Abstract
BACKGROUND
Large artery stiffening is increased in advanced chronic kidney disease (CKD) but likely develops progressively in earlier stages of CKD. Active matrix Gla-protein (MGP) is a potent vitamin K–dependent inhibitor of vascular calcification. A recent animal model demonstrated intrinsic abnormalities in vitamin K metabolism even in early CKD, but whether early human CKD is associated with vascular vitamin K deficiency is unknown.
METHODS
We enrolled 137 adults without HF with varying degrees of renal function: normal estimated glomerular filtration rate (eGFR; >90 ml/min; n = 59), mildly reduced eGFR (stage 2 CKD: eGFR = 60–89 ml/min; n = 53) or at least moderately reduced eGFR (stage 3–5 CKD; eGFR < 60 ml/min; n = 25). Carotid-femoral pulse wave velocity (CF-PWV) was measured with carotid and femoral tonometry. Dephospho-uncarboxylated matrix gla-protein (dp-ucMGP) was measured with enzyme-linked immunosorbent assay (ELISA) (VitaK; Maastricht University; The Netherlands).
RESULT
Dp-ucMGP levels were progressively increased with decreasing renal function (eGFR ≥ 90: 247 pmol/l; eGFR 60–89: 488 pmol/l; eGFR < 60: 953 pmol/l; P < 0.0001). These differences persisted after adjustment for multiple potential confounders (eGFR ≥ 90: 314 pmol/l; eGFR 60–89: 414 pmol/l; eGFR < 60: 770 pmol/l; P < 0.0001). In a multivariable model adjusted for various confounders, dp-ucMGP was a significant independent predictor of CF-PWV (β = 0.21; P = 0.019). In formal mediation analyses, dp-ucMGP mediated a significant relationship between eGFR and higher CF-PWV (β = −0.16; P = 0.005), whereas no significant dp-ucMGP–independent relationship was present (β = −0.02; P = 0.80).
CONCLUSIONS
CKD is associated with increased (inactive) dp-ucMGP, a vitamin K–dependent inhibitor of vascular calcification, which correlates with large artery stiffness. Further studies are needed to assess whether vitamin K2 supplementation represents a suitable therapeutic strategy to prevent or reduce arterial stiffening in CKD.
Keywords: arterial stiffness, blood pressure, chronic kidney disease, hypertension, MGP, pulse wave velocity, vitamin K
Large artery stiffening is increased in advanced chronic kidney disease (CKD) and is thought to causally mediate various cardiovascular complications of CKD. Carotid-femoral pulse wave velocity (CF-PWV), the noninvasive gold-standard measure of large artery stiffness, is strongly associated with increased mortality in advanced CKD. However, large artery stiffening and calcification likely develop progressively in earlier stages of CKD. The identification of pathways related to arterial stiffness in earlier CKD stages may provide opportunities for novel therapeutic strategies to ameliorate the arterial stiffness and calcification process in CKD.
Matrix Gla-protein (MGP) is a protein produced by chondrocytes and vascular smooth muscle cells.1 The inactive form of MGP (dephospho-uncarboxylated MGP [dp-ucMGP]) undergoes serial post-translational carboxylation and phosphorylation to form active MGP. The active form of MGP is a potent inhibitor of vascular calcification. Carboxylation of MGP is dependent upon availability of vitamin K and is thus reduced in vitamin K–deficient states.1 dp-ucMGP is secreted into the circulation, and an increase in its levels indicate deficient MGP maturation/activation. Recent studies indicated that abnormal MGP maturation correlated with arterial stiffness in humans.2,3 A recent animal model demonstrated intrinsic abnormalities in vitamin K metabolism even in early CKD,4 but whether human CKD is associated with vascular vitamin K deficiency in humans, and whether this in turn relates to arterial stiffness, is unknown.
We aimed to assess the relationship between renal dysfunction and the circulating levels of dp-ucMGP (inactive MGP) and to assess the relationship between dp-ucMGP and CF-PWV, the gold-standard noninvasive measure of large artery stiffness.
METHODS
We prospectively enrolled a convenience sample of 137 adults at the Corporal Michael J. Crescenz VA Medical Center. The protocol was approved by the Philadelphia VA Medical Center Institutional Review Board, and all subjects provided written informed consent. We included subjects aged >18 years who were referred to our Department for a cardiac imaging study. Key exclusion criteria were as follows: (i) conditions that could make the measurements of CF-PWV less accurate and/or unreliable (i.e., arrhythmia such as atrial fibrillation), (ii) heart failure with reduced or preserved ejection fraction.
