Abstract
Spinocerebellar ataxia type 1 (SCA1) is caused by the expansion of a trinucleotide repeat that encodes a polyglutamine tract in ataxin-1 (ATXN1). The expanded polyglutamine in ATXN1 increases the protein’s stability and results in its accumulation and toxicity. Previous studies have demonstrated that decreasing ATXN1 levels ameliorates SCA1 phenotypes and pathology in mouse models. We rationalized that reducing ATXN1 levels through pharmacological inhibition of its modulators could provide a therapeutic avenue for SCA1. Here, through a forward genetic screen in Drosophila we identified, p21-activated kinase 3 (Pak3) as a modulator of ATXN1 levels. Loss-of-function of fly Pak3 or Pak1, whose mammalian homologs belong to Group I of PAK proteins, reduces ATXN1 levels, and accordingly, improves disease pathology in a Drosophila model of SCA1. Knockdown of PAK1 potently reduces ATXN1 levels in mammalian cells independent of the well-characterized S776 phosphorylation site (known to stabilize ATXN1) thus revealing a novel molecular pathway that regulates ATXN1 levels. Furthermore, pharmacological inhibition of PAKs decreases ATXN1 levels in a mouse model of SCA1. To explore the potential of using PAK inhibitors in combination therapy, we combined the pharmacological inhibition of PAK with MSK1, a previously identified modulator of ATXN1, and examined their effects on ATXN1 levels. We found that inhibition of both pathways results in an additive decrease in ATXN1 levels. Together, this study identifies PAK signaling as a distinct molecular pathway that regulates ATXN1 levels and presents a promising opportunity to pursue for developing potential therapeutics for SCA1.
Introduction
Spinocerebellar ataxia type 1 (SCA1) (OMIM # 164400) is an adult-onset, autosomal dominant neurodegenerative disease, characterized by degeneration of Purkinje and brain stem neurons, progressive ataxia and premature death. SCA1 is caused by expansion of a CAG repeat in the ATAXIN-1 (ATXN1) gene that encodes a polyglutamine (polyQ) tract (1). SCA1 is one of the nine polyQ expansion disorders: Huntington’s disease, spinal-bulbar muscular atrophy, dentatorubral-pallidoluysian atrophy and six spinocerebellar ataxias (SCA1, 2, 3, 6, 7 and 17). Unfortunately, no disease-modifying treatments are currently available for SCA1 patients.
Several lines of evidence demonstrate that ATXN1 levels are crucial for SCA1 pathogenesis, and that reduction of both wild type and polyQ expanded ATXN1 levels ameliorates SCA1 disease pathology. Haploinsufficiency of an ATXN1 interactor, 14–3-3ε, reduces ATXN1 levels and rescues SCA1 pathology in mice (2). Another study reported that loss of phosphorylation at serine776 (S776) of ATXN1 by a substitution to alanine reduces the stability of ATXN1 and supresses its toxicity (3). Furthermore, an increase in wild-type ATXN1 levels promotes Purkinje cell degeneration and ataxia in mice (4,5). Moreover, partial loss-of-function (LOF) of ATXN1 in mice (50% reduction) is well-tolerated (6). Building on these findings, we focused our efforts on identifying modulators of ATXN1 levels to be used as the potential therapeutic entry point for the treatment of SCA1.
A cross-species forward genetic screen revealed that MSK1 increases ATXN1 protein stability by phosphorylating ATXN1 at S776 (7). Haploinsufficiency of MSK1 and its homolog MSK2 ameliorates SCA1 pathogenesis, providing a potentially druggable target for the treatment of SCA1. Although targeting MSK1/2 is a promising therapeutic avenue, identification of additional modulators of ATXN1 levels that function outside of the RAS-MAPK-MSK pathway may prove therapeutically beneficial for the treatment of SCA1. Successful outcomes of combination therapies in cancer, HIV and antibiotic resistance further motivated us to test such a strategy in SCA1 (8–10). We reason that potent and safe reduction in ATXN1 levels can be achieved via combination drug therapy with additive partial inhibition of multiple signaling pathways that regulate ATXN1. Combination therapy for SCA1 and many other neurodegenerative diseases is yet to be explored. Our study will provide a proof of concept of whether such a strategy is feasible for SCA1 and the broader class of neurodegenerative disorders caused by accumulation of disease-driving proteins.
p21-activated kinases (PAKs) are a family of serine/threonine kinases that regulate diverse cellular functions from actin nucleation to cell survival (11). There are six PAK homologs in humans that are divided into two groups based on their structural similarities and activation mechanisms. Group I PAKs (PAK1, PAK2 and PAK3) have an auto-inhibitory domain (AID) and Group II (PAK4, PAK5 and PAK6) are constitutively active and, in general, lack an AID domain (12,13). A recent cross-species forward genetic screen from our laboratory identified Drosophila Pak3, a mammalian homolog of Group I PAKs, as a potential ATXN1 modulator (7). In Drosophila, there are three PAKs: Pak1 and Pak3 are representative members of Group I and Pak2 is a representative member of Group II (14). All PAKs contain a functional kinase domain that is conserved within their group (80–90% amino acid identity) and between the two groups (50% amino acid identity) (15). Gain-of-function in PAK signaling is implicated in various neurological diseases including Fragile X syndrome and Huntington’s disease (16,17). For instance, a previous study reported that overexpression of PAK1 enhances the disease pathology in Huntington’s disease, a neurodegenerative disorder that is caused by a polyQ expansion in the huntingtin protein (17). However, whether mammalian PAK1 has similar modulatory effect on ATXN1 remains unexplored. Here, we aimed to characterize the role of PAK1 in regulation of ATXN1 levels and toxicity.
Results
Drosophila Pak1 and Pak3 modulate ATXN1 levels and suppress ATXN1 [82Q]-induced neurotoxicity
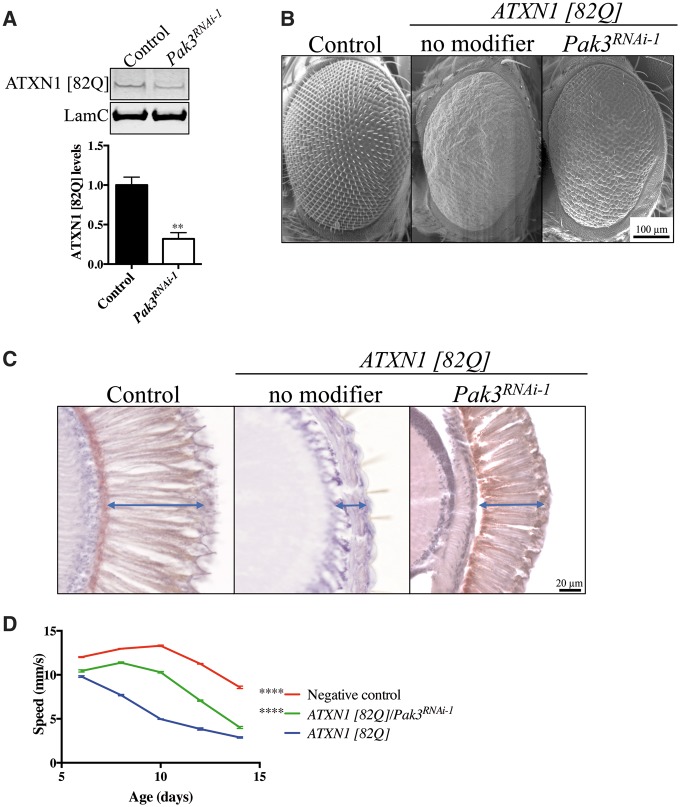
Our previous finding that Drosophila Pak3, a homolog of Group I mammalian PAKs, is a potential suppressor of ATXN1 [82Q]-induced toxicity (7) led us to investigate its modifying effect in more detail. Expression of polyQ expanded ATXN1 [82Q] in the Drosophila eye causes ommatidial abnormalities. Since the severity of this and other ATXN1 [82Q] neurotoxic phenotypes correlates with protein levels (5), we first investigated whether Pak3 regulates ATXN1 [82Q] levels in fruit flies. Immunoblot analysis of SCA1 Drosophila heads shows that decreased function of Pak3 lowers ATXN1 [82Q] protein levels (Fig. 1A and Supplementary Material, Fig. S1B).
