Abstract
BACKGROUND
The 5-year relative survival for prostate cancers diagnosed between 1990 and 1994 in the United States was very high (92%); however, survival in black males was 7% lower compared with white males. The authors updated these findings and examined survival by stage and race.
METHODS
The authors used data from the CONCORD-2 study for males (ages 15–99 years) who were diagnosed with prostate cancer in 37 states, covering 80% of the US population. Survival was adjusted for background mortality (net survival) using state-specific and race-specific life tables and was age-standardized. Data were presented for 2001 through 2003 and 2004 through 2009 to account for changes in collecting SEER Summary Stage 2000.
RESULTS
Among the 1,527,602 prostate cancers diagnosed between 2001 and 2009, the proportion of localized cases increased from 73% to 77% in black males and from 77% to 79% in white males. Although the proportion of distant-stage cases was higher among black males than among white males, they represented less than 6% of cases in both groups between 2004 and 2009. Net survival exceeded 99% for localized stage between 2004 and 2009 in both racial groups. Overall, and in most states, 5-year net survival exceeded 95%.
CONCLUSIONS
Prostate cancer survival has increased since the first CONCORD study, and the racial gap has narrowed. Earlier detection of localized cancers likely contributed to this finding. However, racial disparities also were observed in overall survival. To help understand which factors might contribute to the persistence of this disparity, states could use local data to explore sociodemographic characteristics, such as survivors’ health insurance status, health literacy, treatment decision-making processes, and treatment preferences.
Keywords: cancer registries, early detection of cancer, population-based survival, prevention and control, prostate cancer, Surveillance, Epidemiology, and End Results (SEER) summary stage, therapeutics, trends
INTRODUCTION
Prostate cancer is the second most commonly diagnosed cancer in males worldwide and, in the United States, the most commonly diagnosed invasive cancer in males.1,2 Prostate cancer is also the fifth leading cancer-related cause of death worldwide and, in the United States, the second leading cause of cancer death among males.1–3 In the United States, black males have higher incidence and death rates than white males.2,3 Worldwide, black males have higher prostate cancer incidence and death rates than other males.1 In the United States, the incidence of prostate cancer increased rapidly in the early1990s after widespread adoption of prostate cancer screening using the prostate-specific antigen (PSA) test, peaked in the early 1990s, and then declined sharply thereafter; the decline has been more gradual since 2000.4–6 Prostate cancer death rates increased through the early 1990s and have been gradually declining since the mid-1990s among both white and black males.6
The first CONCORD study provided a systematic comparison of survival for males (ages 15–99 years) who were diagnosed with prostate cancer in 31 countries between 1990 and 1994 and were followed until 1999.7 Data for the United States were included from 21 state-wide and metropolitan-area cancer registries covering 42% of the US population. International differences in age-standardized prostate cancer survival were wide, even after adjustment for differences in mortality from other causes of death, with prostate cancer survival highest in the United States compared with other countries. Five-year survival was higher for white males (92.4%) compared with black males (85.8%) in the United States. This may reflect disparities in the receipt of standard care as well as differences in stage at diagnosis.
The second CONCORD study (CONCORD-2) was undertaken, in part, to update findings from the first CONCORD study and to allow for a more in-depth examination of cancer survival by race and stage.8 For the current study, we used CONCORD-2 data to examine cross-state trends in prostate cancer survival up to 5 years among black and white males by cancer stage.
MATERIALS AND METHODS
Data Source
We used data from 37 state-wide cancer registries that participated in the CONCORD-2 study8 and consented to the inclusion of their data in the more detailed analyses reported here. We analyzed individual tumor records for males (ages 15–99 years) who were diagnosed with prostate cancer between 2001 and 2009 and were followed through December 31, 2009. Cases were identified using International Classification of Diseases for Oncology third edition topography code C61.9 (prostate) and behavior code 3 (malignant).9 We included all cases of cancer originating in the prostate, regardless of a previous cancer diagnosis in the same individual.
