Abstract
We have achieved significant enhancement of gene delivery into livers of large animals using ultrasound (US)-targeted microbubble (MB) destruction methods. An infusion of pGL4 (encoding a luciferase reporter gene) plasmid DNA (pDNA) and MBs into a portal-vein segmental branch of a porcine liver was exposed to US for 4 min. Therapeutic US induced cavitation of MBs to temporarily permeabilize the vascular endothelium and cell membranes, allowing entry of pDNA. We obtained a 64-fold enhancement in luciferase expression in pig livers compared to control without US using an unfocused, dual-element transducer (H105, center frequency [fc] = 1.10 MHz) at 2.7 MPa peak negative pressure (PNP). However, input electrical energy was limited, and modified transducers were designed to have spherical (H185A, fc = 1.10 MHz) or cylindrical foci (H185B, fc = 1.10 MHz; H185D, fc = 1.05 MHz) to enhance PNP output. The revised transducers required less electrical input to achieve 2.7 MPa PNP compared to H105, thereby allowing PNP outputs of up to 6.2 MPa without surpassing the piezo-material limitations. Subsequently, luciferase expression significantly improved up to 9,000-fold compared to controls with minor liver damage. These advancements will allow us to modify our current protocols toward minimally invasive US gene therapy.
Keywords: ultrasound, gene delivery, animal studies, liver, luciferase, ultrasound-mediated gene transfer, nonviral gene transfer, focused ultrasound
Introduction
Therapeutic ultrasound (tUS) has been demonstrated to be a potentially effective method for gene delivery.1, 2, 3, 4, 5 In comparison to viral methods, US-mediated gene transfection is relatively easier and more cost effective; it elicits reduced immunogenic response and prevents random integration into the host genome that can lead to oncogenic events.6 Further, in comparison with other nonviral carriers such as non-echogenic liposomes or polymers, US-mediated gene transfer induces minimal toxicity and can localize the desired effect to a targeted area. In the presence of exogenous cavitation nuclei, such as lipid-shelled microbubbles (MBs), US at certain frequencies and intensities can cause oscillation of these MBs. At the right conditions, these nuclei violently collapse, resulting in temporary permeabilization of the vascular endothelium and cell membranes, allowing entry of plasmid DNA (pDNA) into cells. This technology can allow pDNA or other therapeutic molecules to traverse several layers of barriers that otherwise would prevent their entry.7, 8, 9, 10
We have previously demonstrated successful US-mediated gene transfection: first in small animals, such as mice and rats, followed by in dogs.11, 12, 13 The translation of the methods from these small-animal studies to canine studies was not trivial. It not only involved scaling up volumes and concentrations of pDNA and MBs used in the study, but also adapting surgical methods to re-create the conditions suitable for US-targeted MB destruction (UTMD)-mediated gene transfer. The inferior vena cava (IVC) was clamped to prevent outflow, and a solution of reporter gene plasmid, pGL4, and MBs was injected into a branch of the portal vein (PV) leading to the target lobe. A 1.1-MHz, large-diameter, unfocused transducer (H105) was scanned across the treated lobe to expose the entire lobe to US for 4 min. With its 52-mm aperture, this dual element transducer was appropriately sized, such that it treated a wider area within a short time frame, which is crucial before MBs and plasmids evacuate the treatment site. Using 2.7 MPa peak negative pressure (PNP), significant enhancement in luciferase gene expression with minimal tissue damage was obtained in our canine study.
Our goal is to translate the UTMD gene delivery method to clinical application, requiring an efficient gene transfer with minimally invasive surgical procedure and transcutaneous tUS. Although we have achieved significantly enhanced gene transfer efficiency, the luciferase expression in large animals is still lower than what is achieved in smaller animals. To overcome this, we would like to increase the effective pressure of the transducer to further enhance gene expression. We chose pigs to evaluate different types of transducers to enhance gene transfection because the size of the liver lobe of a small pig is similar to that of the dog and a portion of the human liver, and the venous systems are also very similar among these three species. In addition, more parameters with more animals per group can be examined rapidly, since pigs are less costly and easier to handle than dogs. The increase in PNP could be particularly important for porcine experiments due to greater density of connective tissue in porcine livers and resulting acoustic power loss. However, the planar design of H105 transducer significantly limits the electrical energy input; therefore, its intensity output is restricted. In addition, due to the high peak power requirements, these transducers often experience extremely high physical forces upon the constituent elements and can fail after repeated use over time. A much more stable US source is highly desired.
This paper describes evaluation of transducers designed for effective US-mediated gene therapy in a porcine model. Spherical and cylindrical concave lenses were utilized to focus the acoustic energy and reduce the electrical power requirement, which allowed for exploration of US-mediated gene transfection at higher pressures.
Results
Large Aperture, Multi-lensed Focused Transducers for Gene Delivery
Four different transducers were designed and used in these studies. The H185 series was designed to enhance the acoustic capability of the transducer in comparison to H105. Several acoustic objectives were used to design each H185 iteration: (1) to deliver uniform high-intensity US throughout the acoustic field with slight focusing capability; (2) to eliminate pressure peaks and nulls in the near-field; (3) to develop a pressure focal gain of 2.0–2.8; (4) to sustain constant cross-sectional treatment area throughout the radiating field; and (5) to operate over a ±0.20% operating band from 1.1 MHz both acoustically and electrically. Table 1 summarizes each transducer’s properties and characteristics. The effective treatment area refers to the area within the near-field where the majority of emitted acoustic power is distributed. The relative pressure output of each transducer was measured using a rugged piezoceramic hydrophone placed at each transducer’s focal plane and normalized to 1 MPa peak pressure at acoustic maximum (Figure 1).
Table 1.
