Abstract
In the United States, adolescent and young adult (AYA) patients with cancer have the lowest clinical trial participation rate of all age groups and slower progress in survival improvement than younger patients. Ominously, AYA clinical trial participation has been steadily decreasing since 2010, except in 15–19 year olds and AYAs with acute lymphoblastic leukemia. In order to reverse the accrual trend, multiple changes are necessary, including convincing community oncologists to pursue clinical trials on behalf of their AYA patients and to have the new National Community Oncology Research Program and National Clinical Trials Network lead a coordinated effort to increase accrual.
Keywords: adolescents and young adults, clinical trials, survival progress
1 |. INTRODUCTION
In the United States, adolescent and young adult (AYA) patients with cancer have the lowest participation rate in clinical trials of all age groups, including infants and except for the most elderly (over 85 years of age).1 Because acquisition of clinical specimens for translational research occurs primarily in the setting of clinical trials at academic medical centers, AYAs also have the lowest proportion of specimens available for laboratory and translational research.2 A central issue then is to what extent has the lack of clinical trial activity affected their rate of survival progress and, if substantial, what to do about it.
2 |. METHODS AND MATERIALS
2.1 |. Study cohorts
Incidence, mortality and survival data were obtained from the National Cancer Institute (NCI) Surveillance, Epidemiology and End Results (SEER) program.3,4 Population census data were obtained from the U.S. government census website.5 The NCI Cancer Therapy Evaluation Program (CTEP) sponsors Phase I, II, and III cancer treatment trials conducted by the NCI cooperative groups and NCI-designated cancer centers. Accrual data from these trials were provided by Nita Seibel and Shanda Finnegan of CTEP. A total of 371,302 patient entries during 1997–2009 and 57,701 entries during 2010–2015 were compared with trends in cancer survival as a function of age.
2.2 |. Statistical analysis
Relative survival was used to assess cancer mortality changes over time. Relative survival accounts for competing causes of death as the ratio of observed survival among patients with cancer to expected survival in the overall population of the same age as computed from life tables of mortality in the general population.6 We obtained 5-year relative survival estimates with corresponding 95% confidence intervals (CI) using NCI SEER*Stat software program version 4.2.0.2.7 Average percent change (APC) in survival rate was either provided by SEER, obtained via applying Joinpoint analysis8 provided by the NCI as Join-point Regression Program, Version 4.4.0.0,9 or calculated from log values of survival rates as the exponential of the linear estimate regressions. The test of APC = 0 and other correlations were tested with the ANOVA F-test for regression. All reported P values were two-sided and values ≥0.05 were considered not significant (NS).
3 |. RESULTS
3.1 |. National treatment trial accruals 1997–2015
Figure 1A shows the annual accruals during 2000–2015 onto national treatment trial in patients with cancer of AYAs 15–39 years of age by 5-year age intervals. The dip during 2002–2003 has been attributed to “9–11,”10 after which there was some improvement, especially in 15–19 year olds, until 2010. Since then, however, the accrual steadily declined in all age groups, especially in 30–49 year olds (52–57% during 2010–2015) and least of all in 15–19 year olds (10% during 2010–2015) (Table 1).
FIGURE 1.
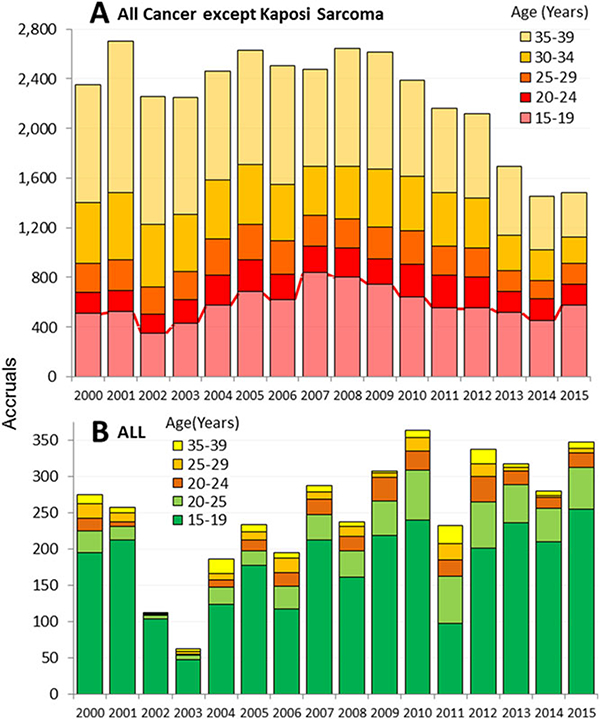
Annual NCI CTEP-sponsored treatment trials accruals of AYAs (age 15–39) during 2000–2015, by calendar year and 5-year age interval. A. All clinical trials except for AIDS-related malignancies (and infections during 2014–2015). B. ALL treatment trials. Accrual data kindly provided by Nita Seibel and Shanda Finnegan, CTEP, NCI
Table 1.