Males were grouped by diagnosis year into 2 calendar periods (2001–2003 and 2004–2009) to reflect changes in the methods used by US cancer registries to collect data on stage at diagnosis. Between 2001 and 2003, most registries coded stage directly from medical records to the Surveillance, Epidemiology, and End Results (SEER) Summary Stage 2000 (SS2000).10 Since 2004, all registries have derived SS2000 using the Collaborative Staging System.11
Survival Analyses
We estimated net survival up to 5 years after diagnosis and 95% confidence intervals (CIs) using the Pohar Perme estimator.12 Net survival is the probability of survival up to a given time since diagnosis, after controlling for other causes of death (background mortality). To control for wide differences in background mortality among participating states, we used a flexible Poisson model to construct life tables of all-cause mortality in the general population of each state from the number of deaths and the population, by single year of age, sex, calendar year, and, where possible, by race (all, black, white).13 Methods for constructing life tables have been published.14
We estimated net survival using the cohort approach for patients diagnosed between 2001 and 2003, because all patients had been followed for at least 5 years by December 31, 2009. We used the complete approach to estimate net survival for patients diagnosed between 2004 and 2009, because 5 years of follow-up data were not available for all patients. We obtained age-standardized survival estimates using International Cancer Survival Standard weights.15 Unstandardized estimates are italicized in the supporting tables. Trends, geographic variations, and differences in age-standardized survival by race are presented graphically in bar charts and funnel plots.16 The funnel plots provide insight into the variability of cancer survival in the United States by race and state and illustrate how much a particular survival estimate deviates from the pooled estimate of US registries, given the precision of each estimate. For additional details on the methods and data quality for this study, see the article by Allemani et al in this Supplement.8
RESULTS
In total, 1,527,602 males with prostate cancer were eligible for analysis, of whom 81.3% were white, and 13.7% were black. The pooled results from the 37 US registries are provided in Tables (1 and 2), and 3; and state-specific results are reported in Supporting Tables 1, 2, and 3. Table 1 details the distribution of SS2000 by race and calendar period.
TABLE 1.
Prostate Cancer: Number of Males (Ages 15–99 Years) Diagnosed Between 2001 and 2009 and Distribution (%) by Surveillance, Epidemiology, and End Results Summary Stage 2000 at Diagnosis by Race and Calendar Period of Diagnosis
| SEER Summary Stage 2000 | 2001–2003 | 2004–2009 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| All Races | White | Black | All Races | White | Black | |
| No. of patients | 494,511 | 410,029 | 64,335 | 1,033,091 | 831,576 | 144,561 |
| Localized, % | 76.5 | 77.2 | 72.8 | 78.2 | 78.8 | 76.8 |
| Regional, % | 8.7 | 8.8 | 8.3 | 9.6 | 10.0 | 8.3 |
| Distant, % | 3.7 | 3.5 | 5.7 | 3.7 | 3.5 | 5.2 |
| Unknown, % | 11.1 | 10.6 | 13.3 | 8.4 | 7.6 | 9.6 |
Abbreviations: SEER, Surveillance, Epidemiology, and End Results program.
TABLE 2.
Prostate Cancer: Age-Standardized Net Survival (%) at 1, 3, and 5 Years for Males (Ages 15–99 Years) Diagnosed Between 2001 and 2009 by Race and Calendar Period of Diagnosis
| Years | 2001–2003 | 2004–2009 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| All Races | White | Black | All Races | White | Black | |||||||
|
|
|
|
|
|
|
|||||||
| NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | |
| 1 | 98.6 | 98.5–98.7 | 98.7 | 98.7–98.8 | 96.8 | 96.6–97.1 | 98.8 | 98.8–98.9 | 98.8 | 98.8–98.9 | 97.3 | 97.1–97.5 |
| 3 | 97.4 | 97.3–97.5 | 97.6 | 97.5–97.8 | 94.2 | 93.8–94.6 | 97.6 | 97.6–97.7 | 97.6 | 97.5–97.7 | 94.7 | 94.4–95.1 |
| 5 | 96.7 | 96.5–96.8 | 96.9 | 96.7–97.1 | 92.4 | 91.8–92.9 | 96.9 | 96.7–97.1 | 96.9 | 96.7–97.1 | 92.7 | 92.1–93.3 |
Abbreviations: CI, confidence interval; NS, net survival.
TABLE 3.
Prostate Cancer: Five-Year, Age-Standardized Net Survival (%) for Males (Ages 15–99 Years) Diagnosed Between 2001 and 2009 by Surveillance, Epidemiology, and End Results Summary Stage 2000 at Diagnosis, Race, and Calendar Period of Diagnosis
| SEER Summary Stage 2000 | 2001–2003 | 2004–2009 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| All Races | White | Black | All Races | White | Black | |||||||
|
|
|
|
|
|
|
|||||||
| NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | |
| All stages | 96.7 | 96.5–96.8 | 96.9 | 96.7–97.1 | 92.4 | 91.8–92.9 | 96.9 | 96.7–97.1 | 96.9 | 96.7–97.1 | 92.7 | 92.1–93.3 |
| Localized | 99.9 | 99.7–100.0 | 99.8 | 99.6–100.0 | 99.5 | 98.8–100.0 | 99.9 | 99.6–100.0 | 99.7 | 99.5–99.9 | 99.6 | 98.8–100.0 |
| Regional | 93.7 | 92.9–94.4 | 93.4 | 92.6–94.2 | 90.8 | 88.3–93.4 | 93.5 | 92.7–94.4 | 93.3 | 92.4–94.2 | 90.7 | 87.8–93.6 |
| Distant | 29.8 | 28.9–30.6 | 29.7 | 28.7–30.7 | 28.6 | 26.8–30.4 | 29.2 | 28.3–30.1 | 28.7 | 27.6–29.8 | 28.4 | 26.6–30.3 |
| Unknown | 88.3 | 87.8–88.7 | 88.3 | 87.8–88.8 | 83.9 | 82.7–85.2 | 88.0 | 87.5–88.5 | 87.3 | 86.7–87.9 | 82.2 | 80.7–83.7 |
Abbreviations: CI, confidence interval; NS, net survival; SEER, Surveillance, Epidemiology, and End Results program.
