Abstract
Chronic Staphylococcus aureus infections are complicated by frequent relapses not only from the development of drug resistance to conventional antibiotics, but also through the formation of persister bacterial cells. Bacterial persisters are in a transient, metabolically inactive state, making conventional antibiotics that target essential cellular growth processes ineffective, resulting in high clinical failure rates of antibiotic chemotherapy. The development of new antibiotics against persistent S. aureus is an urgent issue. Over the last decade, new strategies to identify S. aureus persister-active compounds have been proposed. This review summarizes the proposed targets, antipersister compounds and innovative methods that may augment conventional antibiotics against S. aureus persisters. The reviewed antipersister strategies can be summarized as two broad categories; directly targeting growth-independent targets and potentiating existing, ineffective antibiotics by aiding uptake or accessibility.
Keywords: : antibiotics, drug discover, MRSA, persisters
Staphylococcus aureus is a Gram-positive opportunistic pathogen carried by approximately a third of the human population [1]. It is a leading cause of both hospital-acquired and community-acquired infections, which can range from minor skin infections to life-threatening disease, such as endocarditis and osteomyelitis [2]. Even if patients are treated with a regimen of antibiotics active against S. aureus, they require prolonged therapy and many experience complications due to antibiotic-resistance [3,4], or due to shift to a nongrowing, dormant state that confers a high level of tolerance to most conventional antibiotics [5–11].
Methicillin-resistant S. aureus (MRSA), is resistant to β-lactams, a widely-used class of antibiotics. MRSA is widespread in hospital and community settings and is responsible for approximately 60% of all S. aureus infections in the USA [12,13]. The Centers for Disease Control and Prevention estimates that MRSA causes 80,461 severe infections, 11,285 deaths, and US$3–4 billion in healthcare costs per year in the USA alone [14,15]. Currently, vancomycin is used as an antibiotic of last-resort against MRSA. However, the high selective pressure from increased usage of vancomycin has promoted the emergence of vancomycin-intermediate or -resistant S. aureus [3,16].
In addition to acquiring antibiotic-resistance, as noted above, S. aureus can evade antibiotics by readily entering into nongrowing, dormant states [5,6]. Most antibiotics target biosynthetic processes, such as DNA, protein, and cell wall synthesis during bacterial growth [5,17]. However, these biosynthetic processes are inactive, or significantly minimized in the nondividing, dormant bacteria, known as persisters; therefore current antibiotics are largely ineffective against these cells [5]. S. aureus persisters were first identified in the 1940s, a couple of years after the initial discovery of penicillin-resistant bacteria [18]. In 1944, Bigger found that nongrowing S. aureus was not susceptible to penicillin [19]. He induced S. aureus to enter a nondividing state by culturing at a lower temperature, using 1:800 diluted media, or adding a bacteriostatic agent, boric acid. He discovered that the nondividing cells prepared under each condition were highly tolerant to penicillin, however, unlike resistant mutants, descendants of penicillin-tolerant cells remained susceptible to penicillin. He concluded that the activity of penicillin was bacterial growth-dependent and the tolerance resulted from dormant and nondividing states [19].
Recent studies demonstrate that almost all stationary phase S. aureus cells are persisters, and that they are tolerant to multiple antibiotics, each with a different target, such as gentamicin (protein synthesis inhibitor), ciprofloxacin (DNA replication inhibitor), and vancomycin (cell wall synthesis inhibitor) [7–11]. S. aureus persisters are also found in biofilms, a bacterial complex in which bacteria adhere to surfaces and are encapsulated in a matrix of extracellular polymeric substances [20] and these persisters are, at least partially, responsible for the high antibiotic-tolerance of biofilms [5,21]. Importantly, S. aureus is able to form persisters inside host tissues, and thus avoids host immune systems [9,22]. Several studies have shown that S. aureus persisters are responsible for the recalcitrance of chronic infections [5,23]. For instance, antibiotic treatments have reportedly failed in up to 38% of MRSA bacteremia cases even though the infecting MRSA were susceptible to the same antibiotics in vitro [24,25]. S. aureus persisters in biofilms are associated with high rates of antibiotic treatment failures and recurrence in endocarditis and osteomyelitis [26–28].
Since S. aureus infections often involve both multi-drug resistant and persistent S. aureus, these cases are hard to treat and ultimately cure with conventional antibiotics alone. The development of new therapeutics is urgently needed. In this review, we focus on targets and compounds effective against MRSA persisters and discuss their limitations. We also review new methodologies to kill S. aureus persisters using conventional antibiotics.
Bacterial protease ClpP activators, ADEPs
Bacterial proteases enzymatically cleave peptide bonds to break down proteins and polypeptides, playing important roles in stress responses, pathogenicity, and viability [29]. Bacterial proteases are a promising target for antimicrobial development because deregulation of key proteases results in reduced bacterial survival and virulence [29,30]. A well-known example is the caseinolytic protease system, which consists of a tetradecameric cytoplasmic serine protease (ClpP) and its cognate ATPase chaperones including ClpX, ClpA and ClpC [31,32]. The caseinolytic protease system plays a pivotal role in maintaining protein homeostasis by degrading misfolded and damaged proteins [31,32]. It is also involved in cell division, virulence and the breakdown of short-lived regulatory proteins [31,32]. Because ClpP nonspecifically degrades proteins, its proteolytic activity is strictly regulated by its corresponding cytoplasmic serine protease and its cognate ATPase chaperones in an ATP-dependent manner [29,30]. Briefly, ClpP-ATPases recognize, unfold, and translocate specific proteins into the proteolytic core of ClpP in an energy-dependent manner [32].
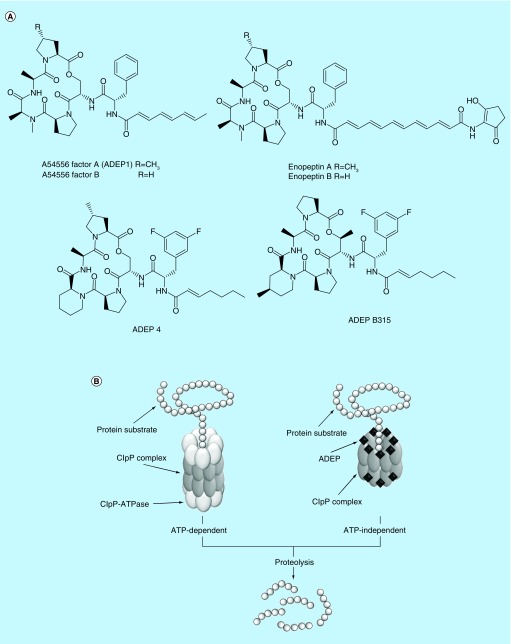
In 1985, the first antimicrobials targeting ClpP were identified by scientists from Eli Lilly and Company [33]. They isolated eight acyldepsipeptides (ADEPs), known as the A54556 complex, with antimicrobial activity against Gram-positive bacteria, including staphylococci and streptococci from Streptomyces hawaiiensis NRRL 15010 [33]. The representative ADEPs in the A54556 complex are A54556 factor A (later designated as ADEP 1) and factor B (Figure 1A). A few years later, Osada et al. isolated other ADEPs, enopeptin A and B (Figure 1A) having antimicrobial activity from Streptomyces sp. RK-1051 [34]. However, their mode of actions remained unknown for two decades. In 2005, Brötz-Oesterhelt et al. elucidated that ADEPs target ClpP by sequencing ADEP 1-resistant Escherichia coli mutants and then confirming the binding between ClpP and ADEP 1 analogs using affinity chromatography and chemical crosslinking [35]. Crystallography studies demonstrated that ADEPs bind at the interfaces between two adjacent ClpP subunits in the ClpP tetradecamer, which also function as the main docking site for the ClpP-ATPases [36,37]. The insertion of ADEPs leads to a change in the quaternary structure of the ClpP tetradecamer complex including the expansion of its axial pores, which subsequently confers the ability to degrade cellular protein without aid of ClpP-ATPases (Figure 1B) [36,37]. The overactivated ClpP protease indiscriminately breaks down oligopeptides and nascent proteins including proteins essential for bacterial survival [35,38].
Figure 1. . Acyldepsipeptides kill MRSA persisters by deregulating the ClpP protease.
Structures of natural acyldepsipeptides and their synthetic analogs (A) and mode of action (B).
