Abstract
Purpose of review
Noncoding RNAs have emerged as important regulators of cellular and systemic lipid metabolism. In particular, the enigmatic class of long noncoding RNAs have been shown to play multifaceted roles in controlling transcriptional and posttranscriptional gene regulation. In this review, we discuss recent advances, current challenges and future opportunities in understanding the roles of lncRNAs in the regulation of lipid metabolism during health and disease.
Recent findings
Despite comprising the majority of the transcriptionally active regions of the human genome, lncRNA functions remain poorly understood, with fewer than 1% of human lncRNAs functionally characterized. Broadly defined as nonprotein coding transcripts greater than 200 nucleotides in length, lncRNAs execute their functions by forming RNA–DNA, RNA–protein, and RNA–RNA interactions that regulate gene expression through diverse mechanisms, including epigenetic remodeling of chromatin, transcriptional activation or repression, posttranscriptional regulation of mRNA, and modulation of protein activity. It is now recognized that in lipid metabolism, just as in other areas of biology, lncRNAs operate to regulate the expression of individual genes and gene networks at multiple different levels.
Summary
The complexity revealed by recent studies showing how lncRNAs can alter systemic and cell-type-specific cholesterol and triglyceride metabolism make it clear that we have entered a new frontier for discovery that is both daunting and exciting.
Keywords: cholesterol, hepatocyte, lincRNA, lipid metabolism, lncRNA, long noncoding RNA, macrophage, triglyceride
INTRODUCTION
For decades, researchers have investigated how protein coding genes and their regulatory networks control lipid metabolism as a gateway to better understanding both homeostatic physiology and disease-causing pathophysiology. Although this approach has yielded a number of major advances, innovations in the postgenomic era have introduced a new variable to the regulation of gene expression and function: noncoding RNAs [1]. It is now recognized that multiple classes of noncoding RNAs, including microRNAs (miRNAs), small nucleolar RNAs (snoRNAs), circular RNAs, and long noncoding RNAs (lncRNAs) possess regulatory functions that feed into the previously described networks controlling cellular lipid metabolism. The study of noncoding RNAs has revealed that they regulate the expression of key genes, as well as networks of genes that function in integrated pathways of cholesterol and fatty acid biosynthesis, reverse cholesterol transport, and lipid storage [2,3]. The complexity already revealed makes it clear that we have entered a new frontier for discovery that is both daunting and exciting.
The vast majority of the transcriptionally active regions of the human genome are now known to produce lncRNAs, which are broadly defined as transcripts greater than 200 nucleotides in length that biochemically resemble mRNAs (transcribed by RNA polymerase II, 5’-capped, 3’-polyadenylated, can undergo splicing), but do not code for proteins [4,5]. Yet, lncRNAs remain the least characterized class of noncoding RNAs: The human genome is estimated to contain on the order of 20 000 lncRNA genes, but only 1% of those have been functionally characterized [6]. Hindering progress is the poorly conserved nature of lncRNAs, their low abundance in the cell, and their ability to function through diverse mechanisms to regulate gene expression or chromosomal architecture [7,8]. The position of lncRNA genes relative to their adjacent protein coding genes is often used an elementary way to classify their transcripts into antisense, sense, bidirectional or intergenic lncRNAs, which can be further distinguished by their abilities to act at genomic loci in cis or in trans [9,10■]. These subclassifications can provide some hints into lncRNA function, but for the most part, unraveling the functions of this intriguing class of noncoding RNAs has proved challenging [11]. However, the rapid evolution of experimental tools for manipulating lncRNA expression (e.g., CRISPR/Cas9 gene-editing and activation tools, antisense oligonucleotides, virus-based overexpression) and for investigating their mechanistic functions [cross-linking immunoprecipitation (CLIP) or analysis of synthetic hybrids (CLASH); chromatin isolation by RNA purification (ChIRP)] is paving the way for discoveries in the area of lipid metabolism, as in all fields of biology.