Subjects were stratified according to estimated glomerular filtration rate (eGFR), as follows: normal GFR (>90 ml/min; n = 59), mildly reduced GFR (stage 2 CKD: GFR = 60–89 ml/min; n = 53), or at least moderately reduced GFR (stage 3–5 CKD; GFR < 60 ml/min; n = 25). GFR was estimated using the creatinine-based CKD-Epidemiology equation.
CF-PWV measurement
CF-PWV, considered the noninvasive “gold-standard” index of large artery stiffness,5,6 was measured using the SphygmoCor system. Briefly, carotid-to-femoral transit time (ΔT) was computed from the foot-to-foot time difference between sequentially acquired carotid and femoral waveforms, using the intersecting tangents method, and the QRS complex of the ECG as a fiducial point. The distance between the sternal notch and the carotid artery was subtracted from the distance between the sternal notch and the femoral artery, in order to estimate the path length (L), and CF-PWV was computed as L/ΔT.
Plasma dp-ucMGP measurement
Citrate tubes were used for collection of venous blood sample at the time of enrollment. The plasma was prepared and stored at −80 °C for batch analysis. A dual-antibody sandwich enzyme-linked immunosorbent assay (ELISA) technique (VitaK; Maastricht University; The Netherlands) was used to measure dp-ucMGP. Intra-assay coefficients of variation for this assay have previously been reported at 3.1% and 5.4% for lower and upper limit of normal. Interassay variation coefficients are 6.9% and 13.6% for lower and upper limits of normal.2
Statistical methods
Continuous and categorical variables were compared between the groups using analysis of variance (ANOVA) and chi-square tests, respectively. Post-hoc analysis was conducted using the Bonferroni correction method. Bivariate and multivariate linear regression models were utilized to assess dp-ucMGP as a predictor of CF-PWV. When required, Box-Cox transformation was applied to normalize regression model residuals. We present standardized regression coefficients for easier comparison of the magnitude of the effect of various predictors on the dependent variable in regression models. All tests were 2-tailed. Statistical significance was defined as an alpha value ≤0.05.
We also performed formal statistical mediation analyses7 when appropriate to assess whether dp-ucMGP mediates a relationship between eGFR and CF-PWV. Mediating variables are “intermediate” factors that act as a link between a dependent variable and an independent variable. Mediation analyses quantify direct and indirect effect sizes that contribute to an observed relationship between the independent variable (in this case, eGFR) and a dependent variable (in this case, CF-PWV) and examine the role of the potential statistical mediator (in this case, dp-ucMGP).7 Estimates of the total, direct, and indirect effect size were computed. Significant mediation is established when the indirect effect is significantly different from zero. Standardized regression coefficients and effect sizes are presented for easier comparison of the magnitude of the relationships of different predictors. All probability values are 2-tailed. Statistical significance was defined as a 2-tailed P value <0.05. We used SPSS v24 for Mac (IBM, Chicago, IL), the Statistics and Machine Learning Toolbox and the M3 mediation toolbox8,9 within Matlab v2016b (The Mathworks; Natick, MA).
RESULTS
Table 1 shows the general characteristics of the study samples stratified by eGFR. The majority of the sample was composed of males. Subjects with eGFR between 60 and 89 and <60 ml/min/1.73 m2 were significantly older than those with eGFR ≥90 ml/min/1.73 m2. African Americans comprised more than half of the group with eGFR >90 ml/min/1.73 m2 but only one-third of the groups with eGFRs of 60–89 ml/min/1.73 m2 and <60 ml/min/1.73 m2. Subjects with eGFR <60 ml/min/1.73 m2 had the highest prevalence of comorbidities such as hypertension (92%) and diabetes mellitus (68%). Furosemide, aspirin, beta blocker, and warfarin were more commonly used by subjects with eGFR <90 ml/min/1.73 m2. There was a trend for increasing CF-PWV with decreasing kidney function. Magnesium levels were lower in subjects with eGFR <60 compared to those with eGFR ≥90 ml/min/1.73 m2, with a trend for lower calcium and higher phosphate levels with decreasing eGFR.
Table 1.