Despite their unique mode of action, natural ADEPs have many drawbacks as drug candidates, including poor solubility and rapid systemic clearance [39]. Further, they demonstrated inefficacy in a mouse model of bacterial infection [39]. To overcome these constraints, structure–activity relationship (SAR) studies and structural optimization were conducted based on ADEP 1. The rigidification of the macrocyclic scaffold by replacing N-methylalanine with pipecolate reduced the entropic energy barrier for binding to ClpP [39]. To improve biological activity and stability, the polyunsaturated side chain was replaced with a heptenoyl moiety; two fluorines were added to the positions 3 and 5 of the phenyl moiety, yielding an improved ADEP 1 analog, termed ADEP 4 (Figure 1A) [39]. Indeed, ADEP 4 showed at least tenfold improved antimicrobial activity against MRSA (MIC 0.05 μg/ml) as compared with ADEP 1 (MIC 6.3 μg/ml). Moreover, it was more efficacious in the mouse model of S. aureus infection than the last resort antibiotic linezolid [35,39].
Recently, further optimization of ADEP 4 has been carried out. Carney et al., hypothesized that an increase in the conformational rigidity of ADEP 4 would improve ClpP binding to and permeability of bacterial membranes [40]. To create more conformationally constrained analogs, they replaced the serine residue of the ADEP 4 macrocycle with allo-threonine and substituted 4-methylpipecoate for the pipecolated residue of ADEP 4. The modified ADEP 4 analog, termed ADEP B315 (Figure 1A) exhibited significantly improved antimicrobial activity against S. aureus with an MIC of 0.024 μg/ml [40]. Arvanitis et al. showed that ADEP B315 was effective in mouse models of both methicillin-sensitive S. aureus (MSSA) and MRSA infections, performing comparable to vancomycin treatment [41]. Further ADEP B315 did not have significant toxicity on murine kidney or liver cells at a dose of 50 mg/kg, while vancomycin caused nephrotoxicity at the same dose [41].
In contrast to conventional antibiotics that mediate antimicrobial activity through inhibiting targets, ADEPs activate their target, resulting in nonspecific protein degradation. Since the ADEP killing mechanism is growth-independent, Conlon et al. reasoned ADEPs could be effective against both growing and dormant persistent bacteria [9]. Indeed, ADEP 4 induced considerable protein degradation in MRSA persister cells, as assessed through proteome analysis [9]. Further, the treatment of 109 CFU/ml stationary-phase MRSA persisters with 5 μg/ml ADEP 4 decreased bacterial viability up to 4 logs in 2 days [9]. Although MRSA developed ADEP4 resistance after 3-days of exposure, 5 μg/ml ADEP 4 combined with 0.4 μg/ml rifampicin completely eradicated 109 CFU/ml MRSA persisters within this time frame [9]. This combination of ADEP 4 and rifampicin also entirely eliminated MRSA persisters in biofilms within 3 days [9]. To test in vivo efficacy of ADEP 4 combined with rifampicin, Conlon et al. used the deep-seated mouse thigh infections model to mimic a chronic MRSA infection [9]. Combined treatment with ADEP 4 and rifampicin completely cleared the MRSA-infected thigh tissues, whereas vancomycin alone, rifampicin alone, or vancomycin and rifampicin could not clear the infection [9]. These results indicate that compounds targeting ClpP are promising antibiotic candidates to treat relapsing chronic infections.
Identification of candidate compounds through interrogation of membrane permeability
The bacterial cell envelope is composed of the lipid bilayer membrane and the cell wall. Since the bacterial cell envelope functions as a protective barrier and includes many proteins essential for cell survival, it is a promising target for novel antibiotics [42]. In fact, evolution tells us the bacterial cell envelope is an excellent target for killing bacteria. For instance, bacteria and bacteriophages produce hydrolytic enzymes, such as lysostaphin [43] and endolysin [44,45], which kills S. aureus by cleaving a major cell wall component peptidoglycan [43,44]. Mammalian immune systems secrete antimicrobial peptides, such as defensin, which inhibit S. aureus by disrupting membrane lipid bilayers [46]. Despite the differences in the specific modes of action, these antimicrobials rapidly permeabilize a part of the bacterial cell envelope [47,48]. Indeed, several antimicrobial peptides (AMPs) and enzymes show potency against bacterial persisters, which has been discussed in several recent reviews [49–53]. Here, we will focus on two membrane-active small molecules that effectively kill MRSA persisters, a recently proposed screen method, and its hit compound.
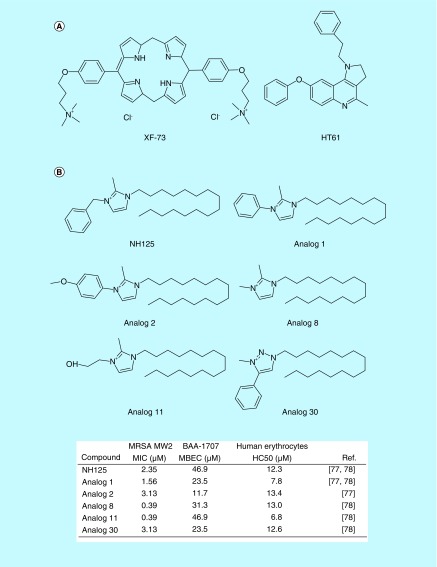
In 2009, Ooi et al. found that a porphyrin antibacterial agent, XF-73 (Figure 2A) exhibited strong bactericidal activity against S. aureus [54]. The MIC of XF-73 against S. aureus was 1 μg/ml, and it killed exponential-phase S. aureus cells faster than daptomycin [54]. Further, Ooi et al. found that XF-73 induced rapid membrane perturbation, which correlated with its bactericidal activity [54]. Based on these results, the authors hypothesized that XF-73 would be able to kill slow-growing and nondividing S. aureus cells by membrane disruption. Indeed, 4X MIC of XF-73 demonstrated bactericidal activity against both nongrowing S. aureus induced by incubation at 4°C and stationary-phase S. aureus cells, which are tolerant to the equivalent concentration of daptomycin [55]. These results provided the important insight that membrane-active small molecules could effectively kill nondividing bacterial persisters. Currently, XF-73 is in Phase II clinical trials for nasal decolonization of S. aureus [56].
Figure 2. . Membrane-active antimicrobials effective against MRSA persisters.
Structures of XF-13 and HT61 (A), and structuresand bioactivities of NH125 and its analogs (B).
MBEC: Minimum biofilm eradication concentration; MIC: Minimum inhibitory concentration; HC50: The concentration at which 50% of erythrocytes are lysed.
Hu et al. conducted a screen assay to discover antimicrobials capable of targeting MRSA persisters [57]. They isolated nonmultiplying S. aureus from long-term stationary phase cultures and screened compounds that killed these nonmultiplying S. aureus cells [57]. The small quinolone-derived compound HT61 (Figure 2A) was identified from this screen. Hu et al. found that HT61 induced rapid membrane permeabilization and cell lysis at over 20 μg/ml. Therefore, they concluded that the membrane disruption is HT61’s major mode of action to kill MRSA persisters. A recent report described the molecular details of this membrane-active mechanisms [58]. Combining cell-based membrane leakage and artificial lipid bilayers assays, Hubbard et al. suggested that HT61 directly interacted with lipid bilayers and subsequently caused unrecoverable membrane damage [58]. Interestingly, although the MIC of HT61 was 8 μg/ml, which is higher than the measured MICs of linezolid (0.8 μg/ml) or daptomycin (1 μg/ml) against S. aureus, HT61 eradicated 107 CFU/ml of persisters formed by both MSSA and MRSA at 10 μg/ml within 6 h [57]. It also exhibited excellent bactericidal activity against persisters formed by a collection of clinical MRSA strains, consisting of 103 isolates [57]. In vivo evaluation of the compound revealed that 1% HT61 gel was significantly efficacious in MRSA mouse skin infection model. Within the effective concentration, a toxicity assessment found that administration of 1–10% HT61 gels for 14 days did not cause adverse effects on mini pig skin [57]. Due to its high efficacy and low skin toxicity, HT61 has progressed to Phase III clinical trials for nasal decolonization of S. aureus [59].
Although XF-73 and HT61 have advanced into clinical trials for topical use, each has limitations when used to treat invasive systemic infections, due to their highly hydrophobic nature. High hydrophobicity often results in high serum binding and low tissue penetration rates. Therefore, XF-73 and HT61 may show lower bioavailability and significantly reduced in vivo efficacy, which is a major obstacle to the development of new membrane-active agents for systemic use [42]. In spite of their limitations, however, these two membrane-active agents can be further optimized as our understanding of the relationship among membrane activity, hydrophobicity, and bioavailability is further developed. Moreover, new drug delivery systems might help address the high serum binding and low tissue penetration issues [60–62]. For example, current drug delivery technologies using nanoparticles and liposomes enable the systemic administration of small molecules having issues of bioavailability, solubility and toxicity [62,63].
