility: stability consideration and practical challenges in amorphous solid dispersion development. J. Pharm. Sci. 2010;99(7):2941. doi: 10.1002/jps.22074. [DOI] [PubMed] [Google Scholar]
- 27.Verreck G, Chun I, Rosenblatt J, et al. Incorporation of drugs in an amorphous state into electrospun nanofibers composed of a water-insoluble, nonbiodegradable polymer. J. Control. Rel. 2003;92(3):349360. doi: 10.1016/s0168-3659(03)00342-0. [DOI] [PubMed] [Google Scholar]
- 28.Peng H, Zhou S, Guo T, et al. In vitro degradation and release profiles for electrospun polymeric fibers containing paracetanol. Colloids Surf. B. Biointerfaces. 2008;66(2):206–212. doi: 10.1016/j.colsurfb.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Huang LF, Tong WQT. Impact of solid state properties on developability assessment of drug candidates. Adv. Drug Del. Rev. 2004;56:321–334. doi: 10.1016/j.addr.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Nagy ZK, Balogh A, Vajna B, et al. Comparison of electrospun and extruded Soluplus®-based solid dosage forms of improved dissolution. J. Pharm. Sci. 2012;101(1):322–332. doi: 10.1002/jps.22731. [DOI] [PubMed] [Google Scholar]
- 31.Kim TG, Lee DS, Park TG. Controlled protein release from electrospun biodegradable fiber mesh composed of poly(epsilon-caprolactone) and poly(ethylene oxide) Int. J. Pharm. 2007;338(1–2):276–283. doi: 10.1016/j.ijpharm.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 32.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26(15):2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 33.Cui W, Li X, Zhu X, Yu G, Zhou S, Weng J. Investigation of drug release and matrix degradation of electrospun poly(DL-lactide) fibers with paracetanol inoculation. Biomacromolecules. 2006;7(5):1623–1629. doi: 10.1021/bm060057z. [DOI] [PubMed] [Google Scholar]
- 34.Yarin AL. Coaxial electrospinning and emulsion electrospinning of core-shell fibers. Polym. Adv. Technol. 2011;22:310–317. [Google Scholar]
- 35.Yoo H, Kim T, Park T. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Del. Rev. 2009;61(12):2997–3017. doi: 10.1016/j.addr.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Falde EJ, Freedman JD, Herrera VL, Yohe ST, Colson YL, Grinstaff MW. Layered superhydrophobic meshes for controlled drug release. J. Control. Rel. 2015;214:23–29. doi: 10.1016/j.jconrel.2015.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong Y, Fujimoto K, Hashizume R, et al. Generating elastic, biodegradable polyurethane/poly(lactide-co-glycolide) fibrous sheets with controlled antibiotic release via two-stream electrospinning. Biomacromolecules. 2008;9(4):1200–1207. doi: 10.1021/bm701201w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang H, Kim S, Huh I, et al. Rapid implantation of dissolving microneedles on an electrospun pillar array. Biomaterials. 2015;64:70–77. doi: 10.1016/j.biomaterials.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 39.Motealleh B, Zahedi P, Rezaeian I, Moghimi M, Abdolghaffari AH, Zarandi MA. Morphology, drug release, antibacterial, cell proliferation, and histology studies of chamomile-loaded wound dressing mats based on electrospun nanofibrous poly (ε-caprolactone)/polystyrene blends. J. Biomed. Mat. Res. 2014;102B:977–987. doi: 10.1002/jbm.b.33078. [DOI] [PubMed] [Google Scholar]
- 40.Chen DW, Liao J-YY, Liu S-JJ, Chan E-CC. Novel biodegradable sandwich-structured nanofibrous drug-eluting membranes for repair of infected wounds: an in vitro and in vivo study. Int. J. Nanomed. 2012;7:763–771. doi: 10.2147/IJN.S29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang G, Wang J, Wang Y, Li L, Guo X, Zhou S. An implantable active-targeting micelle-in-nanofiber device for efficient and safe cancer therapy. ACS Nano. 2015;9(2):1161–1174. doi: 10.1021/nn504573u. [DOI] [PubMed] [Google Scholar]
