Abstract
Aim:
Gallic acid and its ester derivatives have shown antifungal activity in vitro. This study was performed to investigate their activity against Candida albicans and their toxicity in the animal models Caenorhabditis elegans and zebrafish embryos.
Results:
The compounds protected worms from C. albicans infection. The dodecyl gallate was the most effective. In zebrafish embryo, gallic acid and dodecyl gallate were the least toxic.
Conclusion:
Gallic acid and its ester derivatives have potential for in vivo use against C. albicans infection. The antifungal effects and toxicity of gallate esters in these alternative animal models were dependent on carbon chain length.
Keywords: : alternative animal models, antifungal development, gallates, gallic acid, infection
Candida albicans is still the main causative agent of invasive fungal diseases and is associated with high mortality rates (35–50%), especially in immunocompromised patients and patients undergoing high-risk surgeries. Candida infections can be superficial, such as oral or vaginal candidiasis, or systemic [1]. Unfortunately, the current repertoire of antifungal agents is limited and discovery of new antifungal drugs is slow. Furthermore, some antifungals, such as amphotericin B, may be toxic to the host, and fungal resistance to azoles has been described [2]. Thus, interest in discovery of new antifungal agents is increasing.
Gallic acid is a compound derived from the secondary metabolism of various plants. Previous studies have shown that this compound and its alkyl ester derivatives (gallates) possess antioxidant and antimicrobial activities [3,4]. Kubo et al., for example, tested the activity of three gallates against 16 microorganisms and found that octyl gallate showed antifungal activity against Saccharomyces cerevisiae, Zygosaccharomyces bailii, Candida albicans and Aspergillus niger [5]. The in vitro activity of alkyl gallates against human fungal pathogens, including Candida spp., Cryptococcus gattii, Histoplasma capsulatum and Paracoccidioides spp., has also been recently evaluated [6]. In addition, various gallates have shown activity against plant pathogenic fungi [7]. Although the in vitro antifungal activity of gallates has been investigated, little is known about the antifungal efficacy and host toxicity of these compounds in vivo.
Because of ethical reasons, high cost, new requirements for animal protection and consideration of the three Rs (Refinement, Reduction and Replacement), the use of mammals for in vivo tests has become restrictive, which creates the need for standardization of alternative models [8]. Caenorhabditis elegans has been used as an alternative to mammals to study host–pathogen interactions and novel antifungals. Furthermore, C. elegans and zebrafish models are useful for evaluating the toxicity of new drugs.
Caenorhabditis elegans is a free-living nematode found in soil, which presents a number of advantages in experimental assays. Its small size (1 mm), rapid generation and easy laboratory maintenance allow cheap and rapid production [9]. Another advantage is that its genome has been fully sequenced [10]. High genetic homology and conservation of biological functions such as the innate immune response are observed between this nematode and mammals [11]. The use of C. elegans as a host is possible, since C. elegans can consume fungi as a food source, and is susceptible to fungal infection. Studies have shown that human pathogenic fungi such as C. albicans [12], Candida krusei [13], Cryptococcus neoformans [14], H. capsulatum [15] and Penicillium marneffei [16] can lethally infect C. elegans. Additionally, C. elegans has been used to screen for potential antifungal compounds against C. albicans. One previous study examined 1,266 compounds with known pharmaceutical activities and found 15 that prolonged survival of nematodes infected with C. albicans. Two of these compounds, caffeic acid phenethyl ester and fluoroquinolone enoxacin were subsequently tested in a murine model of candidiasis and showed antifungal activity in this model [17]. Another screen was performed using a C. albicans infected C. elegans model that identified 12 saponin derivatives with antifungal activity [18]. Recently, two natural products, magnolol and honokiol have been shown to prolong survival of C. elegans infected with C. albicans [19].
Another alternative animal model is the zebrafish (Danio rerio). Among many applications, this model has been useful for assessing potential toxicities of new compounds toward developing embryos. The use of zebrafish embryos presents advantages including small size and effective compound absorption by embryos and larvae. Other advantages are that embryos are transparent, allowing visualization of their development, and one couple can generate up to 200 embryos [20]. The genome of this vertebrate has been fully sequenced, and 71.4% of zebrafish genes have human orthologs [21]. Thus, the zebrafish has been increasingly accepted as a model for predicting teratogenic effects of new drugs, nanoparticles and agrochemical agents [22].