A systematic strategy to identify membrane-active antipersister agents
The successful identification of the membrane-active small molecules, XF-73 and HT61 inspired our group to seek out additional small molecules that could induce rapid membrane permeability. To identify compounds that permeabilize MRSA persisters, we developed a high-throughput screening method using the membrane-impermeable DNA binding dye, SYTOX Green, as a marker [10]. Membrane-active agents are often nonselective, or indiscriminate between mammalian and bacterial membranes [42]. To identify antimicrobial agents that specifically target bacterial membranes, we used a whole animal Caenorhabditis elegans-based screen of approximately 80,000 small molecules to identify all anti-infectives that prolong the survival of C. elegans infected with a lethal dose of MRSA [10,64]. The major benefit of this screening step is to identify antimicrobial compounds while simultaneously excluding compounds that are toxic to the worm [65,66].
In the subsequent phase, we narrowed our interrogation to 101 compounds identified as hits from the C. elegans-MRSA screen [10]. During this part of the screen, we exposed MRSA persister cells to compounds in the presence of SYTOX Green and used a spectrophotometer to determine if any compounds induced rapid membrane permeabilization. Among the 101 compounds, we identified NH125 (1-Hexadecyl-2-methyl-3-(phenylmethyl)-1H-imidazolium iodide, Figure 2) as a compound that altered membrane permeability [10]. Previously, NH125 was known to kill S. aureus by inhibiting WalK, a histidine kinase highly-conserved in Gram-positive bacteria and essential for survival [67,68]. NH125 is also known to inhibit eukaryotic elongation factor 2 (eEF2) kinase, which contributes to its anticancer and neuromodulatory activities [69–74].
Our findings suggest that NH125 also kills MRSA persisters by inducing rapid membrane permeabilization [10]. It is effective at killing persisters formed by a range of S. aureus strains, including MRSA clinical isolates, at 5 μg/ml [10,11]. When used at 10 μg/ml, NH125 killed over 99% of MRSA persisters formed inside biofilms, and it eliminated 50% of the biomass from mature biofilms. At 80 μg/ml, it completely destroyed established MRSA biofilms [10].
The structure of NH125 is similar to a benzalkonium chloride (BAC), an amphipathic disinfectant that acts on membrane lipid bilayers. Using giant unilamellar vesicles, a widely-used biomembrane-mimicking lipid bilayer system to examine interaction between membrane-active antimicrobials and bacterial lipid bilayers [75,76], we found that like BAC, NH125 exposure led to expansion and bursting of giant unilamellar vesicles [11]. This result demonstrates that the positively charged head of NH125 attaches to the head of the lipid bilayers and enters using hydrophobic interaction between its alkyl chain and the lipid bilayer’s hydrophobic core. The interjection of NH125 into a bilayer results in the full saturation of lipid bilayers. Thus, evidence suggests that the major mechanism of action of NH125 against MRSA persisters is the disruption of lipid bilayers rather than the inhibition of WalK.
Recently, the Huigens group synthesized various NH125 analogs (Figure 2B) to improve its antimicrobial activity and to better understand its SAR [77,78]. The optimal alkyl chain length of NH125 for antimicrobial activity was known to be C16, and analogs with chain lengths of <C8 or >C18 showed no remarkable antibiotic activity [67,68]. Therefore, they focused on head group modifications [77,78]. First, they found that N-arylated NH125 analogs, such as Analogs 1, 2 (Figure 2B) showed significantly enhanced antimicrobial activity against both stationary-phase MRSA persisters and biofilm MRSA persisters, as compared with its parental NH125. Both Analogs 1 and 2 had faster killing kinetics against MRSA persisters than NH125 and also exhibited minimum biofilm eradication concentrations (MBEC) of 23.5 and 11.7 μM against MRSA biofilms, respectively, which is two- to fourfold lower than the MBEC of NH125 (Figure 2B) [77]. Although, alkylated NH125 (Analogs 8 and 11, Figure 2) exhibited four- to sixfold lower MICs against MRSA than NH125, their MBECs against MRSA biofilms were equal or only slightly improved (Figure 2B). Analog 11 alone led to a 4-log reduction in stationary-phase MRSA persisters within 6 h at 50 μM, while NH125 provided only a 2-log reduction at the same concentration [78]. The substitution of triazole for the imidazole in NH125 (Analog 30, Figure 2) exhibited a slightly improved MBEC (23.5–31.3 μM) against MRSA biofilms, compared with NH125 (46.9 μM) (Figure 2B) [78].
In sum, a positively charged head group and an optimized alkyl tail (C16) are essential for the antimicrobial activity of NH125 and its derivatives. This SAR is similar to BAC [79,80]. Therefore, NH125 most likely attaches to a negatively charged bacterial membrane using its positively charged imidazole head, and penetrates into the lipid bilayers through interaction between its alkyl tail and the hydrophobic tails of lipid bilayers.
Although NH125 has excellent antimicrobial activity against both stationary-phase persisters and biofilm persisters, it causes significant hemolysis at high concentration. Its HC50 (the concentration which causes 50% hemolysis) is 38 μg/ml [11]. Despite their improved antimicrobial activity, the NH125 analogs do not exhibit reduced hemolytic activity compared with NH125 (Figure 2B) [77,78]. Thus, due to its narrow therapeutic window, further investigations and improvements in toxicity profiles are required for broader applications to MRSA persistent infections. However, NH125 exhibits higher HC50 than BAC (HC50 = 25 μg/ml), which is currently used as a topical antiseptic and a preservative in eye drops [81]. Therefore, NH125 has the potential to be used for treating skin infections that incorporate S. aureus persisters.
Repurposing of FDA-approved anticancer drugs
New antimicrobial agents need not always be synthesized from scratch or identified among collections of natural materials. Indeed, they can come from drugs that are already approved to treat disease clinically, albeit for different diseases. The search and identification of new uses for existing drugs has become known as drug repurposing [82]. The clinically approved drugs have passed extensive toxicity tests and clinical safety procedures, and their pharmacological properties are well understood. Most have track records in clinical trials with known outcomes and evaluated effects to humans. Thus, drug repositioning allows us to significantly reduce times and costs of drug discovery and development, as compared with de novo drug development [82,83].
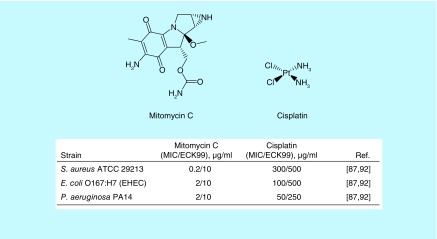
Many US FDA-approved cancer drugs were originally identified as antimicrobial agents from soil-bacteria [84]. For instance, one of the most effective classes of antineoplastic drugs, anthracyclines (for example doxorubicin and daunorubicin), were isolated as antimicrobials from Streptomyces peucetius [85]. These antimicrobial-anticancer agents induce the alkylation or intercalation of DNA, resulting in permanent DNA damage [85,86]. Kwan et al. reasoned that the DNA alkylating or crosslinking antineoplastic agents could be effective against bacterial persisters since this killing mechanism is not growth-dependent [87]. They chose an FDA-approved anticancer drug, mitomycin C (MMC, Figure 3), as a potential antipersister drug because it can passively diffuse into cells and spontaneously causes DNA crosslinking [87].
Figure 3. . Structures and antimicrobial activities of DNA-crosslinking antimicrobial-anticancer drugs.
ECK99: Effective concentration for killing over 99% of persister cells; MIC: Minimum inhibitory concentration.
MMC was originally isolated from Stretpomyces caespitosus as an antimicrobial agent in the 1950s [88]. Since, MMC FDA approval in 1974 for treating gastric and pancreatic cancer, it has been used for treating various cancers, such as breast and bladder cancers [89]. MMC showed excellent bactericidal activity against persisters formed by both Gram-positive and Gram-negative pathogens [87]. MMC completely eradicated 108 CFU/ml S. aureus stationary-phase persisters at 10 μg/ml within 3 h [87]. It eliminated 108 CFU/ml S. aureus persisters formed inside biofilms in 24 h [87]. MMC killed stationary-phase and biofilm persisters of E. coli O157:H7 and P. aeruginosa at 10 μg/ml [87]. Further, MMC was effective in the in vitro wound biofilm model mimicking P. aeruginosa–S. aureus polymicrobial infections and the C. elegans–E. coli O157:H7 infection model [87]. In addition, recent studies demonstrate that MMC also effectively kills Borrelia burgdorferi [90] and Acinetobacter baumannii persister cells [91].