- 42.Luo X, Xie C, Wang H, Liu C, Yan S, Li X. Antitumor activities of emulsion electrospun fibers with core loading of hydroxycamptothecin via intratumoral implantation. Int. J. Pharm. 2012;425:19–28. doi: 10.1016/j.ijpharm.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, Hou Z, Liu B, Huang S, Li C, Lin J. DOX-Cu 9 S 5@ mSiO 2-PG composite fibers for orthotopic synergistic chemo-and photo-thermal tumor therapy. Dalton Transactions. 2015;44:3118–3127. doi: 10.1039/c4dt03113j. [DOI] [PubMed] [Google Scholar]
- 44.Gilchrist SE, Lange D, Letchford K, Bach H. Fusidic acid and rifampicin co-loaded PLGA nanofibers for the prevention of orthopedic implant associated infections. J. Control. Rel. 2013;170:64–73. doi: 10.1016/j.jconrel.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Tseng Y-Y, Kao Y-C, Liao J-Y, Chen W-A, Liu S-J. Biodegradable drug-eluting Poly[lactic-co-glycol acid] nanofibers for the sustainable delivery of vancomycin to brain tissue: in vitro and in vivo studies. ACS Chem. Neurosci. 2013;4(9):1314–1321. doi: 10.1021/cn400108q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yosefifard M, Hassanpour-Ezatti M. Epidural administration of neostigmine-loaded nanofibers provides extended analgesia in rats. DARU J. Pharm. Sci. 2014;22(1):1. doi: 10.1186/s40199-014-0073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Illangakoon UE, Gill H, Shearman GC, et al. Fast dissolving paracetamol/caffeine nanofibers prepared by electrospinning. Int. J. Pharm. 2014;477:369–379. doi: 10.1016/j.ijpharm.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 48.Noolkar SB, Jadhav NR, Bhende SA, Killedar SG. Solid-state characterization and dissolution properties of Meloxicam–Moringa Coagulant–PVP ternary solid dispersions. AAPS PharmSciTech. 2013;14(2):569–577. doi: 10.1208/s12249-013-9941-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen X, Yu D, Zhu L, Branford-White C, White K, Chatterton NP. Electrospun diclofenac sodium loaded Eudragit® L 100– 55 nanofibers for colon-targeted drug delivery. Int. J. Pharm. 2011;408:200–207. doi: 10.1016/j.ijpharm.2011.01.058. [DOI] [PubMed] [Google Scholar]
- 50.Karthikeyan K, Guhathakarta S, Rajaram R, Korrapati PS. Electrospun zein/eudragit nanofibers based dual drug delivery system for the simultaneous delivery of aceclofenac and pantoprazole. Int. J. Pharm. 2012;438:117–122. doi: 10.1016/j.ijpharm.2012.07.075. [DOI] [PubMed] [Google Scholar]
- 51.Li W-JJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002;60(4):613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 52.Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv. Drug Del. Rev. 2007;59:1413–1433. doi: 10.1016/j.addr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 53.Holan V, Chudickova M, Trosan P, et al. Cyclosporine A-loaded and stem cell-seeded electrospun nanofibers for cell-based therapy and local immunosuppression. J. Control. Rel. 2011;156:406–412. doi: 10.1016/j.jconrel.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 54.Jiang Q, Reddy N, Yang Y. Cytocompatible cross-linking of electrospun zein fibers for the development of water-stable tissue engineering scaffolds. Acta Biomater. 2010;6:4042–4051. doi: 10.1016/j.actbio.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 55.Loh XJ, Peh P, Liao S, Sng C, Li J. Controlled drug release from biodegradable thermoresponsive physical hydrogel nanofibers. J. Control. Rel. 2010;143:175–182. doi: 10.1016/j.jconrel.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 56.Li C, Vepari C, Jin H-J, Kim H, Kaplan DL. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27(16):3115–3124. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 57.Ji W, Yang F, Den Beucken J, et al. Fibrous scaffolds loaded with protein prepared by blend or coaxial electrospinning. Acta Biomater. 2010;6:4199–4207. doi: 10.1016/j.actbio.