Figure 1.
Drosophila Pak3 modulates ATXN1 levels and suppresses ATXN1 [82Q]-induced neurotoxicity. (A) Representative immunoblot image and quantification of ATXN1 [82Q] levels in Pak3RNAi-1 (Pak3KK111386) or control Drosophila eyes showing reduction of ATXN1 levels upon Pak3 knockdown. ATXN1 [82Q] levels are normalized to LamC (n = 4, t-test, **P < 0.01. Error bars denote the SEM). (B) Scanning electron microscopy images of wild-type, SCA1 and SCA1/Pak3RNAi-1 (Pak3KK111386) Drosophila eyes. Pak3 knockdown (Pak3KK111386) ameliorates ATXN1 [82Q]-induced toxicity (C) Cross-sections of the eye reveal increased thickness (i.e. less degeneration, arrow) of the retinal cell layer in SCA1 flies upon Pak3 knockdown (Pak3KK111386). (D) Improved motor performance, as assessed by increased speed, of SCA1 flies upon Pak3 knockdown (Pak3KK111386) in the nervous system (n = 30–60, non-linear ANOVA, ****P < 0.0001. Error bars denote the SEM).
Next, we thoroughly investigated whether decreasing Pak3 expression suppresses ATXN1 [82Q]-induced neurotoxicity in the eye (gmr-GAL4) using different readouts. Partial loss of Pak3 function ameliorates both the external eye and the severe retinal degeneration phenotypes caused by ATXN1 [82Q] (Fig. 1B and C and Supplementary Material, Fig. S1C). Last, targeted ATXN1 [82Q] expression to the Drosophila nervous sytem (nrv-GAL4) causes a late-onset, progressive motor impairment phenotype that can be quantified in a climbing assay (18). We used video recordings for quantitative analysis of motor performace of SCA1 fruit flies and SCA1 sibling animals carrying a variety of Pak3 knockdown alleles. In agreement with the protein level and eye neurotoxicity assays, ATXN1 [82Q] flies with either one of multiple Pak3 RNAi or LOF alleles perform significantly better than the control flies as assessed in the motor performance assay (Fig. 1D and Supplementary Material, Fig. S1D).
Since Drosophila Pak1 is also homologous to the mammalian Group I PAKs, we investigated whether it also modulates ATXN1 [82Q] levels and neurotoxicity. Like Pak3, reduced function of Pak1 lowers ATXN1 [82Q] protein levels (Supplementary Material, Fig. S1A), suppresses the retinal degeneration phenotype (Supplementary Material, Fig. S1C) and improves the motor impairments of SCA1 flies (Supplementary Material, Fig. S1D). Together, our results demonstrate that Drosophila Pak1 and Pak3 modulate ATXN1 [82Q] levels and suppress ATXN1 [82Q]-mediated neurotoxicity in Drosophila.
PAK1 regulates ATXN1 levels in mammalian cells
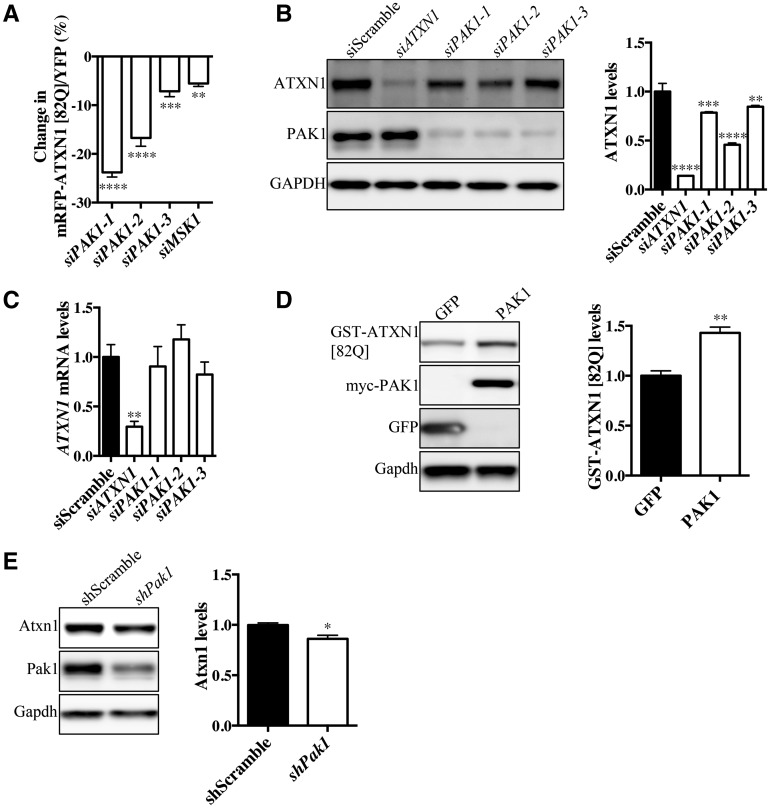
Given that Pak1 and Pak3 modulate ATXN1 [82Q] levels and ameliorate SCA1 pathology in fruit flies, we next set out to investigate whether PAK regulation of ATXN1 is conserved in the mammalian systems. Human PAK1 belongs to Group I PAKs and is closely related to the Drosophila Pak1 and Pak3. To test whether PAK1 modulates ATXN1 levels, we monitored ATXN1 levels in a human medulloblastoma-derived cell line (Daoy) containing a transgene encoding a glutamine-expanded ATXN1 [82Q] fused with a red fluorescent protein (RFP). The tagged transgene is followed by an internal ribosome entry site (IRES) and yellow fluorescent protein (YFP) (mRFP-ATXN1[82Q]-IRES-YFP) (7). Daoy cells express endogenous ATXN1 and its known interactors suggesting that ATXN1 regulatory mechanisms are conserved. In these cells, ATXN1 levels are monitored independently of its transcriptional control, by quantifying mRFP/YFP ratio via flow cytometry. Flow cytometry analyses show that siRNAs targeting PAK1 significantly reduce the mRFP-ATXN1 [82Q]/YFP ratio. In agreement with previous findings, siRNAs targeting MSK1 also decrease the mRFP-ATXN1 [82Q]/YFP ratio (Fig. 2A and Supplementary Material, Fig. S2A).
Figure 2.
PAK1 regulates ATXN1 levels in mammalian cells. (A)PAK1 knockdown using three different siRNAs in mRFP-ATXN1 [82Q]-IRES-YFP Daoy cell line reveals reduction in mRFP-ATXN1 [82Q]/YFP ratio by flow cytometry (n = 3, one-way ANOVA, **P < 0.01, ***P < 0.001 ****P < 0.0001. Error bars denote the SEM). (B) Three different PAK1 siRNAs knockdown PAK1 and significantly reduce endogenous ATXN1 levels in Daoy cells (n = 3, one-way ANOVA, **P < 0.01, ***P < 0.001, ****P < 0.0001. Error bars denote the SEM) (C) Quantification of ATXN1 mRNA levels by qRT-PCR shows no significant change upon PAK1 knockdown (n = 3, t-test, **P < 0.01. Error bars denote the SEM). (D) Co-expression of GST-ATXN1 [82Q] with PAK1 or GFP control significantly increases ATXN1 levels upon PAK1 co-transfection (n = 3, t-test, **P < 0.01. Error bars denote the SEM). (E)PAK1 shRNA-mediated knockdown in mouse primary cerebellar neurons reduces Atxn1 levels (n = 3, t-test, *P < 0.05. Error bars denote the SEM).