Transducers Used and Their Characteristics
| Transducer | No. of Elements/Lenses | Area (in cm2) | Treatment Area (in cm2) (in %) | Focal Depth (in mm) | Focal Gain | Efficiency (in %) | ||
|---|---|---|---|---|---|---|---|---|
| H105 | 2 | – | 25.9 | 18.86 | 74 | 550 | 2 | 85 |
| H185A | 1 | 19 | 18.6 | 0.60 | 4 | 3 | 2.8 | 60 |
| H185B | 1 | 5 | 16.1 | 4.27 | 23 | 15 | 2.6 | 50 |
| H185D | 1 | 3 | 18.9 | 2.15 | 11 | 20 | 4.5 | 57 |
Figure 1.
Relative Pressure Plots from Different Planar and Multi-lensed Therapeutic US Transducers
Relative pressure plots were obtained using a hydrophone placed at the focal plane for each US transducer: at z = 1 mm for H105 (A), at z = 3 for H185A (B), at z = 15 mm for H185B (C), and at z = 20 mm for H185D normalized to up to −20 dB (D), and the relative pressure is encoded in color: warm for higher acoustic pressures and cool colors for lower pressures.
H105 is a 1.1-MHz, dual-element transducer previously used in our dog studies. It has a 52-mm aperture, with an effective treatment area of 18.86 cm2, or about 74% of the transducer face. The transducer was apodized, such that the outer element was only fired at 50% of the inner element, to create a more uniform acoustic field (Figure 1A).
H185A is a 1.1-MHz, single-element transducer, with an aluminum faceplate containing 19 individual 10-mm plano-concave lenses and calculated focal depth of 20 mm. Measured relative pressure output revealed an actual focus at 3 mm. At the focus, there are 19 point foci, with a total effective treatment area of only 0.6 cm2, or 4% of the transducer face (Figure 1B). The design of 19 lenses was intended to emulate the size of the acoustic field achieved by H105. Focusing capability has been added to enhance concentrated regions of acoustic energy.
H185B is also a 1.1-MHz, single-element transducer, with five plano-concave lenses with a calculated cylindrical focus of 20 mm. Measured relative pressure output revealed an actual focus at 15 mm. There are five lines at the focus, with a total effective treatment area of 4.27 cm2, or about 23% of the transducer face (Figure 1C). Cylindrical lens were used as opposed to spherical lens to improve both effective treatment area and focal depth and to maintain focal gain above 2.0.
H185D is a 1.05-MHz, single-element transducer, with three plano-concave cylindrical lenses with a deeper focus of 20 mm. There are three parallel lines at the focus, with a total effective treatment area of 2.15 cm2, or about 11.4% of the face (Figure 1D). Here, both effective treatment area and focal depth were improved relative to H185A and H185B.
These transducers also have high efficiencies (between 50% and 85%) in converting electrical to acoustic energies. Their diameters were kept between 2 and 3 inches to treat a wide area. The pressure plots shown in Figure 1 also shows the effective treatment areas of the transducers, which vary from 74% (H105) to a more focused area representing 4% (H185A). With improved focusing of the H185 transducers, the input electrical power was significantly reduced from 8,500 W (H105) to 300 W (H185D) to achieve 2.7 MPa PNP, and higher focal pressures were investigated.
Enhanced Transfection Results Using the Multi-lensed, Focused Transducers
In our previous study in canine livers, we demonstrated that we can achieve successful US-mediated gene transfection using the H105 transducers. Different US parameters, as well as experimental and surgical conditions were investigated to gradually improve gene transfection. The optimal conditions were used in these subsequent swine studies, and these include MB (0.2 mL/kg) and pGL4 (0.67 mg/kg) concentrations, injection rate of about 10 mL/min, treatment time of 4 min, and US conditions of 20 cycle pulses, 50 Hz pulse repetition frequency, and about 1 MHz fundamental frequency. The pGL4 and MB solution was injected into a PV branch with the IVC occluded, and the transducers were scanned at a rate of 1 cm/s across the targeted liver surface. After 24 hr, the pigs were sacrificed, and the treated lobe (left lateral lobe) and the control lobe (right lateral lobe) were harvested and assayed for luciferase expression.
Figure 2 shows the average luciferase expression using different transducers and PNPs. Each symbol represents the expression from a section of the treated liver, which was processed and assayed, and the horizontal line indicates the average expression. The entire liver lobe was sectioned and spatially mapped, and every other section was assayed (Supplemental Materials and Methods). Sections with the highest level of expression that indicated treatment near or at the focus of the transducer were selected for representation. The sham control is from pig livers injected with pGL4 and MB solution without US treatment. Using 8,500 W to obtain 2.7 MPa PNP with the H105 transducer, the average luciferase expression is 2,420 relative light units (RLU)/mg protein, which is 106-fold better than the sham expression of 22 RLU/mg protein. This is very similar to the enhancement obtained using the same conditions in dog livers.11 The expression was further enhanced using the H185 transducers. With a focal gain of about 2.8, H185A only required 3,000 W to achieve 2.7 MPa and 3,500 W to produce 3.3 MPa, achieving average luciferase expressions of 10,200 RLU/mg protein and 3,940 RLU/mg protein, respectively. This is up to 445-fold better than sham and 4-fold better than using the H105. It is surprising that the expression at 2.7 MPa was relatively higher than at 3.3 MPa PNP. Since H185A is a spherically focused transducer, it is possible when pDNA and MBs were infused into the liver lobes at higher PNPs (3.3 MPa), MBs were immediately destructed and did not have a chance to distribute to other areas, whereas at lower PNPs (2.7 MPa), pDNA and MBs are capable of distributing to larger areas of the liver to generate higher gene expression. The difference could also be due to larger standard deviations due to the limited number of pig experiments.
Figure 2.