Based on this finding, Chowdhury et al. evaluated the antipersister activity of seven additional DNA crosslinking compounds [92]. Among the seven crosslinking agents, they found that cisplatin (Figure 3) was effective against persister cells of E. coli and S. aureus [92]. Like MMC, cisplatin is an FDA-approved antineoplastic drug used for treating various cancers, including testicular, ovarian, cervical, bladder and head/neck tumors [93]. Cisplatin crosslinks DNA by binding between two neighboring guanine bases on the same DNA strand [93]. Cisplatin showed antimicrobial activities against both growing and persistent bacteria at high concentrations. For instance, the MIC of cisplatin against S. aureus was 300 μg/ml. Its MICs against Gram-negative bacteria, such as E. coli and P. aeruginosa were 50–400 μg/ml [92]. Interestingly, cisplatin eradicated the stationary-phase persisters of S. aureus, E. coli O157, and P. aeruginosa at 250–500 μg/ml [92]. In addition to its antimicrobial activity, low-doses of cisplatin (0.1 and 0.5 mg/kg) were found to reduce bacterial burden by enhancing the phagocytosis activity of macrophages in a murine sepsis model [94]. Considering that cisplatin is known to cause nephrotoxicity [95] and high concentrations are required for killing bacterial persisters, most likely clinical development of cisplatin for antimicrobial use will be limited to topical treatment, such as chronic wound infections.
Metabolite-mediated uptake of gentamicin into persisters
In addition to discovering novel antipersister compounds, scientists have explored novel strategies that use existing antibiotics to kill persisters. Although, persisters are in nondividing dormant states, a reduced rate of protein translation does occur [96,97]. Thus, antibiotics targeting translation processes, such as aminoglycosides may have some effect against persisters [8]. However, one such antibiotic, gentamicin does not affect S. aureus persister viability even when provided at 100X MIC [8,10]. Cellular uptake of aminoglycosides requires the proton motive force (PMF), which is generated during bacterial growth [98,99]. Because the PMF is significantly reduced in persisters’ dormant state, aminoglycosides do not enter persisters, resulting in persisters’ high tolerance to aminoglycosides [8]. Allison et al. reasoned that metabolites could stimulate persisters to be susceptible to aminoglycosides [8]. To verify this hypothesis, they screened carbon sources that stimulated persisters to become susceptible to gentamicin. Only particular carbon sources (such as glucose, mannitol, and fructose) combined with gentamicin led to a 3-log decrease in S. aureus persister viability [8]. These carbon sources specifically potentiated gentamicin, but not ampicillin or ofloxacin, suggesting that the carbon sources were not simply converting persisters to growing cells [8]. Since the effect was specific to gentamicin, they hypothesized that these carbon sources were only increasing the persister PMF, which then facilitated the uptake of gentamicin. They proved their hypothesis by the following experimental results.
First, they found that the proton ionophore, carbonyl cyanide m-chlorophenyl hydrazone, which inhibits the PMF, inhibited the uptake of gentamicin and subsequently nullified the carbon source potentiation of gentamicin [8]. Second, they confirmed that the metabolites increased PMF in persisters. This pioneering strategy was remarkably effective to kill both Gram-positive and Gram-negative bacterial persisters [8]. For instance, gentamicin combined with fructose led to a 2-log decrease in S. aureus stationary-phase persisters and a 1.5-log decrease in S. aureus biofilm persisters within 4 h [8]. In addition, gentamicin combined with mannitol reduced the viability of E. coli stationary-phase persisters up to 3-log within 2 h, and more than 4-log within 4 h in E. coli biofilm persisters [8]. Last, the combinatorial treatment of 1 mg/kg gentamicin and 1.5 g/kg mannitol reduced E. coli biofilm persisters in a mouse chronic urinary tract infection model by 1.5-log [8].
Persister membrane permeable tobramycin
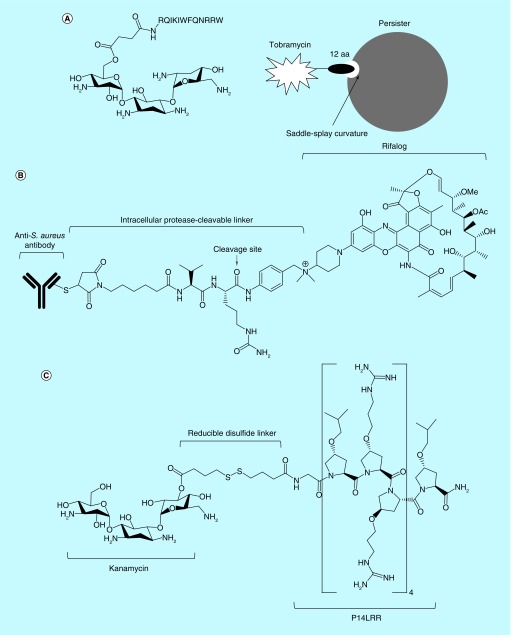
Another approach to augment the uptake of aminoglycoside into persisters was investigated by Schmidt et al. [100]. They focused on the membrane activity of AMPs and cell-penetrating peptides. Although, AMPs and cell-penetrating peptides generally have lower antimicrobial activity than aminoglycosides, they can selectively interact with bacterial membranes, which is independent of bacterial metabolic states [42]. A recent series of studies demonstrated that AMPs and CCPs share a common key functionality of generating saddle-splay (also known as negative Gaussian) curvature in target membranes, which is the topologically required process for membrane destabilization, such as pore formation [101–104]. Arginine, lysine and hydrophobic content in AMPs have been found to play a key role in generating saddle-splay membrane curvature [101,102,104]. Based on these finding, Schmidt et al. hypothesized that a hybrid aminoglycoside conjugated with the specific amino acid sequences that induce saddle-splay membrane curvature could be effective to kill bacterial persisters [100].
To interrogate their hypothesis, Schmidt et al. linked a peptide consisting of 12 amino acid residues, (RQIKIWFQNRRW, originally from penetratin), to the drug tobramycin, and designated the new compound as pentobra (Figure 4A) [100]. Indeed, unlike tobramycin, pentobra permeated and killed S. aureus persisters. Pentobra led to 4-log and 6-log decreases in S. aureus persister viability at 6.4 and 25.7 μM, respectively [100]. Pentobra was also effective at killing E. coli persisters, with 12.8 and 25.7 μM resulting in 1.5 and 1.8-log reductions, respectively. Further, pentobra had high bacterial membrane selectivity, causing no damage to eukaryotic cell membranes or no eukaryotic cytotoxicity up to 100 μM [100].
Figure 4. . Engineered existing antibiotics effectively kill bacterial persisters.
Structures and functional mechanisms of pentobra (A), antibody-antibiotic conjugate (B), and kanamycin-peptide conjugate (C).
Antibody- or peptide-antibiotic conjugates targeting intracellular MRSA persisters
Although, S. aureus is generally regarded as an extracellular pathogen, it does invade and survive inside mammalian cells, including nonprofessional phagocytes [105,106]. The intracellular environment not only provides S. aureus with a protective barrier to extracellular host defenses, but also stimulates the transition from normal dividing-cells to small colony variants and persister cells, both of which have higher tolerances to conventional antibiotics [107,108]. For instance, MRSA sequestered in murine macrophages is known to have high tolerance to last resort antibiotics, such as vancomycin, daptomycin and linezolid [22].
To kill intracellular MRSA, Lehar et al. designed an antibody-antibiotic conjugate (AAC). After evaluating the binding affinity of over 40 anti-S. aureus antibodies, they chose an antibody targeting S. aureus wall-teichoic acids, anionic glycopolymers that connect cell wall peptidoglycan layers [109]. For the antibiotic component, they selected a member of the rifamycin class of antibiotics (RNA polymerase inhibitors), rifalogue due to its high potency, stability in the intracellular environment and feasibility to conjugate the linker [22]. The antibody and rifalogue were conjugated by a cathepsin-cleavable linker (Figure 4B) [22]. Thus, the AAC itself does not have antimicrobial activity and is not toxic to host cells due to its large size. But, once host cells engulf AAC- opsonized bacteria, intracellular proteases break down the linker, and the rifalogue is subsequently released to attack the bacteria inside the host cells (Figure 4B) [22]. Remarkably, AACs were effective to kill MRSA located in various types of host cells including human macrophages, endothelial, and epithelial cell lines [22]. Further, AACs were efficacious at a daily dose of 50 mg/kg in a murine model of bacteremia, while vancomycin failed to cure at twice daily doses of 110 mg/kg [22].