General characteristics of study population
| eGFR < 60 ml/min/1.73 m2 | eGFR = 60–89 ml/min/1.73 m2 | eGFR ≥90 ml/min/1.73 m2 | ||
|---|---|---|---|---|
| n = 25 | n = 53 | n = 59 | P value | |
| Age, years | 64.6 (58.6–70.5) | 62.3 (58.4–66.1) | 55.2 (51.9–58.4) | 0.003a,b |
| Body mass index, kg/m2 | 29.2 (26.9–31.5) | 29.4 (27.8–30.9) | 28.5 (27.1–29.9) | 0.66 |
| Race/ethnicity | ||||
| White | 17 (68.00%) | 37 (69.81%) | 24 (40.68%) | 0.004 |
| African American | 7 (28.00%) | 15 (28.30%) | 33 (55.93%) | |
| Other race | 1 (4.00%) | 1 (1.89%) | 2 (3.39%) | |
| Male sex | 23 (92.00%) | 53 (100.00%) | 53 (89.83%) | 0.064 |
| Hypertension | 23 (92.00%) | 39 (73.58%) | 37 (62.71%) | 0.023 |
| Coronary artery disease | 8 (32.00%) | 11 (20.75%) | 9 (15.25%) | 0.22 |
| Diabetes mellitus | 17 (68.00%) | 18 (33.96%) | 24 (40.68%) | 0.016 |
| Brachial SBP, mm Hg | 146 (138–154) | 144 (139–150) | 142 (137–147) | 0.63 |
| Carotid SBP, mm Hg | 134 (125–142) | 133 (128–139) | 133 (128–138) | 0.99 |
| Diastolic blood pressure, mm Hg | 78.8 (73.8–83.8) | 81.6 (78.1–85.1) | 85.1 (81.9–88.3) | 0.09 |
| Mean artery pressure, mm Hg | 100 (95–106) | 102 (99–106) | 104 (101–107) | 0.47 |
| Carotid pulse pressure, mm Hg | 54 (47.1–60.8) | 50.7 (46.4–55.1) | 47.4 (43.5–51.2) | 0.17 |
| Carotid-radial PWV, mm Hg | 8.9 (8–9.7) | 9.8 (9.2–10.4) | 10 (9.4–10.5) | 0.11 |
| Carotid-femoral PWV, m/s | 10.5 (9.1–11.9) | 9.7 (8.8–10.6) | 8.9 (8.2–9.7) | 0.09 |
| LV ejection fraction, % | 60.9 (56–65.7) | 57.8 (54.5–61.2) | 58.7 (55.5–61.9) | 0.61 |
| Serum calcium | 7.17 (5.45–8.88) | 8.27 (6.98–9.55) | 9.36 (7.96–10.76) | 0.11 |
| Serum phosphate | 3.49 (3.25–3.74) | 3.21 (3.06–3.36) | 3.37 (3.22–3.53) | 0.10 |
| Serum magnesium | 1.7 (1.49–1.9) | 1.92 (1.76–2.07) | 2.03 (1.87–2.18) | 0.043a |
| Medication use | ||||
| Beta blockers | 13 (52.00%) | 27 (50.94%) | 16 (27.12%) | 0.017 |
| Aspirin | 15 (60.00%) | 32 (60.38%) | 22 (37.29%) | 0.029 |
| Clopidogrel | 3 (12.00%) | 4 (7.55%) | 3 (5.08%) | 0.54 |
| ACE inhibitors | 16 (64.00%) | 25 (47.17%) | 21 (35.59%) | 0.054 |
| ARBs | 1 (4.00%) | 5 (9.43%) | 2 (3.39%) | 0.36 |
| Statins | 20 (80.00%) | 38 (71.70%) | 29 (49.15%) | 0.0078 |
| Furosemide | 2 (8.00%) | 0 (0.00%) | 0 (0.00%) | 0.011 |
| Hydralazine | 3 (12.00%) | 2 (3.77%) | 1 (1.69%) | 0.10 |
| Warfarin | 3 (12.00%) | 5 (9.43%) | 0 (0.00%) | 0.036 |
| Calcium-channel blockers | 11 (44.00%) | 15 (28.30%) | 17 (28.81%) | 0.33 |
| Thiazide | 5 (20.00%) | 18 (34.62%) | 12 (20.34%) | 0.17 |
| Insulin | 4 (16.00%) | 4 (7.55%) | 10 (16.95%) | 0.30 |
| Vitamin D | 7 (28.00%) | 15 (28.30%) | 18 (30.51%) | 0.9575 |
| Sevelamer | 1 (4.00%) | 0 (0.00%) | 0 (0.00%) | 0.1047 |
| Calcium supplements | 0 (0.00%) | 0 (0.00%) | 2 (3.39%) | 0.2614 |
Numbers represent mean (95% CI) or count (percentage). Abbreviation: ACE, angiotensin converting enzyme; LV, left ventricle; PWV, pulse wave velocity; SBP, systolic blood Pressure.
a Post-hoc P < 0.05, groups 1 vs. 3.
b Post-hoc P < 0.05, groups 2 vs. 3.