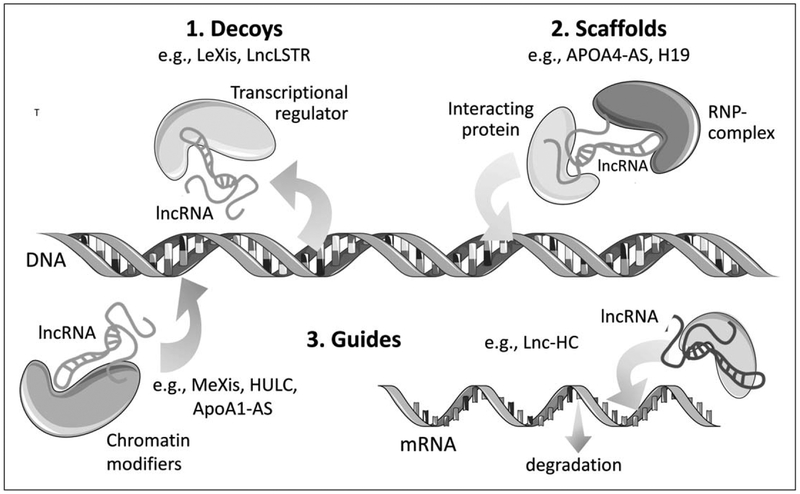
LncRNAs are able to bind to proteins, DNA, RNA, or a combination thereof in order to exert their functions. These actions can be used to classify lncRNAs as: guides, decoys, scaffolds, or enhancers involved in pretranslation and posttranslation regulation of gene expression [12] (Fig. 1). For example, a number of lncRNAs have been shown to mediate transcriptional repression by guiding chromatin modifiers such as the polycomb repressor complex 2 (PRC2) to genomic targets, as has been described for Xist [13]. Fendrr [14], and ANRIL [15]. By contrast, decoying lncRNAs exert their functions by sequestering regulatory factors in the nucleus or cytoplasm: MALAT1, for example, can trap splicing factors in nuclear speckles to regulate premRNA alternative splicing [16]; whereas cytoplasmic lncRNAs, such as CARL or lnc-MD1, can interact with mature miRNAs to block their repression of mRNA translation [17,18]. LncRNAs can also serve as scaffolds that recruit RNA binding proteins into spatial proximity of each other or DNA. Finally, lncRNAs can serve as co-activators or enhancers of target gene activation, as has been described for SRA and LUNAR1, respectively [19,20]. Often a single lncRNA may have more than one function, varying by cell type, stimuli, and/or cellular localization. As the list of functions mediated by lncRNAs continues to grow, it has become clear that they are important regulators of a wide range of biological and cellular processes. Here, we review the roles of lncRNAs in the regulatory networks that control lipid metabolism in hepatocytes and macrophages, and discuss how their dysregulation can contribute to the pathogenesis of diseases, such as atherosclerosis and hepatosteatosis.
FIGURE 1.
Schematic diagram of lncRNA mechanisms of action: (1) LncRNAs can act as molecular decoys to move proteins away from a specific DNA location; (2) LncRNA can serve as molecular scaffolds to bring proteins into stable complexes that can modulate gene expression; (3) LncRNAs can guide chromatin modifiers and transcription factors to DNA to both repress and activate gene expression (left). LncRNAs can also guide proteins to mRNAs and influence the stability of these transcripts. Examples of lncRNAs affecting lipid metabolism are given for each example.
LONG NONCODING RNAS REGULATING THE STEROL REGULATORY ELEMENT BINDING PROTEIN PATHWAY
Recent studies have shown that lncRNAs can regulate the expression, stability and function of the sterol regulatory element binding protein (SREBP) family of transcription factors. The mammalian genome encodes three SREBP isoforms (designated SREBP-1a, SREBP-1c, and SREBP-2) that together regulate cellular and systemic lipid homeostasis by controlling the expression of genes required for cholesterol, fatty acid, and phospholipid synthesis and uptake. SREBP-1c preferentially enhances transcription of genes required for fatty acid synthesis, whereas SREBP-2 preferentially activates transcription of genes involved in cholesterol synthesis. As studies documenting the roles of lncRNAs in controlling SREBP functions have emerged, it is become clear that lncRNAs play fundamental roles in helping SREBPs to execute their transcriptional functions and in regulating transcriptional integration of lipid metabolism through feedback inhibition or cross-talk.