In addition to an antibody-mediated therapy, the Seleem group employed a short antimicrobial peptide that penetrates into mammalian cells to facilitate the uptake of aminoglycosides [110,111]. Previously, this group synthesized a proline-rich short antimicrobial peptide, P14LRR, that effectively penetrated into mammalian membranes without causing cell lysis [112]. They conjugated P14LRR to kanamycin using a reducible disulfide linker and designated this kanamycin–peptide conjugate as P14KanS (Figure 4C) [110,111]. Since, the mammalian cell cytosol is a reducing environment, the disulfide linker is broken down and the components are released [110,111]. Interestingly, P14KanS exhibited superior antimicrobial activity to either P14LRR or kanamycin alone [111]. The MIC of P14KanS against MRSA was 1 μM, which was a 4 and a 64-fold lower than MICs of kanamycin (4 μg/ml) and P14LRR (64 μg/ml), respectively [111]. This improved antimicrobial activity most likely results from enhanced kanamycin uptake due to the membrane-active antimicrobial peptide. P14KanS was also effective at eradicating S. aureus biofilms and destroyed over 65 and 82% of S. aureus biofilms at 1 and 8 μM, respectively [111]. Importantly, P14KanS killed intracellular S. aureus. The treatment of infected human keratinocytes with 15 μM P14KanS for 24 h led to approximately 2.5-log reduction of MSSA viability and approximately 1.24-log reduction of MRSA viability [111]. Further, P14KanS showed efficacy in the C. elegans–S. aureus infection model. Treatment of C. elegans infected with S. aureus with 10 μM P14KanS resulted in 100% C. elegans survival for 72 h [111].
Conclusion & future perspective
Compared to the study of antibiotic-resistant mechanisms and the development of new drugs effective against drug-resistant bacteria, the study of persisters has a relatively short history. Even though persisters were first identified in 1944, intensive investigation on bacterial persisters did not mature until the 2000s, and strategies to treat them were not formulated until the 2010s. In this report, we take the opportunity to review the current targets, their corresponding antimicrobials, and novel strategies effective against S. aureus persisters that have been reported since 2010. To sum up, S. aureus persister eradication strategies can be classified into two categories: directly targeting persisters by disrupting growth-independent targets, and, facilitating the uptake or accessibility of existing antibiotics. Although, each compound discussed here has its own drawbacks, such as cytotoxicity or the potential for developed resistance, the bacterial cell envelope and proteases remain promising targets for inhibiting persisters. Further, the disadvantages of each compound can be lessened, and knowledge from these pioneering approaches can and will be employed as a starting point to develop next generation therapeutics to treat S. aureus persisters infections.
Due to an increasing immunocompromised patient population and medical devices usage, chronic and relapsing infections caused by persisters will continue and most likely become more prevalent. Our current arsenal of antibiotics and means of treating active infections are not sufficient for long term patient care. Thus, anti-infectives that cure chronic infections are urgently needed. As discussed above, membrane-active agents and protease activators are promising candidates to combat persisters. We are provided with insights to discover or rationally design membrane-active antibiotics with high bacterial membrane selectivity through the knowledge gleaned on the currently identified compounds. Further, natural methods employed by AMPs and how they distinguish between bacterial and host membranes have been better elucidated. With a better understanding about the ways to inhibit persister cells and better reduce biofilm accumulation, improved compounds can be generated that will inhibit active infections while offering protection against the development of chronic infections.
Executive summary.
There is urgent need to develop antimicrobials effective against Staphylococcus aureus persisters
In addition to resistance development, S. aureus evade antibiotics by shifting into nondividing, dormant states.
S. aureus persisters are found in stationary-phase planktonic culture, biofilms and host cells and have high tolerance to most of conventional antibiotics.
S. aureus persisters are responsible for relapsing and chronic infections, which are difficult to cure by conventional antibiotics chemotherapy.
Overactivation of ClpP is effective to kill S. aureus persisters
Acyldepsipeptides bind to cytoplasmic serine protease (ClpP) and induces its conformational changes, which subsequently leads to ATP-independent activation of a ClpP protease.
ADEP4-dependent ClpP activation indiscriminately breaks down cellular proteins, resulting in S. aureus persisters death.
Combination of ADEP4 with rifampicin is effective to kill S. aureus persisters and is efficacious in a deep-seated mouse thigh infection model.
Bacterial cell envelope is a promising target for killing S. aureus persisters
The bacterial cell envelope is an evolutionary verified and growth-independent target.
The SYTOX Green-persister membrane permeability screen identifies bacterial envelop targeting antimicrobial agents.
Membrane-active compounds with low membrane selectivity can be excluded by an initial Caenorhabditis elegans-based screen.
XF-73, HT61 and NH125 kills Methicillin-resistant S. aureus (MRSA) persisters by disrupting membrane lipid bilayers.
Novel strategies to augment the potentiation of conventional antibiotics
Metabolites increase proton motive force, facilitating aminoglycoside uptake of into S. aureus persisters.
Gentamicin combined with fructose effectively kills S. aureus persisters.
Tobramycin conjugated with a small peptide inducing saddle-splay membrane curvature (Pentobra) is potent against S. aureus persisters.
An anti-S. aureus antibody conjugated to rifalog effectively kills MRSA persisters formed inside host cells and highly efficacious in a murine model of bacteremia.
A kanamycin-antimicrobial peptide conjugate significantly clears intracellular MRSA.
Footnotes
Financial & competing interests disclosure
This paper was partly supported by NIH grant P01 AI083214 to Mylonakis, Eleftherios. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Gorwitz RJ, Kruszon-Moran D, McAllister SK, et al. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J. Infect. Dis. 2008;197(9):1226–1234. doi: 10.1086/533494. [DOI] [PubMed] [Google Scholar]
- 2.Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015;28(3):603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers HF, DeLeo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009;7(9):629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus . J. Clin. Invest. 2003;111(9):1265–1273. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis K. Persister cells. Annu. Rev. Microbiol. 2010;64(1):357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]; •• Provides an excellent review of the biological properties of persisters and mechanism of persister formation.
- 6.Helaine S, Kugelberg E. Bacterial persisters: formation, eradication, and experimental systems. Trends Microbiol. 2014;22(7):417–424. doi: 10.1016/j.tim.2014.03.008. [DOI] [PubMed] [Google Scholar]; • The authors review current strategies to kill persisters.
- 7.Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K. Persister cells and tolerance to antimicrobials. FEMS Microbiol. Lett. 2004;230(1):13–18. doi: 10.1016/S0378-1097(03)00856-5. [DOI] [PubMed] [Google Scholar]
- 8.Allison KR, Brynildsen MP, Collins JJ. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature. 2011;473(7346):216–220. doi: 10.1038/nature10069. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrates that metabolites make persister cells susceptible to aminoglycosides.
- 9.Conlon BP, Nakayasu ES, Fleck LE, et al. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature. 2013;503(7476):365–370. doi: 10.1038/nature12790. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrates the killing mechanism of acyldepsipeptides 4 (ADEPs) against Staphylococcus aureus persisters.
- 10.Kim W, Conery AL, Rajamuthiah R, Fuchs BB, Ausubel FM, Mylonakis E. Identification of an antimicrobial agent effective against methicillin-resistant Staphylococcus aureus persisters using a fluorescence-based screening strategy. PLoS ONE. 2015;10(6):e0127640. doi: 10.1371/journal.pone.0127640. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The authors provide a fluorescence-based strategy to identify antimicrobial agents effective against methicillin-resistant S. aureus persisters.
- 11.Kim W, Fricke N, Conery AL, et al. NH125 kills methicillin-resistant Staphylococcus aureus persisters by lipid bilayer disruption. Future Med. Chem. 2016;8(3):257–269. doi: 10.4155/fmc.15.189. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The authors identified mode of action of NH125 against methicillin-resistant S. aureus persisters.