Dp-ucMGP levels and renal function
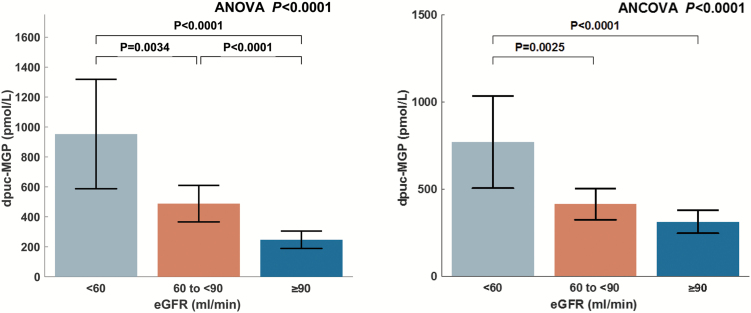
Unadjusted and adjusted comparisons of dp-ucMGP levels between subjects with normal renal function, mildly reduced eGFR, and at least moderately reduced eGFR are shown in Figure 1 and Table 2.
Figure 1.
(A) Unadjusted comparison of dp-ucMGP levels between subjects with normal renal function, mildly reduced eGFR, and at least moderately reduced eGFR; (B) Comparison adjusted for ethnicity, sex, age, presence of hypertension, diabetes mellitus, beta-blocker use, aspirin, angiotensin converting enzyme inhibitor use, furosemide use, and warfarin use.
Table 2.
Unadjusted and adjusted comparisons of dp-ucMGP levels in subjects stratified according to eGFR
| Group 1 | Group 2 | Group 3 | ||
|---|---|---|---|---|
| eGFR (ml/min) < 60 | eGFR (ml/min) = 60–89 | eGFR (ml/min) ≥ 90 | ||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | P value | |
| Unadjusted | ||||
| Dp-ucMGP, pmol/l | 953 (588–1,318) | 488 (366–610) | 247 (189–305) | <0.0001a,b,c |
| Adjusted | ||||
| Dp-ucMGP, pmol/l | 770 (505–1,034) | 414 (324–505) | 314 (247–380) | <0.0001a,b |
Abbreviations: CI, confidence interval; dp-ucMGP, dephospho-uncarboxylated MGP; eGFR, estimated glomerular filtration rate.
a Post-hoc P < 0.05, groups 1 vs. 3.
b Post-hoc P < 0.05, groups 1 vs. 2.
c Post-hoc P < 0.05, groups 2 vs. 3.
Plasma dp-ucMGP was progressively higher with decreasing renal function (GFR ≥ 90: 247 pmol/l; GFR 60–89: 488 pmol/l; GFR < 60: 953 pmol/l; P < 0.0001). In post-hoc pairwise comparisons, all 3 groups were significantly different from each other (Figure 1).
After adjustment for ethnicity, sex, age, presence of hypertension, diabetes mellitus, beta-blocker use, aspirin, angiotensin converting enzyme inhibitor use, furosemide use, and warfarin use, these differences persisted (GFR ≥ 90: 314 pmol/l; GFR 60–89: 414 pmol/l; GFR < 60: 770 pmol/l; P < 0.0001). In post-hoc pairwise comparisons, subjects with at least moderate renal function (eGFR < 60 ml/min) demonstrated significantly greater levels of dp-ucMGP compared to both subjects with mild renal dysfunction and those with normal renal function. No significant difference was found between subjects with normal renal function and those with mild renal dysfunction in this adjusted comparison (Figure 1).
Relationship between dp-ucMGP and CF-PWV
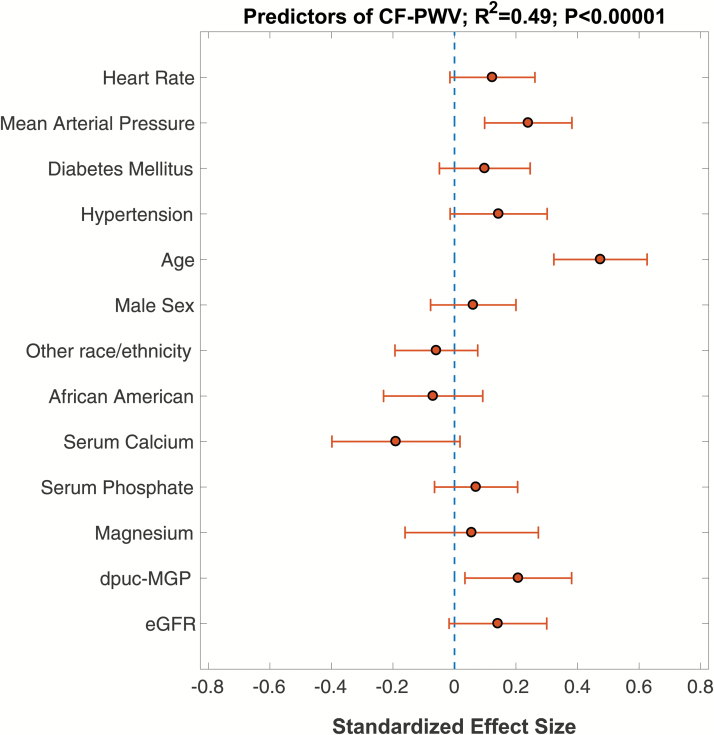
Figure 2 demonstrates the results of a multivariable linear regression in which the independent correlates of CF-PWV were assessed. In this multivariable model, dp-ucMGP was a significant independent predictor of CF-PWV (standardized β = 0.21; 95% CI = 0.03–0.38; P = 0.019). In this model, age (standardized β = 0.47; 95% CI = 0.32–0.63; P < 0.0001) and mean arterial pressure (standardized β = 0.24; 95% CI = 0.10–0.38; P = 0.001) were also significant independent predictors of CF-PWV.