Next, we tested whether knockdown of PAK1 could alter endogenous ATXN1 levels in the Daoy cell line. Indeed, knocking down PAK1 with multiple siRNAs consistently reduces endogenous ATXN1 levels (Fig. 2B). From the same cells, we also measured ATXN1 mRNA expression by quantitative RT-PCR (qRT-PCR). The results indicate that ATXN1 mRNA levels were not changed upon PAK1 knockdown (Fig. 2C), further supporting our findings that PAK1 modulates ATXN1 post-translationally. To test whether increasing PAK1 levels promotes the stability of ATXN1, we co-expressed PAK1 with GST-ATXN1 [82Q] in Neuro2A cells (7,19). We observed that cells over-expressing PAK1 showed a significant increase in ATXN1 [82Q] levels compared with cells expressing the negative control, green fluorescent protein (GFP) (Fig. 2D). Since both PAK2 and PAK3 belong to Group I PAKs, we investigated whether PAK2 and PAK3 modulate ATXN1 levels upon their knockdown. To this end, we knocked down PAK2 in mRFP-ATXN1 [82Q]-IRES-YFP cell line. Our results indicate that substantial reduction of PAK2 levels does not affect mRFP-ATXN1 [82Q] levels (Supplementary Material, Fig. S2B). On the other hand knockdown of PAK3 led to a decrease of mRFP-ATXN1 [82Q] levels (Supplementary Material, Fig. S2C). Since we could not identify an antibody for PAK3, we validated knockdown of PAK3 by qRT-PCR (Supplementary Material, Fig. S2D). Next, we co-expressed Group I PAKs with GST-ATXN1 [82Q] in Neuro2A cells. To our surprise, the entire Group I PAKs can modulate GST-ATXN1 [82Q] levels (Supplementary Material, Fig. S2E). These results demonstrate that although PAK1 is the most potent ATXN1 modulator among Group I, PAK2 and PAK3 may also modulate ATXN1 levels.
To assess whether PAK1 regulates ATXN1 levels in neuronal cells, we cultured primary neurons from the mouse cerebellum. We found that lentiviral-mediated knockdown of Pak1 via shRNA significantly reduced Atxn1 levels in primary cerebellar neurons (Fig. 2E). A similar effect is observed upon knockdown of Pak1 in mouse primary cortical neurons (Supplementary Material, Fig. S2G). Collectively, these results demonstrate that among Group I PAKs, PAK1 potently modulates ATXN1 levels post-transcriptionally across different cell types and species.
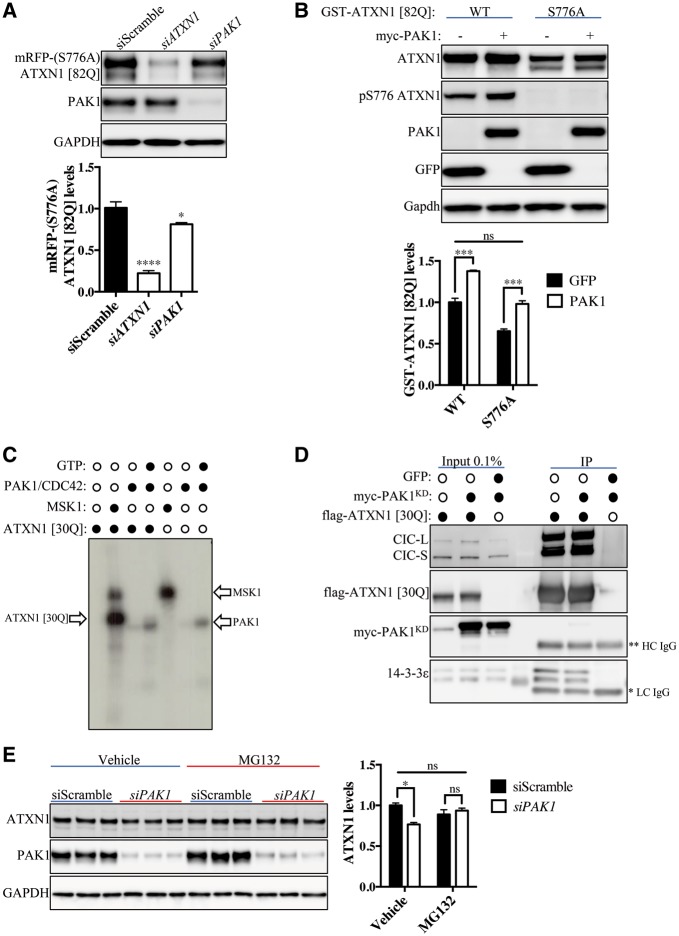
PAK1 modulates ATXN1 independently of S776 phosphorylation
Given that most of the previously identified ATXN1 modulators regulate ATXN1 levels through S776 phosphorylation (2,3,7,19,20), and given that PAK1 is a serine/threonine kinase, we set out to test whether PAK1 modulates ATXN1 levels through phosphorylation of ATXN1 at S776. To address this, we generated a Daoy cell line that stably expresses a transgene encoding the phospho-mutant S776A ATXN1 [82Q] (mRFP-[S776A] ATXN1 [82Q]-IRES-YFP). In these cells, PAK1 is knocked down and mRFP-(S776A) ATXN1 [82Q] levels are analyzed by western blotting. Interestingly, knockdown of PAK1 reduces the levels of the phospho-mutant (S776A) ATXN1 [82Q] (Fig. 3A). To further confirm that PAK1 modulates ATXN1 independently of S776 phosphorylation, we co-expressed wild type or phospho-mutant (S776A) GST-ATXN1 [82Q] with PAK1 or GFP control (3,7). We found that overexpression of ATXN1 with PAK1 increases ATXN1 regardless of its S776 phosphorylation status compared with overexpression of ATXN1 with GFP (Fig. 3B). These results demonstrate that PAK1, unlike many of the identified ATXN1 regulators, modulates ATXN1 independently of S776 phosphorylation.
Figure 3.
PAK1 modulates ATXN1 independently of S776 phosphorylation. (A)PAK1 knockdown using siPAK1-1 significantly reduces mRFP-(S776A) ATXN1 [82Q] levels in a Daoy cell line stably expressing mRFP-(S776A) ATXN1 [82Q]-IRES-YFP by western blot (n = 3, one-way ANOVA, *P < 0.05, ****p < 0.0001. Error bars denote the SEM). (B) Co-expression of ATXN1 [82Q] or phospho-mutant S776A ATXN1 [82Q] with PAK1 or GFP control reveal that PAK1 modulates S776A ATXN1 [82Q] (n = 3, two-way ANOVA, ***P < 0.001; ns, not significant. Error bars denote the SEM). (C) MSK1, but not PAK1, phosphorylates ATXN1 [30Q] in in vitro32P kinase assay. GTP is the co-factor for CDC42 activation that in turn activates PAK1 as depicted by the radioactive signal at a molecular weight below ATXN1 [30Q]. (D) Co-immunoprecipitation of endogenous CIC and 14–3-3ε with transfected flag-ATXN1 [30Q], but not myc-PAK1KD (kinase dead, K299R) in HEK293T cells [*Light chain (LC) specific IgG, **heavy chain (HC) specific IgG]. (E)PAK1 knockdown (siPAK1-3) reduces endogenous ATXN1 levels in Daoy cells. However, upon addition of the proteasome inhibitor, MG132, the PAK1-dependent reduction of ATXN1 levels was abolished (n = 3, two-way ANOVA, *P < 0.05; ns, non-significant. Error bars denote the SEM).