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) was recently shown to regulate hepatic lipid accumulation by increasing the stability of SREBP-1c protein [21■]. MALAT1 is a conserved lncRNA previously implicated in cancer [22] and type 2 diabetes [23,24], that was found to be upregulated in hepatic HepG2 cells and primary murine hepatocytes treated with palmitate [25]. The palmitate-induced increase in MALAT1 expression coincided with an increase in (SREBP)-1c in hepatic cells. Interestingly, knock-out of MALAT1 in mice significantly reduced hepatic lipid levels in vivo, while overexpression of MALAT1 in palmitate-treated HepG2 cells enhanced lipid accumulation. Using RNA immunoprecipitation techniques, investigators demonstrated that binding of MALAT1 to SREBP-1c in the nucleus inhibited its ubiquitination, thereby stabilizing SREBP-1c protein. As a result of this interaction with MALAT1, there was sustained expression of SREBP-1c target genes and consequently, increased cellular lipid accumulation [21■]. A similar function in stabilizing SREBP-1c and promoting lipogenesis has been described for lncRNA H19, which like MALAT1, is upregulated by fatty acids [26■]. H19 was originally reported to function as a protein scaffold in tumor development and osteoblast differentiation [27,28]. Further study showed that in hepatocytes H19 alters SREBP-1c activity at both the mRNA and protein levels. In the cytoplasm, H19 stabilizes the SREBP1c mRNA by facilitating its binding to polypyrimidine tract-binding protein 1 (PTBP1), while in the nucleus H19 promotes the binding of PTBP1 to SREBP-1c protein. Together, these functions increase the transcriptional activity of SREBP-1c and promote hepatic lipogenesis [26■].
Other lncRNAs have been reported to act on the SREBF1 and SREBF2 promoters to repress the expression of these transcription factors. Microarray profiling of human hepatocytes following infection with Hepatitis C Virus (HCV) identified lncHR1 (lncRNA HCV regulated 1) as a regulator of the SREBP-1 promoter. HCV infection is known to induce the expression of SREBP-1c and to promote lipid accumulation in the liver. Levels of lncHR1 were increased upon infection with HCV, yet surprisingly, this lncRNA was shown to repress, not induce, the SREBP-1c promoter. Overexpression of lncHR1 in mice decreased the expression of SREBP-1c and reduced hepatic and circulating triglyceride levels. However, whether lncHR1 exerts its effects upon the SREBP-1c promoter by direct binding to the DNA or through secondary mediators remains to be clarified [29]. The mechanism by which another hepatic lncRNA, LeXis, represses the expression of cholesterol biosynthetic genes in the liver is better understood. LeXis expression is increased in the liver (but not macrophages) upon feeding mice a western diet or pharmacological activation of the liver X receptors (LXR), which are sterol-activated nuclear receptors that regulate the expression of genes involved in cholesterol homeostasis. LeXis overexpression in chow-fed mice leads to reduced serum and hepatic cholesterol levels. Interestingly, LeXis failed to lower serum cholesterol levels in mice with liver specific deletion of the SREBP-2-regulator SCAP, implicating the SREBP-2 pathway in the mechanism underlying LeXis’ function. Subsequent experiments showed that hepatic overexpression of LeXis reduced RNA polymerase II engagement at the promoters of Srebf2 and its target genes through its ability to bind Raly, a ribonucleoprotein that shares homology with the steroid receptor coactivator CoAA [30,31■■]. LeXis-Raly interaction prevented Raly-mediated recruitment of RNA polymerase II to the promoters of cholesterol biosynthesis genes, as Srebf2, Hmgcr, Cyp51, and Fdps [31■■]. To test whether this decoying function of LeXis could be harnessed therapeutically, the investigators used an adeno-associated virus (AAV8)-based gene therapy approach currently in phase II clinical trials [32]. AAV8-mediated delivery of LeXis to Ldlr−/− mice prior to feeding mice an atherogenic western diet for 15 weeks reduced the expression of Srebf2 and its target genes in the liver, and caused a sustained reduction of serum cholesterol and triglyceride levels compared to control AAV8 treated mice. Consequently, AAV8-LeXis treated mice showed reduced atherosclerotic plaque formation in the aorta, compared to control-AAV8 treated mice. These encouraging results are among the first to show that delivery of a lncRNA might be used to treat metabolic diseases. Although LeXis is a mouse lncRNA, a potential orthologue of LeXis is present in humans, but its functional characterization will be required to see if it mediates a similar function in regulating the SREBP-2 pathway.