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen TTT, Ghosh C, Hwang SG, Chanunpanich N, Park JS. Porous core/sheath composite nanofibers fabricated by coaxial electrospinning as a potential mat for drug release system. Int. J. Pharm. 2012;439:296–306. doi: 10.1016/j.ijpharm.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 59.Soliman S, Pagliari S, Rinaldi A, et al. Multiscale three-dimensional scaffolds for soft tissue engineering via multimodal electrospinning. Acta Biomater. 2010;6:1227–1237. doi: 10.1016/j.actbio.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 60.Wu Y, Dong Z, Wilson S, Clark RL. Template-assisted assembly of electrospun fibers. Polymer. 2010;51:3244–3248. [Google Scholar]
- 61.Ionescu LC, Lee GC, Sennett BJ, Burdick JA, Mauck RL. An anisotropic nanofiber/microsphere composite with controlled release of biomolecules for fibrous tissue engineering. Biomaterials. 2010;31:4113–4120. doi: 10.1016/j.biomaterials.2010.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei G, Jin Q, Giannobile WV, Ma PX. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials. 2007;28:2087–2096. doi: 10.1016/j.biomaterials.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fong H, Chun I, Reneker DH. Beaded nanofibers formed during electrospinning. Polymer. 1999;40:4585–4592. [Google Scholar]
- 64.Pillay V, Dott C, Choonara YE, et al. A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J. Nano. 2013;2013:1–22. [Google Scholar]
- 65.Pillay V, Dott C, Choonara YE, et al. A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J. Nano. 2013;2013:2013. [Google Scholar]
- 66.Brettmann BK, Cheng K, Myerson AS, Trout BL. Electrospun formulations containing crystalline active pharmaceutical ingredients. Pharm. Res. 2013;30:238–246. doi: 10.1007/s11095-012-0868-4. [DOI] [PubMed] [Google Scholar]
- 67.Yu D-G, Shen X-X, Branford-White C, White K, Zhu L-M, Bligh ASW. Oral fast-dissolving drug delivery membranes prepared from electrospun polyvinylpyrrolidone ultrafine fibers. Nanotechnology. 2009;20(5):1–9. doi: 10.1088/0957-4484/20/5/055104. [DOI] [PubMed] [Google Scholar]
- 68.Li L, Zhou G, Wang Y, Yang G, Ding S, Zhou S. Controlled dual delivery of BMP-2 and dexamethasone by nanoparticle-embedded electrospun nanofibers for the efficient repair of critical-sized rat calvarial defect. Biomaterials. 2015;37:218. doi: 10.1016/j.biomaterials.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 69.Ma J, Meng J, Simonet M, Stingelin N, Peijs T, Sukhorukov GB. Biodegradable fibre scaffolds incorporating water-soluble drugs and proteins. J. Mater. Sci. Mater. Med. 2015;26(7):5537. doi: 10.1007/s10856-015-5537-9. [DOI] [PubMed] [Google Scholar]
- 70.Qi R, Guo R, Zheng F, Liu H, Yu J, Shi X. Controlled release and antibacterial activity of antibiotic-loaded electrospun halloysite/poly(lactic-co-glycolic acid) composite nanofibers. Colloids Surfaces. B. Biointerfaces. 2013;110:148–155. doi: 10.1016/j.colsurfb.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 71.Song B, Wu C, Chang J. Dual drug release from electrospun poly(lactic-co-glycolic acid)/mesoporous silica nanoparticles composite mats with distinct release profiles. Acta Biomater. 2012;8(5):1901–1907. doi: 10.1016/j.actbio.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 72.Xue J, Niu Y, Gong M, et al. Electrospun microfiber membranes embedded with drug-loaded clay nanotubes for sustained antimicrobial protection. ACS Nano. 2015;9(2):1600–1612. doi: 10.1021/nn506255e. [DOI] [PubMed] [Google Scholar]