In the pooled estimate, the distributions of males diagnosed with localized, regional, and distant prostate cancer were similar during both calendar periods. Between the periods from 2001 to 2003 and from 2004 to 2009, the proportions of cases diagnosed at localized stage increased from 72.8% to 76.8% among black males and from 77.2% to 78.8% among white males. More black males than white males were diagnosed with distant stage prostate cancer between 2001 and 2003 (5.7% vs 3.5%) and between 2004 and 2009 (5.2% vs 3.5%). Similar patterns in the distribution of stage by race were observed in the states during both calendar periods (Supporting Table 1).
Table 2 presents 1-year, 3-year, and 5-year survival estimates by race and calendar period. Overall, survival did not change between the 2 calendar periods. Net survival estimates among males who were diagnosed between 2001 and 2003 were 98.6% at 1 year, 97.4% at 3 years, and 96.7% at 5 years; corresponding estimates among males who were diagnosed between 2004 and 2009 were 98.8%, 97.6%, and 96.9%, respectively. White males had higher 1-year, 3-year, and 5-year net survival than black males. This pattern held true in most states (Supporting Table 2).
Table 3 displays 5-year net survival estimates by race and stage. The pooled estimates for the US registries were comparable between black and white males for localized, regional, and distant prostate cancer during both calendar periods. Between 2004 and 2009, survival exceeded 99% for localized stage and less than 29% for distant stage in both racial groups. However, corresponding estimates varied across the states (Supporting Table 3).
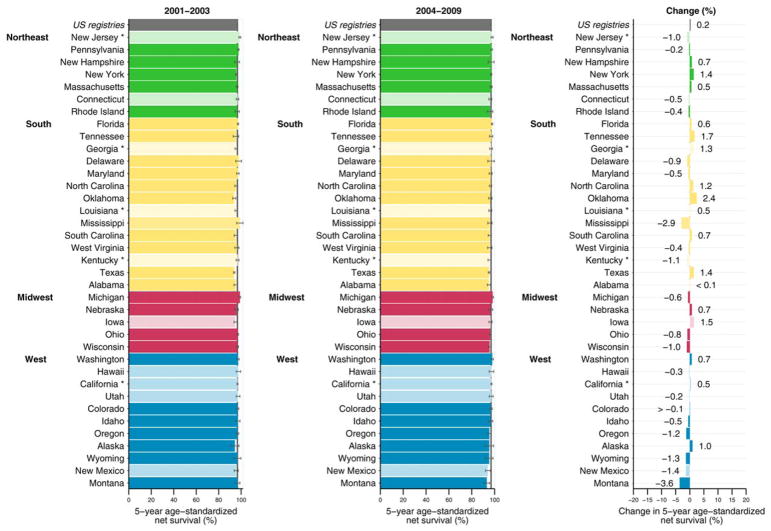
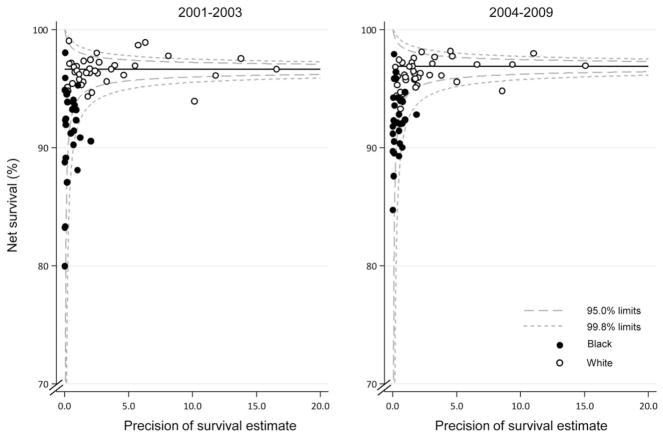
During both calendar periods, as illustrated in Figure 1, the age standardized, 5-year net survival estimates across most states were very high (≥95%) for all races combined. State-specific differences in 5-year net survival were small between males diagnosed between 2001 and 2003 and those diagnosed between 2004 and 2009. Racial and geographic differences in 5-year net survival are displayed graphically in funnel plots (Fig. 2). Survival among black males (Fig. 2, solid circles) was lower compared with that among white males (Fig. 2, open circles), and most estimates were below the pooled estimate. There was less variation around the pooled estimates among white males.