Luciferase Gene Expression following UTMD-Mediated Gene Delivery into the Pig Livers Using Different Transducers
With the IVC clamped, a solution of pGL4 (0.67 mg/kg), neutral MBs (0.2 mL/kg), and 50% glucose (0.2 mL/kg) in PBS (2 mL/kg) was injected into a segmental PV branch with simultaneous exposure of the target liver lobe to tUS (1.05 to 1.1 MHz frequency, 20 cycle pulses, 50 Hz pulse repetition frequency, and 0 to 6.2 MPa PNPs) for 4 min using H105, H185A, H185B, and H185D. A sham-treated pig received an equivalent pGL4 and MB dose but was not exposed to tUS (or 0.0 MPa PNP tUS exposure). “N” denotes the number of pigs used in the experiments. Treated and untreated control lobes were harvested after 24 hr. Each lobe was sectioned (n = 12–15 sections/lobe), and each portion was processed and assayed for luciferase activity, which is shown above as data points. The average luciferase expression for each treated lobe is shown as horizontal lines. * indicates treatment group is statistically different from other groups, *p < 0.05, **p < 0.005, ***p < 0.0005.
H185B required 4,200 W to achieve 2.7 MPa because of the 2.6 focal gain from five cylindrical lenses. Average luciferase expression was 4,600 RLU/mg protein, which is 203-fold better than sham and twice as much as the H105 transducer. Studies were also done at 3.3 MPa, which needed 6,200 W, with enhanced expression averaging 12,320 RLU/mg protein, or 538-fold improvement than sham. However, materials used in the construction of H185A and H185B continued to restrict PNP outputs to an upper limit of 3.3 MPa.
Therefore, new materials and design were used in constructing H185D. It has a better focal gain of 4.5 and was still able to further improve transfection with only three cylindrical foci. With H185D’s improved gain, it only needed 980 W to reach 4.5 MPa pressure. At 4.5 MPa PNP, expression improved and resulted in an average of 25,450 RLU/mg protein, or over 1,110-fold better than control and about 10-fold better than using H105. We were also able to push the transducer more by driving it with 1,930 W to obtain 6.2 MPa at the focus. The result is an average luciferase expression of 37,930 RLU/mg protein, with some sections expressing as high as 198,420 RLU/mg protein—similar to results obtained from our rat studies.13 This is over 1,650-fold enhancement from control, or at least 15-fold better than H105.
A single-factor (or one-way) ANOVA was performed to determine if the average luciferase expression resulting from using different transducers are statistically different. One-way ANOVA analysis revealed evidence that luciferase expression of samples insonated by certain transducers were significantly different from others (p < 0.001). Follow-up testing for individual comparison was performed with correction for multiple comparisons using a Tukey test post-hoc. Use of H185A at 2.7 MPa produced significantly greater gene transfer compared to using H105 at the same PNP (p < 0.005). Gene expression was further significantly enhanced by revising the spherical lens of H185A to cylindrical lens in H185B (p < 0.005). Comparing H185D at 6.2 MPa to all other transducers and PNP settings, gene transfer was significantly greater (p < 0.05–0.0005).
Increasing PNPs Using H185D Can Significantly Improve Luciferase Expression
Similarly, H185D was used to determine whether generating different pressures affects luciferase expression. Figure 3 shows the average luciferase expression at various pressures using H185D (from 0 MPa to 6.2 MPa PNP). With focusing, H185D required approximately 157 W, 482 W, 984 W, and 1,930 W to produce 1.8 MPa, 3.1 MPa, 4.5 MPa, and 6.2 MPa PNPs, respectively. Correspondingly, increasing the focal PNPs significantly enhanced the luciferase expression from 420 RLU/mg protein, 1,480 RLU/mg protein, 17,750 RLU/mg protein and 37,930 RLU/mg protein, respectively (p < 0.001, single-factor ANOVA with Tukey test post-hoc). Since all other US parameters were kept the same, the amplitude or the intensity of the cavitation effects most likely caused this difference in expression. The more violent the cavitation produced, the more perturbations in the cell membranes are created, allowing for pDNA to enter the cells. Higher pressures were not further explored because of the transducer’s limitations.
Figure 3.
Luciferase Gene Expression following UTMD-Mediated Gene Delivery into the Pig Livers at Different Pressures Using H185D
With the IVC clamped, a solution of pGL4 (0.67 mg/kg), neutral MBs (0.2 mL/kg), and 50% glucose (0.2 mL/kg) in PBS (2 mL/kg) was injected into a segmental PV branch with simultaneous exposure of the target liver lobe to tUS (1.05 MHz frequency, 20 cycle pulses, 50 Hz pulse repetition frequency) at 0 MPa (sham), 1.8 MPa, 3.1 MPa, 4.5 MPa, and 6.2 MPa PNPs for 4 min using H185D. A sham-treated pig received an equivalent pGL4 and MB dose but was not exposed to tUS (0 MPa). Three pigs per group (n = 3/group) were used for each US condition. Treated and untreated control lobes were harvested after 24 hr, and each lobe was sectioned (n = 12–15 sections/lobe). Each portion was processed and assayed for luciferase activity, which is shown above as data points, the average luciferase expression for each treated lobe is shown as horizontal lines, and error bars indicate SD. *treatment group is statistically different from other groups, p < 0.001.
Transaminase Levels Mostly Remain within Normal Levels
In order to assess the extent of injury or damage in the liver tissue, levels of transaminase enzymes, including alanine-aminotransaminase (ALT) and aspartate-aminotransaminase (AST), were determined. These enzymes are specifically expressed in hepatocytes and serve as known markers for liver damage or injury.14 Blood samples were collected 24 hr after surgery and were sent to a commercial diagnostic laboratory for analysis. Figure 4A shows the transaminase levels from studies using different transducers (H105, H185A, H185B, and H185D). Normal swine ALT and AST levels are between 60 and 90 U/L. All but one are within the normal ALT and AST levels, indicating that no significant injury is caused by surgery and US exposure from these transducers (p = 0.224 and p = 0.505 for ALT and AST, respectively). Similarly, Figure 4B shows the transaminase levels at different pressures using H185D. All studies are within the normal ALT and AST levels, except for one experiment with slightly higher AST. In this pig, which was exposed to 4.5 MPa pressure using H185D, the AST level was 249 U/L, indicating some minor damage to the liver. The hepatic damage may be related to US or surgical manipulation.