This study was performed to investigate the activity against C. albicans and the host toxicity of gallic acid and its ester derivatives (hexyl gallate, octyl gallate and dodecyl gallate) in C. elegans. We also investigated the embryonic toxicity/teratogenicity of these compounds in zebrafish.
Materials & methods
Fungi
Candida albicans ATCC 90028 was obtained from the collection of the Clinical Mycology Laboratory, School of Pharmaceutical Sciences, UNESP, Araraquara, São Paulo, Brazil, and was maintained in Sabouraud medium at 37°C. For the experiment, C. albicans was cultivated in Sabouraud broth at 37°C with shaking. A total of 50 μl of this culture was inoculated onto brain heart infusion agar supplemented with kanamycin (90 µg/ml) and ampicillin (200 µg/ml) and incubated at 37°C for 24 h.
Drug & compounds
Amphotericin B and gallic acid (3,4,5-trihydroxybenzoic acid) were obtained commercially (Sigma-Aldrich, MO, USA). The ester derivatives (hexyl gallate, octyl gallate and dodecyl gallate) were synthesized according to previously published methods of Morais et al. [23]. Stock solutions of amphotericin B or gallic acid and its derivatives were prepared in DMSO and diluted in M9 buffer (KH2PO4; Na2HPO4; NaCl; MgSO4) for experiments with C. elegans and in embryo medium (NaCl; KCl; CaCl2.2H2O; MgCl2.6H2O) for experiments with zebrafish to make the concentration of DMSO 1%.
C. elegans–C. albicans assay
The C. elegans AU37 (glp-4;sek-1) strain, obtained from the Caenorhabditis Genetics Center, was grown on nematode growth medium agar plates, fed with Escherichia coli OP50 and incubated at 15°C according to standard procedures [24]. For experiments, worms were synchronized by treatment with sodium hypochlorite. Stage L4 worms were added to the center of each plate with an inoculum of C. albicans to induce infection. Plates were incubated at 25°C for 3 h. Worms were then washed with M9 buffer, and about 20 worms each were added to wells of 96-well plates containing 60% M9 buffer, 40% brain heart infusion, 10 µg/ml cholesterol in ethanol, 90 μg/ml kanamycin and 200 mg/ml ampicillin. Different concentrations of the control drug amphotericin B (0.1; 0.5; 1 and 5 µg/ml), or gallic acid and hexyl, octyl and dodecyl gallates (0.1; 1; 5; 10; 15; 30; 60 and 120 µg/ml) were added to the wells. The plates were maintained at 25°C, and worm survival was assessed daily for 4 days (at ∼24 h intervals), based on their mobility and their shape (rod shaped worms were considered dead and sinusoidal worms were considered alive). On the fourth day postinfection, worms were stained with SYTOX® Green (Invitrogen, CA, USA) at a final concentration of 1 µM and were incubated for 15 min at room temperature in a horizontal shaker at 120 r.p.m. Images were captured in the automated microscope IN Cell Analyzer 2000 (GE Healthcare, Buckinghamshire, UK). Three independent experiments were performed with a total of about 60 worms per tested concentration.
Zebrafish embryo assay
Wild-type zebrafish (Danio rerio) were maintained in a temperature-controlled aquarium (28 ± 0.5°C) with a 14 h light/10 h dark cycle. The housing and breeding of the fish used in this study received appropriate national and institutional approvals (Conselho Nacional de Controle de Experimentação Animal – CONCEA and Faculdade de Ciências Farmacêuticas, permit number 01.0082.2014) and were in accordance with the EU Directive 2010/63/EU. Adult zebrafish (male/female ratio of 1:1 or 1:2 or 2:1) were set up for each mating and embryos were collected from the bottom of the tank. Embryos were washed with embryo medium plus 0.00003% methylene blue and placed into Petri dishes. Those with normal fertilization were selected for testing. Embryos were transferred to 96-well plates (2 embryos per well) in 100 µl embryo medium per well. Gallic acid and hexyl, octyl and dodecyl gallates (100 µl) were added to achieve concentrations of 0.1; 1; 5; 10; 15; 30; 60 and 120 µg/ml. Compound exposure was initiated at 2–3 h post-fertilization (hpf). Plates were maintained at 28°C and were observed for phenotypes of malformation at 5, 24 and 48 hpf. Embryos did not reach free-feeding stage during this time. The effect of compounds on the development of embryos was evaluated using an inverted microscope, and images were captured in the automated microscope IN Cell Analyzer 2000 (GE Healthcare). Values for the 50% lethal concentration (LC50) were calculated. Three independent experiments were performed, with a total of 24 embryos per tested concentration.