To test the potential effect of PAK1 on ATXN1 S776 phosphorylation more directly, we performed an in vitro kinase assay. We used recombinant ATXN1 [30Q] and PAK1 and monitored ATXN1 S776 phosphorylation by western blotting (7). Indeed, while MSK1 phosphorylated ATXN1 at the S776 residue, PAK1 showed no detectable signal (Supplementary Material, Fig. S3A). Next, we analyzed whether PAK1 phosphorylates ATXN1 at novel S/T residues to affect ATXN1 levels. To test this, we performed an in vitro32P radioactive kinase assay with recombinant ATXN1 [30Q] and PAK1. Our results show that while PAK1 is auto-phosphorylated, it did not phosphorylate ATXN1 [30Q] (Fig. 3C and Supplementary Material, Fig. S3B). In addition to S776 ATXN1 phosphorylation, ATXN1 interactors can also affect ATXN1 stability (2,21,22). Therefore, we performed co-immunoprecipitation analysis to assess whether PAK1 directly interacts with ATXN1 and, thus, stabilizes its levels. To test whether PAK1 interacts with ATXN1, epitope tagged flag-ATXN1 [30Q] was co-expressed with kinase dead, K299R (23), PAK1 (PAK1KD) or GFP and ATXN1 was immunoprecipitated. We used catalytically inactive PAK1 in our co-immunoprecipitation assay to increase binding affinity with its substrates. Known ATXN1 interactors, such as capicua (CIC) and 14–3-3ε, were co-immunoprecipitated with ATXN1, while myc-PAK1KD was not detected in the immunoprecipitation (Fig. 3D). To test whether PAK1 affects levels of ATXN1 interactors, we performed knockdown of PAK1 and its over-expression and assessed CIC and 14-3-3ε levels. Neither PAK1 knockdown, nor its over-expression affect CIC and 14-3-3ε levels (Supplementary Material, Fig. S3C and D).
Given that PAK1 regulates ATXN1 post-translationally, we investigated whether PAK1 affects ATXN1 degradation. We observed that the effects of PAK1 knockdown on ATXN1 reduction is completely abolished in cells following proteasome inhibition with MG132 (Fig. 3E) (24), indicating that PAK1-mediated stabilization of ATXN1 is dependent on the proteasome pathway. Unlike the proteasome, inhibition of autophagy flux, another major cellular degradation pathway, with Bafilomycin A1 (25) did not affect PAK1-dependent change of ATXN1 levels (Supplementary Material, Fig. S3E). This suggests that PAK1 regulates ATXN1 levels through affecting its proteasome-mediated degradation, but not autophagy. Taken together, our study demonstrates that PAK1 is a novel ATXN1 modulator that regulates ATXN1 levels independently of S776 phosphorylation. This finding opens up the possibility of an additional pathway that can be utilized in SCA1 therapeutic development.
Pharmacological inhibition of PAKs reduces ATXN1 levels
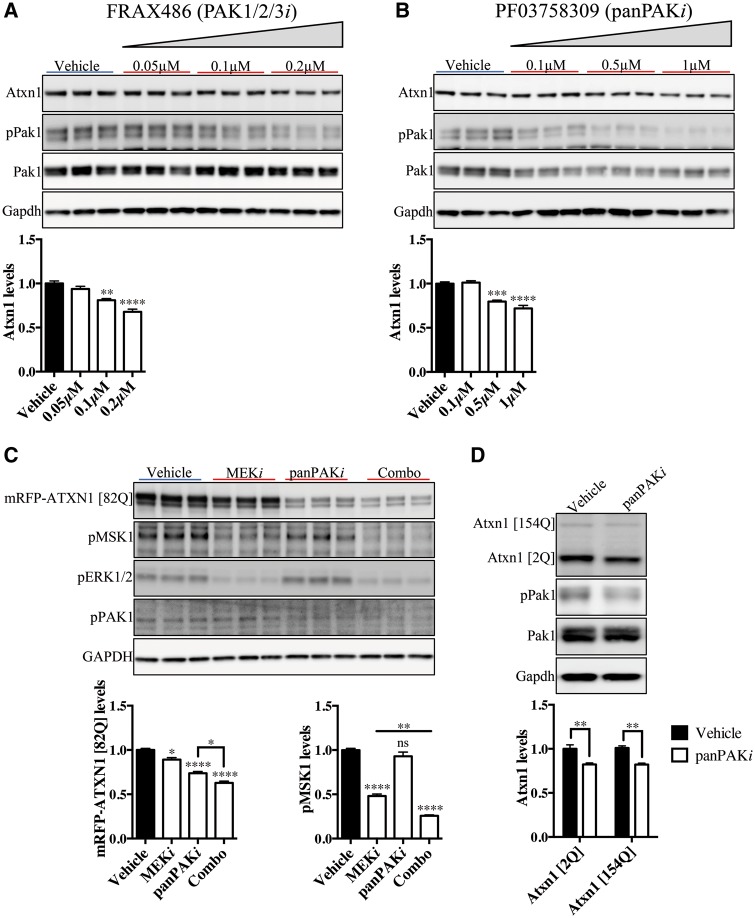
Because genetic perturbation of PAKs results in modulation of ATXN1 levels in fruit flies and mammalian cells, we next set out to investigate whether ATXN1 is modulated by pharmacological inhibition of PAKs. We used commercially available small molecule inhibitors for Group I PAKs to determine whether inhibition of PAK activity alters ATXN1 levels. To inhibit Group I PAKs we utilized FRAX inhibitors and to target the entire family of PAKs we used a panPAK inhibitor (Supplementary Material, Fig. S4A). FRAX486 is a potent ATP-competitive inhibitor for Group I PAKs (26). Mouse primary cerebellar neuronal culture treated with FRAX486 inhibited PAK1 phosphorylation at serine144, suggesting a reduction in PAK1 kinase activity (27). Moreover, primary neuron culture treatment with FRAX486 significantly reduces ATXN1 levels in a concentration-dependent manner (Fig. 4A). We observed similar results when using FRAX597 (28), a FRAX486 derivative with a higher specificity for Group I PAKs (Supplementary Material, Fig. S4B). We also performed similar studies with the panPAK inhibitor, PF03758309, which inhibits all PAK isoforms, albeit with different inhibition constants (Supplementary Material, Fig. S4A) (29). Primary cerebellar neurons treated with the panPAK inhibitor show a significant reduction in ATXN1 levels in a concentration-dependent manner (Fig. 4B). To test whether PAK and MSK inhibition can modulate ATXN1 levels additively, we performed a treatment where we combined the inhibition of both MAP kinase and PAK signaling pathways in the Daoy cell line expressing mRFP-ATXN1 [82Q]-IRES-YFP. Cells treated with panPAK (PF03758309, panPAKi) and MEK (PD0325901, MEKi) inhibitors in combination show an additive and significant reduction in mRFP-ATXN1 [82Q] levels compared with cells treated with either inhibitor alone (Fig. 4C). Although panPAKi slightly inhibits pMSK1 levels (Fig. 4C), we argue that the effect is insignificant given that PAK1, unlike MSK1, modulates ATXN1 levels independently of S776 phosphorylation. Furthermore, the additive effect in ATXN1 reduction is abolished in mRFP-(S776A) ATXN1 [82Q]-IRES-YFP cells (Supplementary Material, Fig. S4C). This result further supports our findings that MSKs regulate ATXN1 through phosphorylation at S776, while PAKs modulation of ATXN1 is S776 phosphorylation-independent. These experiments not only validate our hypothesis that PAKs and MSKs modulate ATXN1 levels through distinct molecular mechanisms, but also provide the framework for pursuing combination therapy for the treatment of SCA1.
Figure 4.