Change in NCI-sponsored treatment trial accruals from 2010 to 2015, by 5-year age intervals, age < 75a
| Age (years) | <5 | 5–9 | 10–14 | 15–19 | 20–24 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | 50–54 | 55–59 | 60–64 | 65–69 | 70–74 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AYA | |||||||||||||||
| Year | Accruals | ||||||||||||||
| 2010 | 1717 | 916 | 811 | 642 | 262 | 265 | 441 | 773 | 1348 | 2217 | 2773 | 3468 | 3420 | 2769 | 1758 |
| 2015 | 1151 | 790 | 693 | 579 | 162 | 170 | 210 | 358 | 599 | 955 | 1528 | 1854 | 2142 | 2239 | 1543 |
| Change | −33% | −14% | −15% | −10% | −38% | −36% | −52% | −54% | −56% | −57% | −45% | −47% | −37% | −19% | −12% |
| Change in average annual accruals to NCI-sponsored treatment trialsa from 2005-2009 to 2011–2015 (the 5 years before and after passage of the ACA) in 19–25 (ACA covered) and 26-64 (ACA not covered) year age groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 19–25 | 26–64 | 26–34 | 35–39 | 40–44 | 45–49 | 50–54 | 55–59 | 60–64 |
| 2004–2009 average | 342 | 18,000 | 666 | 915 | 1,757 | 2,794 | 3,574 | 4,258 | 4,305 |
| 2011–2015 average | 302 | 11,643 | 477 | 561 | 1,043 | 1,656 | 2,261 | 2,746 | 2,898 |
| Change from 2005–2009 to 2011–2015 | −28% | Mean (95% C.I.)b:−51.0% (−26.1% to −75.9%) | −40% | −63% | −69% | −69% | −58% | −55% | −39% |
Data source is the same as in Figure 1.
Excluding AIDS-related entities
Mean (95% confidence interval) of seven age groups between 26 and 64 years, the age range between CHIP and Medicare coverage.
Figure 2 depicts for 2000–2014 both the number of clinical trial accruals and the sum proportion of 14 cancers that have their peak proportion during the AYA years as a function of single patient years of age: AML,CML, Hodgkin lymphoma, non-Hodgkin lymphoma, osteosarcoma, Ewing sarcoma fibromatous sarcoma, Kaposi sarcoma, chondrosarcoma, melanoma, and cancer of testis, thyroid, nasopharynx, and cervix. These cancers accounted for more than 50% of all cancer diagnosed between 15 and 39 years of age, whereas they accounted for only 12% in older (age 40+) patients and 24% of the cancers in younger (age < 15).3 The lower panel (B) shows that the very cancers that have the highest prevalence in AYAs also have hardly had any clinical trial accruals relative to their prevalence and virtually no entries between the ages of 20 and 25. The upper panel (A) also shows a distinct adolescent peak in clinical trial accruals consistent with the accrual trends indicated in Figure 1A and Table 1.
FIGURE 2.
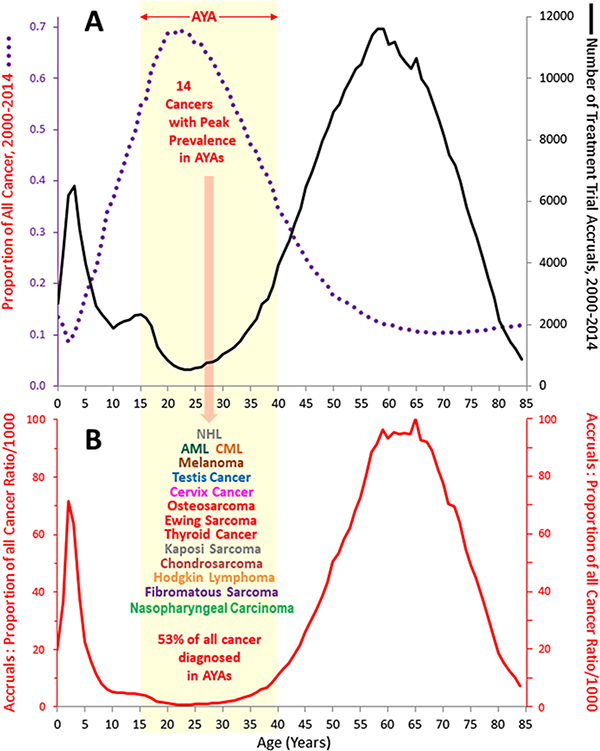
A. Accruals to treatment trials sponsored by National Cancer Institute (NCI)-sponsored cooperative groups and NCI-designated cancer centers (black curve) and proportion of all cancer accounted for by 14 cancers with peak age proportion during AYA years during (purple curve), 2000–2014, by single year of age. B. Ratio of accruals to proportion of the 14 cancers of all cancer (red curve). Accrual data source is the same as in Figure 1. Data on the proportion of all cancer were obtained from incidence data for SEER 18 regions3 and from the U.S. Census Bureau for population data5
3.2 |. Acute lymphoblastic leukemia since 2000
The greatest effort during the last decade to increase accruals in AYAs was directed at ALL, the most common pediatric cancer. New clinical trials in ALL specifically designed for AYAs were launched,11–14 the National Comprehensive Cancer Network (NCTN) released practice guidelines for ALL,15 and an increasing number of presentations and publications on the topic occurred at national meetings and appeared in the peer-reviewed medical literature. Figure 1B shows the annual NCI CTEP-sponsored treatment trial accruals for ALL during 2000–2015. The dramatic decrease during 2002–2003 occurred after “9–11” in 2001,10 since which there has been a steady increase, opposite to the trend for all cancers (Fig. 1A).
Figure 3 shows that both survival and clinical trial accruals for ALL were inversely correlated with age from age 2 to 85 and when analyzed by single years of age, both show a dramatic, cliff-like decrease during the older adolescent years. Joinpoint analysis9 of the 5-year leukemia-specific survival rate for patients with ALL diagnosed during 2000–2014 as a function of single year of age identified two inflections, ages 17 and 20, during which the survival rate decreased 20% in just 3 years of this age range. This “AYA ALL cliff” constituted 26% of the overall decline from 93% at age 5 to 19% at age 70. Joinpoint analysis also identifies ages 16 and 24 as the top and bottom of the accrual cliff. The superimposed “cliff” patterns strongly suggest that the survival cliff is due in large part to the accrual cliff. Other factors such as a switch from pediatric to adult treatment regimens15 contribute to the survival cliff but the accrual coincidence implicates clinical trial participation as a greater factor.16 The inset to Figure 3 shows no correlation of survival when the proportion of patients entered on ALL treatment trials was below 10% (gray zone); above 10% there is a strong positive correlation with accrual proportion (r2 = 0.87, P < 10−12).