Statistical analysis
Statistical analyses were carried out using GraphPad Prism 5 (GraphPad Software Inc., CA, USA). Survival curves for C. elegans worms and zebrafish embryos were plotted using the Kaplan–Meier method and survival differences were analyzed by log-rank (Mantel–Cox). A p-value of <0.05 was considered statistically significant.
Results
Efficacy & toxicity of compounds in the C. elegans–C. albicans model
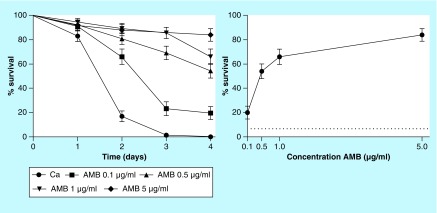
Worms of C. elegans strain AU37 were placed in contact with C. albicans for 3 h and subsequently treated with various concentrations of amphotericin B (control), gallic acid and hexyl, octyl and dodecyl gallates. Survival curves indicated that most of the worms infected with C. albicans died on the second day. The solvent DMSO at 1% showed no toxicity to the worms. All concentrations of amphotericin B significantly increased the survival rate of worms in a concentration-dependent manner (p < 0.0001 in all cases). The percent survival of C. albicans infected worms on the fourth day was 20, 54, 66 and 84% for groups treated with amphotericin B at 0.1, 0.5, 1 and 5 µg/ml, respectively (Figure 1).
Figure 1. . Survival curve and concentration response of amphotericin B from the Caenorhabditis elegans–Candida albicans model.
The graph represents combined data expressed as mean and standard error mean from three independent experiments. Survival curves were plotted using the Kaplan–Meier method, and survival curves were compared by log-rank test. All concentrations of amphotericin B significantly increased the survival rate of worms (p < 0.0001).
AMB: Amphotericin B.
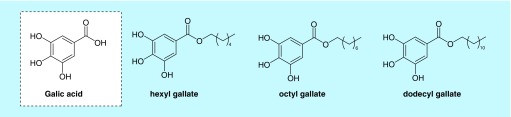
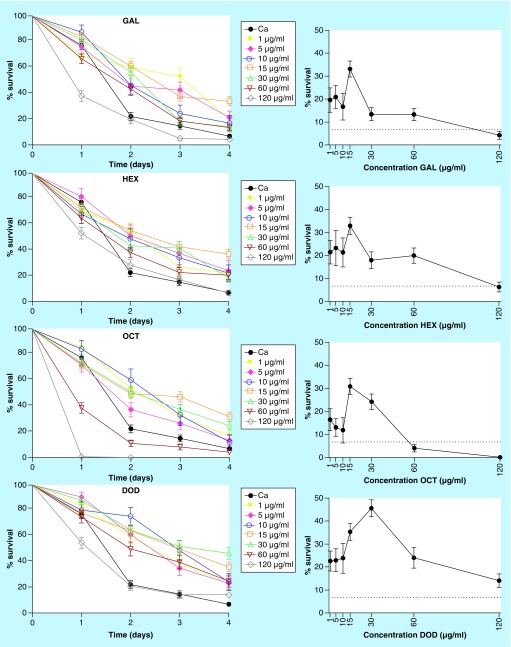
Gallic acid and its ester derivatives were selected for their in vitro antifungal activity [6]. The chemical structures of these compounds are shown in Figure 2. Treatment with the lowest concentration (0.01 µg/ml) of all compounds was not protective against C. elegans infected with C. albicans (data not shown). However, at higher concentrations (1, 5, 10, 15, 30 and 60 µg/ml) gallic acid and its ester derivatives significantly increased survival rates of C. elegans infected with C. albicans (p < 0.05 in all cases). At the end of 4 days, gallic acid, hexyl gallate, octyl gallate and dodecyl gallate increased the survival of worms by 13–33, 18–33, 12–31 and 14–46% at tested concentrations, respectively. Dodecyl gallate was determined to be the most effective compound to protect the worms from fungal infection (Figure 3).
Figure 2. . Chemical structures of gallic acid and its ester derivatives.
Figure 3. . Survival curves and concentration responses of gallic acid, hexyl gallate, octyl gallate and dodecyl gallate from the Caenorhabditis elegans–Candida albicans model.
The graph represents combined data expressed as mean and standard error mean from three independent experiments. Survival curves were plotted using the Kaplan–Meier method. Survival curves were compared with log-rank test. The group of infected but untreated worms is represented by the black line, and the groups treated with different concentrations of the compounds are represented by the colored lines. The gallic acid and its ester derivatives (1–60 µg/ml) significantly increased survival of Caenorhabditis elegans infected with Candida albicans (p < 0.05 in all cases), but compounds became toxic at 120 µg/ml.