Figure 4.
Evaluation of Serum Liver Transaminase Enzymes in Treated Pigs
Blood was collected from all experimental pigs 24 hr post-surgery for a complete blood count and chemistry panel. The plasma levels of liver enzymes, alanine- (ALT) and aspartate-aminotransferase (AST) were examined from pigs exposed to different US transducers and pressures (A) and from pigs exposed to H185D at increasing pressures (B). ALT and AST levels were found to be within normal for all treated pigs, except for one case, which was exposed to 4.5 MPa and was found to be not statistically different. Average values are given and error bars indicate SD.
Nevertheless, a single-factor ANOVA, followed by a Tukey test post-hoc, indicated no difference in ALT and AST levels among sham and US-treated livers using different transducers (p = 0.432). Additionally, there was no difference in ALT and AST levels among sham and H185D-treated livers at increasing pressures (p = 0.791).
Histological Analysis Shows Some Minor Tissue Damage
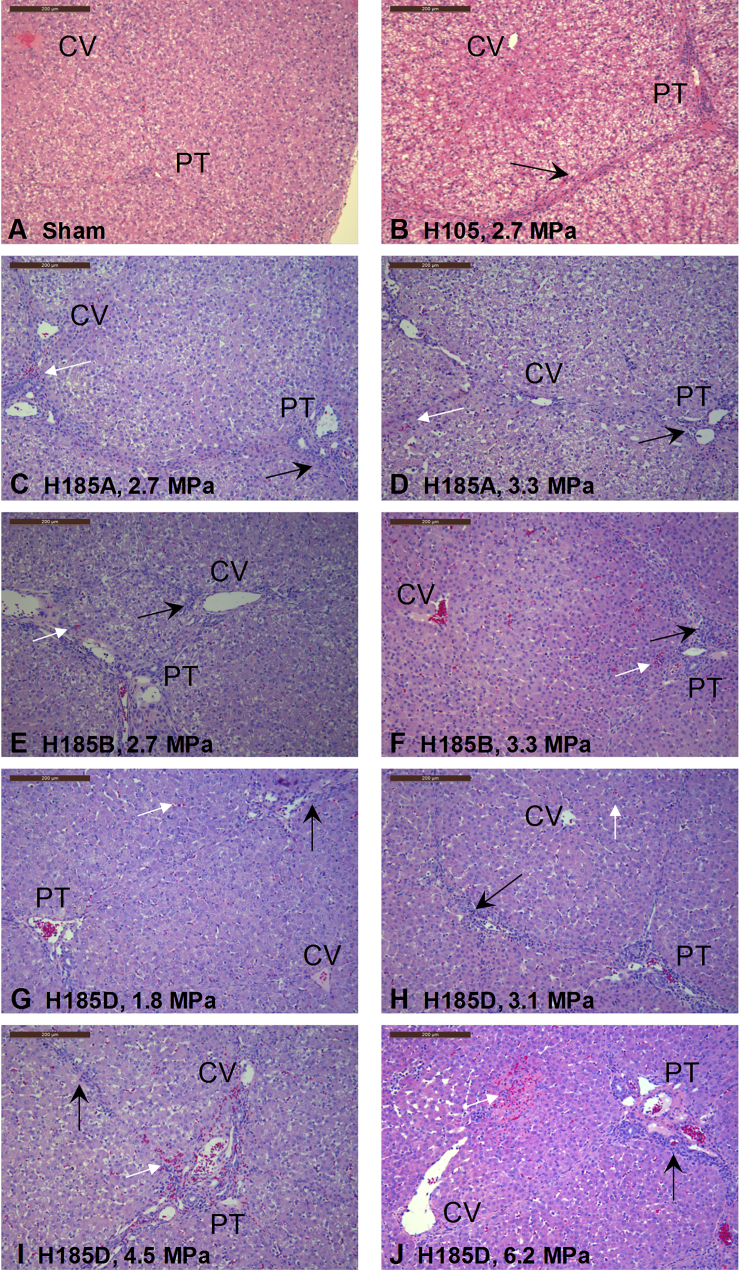
Liver tissue sections were acquired 24 hr after surgery to determine if exposure to US caused any injury or tissue damage. Sections were obtained from the central area of the treated lobe, which received the maximum US exposure, and also from the control lobe, which did not receive any US exposure. Representative images of H&E-stained slides from treated and control livers are shown in Figure 5. These images were obtained from livers exposed to different transducers (H105, H185A, H185B, and H185D) and pressures (0 MPa to 6.2 MPa). Most of them reveal minor tissue damage and portal inflammation (Figure 5, black arrow), which was also observed in the sham control (Figure 5A). In addition, there is focal subcapsular hemorrhage (Figure 5, white arrows) on the treated surface using H105 (Figure 5B), H185A (Figures 5C and 5D), and H185B (Figures 5E and 5F). The studies with higher pressures using H185D, as shown in Figures 5I and 5J, resulted in focal perivascular tissue damage, hemorrhage, and vascular injury that were not seen in livers exposed to lower pressures using H185D (Figures 5G and 5H).
Figure 5.
Histological Analysis of Treated Pig Livers Showed Some Damage Only at Higher Pressures
Representative H&E-stained images from treated pig livers using different US transducers and pressures are shown. (A) Sham control was used for comparison. A transducer was scanned across the treated liver, but no US was applied. Increased leukocyte infiltration was observed as indicated by black arrows, as well as dilatation of the sinusoids. Treated livers using H105 (B), H185A (C and D), and H185B (E and F) at 2.7 MPa and at 3.3 MPa, respectively, showed similar inflammatory response as seen in the sham-treated livers (A), with some focal hemorrhaging as indicated by white arrows. Using H185D at lower pressures, 1.8 MPa (G) and 3.1 MPa (H), also produced minimal damage similar to the other treated livers. But at higher pressures, 4.5 MPa (I) and 6.2 MPa (J), hemorrhaging and disruption of the vessels were much pronounced on the vessel and its surrounding tissue. Incidentally, luciferase expression at these higher pressures was also found to be highest with sections expressing up to 8,600-fold better than control. All images were originally obtained at 10× magnification. Scale bars are 200 μm.