FIGURE 3.
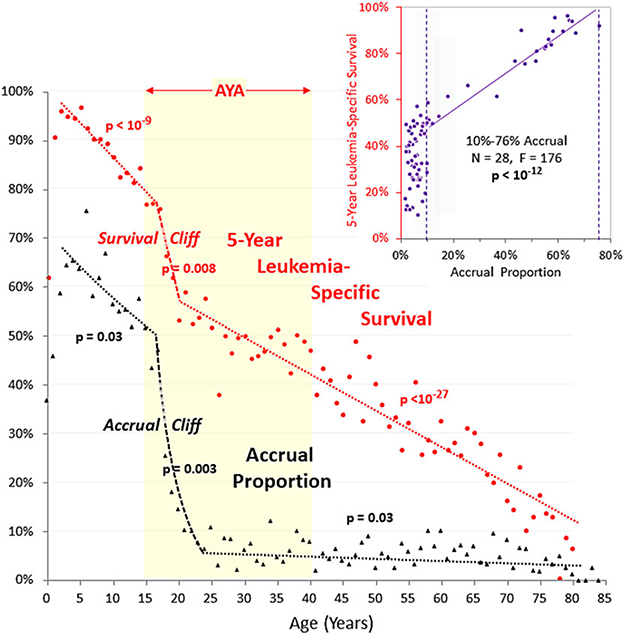
Joinpoint analysis of 5-year leukemia-specific survival of patients with ALL, 2000–2014, SEER18, and estimated treatment trial accrual proportion for 2000–2004, by single year of age. Joinpoint analysis was performed with the National Cancer Institute Joinpoint Regression Program.9 Survival regressions are for 2–17,17–20, and 20–81 years. Accrual proportion regressions are for 2–16,16–23, and 23–80 years. P-values refer to the linear regression of the corresponding age segment. The inset shows all of the annual data of survival rates and accrual proportions as a function of survival with accrual proportion, demonstrating a linear regression for accrual proportion values >10%. Accrual data source is the same as in Figure 1
If for ALL the only increase in treatment trial participation during the last decade occurred in the 10–19 year age group, is there evidence of survival prolongation in the age group and not in younger or older persons? Figure 4 shows that there has been an acceleration of leukemia-specific survival in 10–19 year olds during the years of clinical trial accrual increase since 2004 (APC = 2.44, P < 0.0001) that has been greater than in children <10 years of age and projects that they may have since caught up with the survival rate in children. The trend in 10–19 year olds is in striking contrast to no evidence at all for an improvementin 20–29year olds since 1989 (APC = 0.33, P = NS).The lack of survival improvement in 20–29 year olds corresponds directly and temporally with a negligible accrual increase.
FIGURE 4.
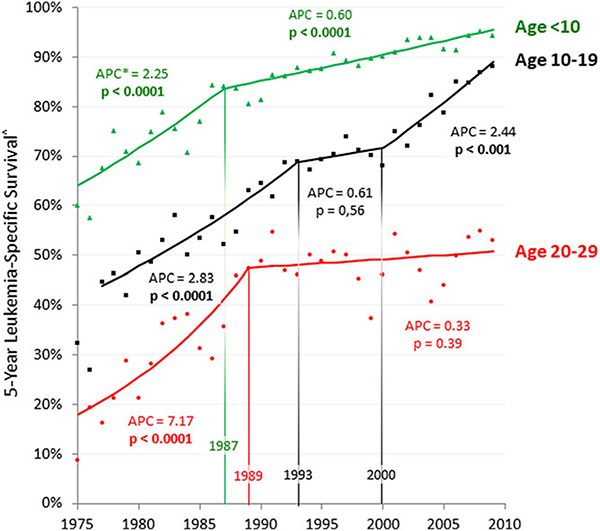
Annual ALL 5-year leukemia-specific survival, age < 30, 1975–2009, SEER, by 10 year age intervals. Joinpoint analysis was performed with the National Cancer Institute Joinpoint Regression Program.9 Survival data were obtained from the SEER program.3 APC, average annual percent change. Annual values are assessed from two consecutive years (year of and year before)
4 |. DISCUSSION
A prior comparison of the APC in the 5-year cancer-specific survival rate from 1985 to 1999 and the accrual rate to national cancer treatment trials during 2001–2006 also showed a nearly 1:1 correlation over the entire age range16 as observed in this study. A similar pattern was also found with respect to cancer mortality, in patients <40 years of age.16 Patients aged 20 to 24 had a particularly poor reduction in cancer mortality, as well as the lowest absolute number of clinical trial accruals and virtually none among the cancers with the highest prevalence during the AYA years. The paradox is that clinical trial accruals have their all-age nadir at precisely the peak age prevalence of AYA cancers. These and the current observations enable three fundamental conclusions: (1) both survival prolongation and mortality reduction in patients with cancer have been directly correlated with clinical trial activity; (2) the dependency of survival prolongation on treatment trial accrual has been apparent at all ages; (3) AYAs have had the least trial participation and a resultant least survival prolongation and mortality reduction, particularly those 20–29 years of age.16
The multiple correlations between national treatment trial accrual and national cancer survival rates explain the slower rate of progress in AYAs than in younger and older patients and underscore the need to increase both the number of clinical trials available to AYAs with cancer and their participation in them. What is clearly ominous, there-fore, is the decline in treatment trial accruals since 2000 in older AYAs. The extra effort that has been expended successfully on increasing the clinical trial participation of adolescents with cancer17–25 and has apparently been successful should be extended to young adults. Reasons for the decline are undoubtedly multifactorial but likely include reorganization of the NCI cooperative group structure, function, and reimbursement that by 2000 was under development and by 2014 was replaced with the NCTN. This entailed reducing the number of adult patient cooperative groups from 10 to 4 and including both the Children’s Oncology Group (COG) and the Canadian clinical trials network, along with some reduction in the total budget spent on cooperative group trials. The NCTN explanation is consistent with the lesser decline in pediatric patient accrual (Table 1) and NCTN’s less effect on pediatric cooperative group structure. Another factor may be the transition of some cooperative group clinical trials to the pharmaceutical industry that become more competitive in funding clinical trials and providing infrastructure support. To the extent that the latter may have occurred, the reduction in overall clinical trial participation by AYAs and older patients may not have been as severe as implied in Figure 1. Among AYAs however, these trials are less common since AYAs have different cancers (Fig. 2) and, since they are a much smaller population than those of older age, fewer drugs are developed for them by the industry.