- 73.Zhang X, Guo R, Xu J, et al. Poly(l-lactide)/halloysite nanotube electrospun mats as dual-drug delivery systems and their therapeutic efficacy in infected full-thickness burns. J. Biomater. Appl. 2015 doi: 10.1177/0885328215593837. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 74.Zhu W, Castro NJ, Cheng X, Keidar M, Zhang LG. Cold atmospheric plasma modified electrospun scaffolds with embedded microspheres for improved cartilage regeneration. PLoS One. 2015;10(7):e0134729. doi: 10.1371/journal.pone.0134729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jayasinghe SN. Cell electrospinning: a novel tool for functionalising fibres, scaffolds and membranes with living cells and other advanced materials for regenerative biology and medicine. Analyst. 2013;138(8):2215–2223. doi: 10.1039/c3an36599a. [DOI] [PubMed] [Google Scholar]
- 76.Nagy ZK, Wagner I, Suhajda A, et al. Nanofibrous solid dosage form of living bacteria prepared by electrospinning. Express Polym. Lett. 2014;8(5):352–361. [Google Scholar]
- 77.Jayasinghe SN, Warnes G, Scotton CJ. Bio-electrosprayed living composite matrix implanted into mouse models. Macromol. Biosci. 2011;11:1364–1369. doi: 10.1002/mabi.201100131. [DOI] [PubMed] [Google Scholar]
- 78.Brettmann BK, Tsang S, Forward KM, Rutledge GC, Myerson AS, Trout BL. Free surface electrospinning of fibers containing microparticles. Langmuir. 2012;28(25):9714–9721. doi: 10.1021/la301422x. [DOI] [PubMed] [Google Scholar]
- 79.Qi H, Hu P, Xu J, Wang A. Encapsulation of drug reservoirs in fibers by emulsion electrospinning: morphology characterization and preliminary release assessment. Biomacromolecules. 2006;7:2327–2330. doi: 10.1021/bm060264z. [DOI] [PubMed] [Google Scholar]
- 80.Briggs T, Arinzeh TL. Examining the formulation of emulsion electrospinning for improving the release of bioactive proteins from electrospun fibers. J. Biomed. Mater. Res. A. 2014;102(3):674–684. doi: 10.1002/jbm.a.34730. [DOI] [PubMed] [Google Scholar]
- 81.Hu J, Prabhakaran MP, Ding X, Ramakrishna S. Emulsion electrospinning of polycaprolactone: influence of surfactant type toward the scaffold properties. J. Biomater. Sci. Polym. Ed. 2015;26(1):57–75. doi: 10.1080/09205063.2014.982241. [DOI] [PubMed] [Google Scholar]
- 82.Hu J, Wei J, Liu W, Chen Y. Preparation and characterization of electrospun PLGA/gelatin nanofibers as a drug delivery system by emulsion electrospinning. J. Biomater. Sci. Polym. Ed. 2013;24(8):972–985. doi: 10.1080/09205063.2012.728193. [DOI] [PubMed] [Google Scholar]
- 83.Tian L, Prabhakaran MP, Ding X, Kai D, Ramakrishna S. Emulsion electrospun vascular endothelial growth factor encapsulated poly(l-lactic acid-co-ε-caprolactone) nanofibers for sustained release in cardiac tissue engineering. J. Materials Sci. 2011;47(7):3272–3281. [Google Scholar]
- 84.Wang Z, Qian Y, Li L, et al. Evaluation of emulsion electrospun polycaprolactone/hyaluronan/epidermal growth factor nanofibrous scaffolds for wound healing. J. Biomater. Appl. 2015 doi: 10.1177/0885328215586907. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 85.Wei K, Li Y, Lei X, et al. Emulsion electrospinning of a collagen-like protein/PLGA fibrous scaffold: empirical modeling and preliminary release assessment of encapsulated protein. Macromol. Biosci. 2011;11(11):1526–1536. doi: 10.1002/mabi.201100141. [DOI] [PubMed] [Google Scholar]
- 86.Briggs T, Arinzeh TL. Examining the formulation of emulsion electrospinning for improving the release of bioactive proteins from electrospun fibers. J. Biomed. Mater. Res. Part A. 2014;102A:674–684. doi: 10.1002/jbm.a.34730. [DOI] [PubMed] [Google Scholar]
- 87.Forward KM, Flores A, Rutledge GC. Production of core/shell fibers by electrospinning from a free surface. Chem. Eng. Sci. 2013;104:250–259. [Google Scholar]
- 88.Zhou Z, Wu X-F, Ding Y, et al. Needleless emulsion electrospinning for scalable fabrication of core-shell nanofibers. J. Appl. Polym. Sci. 2014;131(20):40896. [Google Scholar]
- 89.Yarin AL. Coaxial electrospinning and emulsion electrospinning of core-shell fibers. Polym. Advanced Technologies. 2011;22(3):310–317. [Google Scholar]