Figure 2.
Multivariable model showing predictors of CF-PWV. Standardized regression coefficients and 95% confidence intervals are shown.
Dp-ucMGP, eGFR, and CF-PWV: mediation analyses
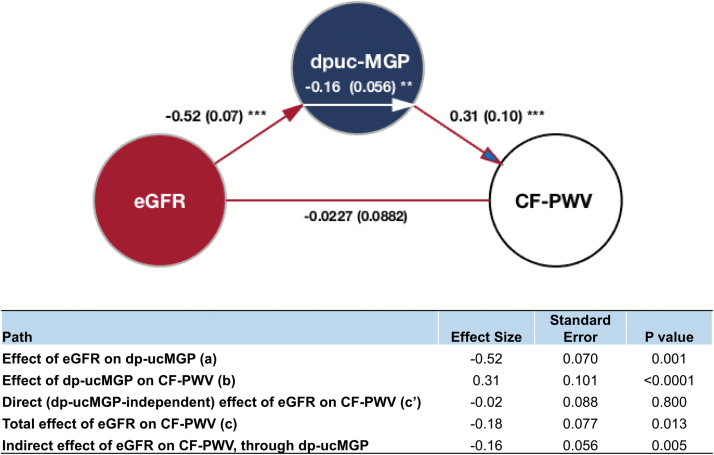
Figure 3 shows the results of formal mediation analyses in which the direct and indirect (dp-ucMGP-mediated) effects of eGFR on CF-PWV were examined. In this model, eGFR demonstrated a significant total effect on CF-PWV (standardized β = −0.18; P = 0.013). There was a significant effect of eGFR on dp-ucMGP (standardized β = −0.52; P = 0.001), as well as a significant effect of dp-ucMGP on CF-PWV (standardized β = 0.31; P < 0.0001). The direct effect of eGFR on CF-PWV, however, was nonsignificant (standardized β = −0.027; P = 0.80), whereas its indirect (dp-ucMGP dependent) effect was significant (standardized β = −0.16; P = 0.005).
Figure 3.
Mediation models examining the direct and indirect (dp-ucMGP-mediated) effects of eGFR on carotid-femoral pulse wave velocity (CF-PWV). Path a (red arrow) represents the effect of eGFR on dp-ucMGP. Path b (red arrow with blue head) represents the effect of dp-ucMGP on CF-PWV. Path c′ (red line) represents the “direct” (dp-ucMGP-independent) effect of eGFR on CF-PWV. The indirect effect of eGFR on CF-PWV (i.e., the effect mediated by dp-ucMGP) is shown as the white arrow within the blue circle. **Significant path with P < 0.01; ***significant path with P < 0.001.
Relationship between dp-ucMGP and carotid-radial PWV
In unadjusted analyses, carotid-radial PWV was negatively associated with carotid-radial PWV (standardized β = −0.21; 95% CI = −0.39 to 0.02; P = 0.03). However, after adjustment for age, sex, race, the presence of hypertension, diabetes mellitus, eGFR, mean arterial pressure, heart rate, serum magnesium, phosphorus, and calcium, dp-ucMGP was not independently associated with CR-PWV (standardized β = 0.18; 95% CI = −0.39 to 0.04; P = 0.11).
Sensitivity analysis
We performed a sensitivity analysis among subjects who were not taking warfarin. In this analysis, observed trends were very similar to the overall analysis regarding differences in dp-ucMGP levels between the 3 eGFR strata in unadjusted and multivariable analyses. Similarly, the results of mediation analyses in this subpopulation regarding the relationship between eGFR, dp-ucMGP, and CF-PWV were similar, demonstrating a significant effect of eGFR on dp-ucMGP (standardized β = −0.365; P < 0.0001), a significant effect of dp-ucMGP on CF-PWV (standardized β = 0.42; P < 0.0001), a significant dp-ucMGP–mediated effect of eGFR on CF-PWV (standardized β = −0.154; P < 0.0001), and a nonsignificant direct effect of eGFR on CF-PWV.