One explanation for the improvement in older adolescents is the cooperation between COG and the adult cooperative groups that study the overlapping AYA age range. In particular, the NCTN has formed a specific intergroup committee representing the AYA interests of the pediatric and adult cooperative groups. In addition, several of the adult cooperative groups have their own AYA committees focused on studying cancers prevalent in the AYAs.
The U.S. Food and Drug Administration (FDA) has recently encouraged the inclusion of 12–17 year olds on disease- and target-appropriate adult oncology trials.26 An FDA Advisory Committee for Pharmaceutical Science and Clinical Pharmacology reviewed this age group in 2012 and recommended that adolescent (>12 years) patients be enrolled on trials for adult patients without the need for a previously dedicated pharmacokinetic study in them,27 a recommendation that has not been enacted. As recently summarized by the FDA,26 inclusion of adolescents in adult oncology trials will require the cooperation of investigators, cooperative groups, industry, institutional review boards, and regulatory agencies to overcome real and perceived barriers.26 Despite the FDA Advisory Committee recommendation, the pharmaceutical industry continues to exclude adolescents from their early drug development clinical trials, primarily because of economic disincentives.
It is particularly gratifying to discover that ALL was one of the cancers that benefited from the adolescent accrual increment, but it is disappointing to find that the benefit is sharply limited at the upper end to age 20. A striking AYA ALL accrual cliff that is coincident with an equally striking AYA survival cliff between the ages of 17 and 20 implicates a strong need to increase clinical trial activity in 20–29 year olds with ALL, as well as those with other types of cancers. Until this is accomplished it is likely that the AYA survival gap will persist. One effort in Ontario, Canada is to specifically study the 15–21 year age interval for determinants of outcome, including access to clinical trials.28
Another barrier to clinical trials accrual in the AYA years is the referral of patients to centers with, or access to, clinical trials. In Utah, with one children’s hospital and one National Comprehensive Cancer Network center, the referral of patients with cancer to either center dropped from 92% at age 14 to 44% by age 17.29–35 By age 20, 2 of every 3 patients stayed in or were referred to private practices and by age 25 less than 30% of the patients were ever seen at the academic medical center.29 Thus a “referral cliff” also exists in AYA oncology.
Prior reports have quantitated AYA patient with cancer participation in clinical trials in the 2–15% range.1,30,31 Multivariate analysis in one study demonstrated that AYA patients with the same cancer diagnosis common to pediatric and AYA populations who were treated by non-pediatric oncologists were less likely to enroll onto clinical trials.31 Efforts by the pediatric oncology research community to recruit more AYAs onto clinical trials have been interpreted by some medical oncologists as an attempt to expand the scope of pediatric practice. In reality, the pediatric oncologists’ goal is to assist in the provision and conduct of such trials, and in aspects of the care of such patients for which they have more extensive experience than their medical colleagues. If the survival improvement versus treatment trial accrual proportion correlation for ALL (Fig. 3) is generalizable, it may take more than 10% accrual proportion of all available AYA patients to achieve significant survival improvement. On the other hand, once the accrual exceeds 10%, the gain may be strongly correlated with increased participation.