Inhibition of PAK signaling reduces ATXN1 levels in cells and mice. (A) Treatment of mouse primary cerebellar neuron culture with FRAX486 (PAK1/2/3i), an inhibitor of Group I PAKs, results in a decrease of pPak1 (S144) and Atxn1 levels in a concentration-dependent manner (n = 3, one-way ANOVA, **P < 0.01, ****P < 0.0001. Error bars denote SEM). (B) PF03758309, panPAK inhibitor (panPAKi), modulates Atxn1 levels in a concentration-dependent manner in mouse cerebellar neuron culture (n = 3, one-way ANOVA, ***P < 0.001, ****P < 0.0001. Error bars denote the SEM). (C) Combinatorial delivery of MEK (PD0325901, MEKi) and panPAK (PF03758309, panPAKi) inhibitors in mRFP-ATXN1 [82Q]-IRES-YFP Daoy cell line additively reduces mRFP-ATXN1 [82Q] levels (n = 3, one-way ANOVA, *P < 0.05, ****P < 0.0001. Error bars denote the SEM). (D) Intraperitoneal administration of 15 mg/kg panPAK inhibitor every 8 h for 5 days in Atxn1154Q/2Q mice reduce expanded Atxn1 [154Q] as wells as wild type, Atxn1 [2Q] levels in the cerebellum (n = 4, two-way ANOVA, **P < 0.01. Error bars denote the SEM).
Since there are multiple members of the PAK family, we reasoned that inhibiting all of them by using panPAK inhibitor might be the most effective approach in lowering ATXN1 levels in vivo. To our knowledge, it is yet to be investigated whether PF03758309 crosses the blood-brain-barrier (BBB); thus, we performed pharmacokinetic studies to determine the penetrance of this drug into the central nervous system. Our studies revealed that PF03758309 was able to cross the BBB, albeit with a short half-life (Supplementary Material, Fig. S4D). Intraperitoneal administration of the panPAK inhibitor to the Atxn1154Q/2Q mouse model of SCA1 results in a significant reduction in both expanded and wild type ATXN1 levels in the cerebellum (Fig. 4D). Together, these results demonstrate that pharmacological inhibition of PAKs potently reduce ATXN1 levels and provide a foundation for the development of combination drug therapy to treat SCA1 patients.
Discussion
In this study, we identified PAK1 as a novel modulator of ATXN1 levels by positively regulating ATXN1 stability and toxicity both in vitro and in vivo. We show that downregulation of Drosophila Pak1 and Pak3 reduces ATXN1 levels and thus ameliorates mutant ATXN1-induced nervous system dysfunction. In mammalian cells, PAK1, a homolog of the Drosophila Pak1 and Pak3, reduces ATXN1 through interefering with its proteasome-mediated degradation and independent of S776 phosphorylation. Administration of a panPAK inhibitor in the mouse model of SCA1 significantly reduces ATXN1 levels. Furthermore, we report that PAKs and MSKs inhibition in combination results in an additive reduction in ATXN1 levels, providing a promising therapeutic entry point for the treatment of SCA1.
In our previous study we identified MSK1 as an ATXN1 modulator (7). In the same screen, Drosophila Pak3, was also identified as a potential suppressor of ATXN1 [82Q]-induced toxicity. The cell-based screen did not uncover PAK1 as an ATXN1 modulator because the siRNAs used against PAK1 did not efficiently knockdown its transcript. We decided to investigate the role of mammalian PAK1 in ATXN1 modulation using effective PAK1 RNAi tools. Here we show that genetic perturbation of PAK1 results in modulation of ATXN1 levels in human cells. We used multiple different PAK1 siRNAs and small molecule inhibitors of PAKs to reduce the possibility of off-target effects. Additionaly, other members of Group I PAKs display regulatory roles on ATXN1 levels. This is not surprising given that the entire Group I PAKs is evolutionary conserved (15). Notably, the effect of PAK1 on ATXN1 levels change is more robust than most previously identified ATXN1 modulators. Additionally, using the Drosophila SCA1 model and mammalian cell lines, we demonstrate that PAK1 modulation of ATXN1 levels persists across species. Furthermore, PAK1 modulates ATXN1 levels through proteasome-mediated degradation, independent of S776 phosphorylation. In line with our study, ATXN1 is reported to undergo ubiquitination followed by proteasome-mediated degradation (30,31). The precise molecular mechanism underlying the PAK1 regulation of ATXN1 clearance, however, still needs to be elucidated. It is possible that PAK1 inhibits a ubiquitin E3 ligase or other factors in the proteasome pathway that normally promote ubiquitination and degradation of ATXN1. Future studies on PAK1 signaling network and on factors in the proteasome pathway that regulate ATXN1 levels may reveal the molecular link between PAK1 and ATXN1.
Pharmacological inhibition of either all PAKs (panPAK inhibitor) or Group I PAKs (FRAX inhibitors) results in reduction of ATXN1 levels in vitro and in vivo. It is noteworthy that we chose to use the panPAK inhibitor in our in vivo studies because of its ability to cross the BBB, and it also eliminates possible compensation that may arise from Group II PAKs in vivo. Since ATXN1 levels affect SCA1 pathogenesis, we sought to determine the therapeutic potential of ATXN1 reduction. Although loss-of-function of MSK1 and MSK2 or PAK1 is viable in mice (32,33), it is worth noting that chronic inhibition of either MSK or PAK signaling may bring undesired effects. SCA1 patients may need to receive chronic MSK inhibition for a few decades. Thus, the discovery of other pathways that modulate ATXN1 levels will provide an opportunity to partially inhibit multiple regulators of ATXN1. The administration of a cocktail of inhibitors, each with reduced on-target inhibition, will potentially reduce the long-term side effects. Such findings open up the possibility of pursuing combination therapy with MSKs and PAKs inhibitors.
In this study, we identified PAK1 as a robust, cross-species ATXN1 modulator that regulates ATXN1 levels independently of S776 phosphorylation. PAKs are attractive druggable targets because of their oncogenic effects in different types of cancers (34). We further expand the necessity to develop medical grade inhibitors for PAKs to potentially treat SCA1. In combination with MSK inhibition, PAK inhibition may prove a promising avenue for the treatment of SCA1.
Materials and Methods
Drosophila Pak1 and Pak3 strains
Pak1KK101874(Vienna Drosophila Resource Center)
Pak1HMS02279(Bloomington Stock Center)
Pak3GL00287 (Bloomington Stock Center)
Pak3KK111386 (Vienna Drosophila Resource Center)
Pak3MI06140(Bloomington Stock Center)
Drosophila SCA1 model and climbing assay
We used the previously reported SCA1 model (5) to drive expression of human polyQ expanded ATXN1 protein in the Drosophila central nervous system using the nrv-Gal4 driver. y, w, UAS-ATXN1(82Q)(line-F7); nrv-GAL4 virgin females were crossed to males carrying the various Pak1 and Pak3 alleles and maintained at 28°C. Non-balancer, female progeny that emerged were collected over an 8-h period and grouped into replicates of fifteen individuals in preparation for the climbing assay, which was performed as previously described in (18). A camera recorded the movements of fruit flies for 10 s and calculated motor performance metrics of each genotype. These climbing assay and the recording were repeated five times in each session, and flies were tested longitudinally until day 14.
Preparation of Drosophila protein lysates and immunoblot
Transgenic expression of ATXN1 [82Q] to the eye was driven by gmr-GAL4. We crossed virgins from this line to males carrying Pak1 and Pak3 alleles and maintained these crosses at 25°C. Non-balancer female progeny were collected and snap-frozen at day 1. NuPAGE LDS sample buffer and reducing buffer (Invitrogen) was used to prepare protein lysates. Each technical replicate contained eight heads per preparation. Protein levels within each sample was analyzed using an immunoblot assay. Primary antibodies used were anti-ATXN1 (11 750, 1:5000) and anti-laminC (Developmental Studies Hybridoma Bank, 1:1000).