Figure 1.
Prostate cancer 5-year, age-standardized net survival (%) for males (ages 15–99 years) diagnosed between 2001 and 2003 and between 2004 and 2009 and absolute change (%) are illustrated. States are grouped by US Census region. Note that data from 37 statewide cancer registries (covering 80.6% of the population) are ranked within US Census Region by the survival estimate for 2004 to 2009. Dark colors denote states affiliated with the National Program of Cancer Registries (NPCR), and pale colors denote states affiliated with the Surveillance, Epidemiology, and End Results (SEER) Program. An asterisk denotes states affiliated with both federal surveillance programs. Change (%) was not plotted if a survival estimate was not available for 1 calendar period or if 1 or more estimates were not age-standardized.
Figure 2.
Prostate cancer 5-year, age-standardized net survival (%) is illustrated for males (ages 15–99 years) by state, race, and calendar period of diagnosis. Note that the pooled US survival estimate for each calendar period is indicated by the horizontal (solid) line with corresponding 95.0% and 99.8% control limits (dashed lines).
DISCUSSION
This study compared prostate cancer stage distribution and survival estimates by race among males who were diagnosed between 2001 and 2003 and between 2004 and 2009 in 37 states, covering 80% of the US population. We observed high percentages of localized prostate cancer among black and white males diagnosed during both calendar periods, whereas black males had slightly higher proportions of distant disease at diagnosis compared with white males. Overall, there was no change in net survival between the 2 calendar periods in either racial group. The high but stable survival estimates in our analysis might reflect trends in prostate cancer incidence, rather than true improvements in survival. The incidence of prostate cancer increased rapidly between the 1980s and the 1990s, largely because of the widespread use of PSA-based screening.17 The increase in incidence was followed by a steady decline in the late 1990s, as the pool of prevalent cases available for detection decreased.18–20 It has been reported that the declines in incidence of localized/regional prostate cancer and of distant prostate cancer started in 2001 and 1995, respectively.17 Another study noted that most decreases in prostate cancer incidence since 2008 have occurred uniformly across age and racial/ethnic groups among males diagnosed at the localized/regional stage.20 Similar to prostate cancer incidence, prostate cancer mortality rates started to decrease in the 1990s.3,18,21–23 The decline has been attributed to several different factors, including PSA screening intensity and improvements in treatment of distant-stage disease.18,21–23
Five-year net survival among black and white males who were diagnosed with localized/regional prostate cancer between 2001 and 2009 approached 99% overall and in most states. Previous studies have reported that racial disparities in prostate cancer survival among males diagnosed at these stages have decreased over time.24–26 During both calendar periods, we observed that 5-year net survival for all other stages was also comparable among black and white males. Across the states, there was more variation in survival among males diagnosed at distant stage than among those diagnosed at other stages; however, these estimates were based on small numbers. State-specific variation in cancer survival might be related to differences in the demographic characteristics of males at risk for prostate cancer, in spending on cancer prevention and control, and in health insurance coverage rates.27–29
The very high 5-year prostate cancer survival that we observed among US males has also been reported among males in several European countries.8 Survival increased in many of these countries between the periods from 1995 to 1999 and from 2005 to 2009; however, increases were smaller in North America, where survival has been very high since the early 1990s.
Clinical Implications
Although adoption of PSA-based prostate cancer screening in the United States over the past 2 decades has led to diagnosis of prostate cancer at earlier stages and improved survival, it has also resulted in overdiagnosis (ie, detection in individuals who would have died of other causes) and over-treatment of many clinically insignificant tumors.30,31 Therefore, as concerns about potential harms of PSA-based screening emerged, some organizations and professional societies recommended against screening males of all ages; however, others emphasized shared decision making and age-specific testing.32–37 Strategies to help clinicians reduce these problems have included updating prostate cancer screening recommendations and using prediction tools to help improve risk stratification.38–40 Tools in use or development to improve clinical stratification for screening and treatment include biomarkers, nomograms, genomic testing, and enhanced imaging.39,40 Increased use of active surveillance for males diagnosed with localized prostate cancer might also relieve some of the burdens associated with early intervention.38
Findings regarding the effect of widespread PSA screening on prostate cancer mortality have been mixed. Some studies have reported that testing reduces the number of deaths from the disease, but others did not report an association.21,41–43 The age-adjusted annual death rate from prostate cancer among black males is 2 to 3 times that of white males, which is caused in part by their higher disease incidence, tumor characteristics, treatment choices, and socioeconomic status.2,44 Deaths have been declining since 1996 at a rate of 3.6% per year among black males and 3.4% among white males.26 However, the reasons for the persistence of the racial disparity are not clear.