DISCUSSION
In this study, we investigated the association between plasma levels of dp-ucMGP, a marker of vitamin K status, renal function, and arterial stiffness. We found that lower kidney function is independently associated with greater levels of plasma dp-ucMGP. In addition, we demonstrated that greater levels of dp-ucMGP are independently associated with increased CF-PWV (a measure of large artery stiffness) but not with carotid-radial PWV (a measure of muscular artery stiffness). We further demonstrate that dp-ucMGP levels mediate a significant relationship between eGFR and higher CF-PWV, whereas no significant dp-ucMGP–independent relationship was present.
A previous study demonstrated increased levels of dp-ucMGP in patients with end-stage kidney disease, but limited data exist in earlier stages of CKD.10 In a previous study in which CKD subjects with a mean eGFR indicative of moderate CKD were included, lower levels of uncarboxylated MGP were reported to be associated with a lower eGFR.11 We hereby demonstrate that a reduced eGFR is associated with a higher level of dp-ucMGP. Since ucMGP is the intermediate product between the precursor (dp-ucMGP) and carboxylated active MGP, its levels do not provide a clear indication of either the level of dp-ucMGP or the level of active MGP. Therefore, nonphosphorylated, noncarboxylated MGP (dp-ucMGP) is now thought to be a better indicator of vascular vitamin K status, as compared with other components of the MGP system. Our findings are also consistent with a recent report that demonstrated that dp-ucMGP levels were associated with proteinuria, inversely related to eGFR and significantly higher in CKD stage 5 patients than those in stage 4.12
In the current study, we also studied the relationship between dp-ucMGP and CF-PWV (a measure of large artery stiffness) and carotid-radial PWV (a measure of medium-sized, muscular artery stiffness). We demonstrated that greater levels of dp-ucMGP were associated with higher CF-PWV but not with carotid-radial PWV. The relationship with CF-PWV persisted after adjustment for multiple confounders in multivariate analysis. Our findings are consistent with accumulating evidence regarding the important role of active MGP as an inhibitor of vascular calcification.13–15
Two recent reports demonstrated a relationship between dp-ucMGP and CF-PWV in a general population sample2 and in a population of patients with type 2 diabetes mellitus.16 However, the relationship between vitamin K status and CF-PWV in early CKD has not been previously reported. Previous studies have demonstrated that higher levels of plasma dp-ucMGP are associated with the severity of aortic calcification in patients with CKD.17,18 However, dp-ucMGP levels were not found to be associated with CF-PWV in a previous study in advanced CKD (dialysis patients).19 Only one previous study assessed the relationship between dp-ucMGP and PWV in stages 3–5 CKD.18 In this study, dp-ucMGP was not significantly related to cardio-ankle PWV (which includes the aortic path and a long muscular segment). Our study separately examined large artery (CF-PWV) and muscular artery (carotid-radial) stiffness while including both subjects with eGFR levels higher than 60 ml/min/1.73 m2 and those with eGFR levels indicative of CKD stages 3–5. Our study not only provides evidence that dp-ucMGP is independently correlated with CF-PWV but also that this relationship is selective for large artery stiffness, without an independent relationship with muscular artery stiffness. It is likely that muscular artery PWV confounded the relationship with large artery stiffness in the previous study. The factors underlying a selective association between dp-ucMGP and large artery stiffness are probably related to the well-known differences in the pathophysiologic determinants of stiffening of elastic arteries vs. muscular arteries. We note that the former, and not the latter, is independently associated with cardiovascular risk.5 Interestingly, in patients with pre-existing cardiovascular disease, circulating dp-ucMGP is associated with mortality and added cardiovascular risk.20,21 It remains to be determined whether dp-ucMGP is related to incident risk independently of CF-PWV.
Using formal statistical mediation analysis, we demonstrate, for the first time, that dp-ucMGP explains the vast majority of the underlying relationship between eGFR and CF-PWV, even after adjustment for confounders. These findings are consistent with paradigm that a poor vitamin K status, which has recently been shown to be caused by CKD in an animal model,4 leads to greater arterial calcification and stiffening. However, there may be bidirectional relationships. In a recent longitudinal study of Flemish subjects, higher circulating dp-ucMGP predicted a greater decline in kidney function.22 Given that large artery stiffness contributes to systolic hypertension, which in turn can promote CKD progression, it is possible that abnormal vitamin K status mediates a vicious circle of arterial calcification/stiffening and worsening CKD. Whether vitamin K supplementation can interrupt this process, and whether it leads to clinical benefit in early CKD, remains to be tested in properly designed clinical trials. Vitamin K2 supplementation has been shown to reduce dp-ucMGP levels, indicating that it exerts a positive effect on MGP maturation/carboxylation, consistent with its known biologic role.12,23 An interesting related issue is that warfarin, a commonly prescribed anticoagulant in CKD patients, inhibits vitamin K reductase, affecting MGP maturation, increasing dp-ucMGP levels, which may contribute to vascular calcification.