DOD: Dodecyl gallate; GAL: Gallic acid; HEX: Hexyl gallate; OCT: Octyl gallate.
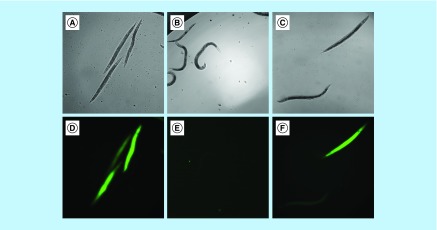
Another point to be considered when analyzing concentration response curves is the toxicity of the compounds. A reduction of larval survival rates at higher concentrations of a tested compound may mean that it is toxic at these concentrations. In the present work, nematode survival was diminished at higher concentrations (60 and 120 µg/ml) for all compounds, but octyl gallate was the most toxic to the worms (Figure 3). Viable and dead worms are shown in Figure 4, and staining with SYTOX Green (Invitrogen) helped distinguish worms killed by infection or treated with drug and compounds.
Figure 4. . Viable and dead Caenorhabditis elegans worms in bright field images (above) and dead worms stained with SYTOX® Green in fluorescence images (below).
Infected and untreated worms (A & D); infected worms treated with amphotericin B at 5 μg/ml (B & E) and infected worms treated with dodecyl gallate at 30 μg/ml (C & F). Images were taken on the fourth day postinfection. Magnification: ×10; Scale bar: 100 µm.
Toxicity of compounds in the zebrafish model
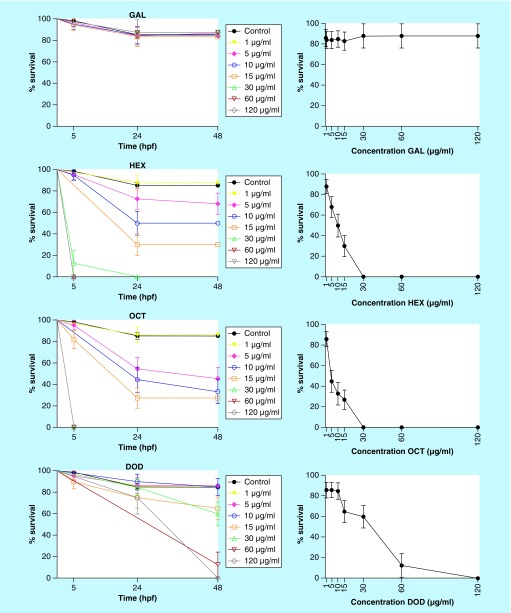
Gallic acid and its derivatives were tested at the same concentrations used in the C. elegans–C. albicans model (0.1, 1, 5, 10, 15, 30, 60 and 120 µg/ml). Based on the percentage of embryos affected at each concentration of each compound, survival curves were plotted (Figure 5). Moreover, LC50 values for embryotoxic effects or lethality were calculated from concentration–response curves plotted at 48 hpf.
Figure 5. . Survival curves and concentration responses for gallic acid, hexyl gallate, octyl gallate and dodecyl gallate from the zebrafish model.
The graph represents combined data expressed as mean and standard error mean from three independent experiments. Survival curves were plotted using the Kaplan–Meier method, and survival curves were compared by log-rank test. The group of untreated embryos is represented by the black line and the different concentrations of compounds are represented by the colored lines. Gallic acid was not toxic to the embryos at all concentrations tested, and gallates were toxic in a concentration-dependent manner. The compounds significantly reduced the survival rate of embryos at concentrations ≥5 μg/ml compared with control (p < 0.05) for hexyl and octyl gallates, and ≥15 μg/ml compared with control (p < 0.05) for dodecyl gallate.
DOD: Dodecyl gallate; GAL: Gallic acid; HEX: Hexyl gallate; OCT: Octyl gallate.