Discussion
US-mediated gene delivery has potential for clinical use to treat genetic diseases such as hemophilia because of its low immunogenicity and spatial and temporal control.11, 12, 15 It makes use of the oscillating and cavitating effects of exogenous cavitation nuclei, such as MBs, when exposed to US to physically create temporary openings in cell membranes and in the vasculature. The intensity of cavitation is dependent on the magnitude of pressure generated by the US transducer and its power source.16
The goal of this project is to not only demonstrate the potential of US-mediated gene delivery, but to further improve gene transfection to levels toward clinical use. Part of this endeavor is to design and develop new therapeutic US transducers that are efficient and effective for successful gene delivery. The initial H105 transducer that was used has a large diameter that covers a wide area and was effective in US-mediated gene delivery in dog livers. With minimal damage incurred to the treated liver, we aimed to investigate gene transfection at higher pressures. But, this was not possible with the H105 transducer. With about 8,500 W electrical power into the H105, it only produces about 2.7 MPa PNP and approaches the maximum power the transducer can convert before mechanical failure.17
The next strategy is to employ high-intensity focused US (HIFU), where it is currently used in minimally or non-invasive clinical applications, such as cancer and tumor ablation, and lithotripsy.18, 19, 20, 21 HIFU therapeutic effects, either thermal or mechanical, can be generated at its focus, which is about the size of a single grain of rice, without any damage to the surrounding tissues. Focusing can be achieved electronically using multiple elements arranged in a phased array or geometrically with the use of lenses or curved transducers. In our case, spherical and cylindrical lenses were employed in constructing the next set of transducers with dimensions similar to the previous planar transducer. These plano-concave lenses were defined using a simple lens formula to calculate an effective focus of 20 mm away from the transducer face. Point spread function simulations were also performed in superimposing the magnitude and phase of each individual lens’ effective focus to determine the expected propagating field. However, pressure maps obtained using a hydrophone reveal that the peak intensities for the H185A and H185B are closer than initially planned, at 3 mm and 15 mm, respectively, while sustaining a pressure focal gain of 2.0 and 2.8. With those focal gains, the electrical power inputs were significantly brought down to generate 2.7 MPa pressure at the focus and were even further driven harder to produce 3.3 MPa. The earliest iteration of the H185 series, H185A, attempted to emulate the acoustic field area achieved by H105 but with added focusing capability. The focal gain was able to be increased, which improved PNP output. Luciferase gene transfection at 2.7 MPa and at 3.3 MPa using H185A and H185B transducers were much improved compared to using H105, even when the effective treatment areas for these transducers were also reduced down from 76% for H105 to 4% for H185A and 23% for H185B of their corresponding transducer’s face. At 3.3 MPa, no significant damage was observed by the transaminase assay as well as analysis of the stained slides. Yet again, the design and the materials used in the construction of these transducers prevented further transfection studies at higher focal pressures, which luciferase gene expression results suggest is required.
A new material was used in constructing the faceplate containing the lenses placed in front of the piezo-ceramic material. Also, the cylindrical lenses were found to have better coverage than the spherical lenses in comparing H185A to H185B, such that the next transducer, H185D, was constructed with three plano-concave cylindrical lenses that focus at 20 mm away from the transducer face. With the new faceplate material, H185D has a pressure focal gain of 4.5 and allowed for conducting luciferase transfection studies at 4.5 MPa and at 6.2 MPa pressures with only a fraction of the initial electrical input power (11.5% and 23.3%). In addition, luciferase expression at these pressures were significantly improved—up to 1,650-fold enhancement from control, and some focal sections had markedly increased luciferase expression comparable to the high expression levels obtained in US-mediated transfection studies in rats. Even when the effective treatment area for H185D is reduced to 11% of its transducer face, the improved focusing allowed for delivery of high acoustic energies necessary for successful gene transfection. When comparing the spread of gene expression data across the different treatment groups, we noticed luciferase expression was more scattered using H105 than when using H185D. This may be due to a difference in the acoustic field of the high-intensity US between H105 and H185D. While the H105 was apodized to minimize near-field transaxial pressure variations, the acoustic field still maintains some non-uniformity and produces some areas with high PNP exposure and others at relatively lower PNPs. However, H185D was designed to deliver a uniform and constant cross-sectional high-intensity US throughout the radiating field.
At high acoustic pressures, it is important to move or scan the transducer across the liver lobe to avoid unnecessary tissue damage. The treated livers exposed to 6.2 MPa pressure showed some visible surface discoloration or bruising during necropsy. Upon further analysis by histology, the extent of damage is indicated by focal areas of necrosis and hemorrhage in the subcapsular and surface areas and transient hepatic distention caused by the occlusion of the IVC, which was also observed in control and sham-treated lobe.22 The tissue damage was not as severe as seen in other cavitation-based HIFU applications, such as lithotripsy and tumor and solid organ ablation, which can produce large areas of coagulative necrosis due to in situ pressures of between 10 and 20 MPa.23, 24 Furthermore, determination of the transaminase enzyme levels, ALT and AST, shows that most of the pigs treated with US in this study have normal levels, indicating that the US exposure induced minimal hepatic injury. In only one instance where a pig was exposed to 4.5 MPa pressure, there was mild elevated AST levels compared to normal ranges, suggesting mild acute hepatic damage. These mild hepatic injuries will probably spontaneously heal within a few days as seen by our long-term rat studies.13 In addition, our lab has recently demonstrated that prolonging the pulse duration can decrease the pressure threshold required for efficient gene transfer, which can further decrease the potential tissue damage.25
In order to achieve efficient US-mediated gene transfer, another important component to consider is the characteristics of the MBs. In the current study, we used homemade RN18 MBs. The MB sizes range from 0.5 μm to 10 μm in diameter.26 The number-weighted size distributions showed that most (>95%) of the MBs were smaller than 2 μm diameter with a peak at ∼0.8 to 0.9 μm. The volume-weighted distributions showed that some larger size MBs existed between 2 μm and 10 μm diameter. Sirsi et al.27, 28 reported that larger MBs cavitate more robustly than smaller MBs. However, larger MBs may not easily pass through the sinusoids. It is believed that at 1 MHz, cavitation of some MBs will need to occur to facilitate the entry of MBs into the extravascular space to target hepatocytes.11, 13 It would be significant to investigate in the area of MB design, including the use of cationic and/or liver-targeted MBs for clinical translation of US-mediated gene transfer.