- 12.Siegel JD, Rhinehart E, Jackson M, Chiarello L Healthcare Infection Control Practices Advisory Committee. Management of multidrug-resistant organisms in health care settings, 2006. Am. J. Infect. Control. 2007;35(10 Suppl. 2):S165–S193. doi: 10.1016/j.ajic.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 14.Fischbach MA, Walsh CT. Antibiotics for emerging pathogens. Science. 2009;325(5944):1089–1093. doi: 10.1126/science.1176667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. www.cdc.gov/drugresistance/threat-report-2013/ [Google Scholar]
- 16.Weigel LM, Clewell DB, Gill SR, et al. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus . Science. 2003;302(5650):1569–1571. doi: 10.1126/science.1090956. [DOI] [PubMed] [Google Scholar]
- 17.Lewis K. Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 2013;12(5):371–387. doi: 10.1038/nrd3975. [DOI] [PubMed] [Google Scholar]
- 18.Abraham EP, Chain E. An enzyme from bacteria able to destroy penicillin. Nature. 1940;146(3713):837–837. [PubMed] [Google Scholar]
- 19.Bigger JW. Treatment of staphylococcal infections with penicillin by intermittent sterilisation. Lancet. 1944;244(6320):497–500. [Google Scholar]
- 20.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 21.Waters EM, Rowe SE, O'Gara JP, Conlon BP. Convergence of Staphylococcus aureus persister and biofilm research: can biofilms be defined as communities of adherent persister cells? PLoS Pathog. 2016;12(12):e1006012. doi: 10.1371/journal.ppat.1006012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehar SM, Pillow T, Xu M, et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus . Nature. 2015;527(7578):323–328. doi: 10.1038/nature16057. [DOI] [PubMed] [Google Scholar]; •• The authors report an innovative strategy to kill MSRA persisters by an antibody–antibiotic conjugate.
- 23.Fisher RA, Gollan B, Helaine S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017;22:417. doi: 10.1038/nrmicro.2017.42. [DOI] [PubMed] [Google Scholar]
- 24.Khatib R, Johnson LB, Sharma M, Fakih MG, Ganga R, Riederer K. Persistent Staphylococcus aureus bacteremia: incidence and outcome trends over time. Scand. J. Infect. Dis. 2009;41(1):4–9. doi: 10.1080/00365540802441711. [DOI] [PubMed] [Google Scholar]
- 25.Chong YP, Park S-J, Kim HS, et al. Persistent Staphylococcus aureus bacteremia: a prospective analysis of risk factors, outcomes, and microbiologic and genotypic characteristics of isolates. Medicine (Baltimore) 2013;92(2):98–108. doi: 10.1097/MD.0b013e318289ff1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364(9431):369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 27.Werdan K, Dietz S, Löffler B, et al. Mechanisms of infective endocarditis: pathogen–host interaction and risk states. Nat. Rev. Cardiol. 2014;11(1):35–50. doi: 10.1038/nrcardio.2013.174. [DOI] [PubMed] [Google Scholar]
- 28.Elgharably H, Hussain ST, Shrestha NK, Blackstone EH, Pettersson GB. Current hypotheses in cardiac surgery: biofilm in infective endocarditis. Semin. Thoracic Surg. 2016;28(1):56–59. doi: 10.1053/j.semtcvs.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Raju RM, Goldberg AL, Rubin EJ. Bacterial proteolytic complexes as therapeutic targets. Nat. Rev. Drug Discov. 2012;11(10):777–789. doi: 10.1038/nrd3846. [DOI] [PubMed] [Google Scholar]
- 30.Culp E, Wright GD. Bacterial proteases, untapped antimicrobial drug targets. J. Antibiot. 2016;70(4):366–377. doi: 10.1038/ja.2016.138. [DOI] [PubMed] [Google Scholar]
- 31.Olivares AO, Baker TA, Sauer RT. Mechanistic insights into bacterial AAA+ proteases and protein-remodelling machines. Nat. Rev. Microbiol. 2016;14(1):33–44. doi: 10.1038/nrmicro.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malik IT, Brotz-Oesterhelt H. Conformational control of the bacterial Clp protease by natural product antibiotics. Nat. Prod. Rep. 2017;24:6227. doi: 10.1039/c6np00125d. [DOI] [PubMed] [Google Scholar]
- 33.Michel KH, Kastner RE. 1985. p. US4492650.
- 34.Osada H, Yano T, Koshino H, Isono K. Enopeptin A, a novel depsipeptide antibiotic with anti-bacteriophage activity. J. Antibiot. 1991;44(12):1463–1466. doi: 10.7164/antibiotics.44.1463. [DOI] [PubMed] [Google Scholar]
- 35.Brötz-Oesterhelt H, Beyer D, Kroll H-P, et al. Dysregulation of bacterial proteolytic machinery by a new class of antibiotics. Nat. Med. 2005;11(10):1082–1087. doi: 10.1038/nm1306. [DOI] [PubMed] [Google Scholar]; •• This is the first paper to report MOA of ADEPs and their in vivo efficacy.
- 36.Li DHS, Chung YS, Gloyd M, et al. Acyldepsipeptide antibiotics induce the formation of a structured axial channel in ClpP: A model for the ClpX/ClpA-bound state of ClpP. Chem. Biol. 2010;17(9):959–969. doi: 10.1016/j.chembiol.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee B-G, Park EY, Lee K-E, et al. Structures of ClpP in complex with acyldepsipeptide antibiotics reveal its activation mechanism. Nat. Struct. Mol. Biol. 2010;17(4):471–478. doi: 10.1038/nsmb.1787. [DOI] [PubMed] [Google Scholar]; • Provides structural insight on the binding of ADEPs to cytoplasmic serine protease.
- 38.Sass P, Josten M, Famulla K, et al. Antibiotic acyldepsipeptides activate ClpP peptidase to degrade the cell division protein FtsZ. Proc. Natl Acad. Sci. USA. 2011;108(42):17474–17479. doi: 10.1073/pnas.1110385108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinzen B, Raddatz S, Paulsen H, et al. Medicinal chemistry optimization of acyldepsipeptides of the enopeptin class antibiotics. ChemMedChem. 2006;1(7):689–693. doi: 10.1002/cmdc.200600055. [DOI] [PubMed] [Google Scholar]; • Reports improved ADEPs.
- 40.Carney DW, Schmitz KR, Truong JV, Sauer RT, Sello JK. Restriction of the conformational dynamics of the cyclic acyldepsipeptide antibiotics improves their antibacterial activity. J. Am. Chem. Soc. 2014;136(5):1922–1929. doi: 10.1021/ja410385c. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The authors report the further optimization of ADEP 4.
- 41.Arvanitis M, Li G, Li D-D, et al. A conformationally constrained cyclic acyldepsipeptide is highly effective in mice infected with methicillin-susceptible and -resistant Staphylococcus aureus . PLoS ONE. 2016;11(4):e0153912. doi: 10.1371/journal.pone.0153912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hurdle JG, O'Neill AJ, Chopra I, Lee RE. Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011;9(1):62–75. doi: 10.1038/nrmicro2474. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reviews membrane-active agents and their possibility for treating persistent infection.