- 90.Angarano M, Schulz S, Fabritius M, et al. Layered gradient nonwovens of in situ crosslinked electrospun collagenous nanofibers used as modular scaffold systems for soft tissue regeneration. Adv. Funct. Mater. 2013;23(26):3277–3285. [Google Scholar]
- 91.Meng L, Arnoult O, Smith M, Wnek GE. Electrospinning of in situ crosslinked collagen nanofibers. J. Mater. Chem. 2012;22(37):19412. [Google Scholar]
- 92.Tang C, Saquing CD, Harding JR, Khan SA. In situ cross-linking of electrospun poly(vinyl alcohol) nanofibers. Macromolecules. 2010;43(2):630–637. [Google Scholar]
- 93.Wang J, Yao HB, He D, Zhang CL, Yu SH. Facile fabrication of gold nanoparticles-poly(vinyl alcohol) electrospun water-stable nanofibrous mats: efficient substrate materials for biosensors. ACS Appl. Mat. Interf. 2012;4(4):1963–1971. doi: 10.1021/am300391j. [DOI] [PubMed] [Google Scholar]
- 94.Kishan AP, Nezarati RM, Radzicki CM, et al. In situ crosslinking of electrospun gelatin for improved fiber morphology retention and tunable degradation. J. Mater. Chem. B. 2015 doi: 10.1039/c5tb00937e. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 95.Gupta P, Trenor SR, Long TE, Wilkes GL. In situ photo-cross-linking of cinnamate functionalized poly(methyl methacrylate-co-2-hydroxyethyl acrylate) fibers during electrospinning. Macromolecules. 2004;37(24):9211–9218. [Google Scholar]
- 96.Mccann JT, Marquez M, Xia Y. Melt coaxial electrospinning: a versatile method for the encapsulation of solid materials and fabrication of phase change nanofibers. Nano Lett. 2006;6(12):2868–2872. doi: 10.1021/nl0620839. [DOI] [PubMed] [Google Scholar]
- 97.Nagy ZK, Balogh A, Dravavolgyi G, et al. Solvent-free melt electrospinning for preparation of fast dissolving drug delivery system and comparison with solvent-based electrospun and melt extruded systems. J. Pharm. Sci. 2013;102(2):508–517. doi: 10.1002/jps.23374. [DOI] [PubMed] [Google Scholar]
- 98.Visser J, Melchels FP, Jeon JE, et al. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 2015;6:6933. doi: 10.1038/ncomms7933. [DOI] [PubMed] [Google Scholar]
- 99.Fang J, Zhang L, Sutton D, Wang X, Lin T. Needleless melt-electrospinning of polypropylene nanofibres. J. Nano. 2012;2012:1–9. [Google Scholar]
- 100.Yarin AL, Zussman E. Upward needleless electrospinning of multiple nanofibers. Polymer. 2004;45(9):2977–2980. [Google Scholar]
- 101.Brettmann BK, Tsang S, Forward KM, Rutledge GC, Myerson AS, Trout BL. Free surface electrospinning of fibers containing microparticles. Langmuir. 2012;28(25):9714–9721. doi: 10.1021/la301422x. [DOI] [PubMed] [Google Scholar]
- 102.Blakney AK, Krogstad EA, Jiang YH, Woodrow KA. Delivery of multipurpose prevention drug combinations from electrospun nanofibers using composite microarchitectures. Int. J. Nanomed. 2014;9:2967–2978. doi: 10.2147/IJN.S61664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brettmann BK, Cheng K, Myerson AS, Trout BL. Electrospun formulations containing crystalline active pharmaceutical ingredients. Pharmaceutical Res. 2013;30(1):238–246. doi: 10.1007/s11095-012-0868-4. [DOI] [PubMed] [Google Scholar]
- 104.Forward KM, Rutledge GC. Free surface electrospinning from a wire electrode. Chem. Eng. J. 2012;183:492–503. [Google Scholar]
- 105.Wang X, Wang X, Lin T. Electric field analysis of spinneret design for needleless electrospinning of nanofibers. J. Mater. Res. 2012;27(23):3013. [Google Scholar]
- 106.Krogstad EA, Woodrow KA. Manufacturing scale-up of electrospun poly(vinyl alcohol) fibers containing tenofovir for vaginal drug delivery. Int. J. Pharm. 2014;475(1–2):282–291. doi: 10.1016/j.ijpharm.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lu B, Wang Y, Liu Y, et al. Superhigh-throughput needleless electrospinning using a rotary cone as spinneret. Small. 2010;6(15):1612–1616. doi: 10.1002/smll.201000454. [DOI] [PubMed] [Google Scholar]
- 108.Wang X, Niu H, Wang X, Lin T. Needleless Electrospinning of uniform nanofibers using spiral coil spinnerets. J. Nano. 2012;2012:1–9. [Google Scholar]