In another recent study in mice, we found that majority of the transgene expression occurs in hepatocytes (S. Song et al., 2014, Int. Soc. Therapeutic Ultrasound, abstract); however, pDNA vector also transfected nonparenchymal cells. It is believed that with cavitation of MBs, the endothelial barrier was broken down to allow pDNA and MB distribution into extravascular space to target hepatocytes. Furthermore, to prepare for clinical translation, we are currently developing minimally invasive surgery procedures for US-mediated gene transfer in large-animal models (D.M. Tran et al., 2018, Am. Soc. Gene Cell Ther., abstract). This procedure will involve occlusion of a main hepatic vein branch via jugular vein access, and therapeutic US is localized at the target tissue site and applied transcutaneously to facilitate pDNA transfer. This minimally invasive technique is highly promising for future clinical translation.
In conclusion, we showed that single-element, multi-lensed transducers, with focal gains between 2.0 and 4.5, reduced the electrical power input to generate high focal pressures necessary for successful gene transfection. Luciferase expression using H185 transducers were significantly improved, with up to 1,650-fold enhancement over controls and focal areas having markedly increased luciferase expression similar to that obtained in our rat studies. Furthermore, focused US can also pave the way toward minimally or non-invasive US-mediated gene therapy, which will be most suitable for clinical applications. Thus, with the new development of transducer design, minimally invasive surgical procedures, and new MBs with enhanced characteristics, significant enhancement of gene transfer efficiencies can be achieved to warrant US-mediated gene transfer a viable and safe clinical approach.
Materials and Methods
Plasmids and MBs
A luciferase reporter plasmid with an SV40 promoter, pGL4.13 (luc2 driven by SV40 promoter) (Promega, Madison, WI) was produced by GenScript (Piscataway, NJ) according to standard techniques.
Preparation and characterization of the MBs were previously described by Sun et al.26 In brief, MBs were prepared by first combining aliquots of three different lipids—1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 1,2-distearoyl-sn-glycero-3-phosphate (DSPA), and N-(carbonyl-methoxypolyethyleneglycol 5000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine (MPEG-5000-DSPE) (Avanti Polar Lipids, Alabaster, AL)—in a 3-mL vial and re-constituting them in PBS solution containing 10% glycerol (Sigma-Aldrich, St. Louis, MO). The vials were capped, and a gas exchange was performed to evacuate the air and replace it with octofluoropropane (American Gas Group, Toledo, OH). MBs were generated by vigorously shaking the vial for 45 s using a Vialmix agitator (Lantheus, N. Billerica, MA). The average MB diameter is about 1.5 μm, and concentration per vial is about 5.0 × 109 MBs/mL.
Transducers and US Systems
Several tUS transducers were designed and built in this study (Sonic Concepts, Bothell, WA). Each transducer was coated with a thin film of epoxy and the housing is plastic. The transducer face, housing, and cable exit are water-tight. The transducer interior was filled with acoustic backing material to make them hermetic throughout. Table 1 lists the various transducers used and their characteristics. H105 is a 1.1-MHz dual-element transducer with an active beam diameter of 57.4 mm and was apodized to reduce near-field interferences and create a more homogeneous acoustic field. The planar H105 transducer was used in successful gene-transfer experiments in dogs, requiring about 8,200 W to obtain a PNP of 2.7 MPa.11 Next, a series of single-element, multi-lensed transducers were designed to reduce the electrical power input and produce higher focal pressures. H185A contains 19 spherical lenses that focus 3 mm away from the transducer face. It has a focal gain of 2.8 and effective treatment area of 13.46 cm2. H185B contains five spherical lenses that focus 15 mm from the transducer face and a focal gain of 2.6. H185C is also spherically focused, having three lenses that focus at 20 mm away, and a focal gain of 4.5.
An HP 4194A Impedance/Gain-Phase Analyzer was used to measure the electrical input impedance of each transducer, and then a 50 ohm radio frequency (RF) matching network was built to minimize reflections and maximize power transfer.
All of the transducers were connected to a power source: a combination pulse generator and radio-frequency power amplifier (RPR-4000-HP pulser/receiver; Ritec, Warwick, RI) that can deliver up to 15 kW electrical power. It is controlled by a custom software interface (Sonic Concepts) to generate US signal.
Pig Surgeries
All procedures were performed according to the guidelines for animal care of both the NIH and Seattle Children’s Research Institute (SCRI), with protocol approval of Institutional Animal Care and Use Committee.