The investigators of the aforementioned study also found that 15 to 19 year-olds had a statistically-significant higher participation rate than any 5-year age range between 20 and 40 years.31 Also apparent was that clinical trial participation varied directly with the quality of health insurance, with those having no insurance have a statistically-significant lower rate of enrollment compared to those with private insurance.31 The clinical trial participation rate was four times higher in the group with private insurance. In the United States, the Patient Protection and Affordable Care Act (ACA) required insurance companies to allow 18–25 year olds to continue to be covered via their parents’ insurance plans. Within 15 months after the ACA was passed in September 2010, the number of newly insured AYAs predicted that there would be an additional 4,150 AYAs diagnosed to have cancer before their 26th birthday who would have been uninsured prior to the ACA.32 By now, 5 years later, that number is well in excess of 15,000. The increase should have allowed more patients of this age group to be better able financially to participate in available clinical trials, and thereby ultimately improve survival and cure rates. Since the ACA was implemented, the 19–25 year age group has had a statistically significantly lower reduction in NCI-sponsored clinical trial accruals in comparison to 26–64-year-old Americans who were not supported by this provision in the ACA (28% vs. 51% reduction) (Table 1). This discrete age-delimited effect indicates that the ACA helped protect this AYA age group from the generalized decline and suggests that the 26-year age cutoff should be increased to an older age.33 Another line of evidence of the ACA benefit for young AYAs is the observation that uninsured AYAs were less likely to enroll in 2006, before the ACA, but not in 2012/2013 after the ACA was implemented.34
Many other factors contribute to lack of participation in clinical trials. In addition to economic and insurance-based factors, these can be classified as issues of continuity of care and philosophy, provider bias, patient/family preferences, and cooperative group and cancer center limitations.35 Specific challenges include lack of clinical trials designed for the AYA cancer population; referral patterns; the nature and number of AYA-specific medical treatment settings available to AYAs; arbitrary and inappropriate age eligibility limitations in clinical trials; perception by AYAs that clinical trials are unsafe/more difficult and more likely to interfere with long term goals; English as a second language; and the additional time and travel commitment required for clinical trial participation.36,37 Of particular concern is that more AYAs with cancer remain in their community oncology setting than any other age group38 with the possible exception of patients in the most elderly age group (>85 years). The national community oncology program (previously known as Community Clinical Oncology Program and now as the National Community Oncology Research Program [NCORP]) has had a progressively lower rate of entering AYAs on clinical trials.38 Another factor is race/ethnicity, particularly in view of evidence that participation of Hispanic, black, and Asian AYAs with cancer worsened from 2006 to 2012/2013.34
Another unfortunate aspect of the accrual gap in 20–30 year olds is the generalizability of their clinical trial results based on an enrollment of only 2% of this population to their counterparts in the rest of the AYA population.39 Does comparing 2% enrolled in clinical trials with a 9–28% “control” population (SEER data) allow generalization to 72–91% of the rest of the country? That for ALL survival, the treatment trial accrual proportion had to be at least 10% before a correlation was noted (Fig. 3 inset) suggests that treatment trials have to enroll 10% or more of eligible patients to have a general impact on survival.
Participation in clinical trials also provides biospecimens that for AYAs, as mentioned above, are under-represented in biobank repositories and have thereby restricted translational research. Also, as mentioned above, the array of cancers in AYAs is distinctly different from that at any other age, and those that appear to be the same as in other age groups are often of a different biology. The host (the AYA) is also clearly different in many ways from children and older adults, including pharmacokinetics, toxicity profiles, dose tolerance, and fertility considerations. The need for translational research is greater and more challenging in AYAs than in any other age group.40 Increased availability of treatment trials with laboratory correlates and accrual to them are essential to improving the outcome of cancer in AYAs. The lack of tumor and tissue specimens obtained via clinical trials has seriously compromised the ability to identify the biologic and histopathologic differences and discover specific treatment approaches for AYAs.
These results should not be interpreted to mean that AYAs and other individuals who participate in clinical trials have a greater probability of survival prolongation. The principle of equipoise in clinical trials means that an adequately designed treatment trial is a trial that tests a new or modified form of therapy that is not known to have that benefit. Otherwise the trial would not be justified. On the other hand, subjects who participate in clinical trials have certain advantages, such as access to standardized protocols with consistent guidelines for dosing and toxicity modification and access to potentially better therapies and less expensive agents, since the agent itself is usually provided at no cost to the patient, and access to professionals and multidisciplinary teams with more expertise in the unique challenges that AYAs face.41 Clinical trials require precise tumor evaluations at initial staging and during/after treatment, as well as respect of time-lines of therapy administration and toxicity evaluation and reporting. The oversight and monitoring of trial patients creates a more assiduous environment for all patients. These advantages are of particular value to AYAs who have more limited financial resources and caregivers in their local community with less experience in managing the trials and tribulations of AYAs.
Recent studies have suggested that effective evidence-based treatment strategies generated in AYA treatment trials may not be rapidly adopted by oncologists.42 In ALL where the evidence is most abundant, only 31% of AYA patients in the greater San Francisco Bay area received a demonstrably superior pediatric type of regimen during 2008–2012, and the rate declined thereafter to 21%.42 Adult facilities treating ≥ 2 AYA ALL patients per year captured in the region were statistically significantly more likely to administer a pediatric regimen than lower volume centers, further indicating the importance of referral patterns.42 Ongoing efforts within NCORP are seeking to further understand factors associated with delayed or limited implementation of effective treatment strategies in AYAs.
Fortunately, NCI-designated cancer centers are evaluating their own AYA referral patterns and clinical trial determinants43 and inter-group efforts are under way within the current organizational structure of the federal clinical trials enterprise, including the NCTN, to create novel opportunities for collaborative AYA oncology research among the pediatric and adult NCTN groups.44,45 Also, a most recent analysis in the United Kingdom documents that sub-groups of AYA patients with advanced solid tumors derive considerable benefit from participating in trials involving novel therapeutics.46 As also noted in England, however, age-specific biology, pharmacology, proteomics, genomics, clinician and patient behavior studies embedded within clinical trials are required to further improve survival for AYAs.47
5 |. CONCLUSIONS AND RECOMMENDATIONS
Table 2 is a summary of recommendations that can potentially improve accrual of AYAs to treatment trials. Most of these have been proffered in prior publications and discussed at workshops.49–54 It is time to pursue these suggestions more vigorously in order to accomplish in older AYAs what has recently been successful in 15–19 year olds with cancer and in children with cancer before that and in whom virtually all progress emanated from research. As reviewed, many factors contribute to lack of participation of AYA patients with cancer in clinical trials. Yet, other countries have been able to overcome many of the limitations.55 American AYAs with cancer deserve better.