LONG NONCODING RNAS REGULATING LIPOPROTEIN METABOLISM
Natural antisense transcripts are lncRNAs that are transcribed from the opposite DNA strand of a protein-coding gene that can either enhance or repress transcription of their partner protein-coding gene. Two such AS-lncRNAs have been reported to regulate expression of apolipoproteins that contribute to the formation and/or functions of plasma lipoproteins: APOA1-AS and APOA4-AS. The human apolipoprotein gene cluster on chromosome 11q23.3 that encodes APOA1, APOC3, APOA4, and APOA5, also contains the antisense transcript, APOA1-AS [33]. APOA1-AS shares a 123 nucleotide-long region that overlaps with the fourth exon of the APOA1 gene, whose product apoA1 is the building block of plasma HDLs [34]. The expression levels of APOA1 and APOA1-AS transcripts were found to be inversely correlated in tissues, particularly in the liver where expression of APOA1 mRNA is high and APOA1-AS is low. Gain-of-function and loss-of-function studies showed that APOA1-AS is a transcriptional switch that facilitates the interaction of repressive chromatin-modifying complexes that bind to the APO gene locus. APOA1-AS exerts this in cis-regulation by physically interacting with SUZ12, a key component of the polycomb repressive PRC2 complex that mediates chromatin silencing [33]. This mechanism is similar to that reported for ANRIL, a lncRNA present in the coronary artery disease susceptibility locus on chromosome 9p21, that mediates silencing of the tumor suppressor gene p15INK4B via recruitment of SUZ12 and repressive H3K27 trimethylation [15]. In vitro silencing of APOA1-AS in hepatic cells using siRNA caused a three to four-fold upregulation of APOA1, APOC3 and APOA4 mRNA, while expression levels of APOA5 were unchanged. In vivo, delivery of APOA1-AS-targeting antisense oligonucleotides (ASOs) to African Green Monkeys increased hepatic APOA1 mRNA three-fold, resulting in a 5% increase in circulating APOA1 proteins levels after 1 week, confirming the inhibitory function of APOA1-AS on the HDL pathway. Interestingly, the apolipoprotein gene cluster contains a second AS-lncRNA that overlaps with APOA4, and is conserved in mice [35■]. Unlike APOA1-AS, levels of APOA4-AS positively correlate with its overlapping protein coding gene, APOA4. Studies in mouse models of obesity and humans with fatty liver disease showed that both APOA4 and APOA4-AS were elevated in these conditions. APOA4-AS was found to interact with the mRNA stabilizing protein HuR to stabilize APOA4 mRNA. In vivo, shRNA targeting of APOA4-AS in the livers of ob/ob mice lead to decreased hepatic levels of Apoa4 mRNA, as well as reductions in plasma levels of cholesterol and triglyceride. These studies highlight the therapeutic potential of AS-lncRNA acting in cis to regulate neighboring protein coding genes.
Like AS-lncRNAs, lncRNAs that overlap with protein coding genes in the sense direction have been reported to act in cis to alter the expression of their neighboring genes. AT102202 is a 303 base pair lncRNA that overlaps exons 4–6 of the HMGCR gene, which codes for the rate-limiting enzyme involved in cholesterol biosynthesis [36]. In HepG2 cells, AT102202 is induced by epigallocatechin-3-gallate (EGCG), one of the key ingredients in green tea, which has been shown to decrease cholesterol levels and reduce risk of cardiovascular disease [37,38]. Notably, AT102202 reciprocally regulates HMGCR to reduce cholesterol synthesis. Loss of function studies in HepG2 cells showed that siRNA knockdown of AT102202 increased the expression of HMGCR mRNA [36], suggesting a cis-regulatory mechanism. It remains to be determined whether AT102202 regulates HMGCR through the recruitment of epigenetic silencing complexes or other mechanisms, and whether manipulation of AAT102202 in vivo can lead to changes in plasma lipoprotein levels. However, not all lncRNAs that overlap protein coding genes regulate their adjacent neighbor. For example, lnc-HC, a hepatic lncRNA induced by high fat diet feeding of rats, partially overlaps with the 3’ UTR of Slc25a15, but this gene is not co-regulated upon high fat diet. Investigators found that the expression of lnc-HC was regulated by a CEBPB binding domain in its promoter region, and upon transcription lnc-HC localized to the nucleus, where it interacted directly with hnRNPA2/B1. The lnc-HC/hnRNPA2/B1 complex was found to bind to Cyp7a1 and Abca1 mRNAs, targeting them for degradation. As a result, lnc-HC represses Cyp7a1 and Abca1, and their functions in reverse cholesterol transport, thereby promoting cholesterol accumulation in hepatocytes [39].