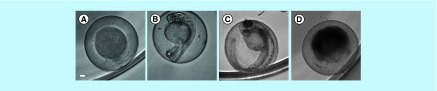
The solvent DMSO at 1% showed no toxic effect in this model. Gallic acid had no toxic effect on zebrafish embryos at up to 120 µg/ml. Due to the lack of toxicity for gallic acid in the range tested, LC50 value can be considered to be >120 µg/ml. For other compounds tested, the number of embryos affected was concentration-dependent. Hexyl gallate and octyl gallate had no toxic effect on zebrafish embryos at up to 1 µg/ml, and presented LC50 values of 10 and 4.5 µg/ml, respectively. Dodecyl gallate had no toxic effect on zebrafish embryos at up to 10 µg/ml, and presented an LC50 of 40 µg/ml. Thus, gallic acid and dodecyl gallate had less embryotoxicity than hexyl and octyl gallates. The effect of the compounds was evaluated at 5 hpf in the gastrula stage and at 24 and 48 hpf. At these times, the parameters recommended by the Organization for Economic Cooperation and Development [25] were quantitated: coagulation of fertilized eggs, lack of somite formation, lack of tail detachment and lack of heart beats. In these assays, the only indicator of toxicity observed was the coagulation of fertilized eggs phenomenon (presented as a black nontransparent ovum), an indicator of lethal toxicity when in contact with toxic concentrations of gallates (Figure 6). The stage of embryonic development that was most sensitive to exposure to the compounds was that at 24 hpf, probably because it is the phase where there is extensive cellular proliferation and organogenesis.
Figure 6. . Representative images of zebrafish embryonic development.
The control group with normal development at 5 hpf (A), 24 hpf (B) and 48 hpf (C), respectively. Example of malformation (coagulation) caused by octyl gallate at 15 μg/ml exposure (D). Magnification: 10×; Scale bar: 100 µm.
Hpf: Hours post fertilization.
Discussion
Candida albicans is the most common fungal pathogen, and development of new antifungal agents is crucial. In this study, we assess the potential of gallic acid and its ester derivatives with different carbon chain lengths (hexyl gallate, octyl gallate and dodecyl gallate) to increase the survival of C. elegans infected with C. albicans. In previous studies, these compounds demonstrated excellent in vitro antifungal activity, including against C. albicans [5,6]. Other studies have demonstrated that gallates had a synergistic effect with imidazole, itraconazole and fluconazole, reducing the MIC of these drugs against C. albicans [26,27]. Fujita and Kubo suggested that gallates are capable of damaging the fungal cell membrane, which gives them their fungicidal effect [28]. The mechanism of action of dodecyl gallate has also been related to its inhibition of oxygen consumption and NADH oxidase in the membrane of Pseudomonas aeruginosa [29].
The nematode C. elegans has been used to evaluate virulence of various fungi [12–16]. Moreover, previous studies have used C. elegans to evaluate conventional and novel antifungal compounds against Candida species [13,17–19]. In this regard, correlation was observed between antifungal activity in C. elegans and in a murine model of candidiasis [17]. We used the glp-4/sek-1 double mutant strain AU37 for analysis of C. elegans survival curves with C. albicans. This strain has been very useful in studies of interaction between C. elegans and fungi, because the glp-4 mutation makes worms unable to reproduce at 25°C, and the sek-1 mutation increases sensitivity to various pathogens [30].
Using the C. elegans infection model, we characterized the antifungal activity of gallic acid and its esters in vivo. All compounds significantly protected C. elegans from C. albicans infection at concentrations above 1 µg/ml. Additionally, dodecyl gallate increased the worms’ survival by 46% at 30 µg/ml, and was the most effective compound. Previous studies showed that alkyl esters had more favorable pharmacological properties than those observed for gallic acid [3,31], which have been correlated to the amphipathic character of these derivatives. The hydrophobic moiety seems to contribute to activity, presumably by increasing affinity for cell membranes and cell permeability [32]. Furthermore, an increase in carbon chain length of the gallates resulted in increased in vitro antifungal activity. The MIC for C. albicans ATCC 90028 of both gallic acid and hexyl gallates was 62.5 µg/ml, whereas the MIC of octyl gallate and dodecyl gallate was 8 and 2 µg/ml, respectively [5,6]. Thus, the in vivo anticandidal profiles of gallic acid and its esters in C. elegans correlated very well with their observed in vitro activity.
Some studies have reported that toxicity can be observed concomitantly with antifungal efficacy in the C. elegans–C. albicans model [17,30]. At high concentrations (60 and 120 µg/ml), it appears that gallic acid and gallates are toxic to the nematode. Octyl gallate was the most toxic compound.