Table 2.
Improving treatment trial accrual of AYAs with cancer
| Challenge to treatment trial accrual | Action to address challenge |
|---|---|
| Low enrollment in existing clinical trials | • Convince community oncologists of the value of clinical trials and the need to pursue clinical trials on behalf of their • AYA patients. |
| • Encourage providers that provide care to AYAs with first cancer symptoms to refer to centers that offer clinical trials. | |
| • Increase adoption of National Comprehensive Cancer Network guidelines on referral of AYAs with cancer. | |
| • Facilitate open enrollment of open trials through partnerships between cooperative groups and community hospitals. | |
| • Increase AYA organization engagement with patients and patient rights groups to encourage health care providers to participate in clinical trials. | |
| Clinical trial availability | • Incorporate AYA-specific aims into cooperative group trials. |
| • For cancers with highly favorable treatment, design new clinical trials with a focus on therapy-related toxicities. | |
| • Increase collaboration between pediatric and adult oncologists to design AYA-focused clinical trials for cancers common among this group. | |
| Physician-related barriers | • Establish an accepted standard of care for AYA cancers among pediatric and adult oncologists. |
| • Increase financial incentives for collaboration between pediatric and adult oncologists. | |
| Institutional barriers | • Increase use of centralized institutional review boards to encourage access and participation in clinical trials. |
| Societal barriers | • Provide health insurance to all AYAs, not just to those less than 25 years of age who are able to continue on the parents’ insurance plan. |
| • Expand and enforce requirements of the health insurance industry to include coverage of clinical trial costs. | |
| • Empower AYAs to expect their medical providers to discuss clinical trials and arrange contact with clinical trial providers. |
Highlights.
AYA patients with cancer in the United States have had since 1980 a slower rate of survival improvement and mortality reduction than those with cancer in younger and older age groups.
Survival prolongation and mortality reduction in patients with cancer is directly correlated with clinical trial activity and apparent at all ages.
AYA patients with cancer have had the lowest participation rate in clinical trials than any other age group.
Even worse, AYAs have had, since 2010, a steady decline in accrual to treatment trials sponsored by National Cancer Institute.
Moreover, clinical trial accruals have their all-age nadir at precisely the peak age prevalence of AYA cancers.
The slower rate of survival improvement and mortality reduction is associated with, and a likely resultant of, their low participation rate in clinical trials and lack of tissue specimens for research.
The problem is particularly obvious in 20–29 year olds with acute lymphoblastic leukemia, who have had little to no improvement in the 5-year survival rate since 1989.
In order to reverse the accrual trend, multiple changes are necessary, including convincing community oncologists to pursue clinical trials on behalf of their AYA patients.
Also the new National Cancer Treatment Network and National Community Oncology Research Program and the Food and Drug Administration should lead a coordinated attack to eliminate the accrual gap.
Other solutions include broader health insurance availability for AYAs and coverage of clinical trials. Increased availability of clinical trials specifically for AYAs with cancer.
Pediatric and adult oncologists should establish a mutual goal that was achieved in children to have clinical trials become a standard of care for AYA cancers.
ACKNOWLEDGEMENTS
The authors thank Nita Seibel and Shanda Finnegan for compiling and providing the data on the CTEP-sponsored treatment trials and the NCI SEER program for its publicly available databases, without either of which little of this report would have been feasible.
Abbreviations:
- ACA
Patient Protection and Affordable Care Act
- ALL
acute lymphoblastic leukemia
- AML
acute myelogenous leukemia
- APC
Average percent change
- AYA
adolescent and young adult
- CI
confidence interval
- CML
chronic myelogenous leukemia
- COG
Children’s Oncology Group
- CTEP
NCI Cancer Therapy Evaluation Program (CTEP)
- FDA
Food and Drug Administration
- NCI
National Cancer Institute
- NCORP
National Community Oncology Research Program
- NCTN
National Cancer Treatment Network
- SEER
Surveillance, Epidemiologyand End Results
Footnotes
CONFLICT OF INTEREST
None of the authors are aware of any conflict of interest, potential or current, with the information in this report or its purpose.
REFERENCES
- 1.Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer: the scope of the problem and criticality of clinical trials. Cancer. 2006;107:1645–1655. [DOI] [PubMed] [Google Scholar]
- 2.Bleyer A, Barr R, Hayes-Lattin B, Thomas D, Ellis C, Anderson B. The distinctive biology of cancer in adolescents and young adults. Nat. Rev. Cancer 2008;8:288–298. [DOI] [PubMed] [Google Scholar]
- 3.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database:Incidence—SEER 9, 13 and 18 Regions Research Data, Nov 2016 Sub (1973–2014) <Katrina/Rita Population Adjustment>-Linked to County Attributes-Total U.S., 1969–2015 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2017, based on the November 2016 submission.
- 4.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality—All COD, Aggregated With State, Total U.S (1969–2014) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released December 2016.
- 5.National Intercensal Estimates (2000–2010). https://www2.census.gov/programs-surveys/popest/tables/2000-2010/intercensal/national/us-est00int-01.xls. Accessed January 28,2016.
- 6.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl. Cancer Inst. Monogr 1961;6:101–121. [PubMed] [Google Scholar]
- 7.SEER*Stat Software, Version 8.3.4-March 23, 2017. https://seer.cancer.gov/seerstat. Accessed April 30,2017.
- 8.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. Correction. [DOI] [PubMed] [Google Scholar]
- 9.Joinpoint Regression Program, Version 4.4.0.0-January 4, 2017, Statistical Research and Applications Branch, National Cancer Institute. http://surveillance.cancer.gov/joinpoint. Accessed February 1, 2017.