- 43.Bastos MCF, Coutinho BG, Coelho MLV. Lysostaphin: a staphylococcal bacteriolysin with potential clinical applications. Pharmaceuticals. 2010;3(4):1139–1161. doi: 10.3390/ph3041139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borysowski J, Weber-Dabrowska B, Górski A. Bacteriophage endolysins as a novel class of antibacterial agents. Exp. Biol. Med. 2006;231(4):366–377. doi: 10.1177/153537020623100402. [DOI] [PubMed] [Google Scholar]
- 45.Gutiérrez D, Ruas-Madiedo P, Martínez B, Rodríguez A, García P. Effective removal of staphylococcal biofilms by the endolysin LysH5. PLoS ONE. 2014;9(9):e107307. doi: 10.1371/journal.pone.0107307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003;3(9):710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 47.Lu X, Wang M, Qi J, et al. Peptidoglycan recognition proteins are a new class of human bactericidal proteins. J. Biol. Chem. 2006;281(9):5895–5907. doi: 10.1074/jbc.M511631200. [DOI] [PubMed] [Google Scholar]
- 48.Zendo T, Yoneyama F, Sonomoto K. Lactococcal membrane-permeabilizing antimicrobial peptides. Appl. Microbiol. Biotechnol. 2010;88(1):1–9. doi: 10.1007/s00253-010-2764-3. [DOI] [PubMed] [Google Scholar]
- 49.Schmelcher M, Loessner MJ. Bacteriophage endolysins: applications for food safety. Curr. Opin. Biotechnol. 2016;37:76–87. doi: 10.1016/j.copbio.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 50.Mohammad H, Thangamani S, Seleem MN. Antimicrobial peptides and peptidomimetics – potent therapeutic allies for staphylococcal infections. Curr. Pharm. Des. 2015;21(16):2073–2088. doi: 10.2174/1381612821666150310102702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mishra B, Reiling S, Zarena D, Wang G. Host defense antimicrobial peptides as antibiotics: design and application strategies. Curr. Opin. Chem. Biol. 2017;38:87–96. doi: 10.1016/j.cbpa.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel S, Akhtar N. Antimicrobial peptides (AMPs): the quintessential “offense and defense” molecules are more than antimicrobials. Biomed. Pharmacother. 2017;95:1276–1283. doi: 10.1016/j.biopha.2017.09.042. [DOI] [PubMed] [Google Scholar]
- 53.Molchanova N, Hansen PR, Franzyk H. Advances in development of antimicrobial peptidomimetics as potential Drugs. Molecules. 2017;22(9):1430. doi: 10.3390/molecules22091430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ooi N, Miller K, Hobbs J, Rhys-Williams W, Love W, Chopra I. XF-73, a novel antistaphylococcal membrane-active agent with rapid bactericidal activity. J. Antimicrob. Chemother. 2009;64(4):735–740. doi: 10.1093/jac/dkp299. [DOI] [PubMed] [Google Scholar]
- 55.Ooi N, Miller K, Randall C, Rhys-Williams W, Love W, Chopra I. XF-70 and XF-73, novel antibacterial agents active against slow-growing and non-dividing cultures of Staphylococcus aureus including biofilms. J. Antimicrob. Chemother. 2010;65(1):72–78. doi: 10.1093/jac/dkp409. [DOI] [PubMed] [Google Scholar]; •• Reports the identification of a membrane-active antimicrobial, XF-73 effective against S. aureus persisters.
- 56.Fowler VG, Proctor RA. Where does a Staphylococcus aureus vaccine stand? Clin. Microbiol. Infect. 2014;20(Suppl. 5):66–75. doi: 10.1111/1469-0691.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu Y, Shamaei-Tousi A, Liu Y, Coates A. A new approach for the discovery of antibiotics by targeting non-multiplying bacteria: a novel topical antibiotic for Staphylococcal infections. PLoS ONE. 2010;5(7):e11818. doi: 10.1371/journal.pone.0011818. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reports the identification of a membrane-active antimicrobial, HT61 effective against S. aureus persisters.
- 58.Hubbard ATM, Barker R, Rehal R, Vandera K-KA, Harvey RD, Coates ARM. Mechanism of action of a membrane-active quinoline-based antimicrobial on natural and model bacterial membranes. Biochemistry. 2017;56(8):1163–1174. doi: 10.1021/acs.biochem.6b01135. [DOI] [PubMed] [Google Scholar]; • Provides molecular details in the interactions between HT61 and lipid bilayers.
- 59.Coates T, Bax R, Coates A. Nasal decolonization of Staphylococcus aureus with mupirocin: strengths, weaknesses and future prospects. J. Antimicrob. Chemother. 2009;64(1):9–15. doi: 10.1093/jac/dkp159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nandi SK, Mukherjee P, Roy S, Kundu B, De DK, Basu D. Local antibiotic delivery systems for the treatment of osteomyelitis – a review. Mater. Sci. Eng. C. 2009;29(8):2478–2485. [Google Scholar]
- 61.Moreno-Sastre M, Pastor M, Salomon CJ, Esquisabel A, Pedraz JL. Pulmonary drug delivery: a review on nanocarriers for antibacterial chemotherapy. J. Antimicrob. Chemother. 2015;70(11):2945–2955. doi: 10.1093/jac/dkv192. [DOI] [PubMed] [Google Scholar]
- 62.Pattni BS, Chupin VV, Torchilin VP. New developments in liposomal drug delivery. Chem. Rev. 2015;115(19):10938–10966. doi: 10.1021/acs.chemrev.5b00046. [DOI] [PubMed] [Google Scholar]
- 63.Torchilin VP. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2012;64:302–315. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 64.Rajamuthiah R, Fuchs BB, Jayamani E, et al. Whole animal automated platform for drug discovery against multi-drug resistant Staphylococcus aureus . PLoS ONE. 2014;9(2):e89189. doi: 10.1371/journal.pone.0089189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moy TI, Ball AR, Anklesaria Z, Casadei G, Lewis K, Ausubel FM. Identification of novel antimicrobials using a live-animal infection model. Proc. Natl Acad. Sci. USA. 2006;103(27):10414–10419. doi: 10.1073/pnas.0604055103. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This is the first report of using C. elegans-infection models for antiinfective drug discovery.
- 66.Moy TI, Conery AL, Larkins-Ford J, et al. High-throughput screen for novel antimicrobials using a whole animal infection model. ACS Chem. Biol. 2009;4(7):527–533. doi: 10.1021/cb900084v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamamoto K, Kitayama T, Ishida N, et al. Identification and characterization of a potent antibacterial agent, NH125 against drug-resistant bacteria. Biosci. Biotechnol. Biochem. 2000;64(4):919–923. doi: 10.1271/bbb.64.919. [DOI] [PubMed] [Google Scholar]; • This is the first paper to identify the activity of NH125, showing it inhibits histidine protein kinases and has antimicrobial activity.
- 68.Yamamoto K, Kitayama T, Minagawa S, et al. Antibacterial agents that inhibit histidine protein kinase YycG of Bacillus subtilis . Biosci. Biotechnol. Biochem. 2001;65(10):2306–2310. doi: 10.1271/bbb.65.2306. [DOI] [PubMed] [Google Scholar]; • The authors report inhibition of WalK (also known as YycG) by NH125.
- 69.Arora S, Yang J-M, Utsumi R, Okamoto T, Kitayama T, Hait WN. P-glycoprotein mediates resistance to histidine kinase inhibitors. Mol. Pharmacol. 2004;66(3):460–467. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y, Cheng Y, Zhang L, et al. Inhibition of eEF-2 kinase sensitizes human glioma cells to TRAIL and down-regulates Bcl-xL expression. Biochem. Biophys. Res. Commun. 2011;414(1):129–134. doi: 10.1016/j.bbrc.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu X-Y, Zhang L, Wu J, et al. Inhibition of elongation factor-2 kinase augments the antitumor activity of temozolomide against glioma. PLoS ONE. 2013;8(11):e81345. doi: 10.1371/journal.pone.0081345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu L, Huang P, Wang Z, et al. Inhibition of eEF-2 kinase sensitizes human nasopharyngeal carcinoma cells to lapatinib-induced apoptosis through the Src and Erk pathways. BMC Cancer. 2016;16(1):813. doi: 10.1186/s12885-016-2853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Autry AE, Adachi M, Nosyreva E, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bender CL, Yang Q, Sun L, Liu SJ. NH125 reduces the level of CPEB3, an RNA binding protein, to promote synaptic GluA2 expression. Neuropharmacology. 2016;101:531–537. doi: 10.1016/j.neuropharm.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walde P, Cosentino K, Engel H, Stano P. Giant vesicles: preparations and applications. ChemBioChem. 2010;11(7):848–865. doi: 10.1002/cbic.201000010. [DOI] [PubMed] [Google Scholar]; • Provides an excellent review of giant unilamellar vesicles and their application in a broad range of fields.
- 76.Kahya N. Protein-protein and protein–lipid interactions in domain-assembly: lessons from giant unilamellar vesicles. Biochim. Biophys. Acta. 2010;1798(7):1392–1398. doi: 10.1016/j.bbamem.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 77.Abouelhassan Y, Basak A, Yousaf H, Huigens RW. Identification of n-arylated NH125 analogues as rapid eradicating agents against MRSA persister cells and potent biofilm killers of Gram-positive pathogens. ChemBioChem. 2017;18(4):352–357. doi: 10.1002/cbic.201600622. [DOI] [PubMed] [Google Scholar]; • The authors demonstrate structure–activity relationship and improved activity of NH125.
- 78.Basak A, Abouelhassan Y, Zuo R, Yousaf H, Ding Y, Huigens RW. Antimicrobial peptide-inspired NH125 analogues: bacterial and fungal biofilm-eradicating agents and rapid killers of MRSA persisters. Org. Biomol. Chem. 2017;40(6):277. doi: 10.1039/c7ob01028a. [DOI] [PubMed] [Google Scholar]; • The authors report further structure–activity relationship studies on NH125.