- 109.He J-X, Qi K, Zhou Y-M, Cui S-Z. Fabrication of continuous nanofiber yarn using novel multi-nozzle bubble electrospinning. Polym. Int. 2014;63(7):1288–1294. [Google Scholar]
- 110.Qiu Y, Yu J, Rafique J, Yin J, Bai X, Wang E. Large-scale production of aligned long boron nitride nanofibers by multijet/multicollector electrospinning. J. Phys. Chem. C. 2009;113(26):11228–11234. [Google Scholar]
- 111.Higham AK, Tang C, Landry AM, et al. Foam electrospinning: a multiple jet, needle-less process for nanofiber production. AIChE J. 2014;60(4):1355–1364. [Google Scholar]
- 112.Yan X, Marini J, Mulligan R, et al. Slit-surface electrospinning: a novel process developed for high-throughput fabrication of core-sheath fibers. PLoS One. 2015;10(5):e0125407. doi: 10.1371/journal.pone.0125407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiang H, Hu Y, Li Y, Zhao P, Zhu K, Chen W. A facile technique to prepare biodegradable coaxial electrospun nanofibers for controlled release of bioactive agents. J. Control. Rel. 2005;108(2–3):237–243. doi: 10.1016/j.jconrel.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 114.Jiang H, Wang L, Zhu K. Coaxial electrospinning for encapsulation and controlled release of fragile water-soluble bioactive agents. J. Control. Rel. 2014;193:296–303. doi: 10.1016/j.jconrel.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 115.Nguyen TT, Ghosh C, Hwang SG, Chanunpanich N, Park JS. Porous core/sheath composite nanofibers fabricated by coaxial electrospinning as a potential mat for drug release system. Int. J. Pharm. 2012;439(1–2):296–306. doi: 10.1016/j.ijpharm.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 116.Yang JM, Zha L, Yu DG, Liu J. Coaxial electrospinning with acetic acid for preparing ferulic acid/zein composite fibers with improved drug release profiles. Colloids Surf. B. Biointerfaces. 2013;102:737–743. doi: 10.1016/j.colsurfb.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 117.Monfared Zanjani JS, Okan BS, Letofsky-Papst I, Menceloglu Y, Yildiz M. Repeated self-healing of nano and micro scale cracks in epoxy based composites by tri-axial electrospun fibers including different healing agents. RSC Adv. 2015;5(89):73133–73145. [Google Scholar]
- 118.Yu DG, Li XY, Wang X, Yang JH, Bligh SW, Williams GR. Nanofibers fabricated using triaxial electrospinning as zero order drug delivery systems. ACS Appl. Materials Interfaces. 2015 doi: 10.1021/acsami.5b06007. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 119.Yang C, Yu D-G, Pan D, et al. Electrospun pH-sensitive core–shell polymer nanocomposites fabricated using a tri-axial process. Acta Biomater. 2016 doi: 10.1016/j.actbio.2016.02.029. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 120.Viry L, Moulton SE, Romeo T, et al. Emulsion-coaxial electrospinning: designing novel architectures for sustained release of highly soluble low molecular weight drugs. J. Mater. Chem. 2012;22(22):11347. [Google Scholar]
- 121.Li D, Wang Y, Xia Y. Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Lett. 2003;3(8):1167–1171. [Google Scholar]
- 122.Zhu W, Masood F, O'brien J, Zhang LG. Highly aligned nanocomposite scaffolds by electrospinning and electrospraying for neural tissue regeneration. Nanomed. Nanotechnol. Biol. Med. 2015;11:693–704. doi: 10.1016/j.nano.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 123.Attout A, Yunus S, Bertrand P. Electrospinning and alignment of polyaniline-based nanowires and nanotubes. Polymer Engineering Sci. 2008;48(9):1661–1666. [Google Scholar]
- 124.Erickson AE, Edmondson D, Chang FC, et al. High-throughput and high-yield fabrication of uniaxially-aligned chitosan-based nanofibers by centrifugal electrospinning. Carbohydr. Polym. 2015;134:467–474. doi: 10.1016/j.carbpol.2015.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rafique J, Yu J, Yu J, et al. Electrospinning highly aligned long polymer nanofibers on large scale by using a tip collector. Appl. Phys. Lett. 2007;91(6):063126. [Google Scholar]