Changes in clinical management of prostate cancer in the United States over the past 3 decades also may have affected survival. A dramatic increase in the receipt of radical prostatectomy overall was reported from the mid-1980s, followed by a plateau in the early 1990s; the rate started to increase again in the early 2000s, but the increase was gradual.21 Among males with small, low-grade, clinical stage T3 (ie, locally advanced) prostate cancer, an increase in the receipt of surgery was reported between 1998 and 2012.45 One study reported that surgery receipt declined between 1995 and 2013 among males with low-risk prostate cancer, whereas it increased among those with intermediate-risk prostate cancer and among older males with low-risk and intermediate-risk disease.46 In another study, however, receipt of radical prostatectomy increased between 2004 and 2010 among males with low-risk and intermediate-risk prostate cancer.47
Demographic disparities have been reported in the receipt of definitive treatment for prostate cancer, with lower rates among black males and variations by insurance type, geographic region, and age group.29,48–55 Differences in treatment preferences may account for some of these disparities. In earlier research examining treatment choice among males with localized prostate cancer, black males reportedly were more likely to select nonsurgical options compared with their white counterparts.49,56,57 A more recent study indicated that, although active surveillance was the most preferred treatment among black and white males with localized prostate cancer, surgery was more common among black males.58 The treatment decision-making process also may differ by race/ethnicity and by other sociodemographic factors. In 1 study of males with localized prostate cancer, white males selected active surveillance/watchful waiting based on cancer risk, but black males did not; this raises concerns about under-treatment and lack of understanding about cancer risk among the latter group.53 A study of treatment choice among males in urban and rural parts of Georgia reported that disparities may be related more to differences in income than differences in race.55 The authors also noted, however, that poor communication with physicians was more prevalent among black patients and was associated with not receiving treatment in rural areas.
Cancer-Control Implications
Monitoring disparities in prostate cancer survival, especially with survival rates for distant stage, requires high-quality surveillance data. To more comprehensively define these disparities, the data could be enhanced with information about socioeconomic factors and other social determinants of health that affect cancer outcomes.59 Lack of insurance, low educational attainment, and poverty status are all associated with increased cancer risk and poor outcomes.25,27,29,50 To help assess prostate cancer outcomes across the states, cancer-control practitioners could continue to work with programs like the Centers for Disease Control and Prevention’s (CDC’s) National Program of Cancer Registries (NPCR) and National Comprehensive Cancer Control Program.60 Other CDC resources available to cancer-control planners include communication materials that explore clinician-patient discussions about prostate cancer screening and treatment, research that has examined enhancement of prostate cancer data in cancer registries and patterns of prostate cancer care, and findings from an active surveillance state-of-science consensus conference organized by the agency. In addition, the CDC has supported projects to explore patient information-seeking behavior postdiagnosis, caregiver and provider involvement in treatment decision making, and patient quality of life after prostate cancer treatment.61 To help reduce disparities in receipt of prostate cancer treatment, cancer-control planners could examine these factors and others, such as patient comorbidities and provider characteristics. They also might assess whether disparities in post-treatment care affect long-term and late effects that prostate cancer survivors may experience.
The current study has notable strengths. The CONCORD-2 study is the largest comparative study of population-based cancer survival in the United States, and it includes high-quality data covering 80% of the US population. Standardized collection, reporting, and analysis of the data ensures the availability of comparable data. A high percentage (>97%) of cases in the United States are microscopically confirmed, and the percentage of cases with unknown stage (11.1% in 2001–2003, 8.4% in 2004–2009) is relatively low.8
However, this study has a few limitations, which might influence interpretation of the results. First, follow-up procedures among cancer registries in the United States differ, depending on federal funding source.60 All SEER registries are required to conduct active follow-up of all registered cases to ascertain vital status. NPCR registries are only funded to ascertain deaths through linkages with state vital records and the National Death Index; therefore, they may overestimate survival time and miss some deaths, because death ascertainment is conducted primarily through data linkages.62 Second, the manner in which SS2000 data were collected and reported changed for all registries in 2004, as described above (see Materials and Methods). The impact of this change was most evident in NPCR-funded registries, where the percentage of cases with unknown stage decreased somewhat when stage was derived rather than manually coded.
Conclusions
Prostate cancer survival remains high among males whose disease is detected early, and the racial gap in survival observed in the first CONCORD study has narrowed. Disparities in receipt of standard and timely care are still being reported, however, particularly among males who might benefit most from treatment.48–52,54 To help ensure that all prostate cancer survivors receive appropriate care, cancer-control planners could optimize their use of local data and resources to explore demographic differences in survivors’ access to health insurance, primary and specialty medical care, and timely receipt of treatment. Clinicians should be aware that, although a recent study of males diagnosed with low-risk prostate cancer indicated that most patients were satisfied with their treatment decision-making discussions with physicians, a patient’s health literacy could affect his understanding of cancer risk and treatment options.58
Supplementary Material
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily reflect the official position of the CDC.