Our study should be interpreted in the context of its strengths and limitations. Strengths of our study include the inclusion of a multiethnic population of patients across the spectrum of early eGFR values, the separate assessment of large artery and muscular artery stiffness, and the use of formal mediation analyses to interrogate underlying statistical relationships. We used high-fidelity carotid and femoral tonometry for measurements of CF-PWV, which is considered the gold-standard noninvasive approach for the noninvasive assessment of large artery stiffness. Our study also has some limitations. Consistent with the demographics of patients in a VA Medical Center, our sample was composed predominantly of males. There was a relatively small number of patients with advanced CKD, and thus, our study should not be considered to address dp-ucMGP levels in advanced CKD. We did not measure vascular calcification, which would have required dedicated imaging modalities (such as computed tomography scans). Finally, this was a cross-sectional investigation which can only provide evidence of associations rather than causal inferences.
In conclusion, lower kidney function is independently associated with greater levels of plasma dp-ucMGP, a marker of poor vitamin K status. In turn, greater levels of dp-ucMGP are independently associated with large artery stiffness but not muscular artery stiffness. Finally, dp-ucMGP levels largely explain the association between a lower CKD and a greater PWV, without evidence of a dp-ucMGP-independent relationship. Our findings should stimulate further studies on dp-ucMGP and trials with vitamin K supplementation in early CKD, to assess whether this intervention can ameliorate the marked, progressive large artery stiffening that occurs with progression to end-stage renal disease.
DISCLOSURES
J.A.C. has received consulting honoraria from Bristol Myers Squibb, OPKO Healthcare, Fukuda-Denshi, Pfizer, Microsoft, Ironwood Pharmaceuticals, Sanifit, and Merck. He received research grants from National Institutes of Health, American College of Radiology Network, Fukuda Denshi, Bristol Myers Squibb, Microsoft and CVRx Inc., and device loans from Atcor Medical. He is named as inventor in a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of Heart Failure and Preserved Ejection Fraction. Other authors declared no conflict of interest.
ACKNOWLEDGMENTS
Dr Chirinos is supported by NIH grants R01 HL-121510-01A1 and R56 HL-124073-01A. J.A.C. is supported by NIH grants R01 HL-121510-01A1 and R56 HL-124073-01A.
REFERENCES
- 1. Dalmeijer GW, van der Schouw YT, Vermeer C, Magdeleyns EJ, Schurgers LJ, Beulens JW. Circulating matrix Gla protein is associated with coronary artery calcification and vitamin K status in healthy women. J Nutr Biochem 2013; 24:624–628. [DOI] [PubMed] [Google Scholar]
- 2. Pivin E, Ponte B, Pruijm M, Ackermann D, Guessous I, Ehret G, Liu YP, Drummen NE, Knapen MH, Pechere-Bertschi A, Paccaud F, Mohaupt M, Vermeer C, Staessen JA, Vogt B, Martin PY, Burnier M, Bochud M. Inactive matrix Gla-protein is associated with arterial stiffness in an adult population-based study. Hypertension 2015; 66:85–92. [DOI] [PubMed] [Google Scholar]
- 3. Mayer O Jr, Seidlerová J, Wohlfahrt P, Filipovský J, Vaněk J, Cífková R, Windrichová J, Topolčan O, Knapen MH, Drummen NE, Vermeer C. Desphospho-uncarboxylated matrix Gla protein is associated with increased aortic stiffness in a general population. J Hum Hypertens 2016; 30:418–423. [DOI] [PubMed] [Google Scholar]
- 4. McCabe KM, Booth SL, Fu X, Ward E, Adams MA, Holden RM. Vitamin K metabolism in a rat model of chronic kidney disease. Am J Nephrol 2017; 45:4–13. [DOI] [PubMed] [Google Scholar]
- 5. Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension . Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 2015; 66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 7. Hayes A. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Guilford Press: New York, 2013. [Google Scholar]
- 8. Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: part I: reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage 2009; 47:821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 2008; 59:1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cranenburg EC, Koos R, Schurgers LJ, Magdeleyns EJ, Schoonbrood TH, Landewé RB, Brandenburg VM, Bekers O, Vermeer C. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost 2010; 104:811–822. [DOI] [PubMed] [Google Scholar]