Evaluation of developmental and reproductive toxicity is also important in drug discovery. For this purpose, the zebrafish has been used as a rapid, easy and efficient animal model, which combines the biological complexity of vertebrate systems with the possibility of high-throughput screening, and can detect potential teratogenic effects of new drugs [33,34]. Moreover, studies have demonstrated that zebrafish responses to compound toxicities are predictive of mammalian responses [34–36]. In addition, zebrafish, especially transgenic lines, have emerged as a useful model for evaluating mechanisms of specific diseases such as cancer and infection. In this respect, the transparency of zebrafish embryos and the use of fluorescent reporter techniques allow observation of tumor formation or microorganism colonization and immune system action. In addition to providing detailed understanding of the mechanisms of a disease, zebrafish could be an in vivo drug screening model to identify targets and determine efficacy, contributing to development of preclinical agents [37–39].
Taking advantage of the zebrafish model, we evaluated the effects of gallic acid and its esters in embryo assays. Our results demonstrated that gallic acid has no toxic effect on zebrafish embryos at all concentrations tested (up to 120 µg/ml). However, gallates demonstrated clear concentration-dependent toxicities that were significantly influenced by their carbon chain length. Gallate with eight carbon atoms in the side chain (octyl gallate) was more toxic than gallate with six carbon atoms (hexyl gallate) in zebrafish. However, when the alkyl chain was longer (dodecyl gallate), the ester compound was less toxic, suggesting a cut-off phenomenon for chain length [5,6]. The LC50 of dodecyl gallate was 40 µg/ml, which is 20× higher than its MIC against C. albicans (2 µg/ml), providing a good therapeutic window.
The C. elegans–C. albicans and zebrafish embryo assays are shown to be suitable as screening tool intermediates between preliminary in vitro evaluation and more conclusive analyses in mice and rats. The observed antifungal and toxic effects of gallates in alternative animal models depend on carbon chain length. Dodecyl gallate demonstrated good C. elegans protection by inhibiting C. albicans growth. Moreover, the toxic concentrations in nematode and zebrafish were much higher than the MIC value. Thus, dodecyl gallate should be a potential anticandidal compound in vivo.
Future perspective
There are good evidences supporting alternative animal models as useful, facile and inexpensive models to evaluate toxicity and efficacy of antifungals. Furthermore, they can reduce the number of mammals needed for in vivo experimentation. In C. elegans and zebrafish models, dodecyl gallate was potent against C. albicans and showed low embryotoxicity. Thus, the compound can be considered a candidate antifungal agent.
Executive summary.
Candidiasis
Candida albicans is the major cause of invasive fungal disease.
Antifungal agents
The repertoire of antifungal agents is limited; toxic effects on the host and selection of resistant strains are reported. Thus, interest in discovery of new antifungal agents is increasing.
Antifungal activity of gallic acid and its ester derivatives was confirmed in several in vitro studies.
Alternative animal models
Caenorhabditis elegans and zebrafish (Danio rerio) have been useful, rapid and cheap models to study fungal infection and drug efficacy and toxicity.
In vivo effect of gallic acid and its esters derivatives
The carbon chain length of gallates is responsible for the in vivo antifungal activity and toxicity of the compound, in which dodecyl gallate appears to be the most interesting compound.
Footnotes
Financial & competing interests disclosure
We thank the Caenorhabditis Genetics Center (CGC), which is funded by the NIH Office of Research Infrastructure Programs, for providing C. elegans strain AU37. This work was supported by Conselho Nacional de Pesquisa e Desenvolvimento (CNPq), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) [2015/03700–9, 2014/10446–9 and 2013/10917–9], Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Programa de Apoio ao Desenvolvimento Científico da Faculdade de Ciências Farmacêuticas da UNESP (PADC/FCF). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Cui J, Ren B, Tong Y, Dai H, Zhang L. Synergistic combinations of antifungals and anti-virulence agents to fight against Candida albicans . Virulence. 2015;6(4):362–371. doi: 10.1080/21505594.2015.1039885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kauffman CA. Fungal infections. Proc. Am. Thorac. Soc. 2006;3(1):35–40. doi: 10.1513/pats.200510-110JH. [DOI] [PubMed] [Google Scholar]
- 3.Savi LA, Leal PC, Vieira TO, et al. Evaluation of anti-herpetic and antioxidant activities, and cytotoxic and genotoxic effects of synthetic alkyl-esters of gallic acid. Arzneimittelforschung. 2005;55(1):66–75. doi: 10.1055/s-0031-1296825. [DOI] [PubMed] [Google Scholar]
- 4.Król E, De Sousa Borges A, Da Silva I, et al. Antibacterial activity of alkyl gallates is a combination of direct targeting of FtsZ and permeabilization of bacterial membranes. Front. Microbiol. 2015;6:390. doi: 10.3389/fmicb.2015.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubo I, Xiao P, Fujita K. Antifungal activity of octyl gallate: structural criteria and mode of action. Bioorg. Med. Chem. Lett. 2001;11(3):347–350. doi: 10.1016/s0960-894x(00)00656-9. [DOI] [PubMed] [Google Scholar]; • Presents the potential of gallates toward a broad spectrum of fungi.