- 10.Bleyer A Effect of 9–11 on U.S. national cancer clinical trials accrual and lack of effect of National Cancer Institute budget increases (and impact-benefit of the Adolescent and Young Adult Initiative. J Clin Oncol. 2007;25:334s. [Google Scholar]
- 11.Breitenbach K, Stock W. Intergroup Trial C10403: a pediatric treatment approach to improve outcomes in adolescents and young adults with acute lymphoblastic leukemia. J Adolesc Young Adult Oncol. 2011;1:107–108. [DOI] [PubMed] [Google Scholar]
- 12.DeAngelo DJ, Stevenson KE, Dahlberg SE, et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18–50 years Improving treatment trial accrual of AYAs with cancer with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2015. March;29:526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chemotherapy with liposomal cytarabine CNS prophylaxis for adult acute lymphoblastic leukemia & lymphoblastic lymphoma. https://clinicaltrials.gov/ct2/show/NCT02043587?term=acute+lymphoblastic+leukemia+wieduwilt&rank=1. Accessed January 28, 2016.
- 14.Bleyer A How NCCN guidelines can help young adults and older adolescents with cancer and the professionals who care for them. J Natl ComprCanc Netw. 2012;10:1065–1071. [DOI] [PubMed] [Google Scholar]
- 15.Siegel SE, Stock W, Johnson RH, et al. Pediatric-inspired treatment regimens for adolescents and young adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: a review. JAMA Oncol. Published online February 15, 2018. 10.1001/jamaoncol.2017.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unger J, Cook E, Bleyer A. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book. 2016;35:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tai E, Beaupin L, Bleyer A. Clinical trial enrollment among adolescents with cancer: supplement overview. Pediatrics. 2014;133(suppl 3):S85–S90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tai E, Buchanan N, Westervelt L, Elimam D, Lawvere S. Treatment setting, clinical trial enrollment, and subsequent outcomes among adolescents with cancer: a literature review. Pediatrics. 2014;133(suppl 3):S91–S97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tai E, Buchanan N, Eliman D, et al. Understanding and addressing the lack of clinical trial enrollment among adolescents with cancer. Pediatrics. 2014; 133(suppl 3):S98–S103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albritton KH, Coccia P. Influencing referral of adolescents and young adults with cancer to sites with higher rates of trial enrollment. Pediatrics. 2014;133(suppl 3):S104–S108. [DOI] [PubMed] [Google Scholar]
- 21.Shaw PH, Hayes-Lattin B, Johnson R, Bleyer A. Improving enrollment in clinical trials for adolescents with cancer. Pediatrics. 2014;133(suppl 3):S109–S113. [DOI] [PubMed] [Google Scholar]
- 22.Gupta AA, Indelicato DJ. Increasing the number of clinical trials available to adolescents diagnosed with cancer. Pediatrics. 2014;133(suppl 3):S114–S118. [DOI] [PubMed] [Google Scholar]
- 23.Felgenhauer J, Hooke MC. Regulatory barriers to clinical trial enrollment of adolescent and young adult oncology patients. Pediatrics. 2014;133(suppl 3). S119–S122. [DOI] [PubMed] [Google Scholar]
- 24.Buchanan ND, Block R, Smith AW, Tai E. Psychosocial barriers and facilitators to clinical trial enrollment and adherence for adolescents with cancer. Pediatrics. 2014;133(sup 3):S123–S130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw PH, Hayes-Lattin B, Johnson R, Bleyer A. Improving enrollment in clinical trials for adolescents with cancer. Pediatrics. 2014;133( 3):S109–S113. [DOI] [PubMed] [Google Scholar]
- 26.Chuk MK, Mulugeta Y, Roth-Cline M, Mehrotra N, Reaman GH. Enrolling adolescents in disease/target-appropriate adult oncology clinical trials of investigational agents. Clin Cancer Res. 2017;23:9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Food and Drug Administration Center for Drug Evaluation and Research Summary Minutes of the Advisory Committee for Pharmaceutical Science and Clinical Pharmacology, March 14, 2012. https://wayback.archive-it.org/7993/20170404154932/https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AdvisoryCommitteeforPharmaceuticalScienceandClinicalPharmacology/UCM303015.pdf. pages 41–50 and https://wayback.archive-it.org/7993/20170404154932/https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AdvisoryCommitteeforPharmaceuticalScienceandClinicalPharmacology/UCM303015.pdf. Accessed March 30,2018.