This Supplement edition of Cancer has been sponsored by the U.S. Centers for Disease Control and Prevention (CDC), an Agency of the Department of Health and Human Services.
The CONCORD-2 study was approved by the Ethics and Confidentiality Committee of the UK’s statutory National Information Governance Board (now the Health Research Authority) (ref ECC 3-04(i)/2011) and by the National Health Service Research Ethics Service (Southeast; 11/LO/0331).
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
AUTHOR CONTRIBUTIONS
C. Brooke Steele: Writing–original draft, and supervision. Jun Li: Writing–review and editing. Bin Huang: Writing–review and editing. Hannah K. Weir: Conceptualization, methodology, writing–review and editing, visualization.
FUNDING SUPPORT
No specific funding was disclosed.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.US Cancer Statistics (USCS) Working Group. US Cancer Statistics: 1999–2013 Incidence and Mortality Web-Based Report. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control, Prevention and National Cancer Institute; 2016. [Accessed April 1, 2016]. Available at: www.cdc.gov/uscs. [Google Scholar]
- 3.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cancer Institute. Prostate specific antigen (PSA) test. Bethesda, MD: National Cancer Institute; 2016. [Accessed March 31, 2016]. Available at: http://www.cancer.gov/types/prostate/psa-fact-sheet. [Google Scholar]
- 5.Hankey BF, Feuer EJ, Clegg LX, et al. Cancer surveillance series: interpreting trends in prostate cancer—Part I: evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. 1999;91:1017–1024. doi: 10.1093/jnci/91.12.1017. [DOI] [PubMed] [Google Scholar]
- 6.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2013. Bethesda, MD: National Cancer Institute; 2016. [Accessed April 1, 2016]. Available at: http://seer.cancer.gov/csr/1975_2013/ ( http://seer.cancer.gov/csr/1975_2013), based on November 2015 SEER data submission, posted to the SEER web site, April 2016. [Google Scholar]
- 7.Coleman MP, Quaresma M, Berrino F, et al. CONCORD Working Group. Cancer survival in 5 continents: a worldwide population-based study (CONCORD) Lancet Oncol. 2008;9:730–756. doi: 10.1016/S1470-2045(08)70179-7. [DOI] [PubMed] [Google Scholar]
- 8.Allemani C, Harewood R, Johnson CJ, et al. Population-based cancer survival in the United States: data, quality control, and statistical methods. Cancer. 2017;123:4982–4993. doi: 10.1002/cncr.31025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritz AG, Percy C, Jack A, et al., editors. International Classification of Diseases for Oncology (ICD-O) 3. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 10.Young JL Jr, Roffers SD, Ries LAG, Fritz AG, Hurlbut AA, editors. SEER Summary Staging Manual-2000: Codes and Coding Instructions. Bethesda, MD: National Cancer Institute; 2001. NIH Pub. No. 01-4969. [Google Scholar]
- 11.Surveillance Epidemiology and End Results (SEER) Program. Collaborative Stage. Bethesda, MD: National Cancer Institute; 2004. [Accessed April 1, 2016]. Available at: http://seer.cancer.gov/tools/collabstaging/ [Google Scholar]
- 12.Pohar Perme M, Stare J, Esteve J. On estimation in relative survival. Biometrics. 2012;68:113–120. doi: 10.1111/j.1541-0420.2011.01640.x. [DOI] [PubMed] [Google Scholar]
- 13.Rachet B, Maringe C, Woods LM, Ellis L, Spika D, Allemani C. Multivariable flexible modelling for estimating complete, smoothed life tables for sub-national populations [serial online] BMC Public Health. 2015;15:1240. doi: 10.1186/s12889-015-2534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spika D, Bannon F, Bonaventure A, et al. Life tables for global surveillance of cancer survival (the CONCORD programme): data sources and methods [serial online] BMC Cancer. 2017;17:159. doi: 10.1186/s12885-017-3117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corazziari I, Quinn MJ, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer. 2004;40:2307–2316. doi: 10.1016/j.ejca.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Quaresma M, Coleman MP, Rachet B. Funnel plots for population-based cancer survival: principles, methods and applications. Stat Med. 2014;33:1070–1080. doi: 10.1002/sim.5953. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman RM, Meisner AL, Arap W, et al. Trends in United States prostate cancer incidence rates by age and stage, 1995–2012. Cancer Epidemiol Biomarkers Prev. 2015;25:259–263. doi: 10.1158/1055-9965.EPI-15-0723. [DOI] [PubMed] [Google Scholar]
- 18.Singh SD, Henley SJ, Ryerson AB. Summary of notifiable noninfectious conditions and disease outbreaks—United States, 2011. MMWR Morb Mortal Wkly Rep. 2015;62:11–51. doi: 10.15585/mmwr.mm6254a3. [DOI] [PubMed] [Google Scholar]