Cell culture and transfections
All cell lines described in this article (except primary neuron culture) were cultured in Dulbecco's Modified Eagle Medium (DMEM) (Invitrogen) with 10% FBS (Invitrogen). mRFP-(S776A) ATXN1 [82Q]-IRES-YFP stable expressing cell line was generated as previously reported in (7). Cells were transfected with siRNA using DharmaFECT (Dharmacon) and incubated for 3 days prior to analysis. All cDNA constructs were transfected into cells using Lipofectamine2000 (Invitrogen) and cells were incubated for 2 days, post-transfection prior to analysis. FRAX486 (Tocris), FRAX597, PF03758309 and PD0325901 (Selleckchem) were solubilized in dimethyl sulfoxide (DMSO) (Sigma) and added to Daoy cells or primary neurons for the culture at 2 and 8 days, respectively.
siRNAs, shRNAs and plasmids
siPAK1–1: (Sigma, SASI_Hs01_00087970)
siPAK1–2: (Sigma, SASI_Hs01_00087971)
siPAK1–3: (Sigma, SASI_Hs01_00087972)
siPAK2–1: (Sigma, SASI_Hs01_00014671)
siPAK2–2: (Sigma, SASI_Hs01_00014672)
siPAK2–3: (Sigma, SASI_Hs01_00014673)
siPAK3–1: (Sigma, SASI_Hs02_00330666)
siPAK3–2: (Sigma, SASI_Hs02_00330667)
siPAK3–3: (Sigma, SASI_Hs02_00330668)
siScramble: (Dharmacon, non-targeting siRNA no. 3)
psPAX2 (Addgene, 12260)
pMD2.G (Addgene, 12259)
shScramble (Dharmacon, RHS4348)
shPak1: (Dharmacon, V2LMM_63190)
GST-ATXN1 [82Q]: [cloned in our lab (19)]
flag-ATXN1 [30Q]: (Addgene, 33236)
myc-PAK1: (Addgene, 12209)
myc-PAK1KD (K299R): (Addgene, 12210)
flag-PAK2: (Addgene, 31663)
myc-Pak3: (Addgene, 99510)
GFP: (Addgene, 11153)
Cell lysate preparation and antibodies
Cells were washed with PBS and lysed with radioimmunoprecipitation assay (RIPA) buffer (50 mm Tris HCl pH 8, 150 mm NaCl, 0.1% SDS, 1% Igel CA630, 0.5% sodium deoxycholate with Xpert phosphatase inhibitor cocktail and Xpert protease cocktail [Gendepot]). The lysates were sonicated and centrifuged at 13 000g for 20 min. The supernatant was collected and protein was quantified by Bicinchninic acid assay (Fisher). Proteins were separated using SDS-PAGE gels (Invitrogen). Primary antibody used: anti-ATXN1 [11 750 (35), 1:5000], anti-pS776 ATXN1 [PN1248 (3), 1:2000], anti-CIC [126 (22), 1:1000], anti-GAPDH (Advanced ImmunoChemical, 1:20 000), anti-PAK1 (Cell Signaling, 1:1000), anti-pPAK1 (Cell Signaling, 1:800), anti-PAK2 (Cell Signaling, 1:1000), anti-pERK1/2 (Cell Signaling, 1:1000), anti-MSK1 (R&D Systems, 1:1000), anti-pMSK1 (Abcam, 1:800), anti-14–3-3ε (Santa Cruz, 1:800), anti-LC3 (MBL, 1:800) and anti-GFP (Genetex, 1:5000). After the incubation of western blots with primary antibody, a species-specific, HRP-conjugated antibodies were added: goat anti-rabbit (Bio-rad, 1:10 000), donkey anti-mouse (Jackson ImmunoResearch Labs, 1:10 000) and donkey anti-goat (Jackson ImmunoResearch Labs 1: 10 000). For western imaging an HRP substrate was used (Amersham ECL Prime Reagent, Fisher).
Immunoblot imaging and analysis
The immunoblots were imaged and analyzed using ImageQuant LAS 4000 (GE) western imager. Development of the image proceeded in progressive time and was terminated upon image saturation. The image densitrometry is quantified using GE imager software as it autonomously identifies bands on an western image and quantifies the signal. Densitometry of a target band was normalized with densitometry of its corresponding loading control, GAPDH. The resulting values were plotted in GraphPrism and analyzed with the appropriate statistical analysis.
Animal handling
All procedures for mouse use were approved by the institutional Animal Care and Use Committee for Baylor College of Medicine and Affiliates. Atxn1154Q/2Q mouse model, which expresses expanded form of ATXN1 [154Q] has been previously described in (36), and it has been back-crossed to C57BL/6 for more than 10 generations. For intraperitoneal panPAK inhibitor administration to the Atxn12Q/154Q mice, panPAKi was solubilized in 5% N-Methyl-2-pyrrolidone (NMP), 5% Solutol HS-15 in normal saline and administrated every 8 h for 5 days.
In vivo pharmacokinetic study
The pharmacokinetic study was performed by Sai Life Sciences Limited. Briefly, 18 male C57BL/6 mice were used for a single intravenous or intraperitoneal administration of the PF03758309 (panPAK) inhibitor. The compound was solubilized in 5% NMP, 5% Solutol HS-15 in normal saline. The blood samples and brain samples were collected at 1-, 8- and 24-h post-injection and panPAK inhibitor concentration was interrogated by LC/MS/MS method.
Mouse primary cerebellar neuron isolation and culture
Cerebelli from C57BL/6 wild type pups of 8 days of age were isolated and cells were separated by trypsin digestion (Invitrogen). Cells were plated on poly-L-lysine (Sigma) coated 12-well plates and cultured in primary cerebellar neuron media (Neurobasal media (Invitrogen), Penicillin/Streptomycin (Invitrogen), B27 (Invitrogen), Insulin/transferring/selenite (Invitrogen), Sodium Pyruvate (Invitrogen), 2 mm glutamine (Invitrogen), 0.45% Glucose (Sigma), Linoleic acid/albumin (Invitrogen), 16 µg/ml N-acetyl-cysteine (Sigma), 25 ml potassium chloride (Invitrogen)). shRNA lentiviral particles were added to neurons on the first day of the culture, while inhibitors were added on the third day. The cells were subsequently cultured for total of 12 days.
Mouse primary cortical neuron isolation and culture
Mouse cortical neurons were prepared from postnatal day 0–1 FVB/N mice using papain dissociation system (Worthington) and cultured in poly-D-lysine coated 12-well plates (5 × 105 per well) in Neurobasal medium supplemented with GlutaMAX (Invitrogen), B27 and antibiotics (Penicillin/Streptomycin).
Lentivirus generation
Letiviral particles for cell induction were generated as previously described in (37). Briefly, packaging vectors, psPAX2 and pMD2.G, were cotransfected with shScrambe or shPAK1 plasmid in HEK293T cells at 4:3:1 molar ratio, respectively. About 16-h post-transfection, the media was removed and 5 ml of new media was added to 10-cm dish. The media was collected 48 h after transfection and 5 ml of new media was added. After 72 h of transfection the rest of the media was collected and cleared of cell debris. Lentiviruses were further concentrated using Lenti-X concentrator (Clontech).
MG132 and bafilomycin A1 administration in cells
Daoy cells were plated and the next day transfected with siPAK1–3. After 39 h of siRNA transfection, vehicle (DMSO, Sigma), MG132 (EMD Biosciences, 5 µm) or Bafilomycin A1 (Sigma, 150 nm) were added to the culture. The cells were left for additional 9 h, and then the cells were lysed as stated above and ATXN1 levels was assessed by western blot.
In vitro kinase assay
One microgram of recombinant substrate was combined with 250 ng of active kinase [PAK1/CDC42 (SignalChem) and 100 µm GTP (Thermo Fisher)] and incubated in kinase buffer [50 mm PO4 pH 7.4, 150 mm NaCl, 20 mm MgCl2, 0.1 mg/ml BSA, 1 mm dithiothreitol] with phosphatase inhibitor (Roche), 20 µm cold adenosine tryphosphate (ATP) (Invitrogen) and 1.2 µl of 0.01 mCi/µl 32P ATP (PerkinElmer) for 1 h at 30°C. The kinase reaction was terminated by the addition of NuPAGE LDS sample buffer and sample reducing agent (Invitorgen) followed by boiling for 15 min. The samples were ran on a NuPAGE 4–12% Bis-Tris Gel (Invitrogen). The gel was coomassie stained (InstantBlue, VWR) for 20 min and exposed to X-ray film (GE) for 1 h.