- 126.Ali U, Zhou Y, Wang X, Lin T. Direct electrospinning of highly twisted, continuous nanofiber yarns. J. Textile Institute. 2012;103(1):80–88. [Google Scholar]
- 127.Niu H, Gao W, Lin T, Wang X, Kong L. Composite yarns fabricated from continuous needleless electrospun nanofibers. Polym. Engin. Sci. 2014;54(7):1495–1502. [Google Scholar]
- 128.Wang L, Wu Y, Guo B, Ma PX. Nanofiber yarn/hydrogel core-shell scaffolds mimicking native skeletal muscle tissue for guiding 3D myoblast alignment, elongation, and differentiation. ACS nano. 2015 doi: 10.1021/acsnano.5b03644. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 129.Wu S-H, Qin X-H. Uniaxially aligned polyacrylonitrile nanofiber yarns prepared by a novel modified electrospinning method. Mater. Lett. 2013;106:204–207. [Google Scholar]
- 130.He J, Qi K, Wang L, Zhou Y, Liu R, Cui S. Combined application of multinozzle air-jet electrospinning and airflow twisting for the efficient preparation of continuous twisted nanofiber yarn. Fibers Polym. 2015;16(6):1319–1326. [Google Scholar]
- 131.Li N, Hui Q, Xue H, Xiong J. Electrospun Polyacrylonitrile nanofiber yarn prepared by funnel-shape collector. Mater. Lett. 2012;79:245–247. [Google Scholar]
- 132.Blakeney BA, Tambralli A, Anderson JM, et al. Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomaterials. 2011;32(6):1583–1590. doi: 10.1016/j.biomaterials.2010.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cai S, Xu H, Jiang Q, Yang Y. Novel 3D electrospun scaffolds with fibers oriented randomly and evenly in three dimensions to closely mimic the unique architectures of extracellular matrices in soft tissues: fabrication and mechanism study. Langmuir. 2013;29(7):2311–2318. doi: 10.1021/la304414j. [DOI] [PubMed] [Google Scholar]
- 134.Li D, Wu T, He N, et al. Three-dimensional polycaprolactone scaffold via needleless electrospinning promotes cell proliferation and infiltration. Colloids Surfaces. B, Biointerf. 2014;121:432–443. doi: 10.1016/j.colsurfb.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 135.Hayes TR, Hosie IC. Turning nanofibres into products: electrospinning from a manufacturer's perspective. In: Macagnano A, Zampetti E, Kny E, editors. Electrospinning for High Performance Sensors. Springer International Publishing, Cham; 2015. pp. 305–329. [Google Scholar]
- 136.Blakeney BA, Tambralli A, Anderson JM, et al. Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomaterials. 2011;32(6):1583–1590. doi: 10.1016/j.biomaterials.2010.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jiang H, Wang L, Zhu K. Coaxial electrospinning for encapsulation and controlled release of fragile water-soluble bioactive agents. J. Control. Rel. 2014;193:296–303. doi: 10.1016/j.jconrel.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 138.Petrik S, Maly M. MRS Fall Meeting. MA, USA: 30 November 30 – 4 December 4 (2009). Production nozzle-less electrospinning nanofiber technology. Presented at. [Google Scholar]
- 139.Krogstad EA, Woodrow KA. Manufacturing scale-up of electrospun poly(vinyl alcohol) fibers containing tenofovir for vaginal drug delivery. Int. J. Pharmaceut. 2014;475(12):282–291. doi: 10.1016/j.ijpharm.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li D, Wu T, He N, et al. Three-dimensional polycaprolactone scaffold via needleless electrospinning promotes cell proliferation and infiltration. Colloids Surfaces B: Biointerfaces. 2014;121:432–443. doi: 10.1016/j.colsurfb.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 141.Nagy ZK, Balogh A, Drávavölgyi G, et al. Solvent-free melt electrospinning for preparation of fast dissolving drug delivery system and comparison with solvent-based electrospun and melt extruded systems. J. Pharm. Sci. 2013;102(2):508–517. doi: 10.1002/jps.23374. [DOI] [PubMed] [Google Scholar]