- 19.Zhou CK, Check DP, Lortet-Tieulent J, et al. Prostate cancer incidence in 43 populations worldwide: an analysis of time trends overall and by age group. Int J Cancer. 2015;138:1388–1400. doi: 10.1002/ijc.29894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Etzioni R, Gulati R. Recent trends in PSA testing and prostate cancer incidence: a look at context. JAMA. 2015;314:2054–2061. doi: 10.1001/jamaoncol.2015.6310. [DOI] [PubMed] [Google Scholar]
- 21.Collin SM, Martin RM, Metcalfe C, et al. Prostate cancer mortality in the USA and UK in 1975–2004. Lancet Oncol. 2008;9:445–452. doi: 10.1016/S1470-2045(08)70104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;45:152–156. doi: 10.1093/jncimonographs/lgs035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 24.Zeng C, Wen W, Morgans AK, Pao W, Shu XO, Zheng W. Disparities by race, age, and sex in the improvement of survival for major cancers. JAMA Oncol. 2015;1:88–96. doi: 10.1001/jamaoncol.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 26.DeSantis CE, Siegel RL, Sauer AG, et al. Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016;66:290–308. doi: 10.3322/caac.21340. [DOI] [PubMed] [Google Scholar]
- 27.Kish JK, Yu M, Percy-Laurry A, Altekruse SF. Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in Surveillance, Epidemiology, and End Results (SEER) registries. J Natl Cancer Inst Monogr. 2014;49:236–243. doi: 10.1093/jncimonographs/lgu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooks GA, Li L, Sharma DB, et al. Regional variation in spending and survival for older adults with cancer. J Natl Cancer Inst. 2013;105:634–642. doi: 10.1093/jnci/djt025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niu X, Roche LM, Pawlish KS, Henry KA. Cancer survival disparities by health insurance status. Cancer Med. 2013;2:403–411. doi: 10.1002/cam4.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loeb S, Bjurlin M, Tammela TL, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65:1046–1055. doi: 10.1016/j.eururo.2013.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from US prostate cancer incidence trends. J Natl Cancer Inst. 2002;94:981–990. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 32.Moyer VA. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 33.Livingston CJ, Freeman RJ, Mohammad A, et al. Choosing Wisely in preventive medicine: the American College of Preventive Medicine’s top 5 list recommendations. Am J Prev Med. 2016;51:141–149. doi: 10.1016/j.amepre.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 34.American Academy of Family Physicians. Prostate Cancer: Clinical Preventive Services Recommendation. Leawood, KS: American Academy of Family Physicians; 2012. [Accessed April 1, 2016]. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/prostate-cancer.html. [Google Scholar]
- 35.American Urological Association (AUA) Early Detection of Prostate Cancer: AUA Guideline. Seaford, NY: AUA; 2013. [Accessed April 1, 2016]. Available at: https://www.auanet.org/education/guidelines/prostate-cancer-detection.cfm. [Google Scholar]
- 36.Wilt TJ, Harris RP, Qaseem A. Screening for cancer: advice for high-value care from the American College of Physicians. Ann Intern Med. 2015;162:718–725. doi: 10.7326/M14-2326. [DOI] [PubMed] [Google Scholar]
- 37.National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer Early Detection, Version 2.2016. Fort Washington, PA: NCCN; 2016. [Accessed April 1, 2016]. Available at: https://www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf. [Google Scholar]
- 38.Pinsky PF, Prorok PC, Kramer BS. Prostate cancer screening—a perspective on the current state of the evidence. N Engl J Med. 2017;376:1285–1289. doi: 10.1056/NEJMsb1616281. [DOI] [PubMed] [Google Scholar]
- 39.Lee DJ, Mallin K, Graves AJ, et al. Recent changes in prostate cancer screening practices and prostate cancer epidemiology [published online ahead of print May 25, 2017] J Urol. doi: 10.1016/j/juro.2017.05.074. [DOI] [PubMed] [Google Scholar]
- 40.Rodgers L, Peer CJ, Figg WD. Diagnosis, staging, and risk stratification in prostate cancer: utilizing diagnostic tools to avoid unnecessary therapies and side effects [published online ahead of print May 5, 2017] Cancer Biol Ther. doi: 10.1080/15384047.2017.1323600. [DOI] [PMC free article] [PubMed]