- 79.McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 1999;12(1):147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gilbert P, Moore LE. Cationic antiseptics: diversity of action under a common epithet. J. Appl. Microbiol. 2005;99(4):703–715. doi: 10.1111/j.1365-2672.2005.02664.x. [DOI] [PubMed] [Google Scholar]
- 81.Müller G, Kramer A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J. Antimicrob. Chemother. 2008;61(6):1281–1287. doi: 10.1093/jac/dkn125. [DOI] [PubMed] [Google Scholar]
- 82.Sisignano M, Parnham MJ, Geisslinger G. Drug repurposing for the development of novel analgesics. Trends Pharmacol. Sci. 2016;37(3):172–183. doi: 10.1016/j.tips.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 83.Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004;3(8):673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 84.Pettit RK. Soil DNA libraries for anticancer drug discovery. Cancer Chemother. Pharmacol. 2004;54(1):1–6. doi: 10.1007/s00280-004-0771-8. [DOI] [PubMed] [Google Scholar]
- 85.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004;56(2):185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 86.Deans AJ, West SC. DNA interstrand crosslink repair and cancer. Nat. Rev. Cancer. 2011;11(7):467–480. doi: 10.1038/nrc3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kwan BW, Chowdhury N, Wood TK. Combatting bacterial infections by killing persister cells with mitomycin C. Environ. Microbiol. 2015;17(11):4406–4414. doi: 10.1111/1462-2920.12873. [DOI] [PubMed] [Google Scholar]; •• This is the first report to describe a DNA crosslinking anticancer drug is effective to kill persisters.
- 88.Wakaki S, Marumo H, Tomioka K, et al. Isolation of new fractions of antitumor mitomycins. Antibiot. Chemother. (Northfield) 1958;8(5):228–240. [PubMed] [Google Scholar]
- 89.Doll DC, Weiss RB, Issell BF. Mitomycin: ten years after approval for marketing. J. Clin. Oncol. 1985;3(2):276–286. doi: 10.1200/JCO.1985.3.2.276. [DOI] [PubMed] [Google Scholar]
- 90.Sharma B, Brown AV, Matluck NE, Hu LT, Lewis K. Borrelia burgdorferi, the causative agent of lyme disease, forms drug-tolerant persister cells. Antimicrob. Agents Chemother. 2015;59(8):4616–4624. doi: 10.1128/AAC.00864-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cruz-Muñiz MY, López-Jacome LE, Hernández-Durán M, et al. Repurposing the anticancer drug mitomycin C for the treatment of persistent Acinetobacter baumannii infections. Int. J. Antimicrob. Agents. 2017;49(1):88–92. doi: 10.1016/j.ijantimicag.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 92.Chowdhury N, Wood TL, Martínez Vázquez M, García Contreras R, Wood TK. DNA-crosslinker cisplatin eradicates bacterial persister cells. Biotechnol. Bioeng. 2016;113(9):1984–1992. doi: 10.1002/bit.25963. [DOI] [PubMed] [Google Scholar]; • Describes antipersister activity of a DNA crosslinking anticancer drug, cisplatin.
- 93.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer. 2007;7(8):573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 94.Li Y, Wang Z, Ma X, et al. Low-dose cisplatin administration to septic mice improves bacterial clearance and programs peritoneal macrophage polarization to M1 phenotype. Pathog. Dis. 2014;72(2):111–123. doi: 10.1111/2049-632X.12189. [DOI] [PubMed] [Google Scholar]
- 95.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2010;2(11):2490–2518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shah D, Zhang Z, Khodursky A, Kaldalu N, Kurg K, Lewis K. Persisters: a distinct physiological state of E. coli . BMC Microbiol. 2006;6(1):53. doi: 10.1186/1471-2180-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gefen O, Gabay C, Mumcuoglu M, Engel G, Balaban NQ. Single-cell protein induction dynamics reveals a period of vulnerability to antibiotics in persister bacteria. Proc. Natl Acad. Sci. USA. 2008;105(16):6145–6149. doi: 10.1073/pnas.0711712105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mates SM, Eisenberg ES, Mandel LJ, Patel L, Kaback HR, Miller MH. Membrane potential and gentamicin uptake in Staphylococcus aureus . Proc. Natl Acad. Sci. USA. 1982;79(21):6693–6697. doi: 10.1073/pnas.79.21.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fraimow HS, Greenman JB, Leviton IM, Dougherty TJ, Miller MH. Tobramycin uptake in Escherichia coli is driven by either electrical potential or ATP. J. Bacteriol. 1991;173(9):2800–2808. doi: 10.1128/jb.173.9.2800-2808.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schmidt NW, Deshayes S, Hawker S, Blacker A, Kasko AM, Wong GCL. Engineering persister-specific antibiotics with synergistic antimicrobial functions. ACS Nano. 2014;8(9):8786–8793. doi: 10.1021/nn502201a. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The authors report an innovative strategy to augment the uptake of gentamicin into persisters by conjugating with a small peptide inducing saddle-splay curvature.
- 101.Mishra A, Lai GH, Schmidt NW, et al. Translocation of HIV TAT peptide and analogues induced by multiplexed membrane and cytoskeletal interactions. Proc. Natl Acad. Sci. USA. 2011;108(41):16883–16888. doi: 10.1073/pnas.1108795108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schmidt NW, Mishra A, Lai GH, et al. Criterion for amino acid composition of defensins and antimicrobial peptides based on geometry of membrane destabilization. J. Am. Chem. Soc. 2011;133(17):6720–6727. doi: 10.1021/ja200079a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schmidt NW, Lis M, Zhao K, et al. Molecular basis for nanoscopic membrane curvature generation from quantum mechanical models and synthetic transporter sequences. J. Am. Chem. Soc. 2012;134(46):19207–19216. doi: 10.1021/ja308459j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schmidt NW, Wong GCL. Antimicrobial peptides and induced membrane curvature: geometry, coordination chemistry, and molecular engineering. Curr. Opin. Solid State Mater. Sci. 2013;17(4):151–163. doi: 10.1016/j.cossms.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garzoni C, Kelley WL. Staphylococcus aureus: new evidence for intracellular persistence. Trends Microbiol. 2009;17(2):59–65. doi: 10.1016/j.tim.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 106.Löffler B, Tuchscherr L, Niemann S, Peters G. Staphylococcus aureus persistence in non-professional phagocytes. Int. J. Med. Microbiol. 2014;304(2):170–176. doi: 10.1016/j.ijmm.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 107.Vesga O, Groeschel MC, Otten MF, Brar DW, Vann JM, Proctor RA. Staphylococcus aureus small colony variants are induced by the endothelial cell intracellular milieu. J. Infect. Dis. 1996;173(3):739–742. doi: 10.1093/infdis/173.3.739. [DOI] [PubMed] [Google Scholar]
- 108.Helaine S, Cheverton AM, Watson KG, Faure LM, Matthews SA, Holden DW. Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science. 2014;343(6167):204–208. doi: 10.1126/science.1244705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weidenmaier C, Peschel A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat. Rev. Microbiol. 2008;6(4):276–287. doi: 10.1038/nrmicro1861. [DOI] [PubMed] [Google Scholar]
- 110.Brezden A, Mohamed MF, Nepal M, et al. Dual targeting of intracellular pathogenic bacteria with a cleavable conjugate of kanamycin and an antibacterial cell-penetrating peptide. J. Am. Chem. Soc. 2016;138(34):10945–10949. doi: 10.1021/jacs.6b04831. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The authors report the development of a peptide–kanamycin conjugate effective against intracellular pathogens.
- 111.Mohamed MF, Brezden A, Mohammad H, Chmielewski J, Seleem MN. Targeting biofilms and persisters of ESKAPE pathogens with P14KanS, a kanamycin peptide conjugate. Biochim. Biophys. Acta. 2017;1861(4):848–859. doi: 10.1016/j.bbagen.2017.01.029. [DOI] [PubMed] [Google Scholar]; •• The authors report an innovative strategy to kill S. aurues persisters by a peptide–antibiotic conjugate.
- 112.Kuriakose J, Hernandez-Gordillo V, Nepal M, et al. Targeting intracellular pathogenic bacteria with unnatural proline-rich peptides: coupling antibacterial activity with macrophage penetration. Angew. Chem. Int. Ed. Engl. 2013;52(37):9664–9667. doi: 10.1002/anie.201302693. [DOI] [PubMed] [Google Scholar]