- 142.Fang J, Zhang L, Sutton D, Wang X, Lin T. Needleless melt-electrospinning of polypropylene nanofibres. J. Nanomaterials. 2012;2012:1–9. [Google Scholar]
- 143.Mary LA, Senthilram T, Suganya S, et al. Centrifugal spun ultrafine fibrous web as a potential drug delivery vehicle. eXPRESS Polym. Lett. 2013;7(3):238–248. [Google Scholar]
- 144.Edmondson D, Cooper A, Jana S, Wood D, Zhang M. Centrifugal electrospinning of highly aligned polymer nanofibers over a large area. J. Mater. Chem. 2012;22(35):18646–18652. [Google Scholar]
- 145.Erickson AE, Edmondson D, Chang F-C, et al. High-throughput and high-yield fabrication of uniaxially-aligned chitosan-based nanofibers by centrifugal electrospinning. Carbohydr. Polym. 2015;134:467–474. doi: 10.1016/j.carbpol.2015.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jayasinghe SN. Cell electrospinning: a novel tool for functionalising fibres, scaffolds and membranes with living cells and other advanced materials for regenerative biology and medicine. Analyst. 2013;138(8):2215–2223. doi: 10.1039/c3an36599a. [DOI] [PubMed] [Google Scholar]
- 147.Liu S-L, Long Y-Z, Zhang Z-H, et al. Assembly of oriented ultrafine polymer fibers by centrifugal electrospinning. J. Nano. 2013;2013(2514103):8. [Google Scholar]
- 148.Tao X, Shi ZD, Xiong L, et al. In-vitro and clinical study on a novel synthetic absorbable dural substitute. J. Neurological Surgery B. 2014;75:a192. [Google Scholar]
- 149.Patel S, Kurpinski K, Quigley R, et al. Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 2007;7(7):2122–2128. doi: 10.1021/nl071182z. [DOI] [PubMed] [Google Scholar]
- 150.Hayes TR, Hosie IC. Electrospinning for High Performance Sensors. 2015. Turning nanofibres into products: electrospinning from a manufacturer's perspective; pp. 305–329. [Google Scholar]
- 151.Zhu Y, Wang A, Patel S, et al. Engineering bi-layer nanofibrous conduits for peripheral nerve regeneration. Tissue Engineer. 2011;17(7):705–715. doi: 10.1089/ten.tec.2010.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Heunis TDJ, Smith C, Dicks LMT. Evaluation of a nisin-eluting nanofiber scaffold to treat Staphylococcus aureus-induced skin infections in mice. Antimicrob. Agents Chemother. 2013;57(8):3298–3935. doi: 10.1128/AAC.00622-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Weldon CB, Tsui JH, Shankarappa SA, et al. Electrospun drug-eluting sutures for local anesthesia. J. Control. Rel. 2012;161(3):903–909. doi: 10.1016/j.jconrel.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rujitanaroj PO, Aid-Launais R, Chew SY, Le Visage C. Polysaccharide electrospun fibers with sulfated poly (fucose) promote endothelial cell migration and VEGF-mediated angiogenesis. Biomaterials Sci. 2014;2:843. doi: 10.1039/c3bm60245a. [DOI] [PubMed] [Google Scholar]
- 155.Uppal R, Ramaswamy GN, Arnold C, Goodband R, Wang Y. Hyaluronic acid nanofiber wound dressing – production, characterization, and in vivo behavior. J. Biomed. Materials Res. Part B: Applied Biomaterials. 2011;97B(1):20–29. doi: 10.1002/jbm.b.31776. [DOI] [PubMed] [Google Scholar]
- 156.Phipps MC, Clem WC, Grunda JM, Clines GA, Bellis SL. Increasing the pore sizes of bone-mimetic electrospun scaffolds comprised of polycaprolactone, collagen I and hydroxyapatite to enhance cell infiltration. Biomaterials. 2012;33(2):524–534. doi: 10.1016/j.biomaterials.2011.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schulz S, Angarano M, Fabritius M, et al. Nonwoven-based gelatin/polycaprolactone membrane proves suitability in a preclinical assessment for treatment of soft tissue defects. Tissue Engineer. 2014;20(13–14):1935–1947. doi: 10.1089/ten.tea.2013.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]