- 41.Etzioni R, Gulati R, Tsodikov A, et al. The prostate cancer conundrum revisited: treatment changes and prostate cancer mortality declines. Cancer. 2012;118:5955–5963. doi: 10.1002/cncr.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinsky PF, Prorok PC, Yu K, et al. Extended mortality results for prostate cancer screening in the PLCO trial with median follow-up of 15 years. Cancer. 2017;1123:592–599. doi: 10.1002/cncr.30474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroder FH, Hugosson J, Roobol MJ, et al. ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 44.Taksler GB, Keating NL, Cutler DM. Explaining racial differences in prostate cancer mortality. Cancer. 2012;118:4280–4289. doi: 10.1002/cncr.27379. [DOI] [PubMed] [Google Scholar]
- 45.Nezolosky MD, Dinh KT, Muralidhar V, et al. Significant increase in prostatectomy and decrease radiation for clinical T3 prostate cancer from 1998 to 2012. Urol Oncol. 2016;34:57.e15–57.e22. doi: 10.1016/j.urolonc.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990–2013. JAMA. 2015;314:80–82. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 47.Weiner AB, Patel SG, Etzioni R, Eggener SE. National trends in the management of low and intermediate risk prostate cancer in the United States. J Urol. 2014;193:95–102. doi: 10.1016/j.juro.2014.07.111. [DOI] [PubMed] [Google Scholar]
- 48.Schmid M, Meyer CP, Reznor G, et al. Racial differences in the surgical care of Medicare beneficiaries with localized prostate cancer. JAMA Oncol. 2016;2:85–93. doi: 10.1001/jamaoncol.2015.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moses KA, Paciorek AT, Penson DF, Carroll PR, Master VA. Impact of ethnicity on primary treatment choice and mortality in men with prostate cancer: data from CaPSURE. J Clin Oncol. 2010;28:1069–1074. doi: 10.1200/JCO.2009.26.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chornokur Dalton K, Borysova ME, Kumar NB. Disparities at prevention, diagnosis, treatment, and survival in African American men affected by prostate cancer. Prostate. 2011;71:985–997. doi: 10.1002/pros.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Underwood W, 3rd, Jackson J, Wei JT, et al. Racial treatment trends in localized/regional prostate carcinoma: 1992–1999. Cancer. 2005;103:538–545. doi: 10.1002/cncr.20796. [DOI] [PubMed] [Google Scholar]
- 52.Cary KC, Punnen S, Odisho AY, et al. Nationally representative trends and geographic variation in treatment of localized prostate cancer: the Urologic Diseases in America. Prostate Cancer Prostatic Dis. 2015;18:149–154. doi: 10.1038/pcan.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu J, Janisse J, Ruterbusch J, Ager J, Schwartz KL. Racial differences in treatment decision-making for men with clinically localized prostate cancer: a population-based study. J Racial Ethn Health Disparities. 2016;3:35–45. doi: 10.1007/s40615-015-0109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Presley CJ, Raldow AC, Cramer LD, et al. A new approach to understanding racial disparities in prostate cancer treatment. J Geriatr Oncol. 2013;4:1–8. doi: 10.1016/j.jgo.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steenland K, Goodman M, Liff J, et al. The effect of race and rural residence on prostate cancer treatment choice among men in Georgia. Urology. 2011;77:581–587. doi: 10.1016/j.urology.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 56.Hoffman RM, Harlan LC, Klabunde CN, et al. Racial differences in initial treatment for clinically localized prostate cancer. J Gen Intern Med. 2003;18:845–853. doi: 10.1046/j.1525-1497.2003.21105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denberg TD, Beaty BL, Kim FJ, Steiner JF. Marriage and ethnicity predict treatment in localized prostate carcinoma. Cancer. 2005;103:1819–1825. doi: 10.1002/cncr.20982. [DOI] [PubMed] [Google Scholar]
- 58.Hoffman RM, Van Den Eeden SK, Davis KM, et al. Decision-making processes among men with low-risk prostate cancer: a survey study [published online ahead of print June 13, 2017] Psychooncology. doi: 10.1002/pon.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brawley OW. Some thoughts on health surveillance data, race, and population categorization. CA Cancer J Clin. 2016 May;66:179–181. doi: 10.3322/caac.21346. [DOI] [PubMed] [Google Scholar]
- 60.White MC, Babcock F, Hayes NS, et al. The history and use of cancer registry data by public health cancer control programs in the United States. Cancer. 2017;123:4969–4976. doi: 10.1002/cncr.30905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hall IJ, Lee Smith J. Evolution of a CDC public health research agenda for low-risk prostate cancer. Am J Prev Med. 2015;49(6 suppl 5):S483–S488. doi: 10.1016/j.amepre.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson CJ, Weir HK, Fink AK, et al. Accuracy of Cancer Mortality Study Group. The impact of National Death Index linkages on population-based cancer survival rates in the United States. Cancer Epidemiol. 2013;37:20–28. doi: 10.1016/j.canep.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.