- 11. Parker BD, Ix JH, Cranenburg EC, Vermeer C, Whooley MA, Schurgers LJ. Association of kidney function and uncarboxylated matrix Gla protein: data from the Heart and Soul Study. Nephrol Dial Transplant 2009; 24:2095–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kurnatowska I, Grzelak P, Masajtis-Zagajewska A, Kaczmarska M, Stefańczyk L, Vermeer C, Maresz K, Nowicki M. Plasma desphospho-uncarboxylated matrix Gla protein as a marker of kidney damage and cardiovascular risk in advanced stage of chronic kidney disease. Kidney Blood Press Res 2016; 41:231–239. [DOI] [PubMed] [Google Scholar]
- 13. Spronk HM, Soute BA, Schurgers LJ, Cleutjens JP, Thijssen HH, De Mey JG, Vermeer C. Matrix Gla protein accumulates at the border of regions of calcification and normal tissue in the media of the arterial vessel wall. Biochem Biophys Res Commun 2001; 289:485–490. [DOI] [PubMed] [Google Scholar]
- 14. Schurgers LJ, Uitto J, Reutelingsperger CP. Vitamin K-dependent carboxylation of matrix Gla-protein: a crucial switch to control ectopic mineralization. Trends Mol Med 2013; 19:217–226. [DOI] [PubMed] [Google Scholar]
- 15. van den Heuvel EG, van Schoor NM, Lips P, Magdeleyns EJ, Deeg DJ, Vermeer C, den Heijer M. Circulating uncarboxylated matrix Gla protein, a marker of vitamin K status, as a risk factor of cardiovascular disease. Maturitas 2014; 77:137–141. [DOI] [PubMed] [Google Scholar]
- 16. Sardana M, Vasim I, Varakantam S, Kewan U, Tariq A, Koppula MR, Syed AA, Beraun M, Drummen NE, Vermeer C, Akers SR, Chirinos JA. Inactive matrix Gla-protein and arterial stiffness in type 2 diabetes mellitus. Am J Hypertens 2017; 30:196–201. [DOI] [PubMed] [Google Scholar]
- 17. Schurgers LJ, Barreto DV, Barreto FC, Liabeuf S, Renard C, Magdeleyns EJ, Vermeer C, Choukroun G, Massy ZA. The circulating inactive form of matrix Gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol 2010; 5:568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thamratnopkoon S, Susantitaphong P, Tumkosit M, Katavetin P, Tiranathanagul K, Praditpornsilpa K, Eiam-Ong S. Correlations of plasma desphosphorylated uncarboxylated matrix Gla protein with vascular calcification and vascular stiffness in chronic kidney disease. Nephron 2017; 135:167–172. [DOI] [PubMed] [Google Scholar]
- 19. Hermans MM, Vermeer C, Kooman JP, Brandenburg V, Ketteler M, Gladziwa U, Rensma PL, Leunissen KM, Schurgers LJ. Undercarboxylated matrix GLA protein levels are decreased in dialysis patients and related to parameters of calcium-phosphate metabolism and aortic augmentation index. Blood Purif 2007; 25:395–401. [DOI] [PubMed] [Google Scholar]
- 20. Mayer O Jr, Seidlerová J, Bruthans J, Filipovský J, Timoracká K, Vaněk J, Cerná L, Wohlfahrt P, Cífková R, Theuwissen E, Vermeer C. Desphospho-uncarboxylated matrix Gla-protein is associated with mortality risk in patients with chronic stable vascular disease. Atherosclerosis 2014; 235:162–168. [DOI] [PubMed] [Google Scholar]
- 21. Mayer O Jr, Seidlerová J, Vaněk J, Karnosová P, Bruthans J, Filipovský J, Wohlfahrt P, Cífková R, Windrichová J, Knapen MH, Drummen NE, Vermeer C. The abnormal status of uncarboxylated matrix Gla protein species represents an additional mortality risk in heart failure patients with vascular disease. Int J Cardiol 2016; 203:916–922. [DOI] [PubMed] [Google Scholar]
- 22. Wei FF, Trenson S, Thijs L, Huang QF, Zhang ZY, Yang WY, Moliterno P, Allegaert K, Boggia J, Janssens S, Verhamme P, Vermeer C, Staessen JA. Desphospho-uncarboxylated matrix Gla protein is a novel circulating biomarker predicting deterioration of renal function in the general population. Nephrol Dial Transplant 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schlieper G, Westenfeld R, Krüger T, Cranenburg EC, Magdeleyns EJ, Brandenburg VM, Djuric Z, Damjanovic T, Ketteler M, Vermeer C, Dimkovic N, Floege J, Schurgers LJ. Circulating nonphosphorylated carboxylated matrix Gla protein predicts survival in ESRD. J Am Soc Nephrol 2011; 22:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]