LONG NON-CODING RNAS REGULATING TRIGLYCERIDE METABOLISM
The lncRNA HULC (Highly Upregulated in Liver Cancer) is dysregulated in hepatocellular carcinomas and has been shown to affect a multitude of cellular functions, including hepatoma cell proliferation, survival, migration, and invasion [40,41]. Interestingly, HULC expression is induced by cholesterol in hepatoma cells via the retinoic receptor RXRA, and can promote lipogenesis by suppressing miR-9 targeting of the nuclear hormone receptor PPARA [42]. HULC exerts its effect on miR-9 expression by epigenetically silencing the miR-9 promoter through methylation of the CpG islands. As a consequence of decreased miR-9 levels, HULC increases the expression of PPARA and thus, transcription of the acyl-CoA synthetase long-chain family member (ACSL1), an enzyme involved in fatty acid metabolism that converts free fatty acids into acyl-CoAs. HULC overexpression in HepG2 and Huh7 cells increased cell triglyceride and cholesterol content and promoted proliferation. Notably, siRNA silencing of ACSL1 in HepG2 cells abolished HULC’s effects on proliferation in vitro and in vivo. Thus, HULC appears to enhance lipogenesis in hepatoma cells, which supports tumor growth.
Triglycerides are a key energy source made up of free fatty acids that are ester-linked to a glycerol backbone. Triglycerides are first synthesized in intestinal and liver cells and are then transported to the plasma for further processing or storage. Elevated triglyceride levels in the circulation are associated with metabolic syndrome and cardiovascular disease [43]. LncRNAs regulating the synthesis and clearance of triglycerides are thus of interest for the generation of novel therapeutic approaches to treat dyslipidemias [29].
LncRNA Liver-Specific Triglyceride Regulator (lncLSTR) is an intergenic lncRNA that was identified by lncRNA profiling of murine livers after subjecting mice to fasting and refeeding [44]. LncLSTR expression rapidly declined upon fasting and was restored after refeeding, suggesting a putative role of lncLSTR in energy metabolism. Indeed, in vivo depletion of lncLSTR using adenovirus-mediated delivery of two independent shRNAs targeting lncLSTR in wild type or Apoe−/− mice markedly reduced plasma triglyceride, but not cholesterol, levels. Investigation of the underlying mechanism showed that lncLSTR knockdown enhanced plasma triglyceride clearance via an increase in ApoC2 expression and LPL activity. Interestingly, lncLSTR does not regulate ApoC2 in a cell-autonomous manner, but rather alters expression of Cyp8b1, one of two enzymes that are rate-limiting in bile acid synthesis [45]. The subsequent shift in bile acid ratio activates FXR, which in turn elevates ApoC2 expression, leading to enhanced triglyceride clearance from the circulation [46]. Notably, lncLSTR was found to interact with TDP-43, a known RNA and DNA binding protein and transcriptional repressor [44], prohibiting TDP-43 binding at the Cyp8b1 promoter to attenuate its expression. Although a human homologue of lncLSTR has not yet been identified, this study highlights the therapeutic potential of targeting such a lncRNA to regulate plasma triglyceride levels.
The genomic region surrounding the TRIB1 (Tribbles homolog 1) gene locus has been associated with high circulating triglyceride levels in several genome-wide association studies (GWAS) [47,48], and it harbors a lncRNA with multiple splice variants that was named TRIBAL (TRIB1 Associated Locus). The promoter region of TRIBAL is altered by a single nucleotide polymorphism that is associated with plasma triglyceride concentrations and TRIB1 expression in blood cells. However, reducing TRIBAL levels in vitro using ASOs led to only a small decrease in the expression TRIB1, and overexpression of TRIBAL had no effect on TRIB1 levels. These findings argue against cis-regulation of TRIB1 by TRIBAL. Furthermore, it was shown that other lipid genes potentially affecting triglyceride levels (e.g., ABCA1, APOB, FASN) were similarly unaffected by manipulation of TRIBAL expression. Transcriptional analyses of cells with TRIBAL downregulation and upregulation failed to identify changes in protein coding transcripts, and minimal changes in lncRNAs and piwi-RNAs were observed. As such, despite GWAS evidence, a distinct role for TRIBAL in triglyceride synthesis, remains to be elucidated [49].