- 28.Baxter NN, Daly C, Gupta S, et al. The Initiative to Maximize Progress in Adolescent and Young Adult Cancer Therapy (IMPACT) Cohort Study: a population-based cohort of young Canadians with cancer. BMC Cancer. 2014. November 3;14:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albritton KH, Wiggins CH, Nelson HE, Weeks JC. Site of oncologic specialty care for older adolescents in Utah. J Clin Oncol. 2007;25:4616–4621. [DOI] [PubMed] [Google Scholar]
- 30.Fern LA, Whelan JS. Recruitment of adolescents and young adults to cancer clinical trials-international comparisons, barriers, and implications. Semin Oncol. 2010;37:e1–e8. [DOI] [PubMed] [Google Scholar]
- 31.Parsons HM, Harlan LC, Seibel NL, Stevens JL, Keegan TH. Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference?. J Clin Oncol. 2011;29:4045–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bleyer A, Ulrich C, Martin S. Young adults, cancer, health insurance, socioeconomic status, and the Affordable Care Act. Cancer. 2012;118:6018–6021. [DOI] [PubMed] [Google Scholar]
- 33.Bleyer A Potential favorable impact of the Affordable Care Act of 2010 on cancer in young adults in the United States. The Cancer J. 2010;16:563–573. [DOI] [PubMed] [Google Scholar]
- 34.Keegan T, Penn D, Li Q, et al. Changes in clinical trial participation among adolescent and young adult (AYA) cancer patients from 2006 to 2013 in the United States. J Clin Oncol. 2017;35(suppl): e18037. [Google Scholar]
- 35.Bleyer A, Budd T, Montello M. Lack of clinical trial participation and of progress in older adolescents and young adults with cancer. Curr Probl Pediatr Adolesc Health Care. 2005;35:186–195. [Google Scholar]
- 36.Shaw PH, Hayes-Lattin B, Johnson R, Bleyer A. Improving enrollment in clinical trials for adolescents with cancer. Pediatrics. 2014;133(suppl 3):S109–S113. [DOI] [PubMed] [Google Scholar]
- 37.Gupta AA, Bell AH, Wang K, et al. Evaluation of adolescents and young adults (AYA) attitudes towards participation in cancer clinical trials. J Clin Oncol. 2017;35(suppl):10047. [DOI] [PubMed] [Google Scholar]
- 38.Roth ME, O’Mara AM, Seibel NL, et al. Low enrollment of adolescents and young adults onto cancer trials: insights from the Community Clinical Oncology Program. JOP. April 2016:e388–e395. Published online March 29, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bleyer A In and out, good and bad news, of generalizability of SWOG treatment trial results. JNCI. 2014;106(3):dju027. doi: 10.1093/jnci/dju027 [DOI] [PubMed] [Google Scholar]
- 40.Taylor RM, Solanki A, Aslam N, Whelan JS, Fern LA. A participatory study of teenagers and young adults views on access and participation in cancer research. Eur J Oncol Nurs. 2016;20:156–164. [DOI] [PubMed] [Google Scholar]
- 41.Johnson RH, Macpherson CF, Smith AW, Block RG, Keyton J. Facilitating teamwork in adolescent and young adult oncology. J Oncol Pract. 2016;12:1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muffly L, Lichtensztajn D, Shiraz P, et al. Adoption of pediatric-inspired acute lymphoblastic leukemia regimens by adult oncologists treating adolescents and young adults: a population-based study. Cancer. 2017;123:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins CL, Malvar J, Hamilton AS, Deapen DM, Freyer DR. Case-linked analysis of clinical trial enrollment among adolescents and young adults at a National Cancer Institute-designated comprehensive cancer center. Cancer. 201515;121:4398–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freyer DR, Felgenhauer J, Perentesis J. COG Adolescent and Young Adult Oncology Discipline Committee. Children’s Oncology Group’s 2013 blueprint for research: adolescent and young adult oncology. Pediatr Blood Cancer. 2013;60:1055–1058. 10.1002/pbc.24431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss AR, Nichols CR, Freyer DR. Enhancing adolescent and young adult oncology research within the National Clinical Trials Network: rationale, progress, and emerging strategies. Semin Oncol. 2015;42:740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sundar R, McVeigh P, Petruckevitch A, et al. Clinical outcomes of adolescents and young adults (AYA) with advanced solid tumors participating in phase I trials. J Clin Oncol. 2017;35(suppl):10536. [Google Scholar]
- 47.Stark D, Bowen D, Dunwoodie E, et al. Survival patterns in teenagers and young adults with cancer in the United Kingdom: comparisons with younger and older age groups. Eur J Cancer. 2015;51:2643–2654. [DOI] [PubMed] [Google Scholar]
- 48.Albritton KH, Coccia P. Influencing referral of adolescents and young adults with cancer to sites with higher rates of trial enrollment. Pediatrics. 2014;133(suppl 3):S104–S108. [DOI] [PubMed] [Google Scholar]
- 49.Gupta AA, Indelicato DJ. Increasing the number of clinical trials available to adolescents diagnosed with cancer. Pediatrics. 2014;133(suppl 3):S114–S118. [DOI] [PubMed] [Google Scholar]
- 50.Shaw PH, Hayes-Lattin B, Johnson R, Bleyer A. Improving enrollment in clinical trials for adolescents with cancer. Pediatrics. 2014;133(suppl 3):S109–S113. [DOI] [PubMed] [Google Scholar]
- 51.Tai E, Beaupin L, Bleyer A. Clinical trial enrollment among adolescents with cancer: supplement overview. Pediatrics. 2014;133(suppl 3):S85–S90.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tai E, Buchanan N, Eliman D, et al. Understanding and addressing the lack of clinical trial enrollment among adolescents with cancer. Pediatrics. 2014;133(suppl 3):S98–S103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sender L, Zabokrtsky KB. Adolescent and young adult patients with cancer: a milieu of unique features. Nat Rev Clin Oncol. 2015;12:465–480. [DOI] [PubMed] [Google Scholar]
- 54.Fern LA, Lewandowski JA, Coxon KM, Whelan J; National Cancer Research Institute Teenage and Young Adult Clinical Studies Group, UK. Available, accessible, aware, appropriate, and acceptable: a strategy to improve participation of teenagers and young adults in cancer trials. Lancet Oncol. 2014;15:e341–e350. [DOI] [PubMed] [Google Scholar]
- 55.Fern LA, Whelan JS. Recruitment of adolescents and young adults to cancer clinical trials-international comparisons, barriers, and implications. Semin Oncol. 2010;37:e1–e8. [DOI] [PubMed] [Google Scholar]