- 6.De Paula E, Silva AC, Costa-Orlandi CB, Gullo FP, et al. Antifungal activity of decyl gallate against several species of pathogenic fungi. Evid. Based Complement. Alternat. Med. 2014;2014:506273. doi: 10.1155/2014/506273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito S, Nakagawa Y, Yazawa S, Sasaki Y, Yajima S. Antifungal activity of alkyl gallates against plant pathogenic fungi. Bioorg. Med. Chem. Lett. 2014;24(7):1812–1814. doi: 10.1016/j.bmcl.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Hartung T. Lessons learned from alternative methods and their validation for a new toxicology in the 21st century. J. Toxicol. Environ. Health. B Crit. Rev. 2010;13(2–4):277–290. doi: 10.1080/10937404.2010.483945. [DOI] [PubMed] [Google Scholar]
- 9.Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 2006;5(5):387–398. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- 10.Consortium CES. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282(5396):2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- 11.Millet AC, Ewbank JJ. Immunity in Caenorhabditis elegans . Curr. Opin. Immunol. 2004;16(1):4–9. doi: 10.1016/j.coi.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Pukkila-Worley R, Peleg AY, Tampakakis E, Mylonakis E. Candida albicans hyphal formation and virulence assessed using a Caenorhabditis elegans infection model. Eukaryot. Cell. 2009;8(11):1750–1758. doi: 10.1128/EC.00163-09. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Presents the use of invertebrate models to evaluate the fungi virulence and the efficacy of antifungal agents.
- 13.Scorzoni L, De Lucas MP, Mesa-Arango AC, et al. Antifungal efficacy during Candida krusei infection in non-conventional models correlates with the yeast in vitro susceptibility profile. PLoS ONE. 2013;8(3):e60047. doi: 10.1371/journal.pone.0060047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mylonakis E, Ausubel FM, Perfect JR, Heitman J, Calderwood SB. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc. Natl Acad. Sci. USA. 2002;99(24):15675–15680. doi: 10.1073/pnas.232568599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson CH, Ayyadevara S, Mcewen JE, Shmookler Reis RJ. Histoplasma capsulatum and Caenorhabditis elegans: a simple nematode model for an innate immune response to fungal infection. Med. Mycol. 2009;47(8):808–813. doi: 10.3109/13693780802660532. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Li D, Xi L, Mylonakis E. Caenorhabditis elegans: a simple nematode infection model for Penicillium marneffei . PLoS ONE. 2014;9(9):e108764. doi: 10.1371/journal.pone.0108764. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Presents the use of Caenorhabditis elegans as an alternative model to evaluate the efficacy of antifungal compounds.
- 17.Breger J, Fuchs BB, Aperis G, Moy TI, Ausubel FM, Mylonakis E. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 2007;3(2):e18. doi: 10.1371/journal.ppat.0030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman JJ, Okoli I, Tegos GP, et al. Characterization of plant-derived saponin natural products against Candida albicans . ACS Chem. Biol. 2010;5(3):321–332. doi: 10.1021/cb900243b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun L, Liao K, Wang D. Effects of magnolol and honokiol on adhesion, yeast-hyphal transition, and formation of biofilm by Candida albicans . PLoS ONE. 2015;10(2):e0117695. doi: 10.1371/journal.pone.0117695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat. Rev. Genet. 2007;8(5):353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- 21.Howe K, Clark MD, Torroja CF, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Discusses the use of zebrafish as an alternative model to evaluate the drug toxicity.