LONG NON-CODING RNAS REGULATING MACROPHAGE CHOLESTEROL UPTAKE AND EFFLUX
During hypercholesterolemia, LDL particles are retained in the subendothelial space, where they are susceptible to modifications that render them proinflammatory. This elicits the recruitment of immune cells, particularly monocyte-derived macrophages, and sets off the chronic inflammation that fuels the formation of atherosclerotic plaques. Macrophages in the arterial intima take up modified LDL particles, and readily become laden with cholesterol-rich and triglyceride-rich lipid droplets, giving these cells a ‘foamy’ appearance. These macrophage foam cells have been shown to play central roles in the initiation and progression of atherosclerotic plaques through their secretion of inflammatory mediators, including cytokines, chemokines, proteases, and tissue factor, among others. Several studies have been performed to screen for lncRNAs induced during macrophage foam cell formation after incubation with oxidized or acetylated LDL in vitro. Transcriptome analysis of in vitro formed macrophage foam cells identified several lncRNAs that were upregulated, including RP5–833A20.1 [50], H19 [51], and lincRNA-DYNLRB2–2 [52]. Overexpression of RP5–833A20.1 in THP-1 macrophages was shown to promote the uptake of oxidized LDL and to decrease cholesterol efflux, resulting in the increased expression of inflammatory mediators such as TNFα, IL-1β and IL-6. Interestingly, RP5–833A20.1 is located within the second intron of the Nuclear Factor I A (NFIA) gene, and mechanistic studies suggested that RP5–833A20.1 exerts its effects on macrophage cholesterol content by down-regulating NFIA, in part via miR-382–5p [50]. Like RP5–833A20.1, lncRNA H19 was found to be upregulated in oxidized LDL-treated macrophages, and to correlate with cellular lipid content. Silencing of H19 using shRNAs decreased macrophage foam cell formation and inflammatory cytokine expression [51]. By contrast, upregulation of lincRNA-DYNLRB2–2 in macrophage foam cells was shown to reduce macrophage cholesterol content by upregulating the expression of GPR119 and the cholesterol transporter ABCA1 [52]. These studies suggest that multiple lncRNAs are upregulated during macrophage foam cell formation that contribute to the regulation of macrophage cholesterol accumulation and inflammatory response.
The LXR transcription factors act as cholesterol sensors to mediate the response to cholesterol excess and upon activation, induce the transcription of a program of genes involved in cholesterol efflux and reverse cholesterol transport. A recent study of LXR-induced lncRNAs in mouse peritoneal macrophages identified MeXis as a key regulator of the cholesterol efflux protein ABCA1 [53■■]. MeXis is located in close proximity to Abca1 and LeXis loci, and like its neighboring genes, MeXis is induced by LXR-agonist stimulation. Targeting of MeXis using ASOs reduced levels of Abca1 in macrophages, while its overexpression increased Abca1 expression and cholesterol efflux to apoA1, suggesting that MeXis works in cis to regulate Abca1. Mechanistic studies showed that MeXis amplifies LXR-dependent transcription of Abca1 in macrophages, but not hepatocytes, by altering the chromosomal architecture surrounding the Abca1 locus. Using ATAC-Seq and mass spectrometry in MeXis−/− macrophages investigators showed that MeXis interacts with the RNA helicase DDX17 and guides this transcriptional coactivator to the Abca1 promoter. MeXis−/− mice showed reduced Abca1 expression in macrophages, heart and kidney (but not liver), but little change in other LXR-target genes. Consistent with the atheroprotective role of ABCA1 in macrophages, MeXis−/− mice fed a western diet showed increased foam cell formation and accelerated progression of atherosclerosis, compared to their wild type counterparts. While this initial study was conducted in mice, the LXR-MeXis axis is functionally conserved in humans, making it an attractive therapeutic target to increase cholesterol efflux and reverse cholesterol transport.
EMERGING AREAS OF STUDY: ENHANCER RNAS AND COMPETING ENDOGENOUS RNAS
Although not yet well understood, enhancer RNAs (eRNAs) represent an exciting new area of investigation into lncRNA functions. eRNAs are transcribed from enhancer regions encoded within the DNA sequence. Enhancer regions bind transcription factors and other components of the basal transcription machinery, creating loops in chromatin, and modulating the transcription of distant genes in a cis-acting manner [54]. Genome-wide techniques such as RNA-Seq and ChIP-Seq have detected extensive RNA polymerase II binding to these enhancer sites, indicating putative noncoding RNA transcription of eRNAs [55,56]. The biological significance of eRNAs is still unclear, although eRNA synthesis may be required to generate and/or maintain certain chromatin landscapes [57] or, as some evidence suggests, eRNA transcripts themselves may be biologically relevant [58]. As of yet, no eRNAs affecting lipid metabolism have been described.