Statistical analyses
Data collection and experimental assays were performed with experimenters blinded to the treatments. GraphPad Prism software was used with the appropriate statistical method to obtain the P-values. Student’s t-test was used for comparisons of two groups and for multiple comparisons we used analysis of variants (ANOVA) with appropriate post hoc analysis. For analysis of longitudinal Drosophila motor performance according to genotype, we used longitudinal mixed effects models with smoothing splines to capture non-linear trends over time. Statistical analysis was performed in R using the lme4 package. Detailed statistical results are included in the resource data section posted online. Data are presented as mean with standard error of the mean (SEM), unless specified otherwise (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Quantitative RT-PCR
miRNeasy kit (Qiagen) was used to obtain total RNA according with the manufacturer’s instructions. cDNA was synthesized using the Superscript III kit (Invitrogen). Quantitive RT-PCR was performed using Perfecta SYBR Green FastMix (Quanta Biosciences). qRT-PCR results were analyzed using the comparative Ct method and normalized against GAPDH.
Primer sequences are the following:
huATXN1-Frwd: GGATTGAAGACAGCCATAGCC
huATXN1-Rev: CCAAAACTTCAACGCTGACC
huPAK3-Frwd: ACCACAGCTCCAAACCACTT
huPAK3-Rev: GGCGCTCTTTCTCCTTCTTC
huGAPDH-Frwd: AGAAGGCTGGGGCTCATTTG
huGAPDH-Rev: AGGGGCCATCCACAGTCTTC
Mouse brain lysate preparation and immunoblot analysis
Mouse cerebelli were dissected and sonicated in RIPA buffer [50 mm Tris HCl pH 8, 150 mm NaCl, 0.1% SDS, 1% Igel CA630, 0.5% sodium deoxycholate with Xpert phosphatase inhibitor cocktail (Gendepot) and Xpert protease cocktail (Gendepot)]. The samples were incubated on ice for 15 min and centrifuged at 13 000g for 20 min. The samples were then treated with NuPAGE LDS and sample reducing agent (Invitrogen) and boiled for 15 min. Samples were analyzed by immunoblot assay using SDS-PAGE gel.
Purifying recombinant ATXN1
Three gBlocks were designed to encode human ATXN1 [30Q] with codons optimized for expression in Escherichia coli K-12 (IDT technologies). Gibson cloning was performed using the pET28a vector and the three gBlocks to generate a protein expression vector with a 6X-His tag and a TEV cleavage site. This construct was transformed in BL21AI One Shot E. coli and ATXN1 [30Q] expression was induced with 0.5 mM IPTG and 0.2% L-arabinose for 4 h at 37°C. The bacteria were lysed using sonication and 1% Triton X-100 in lysis buffer (6 M urea, 20 mm BME, 0.5 m NaCl, 30 mm Imidazole and 50 mm NaPO4 pH 7.4). The supernatant was then loaded onto a pre-packed 5 ml high performance Ni sepharose column (GE healthcare). Elution was performed using a step gradient of 330 mm imidazole in lysis buffer. Fractions with >80% purity were used in the kinase assays.
PAK1 co-immunoprecipitation
HEK293T cells were transfected (Lipofectamine2000, Invitrogen) with flag-ATXN1 [30Q] and myc-PAK1 or GFP and the cells were cultured for 3 days. Cells were lysed in lysis buffer [50 mM Tris pH 7.5, 0.5% NP-40, 150 mm NaCl and 1 mm EDTA with protease and phosphatase inhibitors (Xpert)]. The cell lysates were centrifuged at 100 000g for 20 min and 5 µl of M2 flag antibody (Sigma) with 20 µl of Protein-A bead slurry (Fisher) were added to the supernatant. The samples were incubated for 1 h and then washed three times with the lysis buffer. NuPAGE LDS and reducing buffer (Invitrogen) were added to the samples and then boiled for 5 min. The co-immunoprecipitation was assessed by western blotting.
Supplementary Material
Supplementary Material is available at HMG online.
Funding
This project was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) 2R37NSO27699 (H.Y.Z.); NIH/NINDS F32 NS083091 (Q.T.); Baylor College of Medicine IDDRC grant 1 U54HD083092 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represents the official views of the National Institutes of Health (administrative and microscopy cores). H.Y.Z. is an investigator of the Howard Hughes Medical Institute. The authors would like to thank Rituraj Pal and Maxime W. C. Rousseaux for experimental suggestions and critical reading of the article.
Conflict of Interest statement. None declared.
Supplementary Material
References
- 1. Orr H.T., Chung M-y., Banfi S., Kwiatkowski T.J., Servadio A., Beaudet A.L., McCall A.E., Duvick L.A., Ranum L.P.W., Zoghbi H.Y. (1993) Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat. Genet., 4, 221–226. [DOI] [PubMed] [Google Scholar]
- 2. Jafar-Nejad P., Ward C.S., Richman R., Orr H.T., Zoghbi H.Y. (2011) Regional rescue of spinocerebellar ataxia type 1 phenotypes by 14-3-3ε haploinsufficiency in mice underscores complex pathogenicity in neurodegeneration. Proc. Natl. Acad. Sci. USA, 108, 2142–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Emamian E.S., Kaytor M.D., Duvick L.A., Zu T., Tousey S.K., Zoghbi H.Y., Clark H.B., Orr H.T. (2003) Serine 776 of ataxin-1 is critical for polyglutamine-induced disease in SCA1 transgenic mice. Neuron, 38, 375–387. [DOI] [PubMed] [Google Scholar]
- 4. Gennarino V.A., Singh R.K., White J.J., De Maio A., Han K., Kim J.-Y., Jafar-Nejad P., di Ronza A., Kang H., Sayegh L.S. (2015) Pumilio1 haploinsufficiency leads to SCA1-like neurodegeneration by increasing wild-type ataxin1 levels. Cell, 160, 1087–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernandez-Funez P., Nino-Rosales M.L., de Gouyon B., She W.C., Luchak J.M., Martinez P., Turiegano E., Benito J., Capovilla M., Skinner P.J.. et al. (2000) Identification of genes that modify ataxin-1-induced neurodegeneration. Nature, 408, 101–106. [DOI] [PubMed] [Google Scholar]
- 6. Matilla A., Roberson E.D., Banfi S., Morales J., Armstrong D.L., Burright E.N., Orr H.T., Sweatt J.D., Zoghbi H.Y., Matzuk M.M. (1998) Mice lacking ataxin-1 display learning deficits and decreased hippocampal paired-pulse facilitation. J. Neurosci., 18, 5508–5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park J., Al-Ramahi I., Tan Q., Mollema N., Diaz-Garcia J.R., Gallego-Flores T., Lu H.-C., Lagalwar S., Duvick L., Kang H.. et al. (2013) RAS-MAPK-MSK1 pathway modulates ataxin 1 protein levels and toxicity in SCA1. Nature, 498, 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bock C., Lengauer T. (2012) Managing drug resistance in cancer: lessons from HIV therapy. Nat. Rev. Cancer, 12, 494–501. [DOI] [PubMed] [Google Scholar]
- 9. Gonzales P.R., Pesesky M.W., Bouley R., Ballard A., Biddy B.A., Suckow M.A., Wolter W.R., Schroeder V.A., Burnham C.A., Mobashery S.. et al. (2015) Synergistic, collaterally sensitive [beta]-lactam combinations suppress resistance in MRSA. Nat. Chem. Biol., 11, 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Darbyshire J.H. (1996) Delta: a randomised double-blind controlled trial comparing combinations of zidovudine plus didanosine or zalcitabine with zidovudine alone in HIV-infected individuals. Delta Coordinating Committee. Lancet, 348, 283–291. [PubMed] [Google Scholar]