Domestic swine (8–15 kg) were obtained from Washington State University (Pullman, WA). These pigs were acclimated for at least 3 days prior to surgery. After induction of general anesthesia (ketamine and xylazine subcutaneously, and 3% isofluorane by inhalation), each animal was placed in supine position. The abdomen was shaved, prepped and draped in a sterile fashion. A midline incision was made, and a balfour retractor was used to further expose the liver. The left lateral liver lobe was flipped over to expose the major vessels on the dorsal surface. A 20G × 1.25-inch angiocath (Becton Dickenson, Franklin Lakes, NJ) was inserted in the PV segmental branch leading to the left lateral lobe. The pGL4 and MB solution was drawn into a syringe and attached to the angiocath via an extension set. The IVC was occluded prior to injection of pGL4 and MB solution (2 mL/kg solution containing 0.67 mg/kg pGL4, 0.2 mL/kg MBs, 0.2 mL/kg 50% glucose, and enough PBS to total volume) to improve localization of the pGL4 and MBs in the liver. Shortly after occlusion, tUS was applied simultaneously with this injection on the liver surface for 4 min (1.05 to 1.1 MHz frequency, 20 cycle pulses, 50 Hz pulse repetition frequency). The MB distribution in the target liver lobe was visualized using a 4V1 vector array transducer connected to an Acuson Sequoia C512 imaging system (Siemens, Mountain View, CA) before and after tUS treatment (see Videos S1, S2, and S3 of Expt 73). This allowed us to verify that the pGL4 and MB solution was going into the target liver lobe and also to see if the MBs were retained after tUS application, after which cannulation sites were repaired, and the incision was closed using sutures and surgical staples. Post-operative local (lidocaine) and systemic (ketoprofen) analgesics were also administered. The pigs were recovered, and after 24 hours, they were sacrificed, and the treated and control lobes were sectioned and processed for luciferase expression. Blood and tissue samples were collected for liver enzyme tests and histological analysis.
Gene Expression Evaluation by Luciferase Assay
Harvested lobes (right and left lateral lobes) were resected following euthanasia. The right lateral lobe, which was not directly injected with the pGL4 and MB solution and was not exposed to tUS, was collected as control for comparison with the treated lobe (left lateral lobe). The treated and control lobes were then sectioned into smaller pieces (∼2 to 3 g), which were then assayed for luciferase expression (Supplemental Materials and Methods). Tissues were homogenized at a ratio of 3 mL/g in reporter lysis buffer (Promega, Madison, WI) and were exposed to three freeze-thaw cycles to completely release the luciferase protein. The homogenates were vortexed and centrifuged at 18,000 × g for 5 min, and the supernatant was collected and stored in −80°C until measurement. A commercially available kit was used to perform the luciferase assay (Luciferase Assay System, cat. E1500, Promega). The light produced by the oxidation of luciferin was measured by a luminometer (Victor 3; PerkinElmer, Wellesley, MA), followed by a protein assay to normalize luciferase activity to the total protein content (RLU per mg protein).
Blood Analysis
Collected blood samples were sent to a commercial veterinary diagnostic laboratory (Phoenix Central Laboratory, Mukilteo, WA) for a complete blood count and a chemistry panel including alanine- and aspartate-aminotransferase, ALT and AST, respectively, for determination of liver damage.
Histological Analysis
Immediately after harvesting the treated and control lobes of the liver, sections were fixed in 10% neutral buffered formalin. Tissues were sent to a pathology lab (Seattle Children’s Hospital) for processing and embedding in paraffin. Sections were stained with H&E and Masson’s trichrome to determine hepatocyte abnormalities and cellular changes and evaluated by a surgical pathologist (K.R.L.).
Statistical Analysis
A single-factor ANOVA was performed using a statistical package (MiniTab 16, MiniTab, State College, PA) to evaluate the luciferase gene expression from each treatment group. If statistical difference was found, a Tukey test was also performed post-hoc for multiple pairwise comparisons. Similarly, ALT and AST enzyme levels were evaluated using single-factor ANOVA. For all analyses, a p < 0.05 was deemed to be statistically significant.
Author Contributions
M.L.N.-V. designed and performed research, analyzed data, and wrote the paper. S.S., R.R.S., and K.R.L. performed research. D.M.T. analyzed data and revised the paper. K.P.M. and G.W.K. designed and made the transducer. C.H.M. designed the project, performed research, analyzed data, and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
We would like to thank Dr. Ida Washington, Marla Paun, Luping Fan, Devin Margolies, and Jennifer Jagielski for their assistance with the pig surgeries and Drs. Colleen OKelly-Priddy and Robert DiBlasi for helping with the initial setup of these experiments. This work is supported by grants from the NIH-NHLBI: R01 HL69049 and R21/33 HL089038.
Footnotes
Supplemental Information includes one figure and three videos and can be found with this article online at https://doi.org/10.1016/j.omtm.2018.06.008.