- 22.Kanungo J, Cuevas E, Ali SF, Paule MG. Zebrafish model in drug safety assessment. Curr. Pharm. Des. 2014;20(34):5416–5429. doi: 10.2174/1381612820666140205145658. [DOI] [PubMed] [Google Scholar]
- 23.Morais MC, Luqman S, Kondratyuk TP, et al. Suppression of TNF-α induced NFκB activity by gallic acid and its semi-synthetic esters: possible role in cancer chemoprevention. Nat. Prod. Res. 2010;24(18):1758–1765. doi: 10.1080/14786410903335232. [DOI] [PubMed] [Google Scholar]
- 24.Wood WB. The Nematode Caenorhabditis Elegans (Volume 17) The Cold Spring Harbor Laboratory; NY, USA: 1988. [Google Scholar]
- 25.Oecd. Guideline for Testing of Chemicals, 236. Fish Embryo Acute Toxicity (FET) Test. 2013. www.oecd.org/chemicalsafety/testing/36817070.pdf
- 26.D'auria FD, Tecca M, Strippoli R, Simonetti N. In vitro activity of propyl gallate-azole drug combination against fluconazole- and itraconazole-resistant Candida albicans strains. Lett. Appl. Microbiol. 2001;32(4):220–223. doi: 10.1046/j.1472-765x.2001.00893.x. [DOI] [PubMed] [Google Scholar]
- 27.Strippoli V, Dauria FD, Tecca M, Callari A, Simonetti G. Propyl gallate increases in vitro antifungal imidazole activity against Candida albicans . Int. J. Antimicrob. Agents. 2000;16(1):73–76. doi: 10.1016/s0924-8579(00)00200-4. [DOI] [PubMed] [Google Scholar]; • Discusses the mechanism of action of gallate on fungi.
- 28.Fujita K, Kubo I. Plasma membrane injury induced by nonyl gallate in Saccharomyces cerevisiae . J. Appl. Microbiol. 2002;92(6):1035–1042. doi: 10.1046/j.1365-2672.2002.01614.x. [DOI] [PubMed] [Google Scholar]
- 29.Kubo I, Fujita K, Nihei K, Masuoka N. Non-antibiotic antibacterial activity of dodecyl gallate. Bioorg. Med. Chem. 2003;11(4):573–580. doi: 10.1016/s0968-0896(02)00436-4. [DOI] [PubMed] [Google Scholar]
- 30.Okoli I, Coleman JJ, Tampakakis E, et al. Identification of antifungal compounds active against Candida albicans using an improved high-throughput Caenorhabditis elegans assay. PLoS ONE. 2009;4(9):e7025. doi: 10.1371/journal.pone.0007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Locatelli C, Rosso R, Santos-Silva MC, et al. Ester derivatives of gallic acid with potential toxicity toward L1210 leukemia cells. Bioorg. Med. Chem. 2008;16(7):3791–3799. doi: 10.1016/j.bmc.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 32.Saeki K, Yuo A, Isemura M, Abe I, Seki T, Noguchi H. Apoptosis-inducing activity of lipid derivatives of gallic acid. Biol. Pharm. Bull. 2000;23(11):1391–1394. doi: 10.1248/bpb.23.1391. [DOI] [PubMed] [Google Scholar]
- 33.Rizzo LY, Golombek SK, Mertens ME, et al. In vivo nanotoxicity testing using the zebrafish embryo assay. J. Mater. Chem. B Mater. Biol. Med. 2013;1:10. doi: 10.1039/C3TB20528B. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Argues on the use of zebrafish model in assessing the toxicity compared with conventional mammal testing.
- 34.He JH, Gao JM, Huang CJ, Li CQ. Zebrafish models for assessing developmental and reproductive toxicity. Neurotoxicol. Teratol. 2014;42:35–42. doi: 10.1016/j.ntt.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Mcgrath P, Li CQ. Zebrafish: a predictive model for assessing drug-induced toxicity. Drug Discov. Today. 2008;13(9–10):394–401. doi: 10.1016/j.drudis.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Parng C, Seng WL, Semino C, Mcgrath P. Zebrafish: a preclinical model for drug screening. Assay Drug Dev. Technol. 2002;1(1 Pt 1):41–48. doi: 10.1089/154065802761001293. [DOI] [PubMed] [Google Scholar]
- 37.Novoa B, Figueras A. Zebrafish: model for the study of inflammation and the innate immune response to infectious diseases. Adv. Exp. Med. Biol. 2012;946:253–275. doi: 10.1007/978-1-4614-0106-3_15. [DOI] [PubMed] [Google Scholar]
- 38.Xie X, Ross JL, Cowell JK, Teng Y. The promise of zebrafish as a chemical screening tool in cancer therapy. Future Med. Chem. 2015;7(11):1395–1405. doi: 10.4155/fmc.15.73. [DOI] [PubMed] [Google Scholar]
- 39.Shull AY, Hu CA, Teng Y. Zebrafish as a model to evaluate peptide-related cancer therapies. Amino Acids. 2017 doi: 10.1007/s00726-017-2388-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]