- 11. Radu M., Semenova G., Kosoff R., Chernoff J. (2014) PAK signalling during the development and progression of cancer. Nat. Rev. Cancer, 14, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ha B.H., Morse E.M., Turk B.E., Boggon T.J. (2015) Signaling, regulation, and specificity of the type II p21-activated kinases. J. Biol. Chem., 290, 12975–12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eswaran J., Soundararajan M., Kumar R., Knapp S. (2008) UnPAKing the class differences among p21-activated kinases. Trends Biochem. Sci., 33, 394–403. [DOI] [PubMed] [Google Scholar]
- 14. Mentzel B., Raabe T. (2005) Phylogenetic and structural analysis of the Drosophila melanogaster p21-activated kinase DmPAK3. Gene, 349, 25–33. [DOI] [PubMed] [Google Scholar]
- 15. Rudolph J., Crawford J.J., Hoeflich K.P., Wang W. (2015) Inhibitors of p21-Activated Kinases (PAKs) Miniperspective. J. Med. Chem., 58, 111–129. [DOI] [PubMed] [Google Scholar]
- 16. Hayashi M.L., Rao B.S.S., Seo J.-S., Choi H.-S., Dolan B.M., Choi S.Y., Chattarji S., Tonegawa S. (2007) Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc. Natl. Acad. Sci. USA, 104, 11489–11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luo S., Mizuta H., Rubinsztein D.C. (2008) p21-activated kinase 1 promotes soluble mutant huntingtin self-interaction and enhances toxicity. Hum. Mol. Genet., 17, 895–905. [DOI] [PubMed] [Google Scholar]
- 18. Al-Ramahi I., Pérez A.M., Lim J., Zhang M., Sorensen R., de Haro M., Branco J., Pulst S.M., Zoghbi H.Y., Botas J. (2007) dAtaxin-2 mediates expanded Ataxin-1-induced neurodegeneration in a Drosophila model of SCA1. PLoS Genet., 3, e234–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lai S., O'Callaghan B., Zoghbi H.Y., Orr H.T. (2011) 14-3-3 Binding to ataxin-1(ATXN1) regulates its dephosphorylation at Ser-776 and transport to the nucleus. J. Biol. Chem., 286, 34606–34616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jorgensen N.D., Andresen J.M., Lagalwar S., Armstrong B., Stevens S., Byam C.E., Duvick L.A., Lai S., Jafar-Nejad P., Zoghbi H.Y., Clark H.B., Orr H.T. (2009) Phosphorylation of ATXN1 at Ser776 in the cerebellum. J. Neurochem., 110, 675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fryer J.D., Yu P., Kang H., Mandel-Brehm C., Carter A.N., Crespo-Barreto J., Gao Y., Flora A., Shaw C., Orr H.T., Zoghbi H.Y. (2011) Exercise and genetic rescue of SCA1 via the transcriptional repressor capicua. Science, 334, 690–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lam Y.C., Bowman A.B., Jafar-Nejad P., Lim J., Richman R., Fryer J.D., Hyun E.D., Duvick L.A., Orr H.T., Botas J., Zoghbi H.Y. (2006) ATAXIN-1 Interacts with the repressor capicua in its native complex to cause SCA1 neuropathology. Cell, 127, 1335–1347. [DOI] [PubMed] [Google Scholar]
- 23. Sells M.A., Knaus U.G., Bagrodia S., Ambrose D.M., Bokoch G.M., Chernoff J. (1997) Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol., 7, 202–210. [DOI] [PubMed] [Google Scholar]
- 24. Tsvetkov A.S., Arrasate M., Barmada S., Ando D.M., Sharma P., Shaby B.A., Finkbeiner S. (2013) Proteostasis of polyglutamine varies among neurons and predicts neurodegeneration. Nat. Chem. Biol., 9, 586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamamoto A., Tagawa Y., Yoshimori T., Moriyama Y., Masaki R., Tashiro Y. (1998) Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct. Funct., 23, 33–42. [DOI] [PubMed] [Google Scholar]
- 26. Dolan B.M., Duron S.G., Campbell D.A., Vollrath B., Shankaranarayana Rao B.S., Ko H.Y., Lin G.G., Govindarajan A., Choi S.Y., Tonegawa S. (2013) Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by the small-molecule PAK inhibitor FRAX486. Proc. Natl. Acad. Sci. USA, 110, 5671–5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chong C., Tan L., Lim L., Manser E. (2001) The mechanism of PAK activation. Autophosphorylation events in both regulatory and kinase domains control activity. J. Biol. Chem., 276, 17347–17353. [DOI] [PubMed] [Google Scholar]
- 28. Licciulli S., Maksimoska J., Zhou C., Troutman S., Kota S., Liu Q., Duron S., Campbell D., Chernoff J., Field J., Marmorstein R., Kissil J.L. (2013) FRAX597, a small molecule inhibitor of the p21-activated kinases, inhibits tumorigenesis of neurofibromatosis type 2 (NF2)-associated Schwannomas. J. Biol. Chem., 288, 29105–29114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murray B.W., Guo C., Piraino J., Westwick J.K., Zhang C., Lamerdin J., Dagostino E., Knighton D., Loi C.-M., Zager M.. et al. (2010) Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc. Natl. Acad. Sci. USA, 107, 9446–9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cummings C.J., Reinstein E., Sun Y., Antalffy B., Jiang Y-H., Ciechanover A., Orr H.T., Beaudet A.L., Zoghbi H.Y. (1999) Mutation of the E6-AP ubiquitin ligase reduces nuclear inclusion frequency while accelerating polyglutamine-induced pathology in SCA1 mice. Neuron, 24, 879–892. [DOI] [PubMed] [Google Scholar]
- 31. Kang A., Park S.H., Lee S., Choi D.Y., Kim K.P., Song H.K., Hong S., Kang S. (2015) A key lysine residue in the AXH domain of ataxin-1 is essential for its ubiquitylation. Biochim. Biophys. Acta, 1854, 356–364. [DOI] [PubMed] [Google Scholar]
- 32. Chandramohan Y., Droste S.K., Arthur J.S.C., Reul J.M. (2008) The forced swimming‐induced behavioural immobility response involves histone H3 phospho‐acetylation and c‐Fos induction in dentate gyrus granule neurons via …. Eur. J. Neurosci., 27, 2701–2713. [DOI] [PubMed] [Google Scholar]
- 33. McDaniel A.S., Allen J.D., Park S.J., Jaffer Z.M., Michels E.G., Burgin S.J., Chen S., Bessler W.K., Hofmann C., Ingram D.A., Chernoff J., Clapp D.W. (2008) Pak1 regulates multiple c-Kit mediated Ras-MAPK gain-in-function phenotypes in Nf1+/− mast cells. Blood, 112, 4646–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kumar R., Gururaj A.E., Barnes C.J. (2006) p21-activated kinases in cancer. Nat. Rev. Cancer, 6, 459–471. [DOI] [PubMed] [Google Scholar]
- 35. Burright E.N., Clark H.B., Servadio A., Matilla T., Feddersen R.M., Yunis W.S., Duvick L.A., Zoghbi H.Y., Orr H.T. (1995) SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell, 82, 937–948. [DOI] [PubMed] [Google Scholar]
- 36. Watase K., Weeber E.J., Xu B., Antalffy B., Yuva-Paylor L., Hashimoto K., Kano M., Atkinson R., Sun Y., Armstrong D.L.. et al. (2002) A long CAG repeat in the mouse Sca1 locus replicates SCA1 features and reveals the impact of protein solubility on selective neurodegeneration. Neuron, 34, 905–916. [DOI] [PubMed] [Google Scholar]
- 37. Rousseaux M.W.C., de Haro M., Lasagna-Reeves C.A., De Maio A., Park J., Jafar-Nejad P., Al-Ramahi I., Sharma A., See L., Lu N.. et al. (2016) TRIM28 regulates the nuclear accumulation and toxicity of both alpha-synuclein and tau. eLife, 5, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.