Supplemental Information
References
- 1.Miao C., Brayman A.A. Ultrasound-mediated gene delivery. In: Yuan X., editor. Non-Viral Gene Therapy. Intech; 2011. pp. 213–242. [Google Scholar]
- 2.Chen Z.Y., Lin Y., Yang F., Jiang L., Ge Sp. Gene therapy for cardiovascular disease mediated by ultrasound and microbubbles. Cardiovasc. Ultrasound. 2013;11:11. doi: 10.1186/1476-7120-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman C.M., Bettinger T. Gene therapy progress and prospects: ultrasound for gene transfer. Gene Ther. 2007;14:465–475. doi: 10.1038/sj.gt.3302925. [DOI] [PubMed] [Google Scholar]
- 4.Cao W.J., Matkar P.N., Chen H.H., Mofid A., Leong-Poi H. Microbubbles and Ultrasound: Therapeutic Applications in Diabetic Nephropathy. Adv. Exp. Med. Biol. 2016;880:309–330. doi: 10.1007/978-3-319-22536-4_17. [DOI] [PubMed] [Google Scholar]
- 5.Negishi Y., Endo-Takahashi Y., Maruyama K. Gene delivery systems by the combination of lipid bubbles and ultrasound. Drug Discov. Ther. 2016;10:248–255. doi: 10.5582/ddt.2016.01063. [DOI] [PubMed] [Google Scholar]
- 6.Nayak S., Herzog R.W. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17:295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang H.D., Tang J., Halliwell M. Sonoporation, drug delivery, and gene therapy. Proc. Inst. Mech. Eng. H. 2010;224:343–361. doi: 10.1243/09544119JEIM565. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki R., Oda Y., Utoguchi N., Maruyama K. Progress in the development of ultrasound-mediated gene delivery systems utilizing nano- and microbubbles. J. Control. Release. 2011;149:36–41. doi: 10.1016/j.jconrel.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Stride E. Physical principles of microbubbles for ultrasound imaging and therapy. Cerebrovasc. Dis. 2009;27(Suppl 2):1–13. doi: 10.1159/000203122. [DOI] [PubMed] [Google Scholar]
- 10.Delalande A., Kotopoulis S., Postema M., Midoux P., Pichon C. Sonoporation: mechanistic insights and ongoing challenges for gene transfer. Gene. 2013;525:191–199. doi: 10.1016/j.gene.2013.03.095. [DOI] [PubMed] [Google Scholar]
- 11.Noble M.L., Kuhr C.S., Graves S.S., Loeb K.R., Sun S.S., Keilman G.W., Morrison K.P., Paun M., Storb R.F., Miao C.H. Ultrasound-targeted microbubble destruction-mediated gene delivery into canine livers. Mol. Ther. 2013;21:1687–1694. doi: 10.1038/mt.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song S., Shen Z., Chen L., Brayman A.A., Miao C.H. Explorations of high-intensity therapeutic ultrasound and microbubble-mediated gene delivery in mouse liver. Gene Ther. 2011;18:1006–1014. doi: 10.1038/gt.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song S., Noble M., Sun S., Chen L., Brayman A.A., Miao C.H. Efficient microbubble- and ultrasound-mediated plasmid DNA delivery into a specific rat liver lobe via a targeted injection and acoustic exposure using a novel ultrasound system. Mol. Pharm. 2012;9:2187–2196. doi: 10.1021/mp300037t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green R.M., Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367–1384. doi: 10.1053/gast.2002.36061. [DOI] [PubMed] [Google Scholar]
- 15.Miao C.H., Brayman A.A., Loeb K.R., Ye P., Zhou L., Mourad P., Crum L.A. Ultrasound enhances gene delivery of human factor IX plasmid. Hum. Gene Ther. 2005;16:893–905. doi: 10.1089/hum.2005.16.893. [DOI] [PubMed] [Google Scholar]
- 16.Azmin M., Harfield C., Ahmad Z., Edirisinghe M., Stride E. How do microbubbles and ultrasound interact? Basic physical, dynamic and engineering principles. Curr. Pharm. Des. 2012;18:2118–2134. doi: 10.2174/138161212800099955. [DOI] [PubMed] [Google Scholar]
- 17.Morrison K.P., Keilman G.W., Noble M.L., Brayman A.A., Miao C.H. High intensity ultrasound transducer used in gene transfection. AIP Conf. Proc. 2012;1503:288. [Google Scholar]
- 18.Miller D.L., Smith N.B., Bailey M.R., Czarnota G.J., Hynynen K., Makin I.R., Bioeffects Committee of the American Institute of Ultrasound in Medicine Overview of therapeutic ultrasound applications and safety considerations. J. Ultrasound Med. 2012;31:623–634. doi: 10.7863/jum.2012.31.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenne J.W., Preusser T., Günther M. High-intensity focused ultrasound: principles, therapy guidance, simulations and applications. Z. Med. Phys. 2012;22:311–322. doi: 10.1016/j.zemedi.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Foldager C.B., Kearney C., Spector M. Clinical application of extracorporeal shock wave therapy in orthopedics: focused versus unfocused shock waves. Ultrasound Med. Biol. 2012;38:1673–1680. doi: 10.1016/j.ultrasmedbio.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Wijlemans J.W., Bartels L.W., Deckers R., Ries M., Mali W.P., Moonen C.T., van den Bosch M.A. Magnetic resonance-guided high-intensity focused ultrasound (MR-HIFU) ablation of liver tumours. Cancer Imaging. 2012;12:387–394. doi: 10.1102/1470-7330.2012.9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cullen J.M., Van den Ingh T.S., Bunch S.E., Rothuizen J., Washabau R.J., Desmet V.J. Morphological classification of circulatory disorders of the canine and feline livers. In: Group W.L.S., editor. WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Disease. Saunders Elsevier; 2006. pp. 41–59. [Google Scholar]
- 23.Cheung T.T., Fan S.T., Chan S.C., Chok K.S., Chu F.S., Jenkins C.R., Lo R.C., Fung J.Y., Chan A.C., Sharr W.W. High-intensity focused ultrasound ablation: an effective bridging therapy for hepatocellular carcinoma patients. World J. Gastroenterol. 2013;19:3083–3089. doi: 10.3748/wjg.v19.i20.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vlaisavljevich E., Kim Y., Allen S., Owens G., Pelletier S., Cain C., Ives K., Xu Z. Image-guided non-invasive ultrasound liver ablation using histotripsy: feasibility study in an in vivo porcine model. Ultrasound Med. Biol. 2013;39:1398–1409. doi: 10.1016/j.ultrasmedbio.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran D.M., Harrang J., Song S., Chen J., Smith B.M., Miao C.H. Prolonging pulse duration in ultrasound-mediated gene delivery lowers acoustic pressure threshold for efficient gene transfer to cells and small animals. J. Control. Release. 2018;279:345–354. doi: 10.1016/j.jconrel.2018.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun R.R., Noble M.L., Sun S.S., Song S., Miao C.H. Development of therapeutic microbubbles for enhancing ultrasound-mediated gene delivery. J. Control. Release. 2014;182:111–120. doi: 10.1016/j.jconrel.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sirsi S., Feshitan J., Kwan J., Homma S., Borden M. Effect of microbubble size on fundamental mode high frequency ultrasound imaging in mice. Ultrasound Med. Biol. 2010;36:935–948. doi: 10.1016/j.ultrasmedbio.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sirsi S.R., Borden M.A. Advances in ultrasound mediated gene therapy using microbubble contrast agents. Theranostics. 2012;2:1208–1222. doi: 10.7150/thno.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.