Cytoplasmic competing endogenous RNAs (ceRNAs)have been described to contain multiple miRNA response elements that act as sinks for active miRNAs, sequestering them away from their mRNA target networks [59,60]. This mechanism is thought to contribute to homeostatic maintenance by rapidly curtailing miRNA activity when perturbations are detected, liberating mRNA target genes from miRNA-induced silencing to return gene expression to homeostatic levels [59]. This ceRNA hypothesis has recently been challenged, however, as computational analyses indicate that miRNA response elements in mRNA sequences are in large excess over their lesser expressed lncRNA regulators [61]. However, evidence of lncRNAs being able to sponge and sequester miRNAs with regulatory effects continues to accumulate [62], suggesting that the sequestration of miRNAs by ceRNAs may be supported by factors (e.g., RNA binding proteins) and/or RNA modifications that increase the affinity of these interactions. To date, no direct evidence for ceRNAs in lipid metabolism has been found, yet given the pace of discovery in other fields of study and the emergence of bioinformatics techniques that are publicly accessible, it is likely lncRNAs with ceRNA or eRNA functions affecting lipid metabolism will soon be reported.
CONCLUSION
Collectively, the studies cited above show that lncRNAs can coordinate diverse aspects of lipid metabolism (listed in Table 1 and represented in Fig. 1). To date, there is a large discrepancy between the number lncRNAs being discovered and the number of lncRNAs being functionally described in detail. This is due, in part, to the fact that lncRNAs act through diverse and complex mechanisms, and there is currently a lack of prediction algorithms that could accelerate functional characterization of lncRNAs. A current challenge in all fields of biology is to translate the observed changes in expression and localization of lncRNAs to the discovery of their functions. Nonetheless, it is clear already that lncRNAs will emerge as important new therapeutic targets for the treatment of metabolic disorders, including dyslipidemias, hepatosteatosis and atherosclerosis. ASO-based therapies to inhibit mRNAs and lncRNAs are actively being pursued, with mipomersen, the apoB-targeting ASO for treatment of familial hypercholesterolemia leading the way with FDA-approval [63,64]. As the mysteries of lncRNAs continue to be unraveled, parallel improvements of therapeutic tools to target non-coding RNAs in tissue-specific ways will no doubt accelerate the development future RNA-based therapies for diseases in the area of lipid metabolism and beyond.
Table 1.
List of lncRNA involved in lipid metabolism
| lncRNA | Cell-type | Loss-of-function phenotype | Gain-of-function phenotype | Interaction | References |
|---|---|---|---|---|---|
| MALAT1 | Hepatocytes | Reduces hepatic lipid levels in mice | Enhances lipid accumulation in HepG2 cells |
lncRNA / protein Inhibits ubiquitination of SREBP-1c |
[21■] |
| H19 | Hepatocytes | Abolishes high-fat and high-sucrose diet-induced steatosis |
Promotes hepatic lipogenesis | lncRNA / protein, lncRNA / RNA Stabilizes both SREBP1c mRNA and SREBP1c protein via PTBP1 |
[26■] |
| LncHR1 | Hepatocytes | Increases SREBP-1c mRNA and protein in Huh7 cells |
Reduces SREBP-1c, decreases hepatic and plasma triglycerides |
unknown interactions Interacts with SREBP-1c promoter |
[29] |
| LeXis | Hepatocytes | Increases serum and hepatic cholesterol in mice |
Reduces cholesterol levels Reduces plaque formation |
lncRNA / protein Interacts with Raly to block trans cription of cholesterol biosynthesis genes |
[31■■] |
| APOA1-AS | Hepatocytes | Upregulates AAPOA1, APOC3 and APOA4 mRNA and plasma levels of APOA1 |
lncRNA / protein Interacts with SUZ12/PRC2 to mediate chromatin silencing |
[33] | |
| APOA4-AS | Hepatocytes | Decreases levels of APOA4 and plasma cholesterol and triglycerides in vivo |
lncRNA / protein Interacts with HuR to stabilize APOA4 mRNA |
[35■] | |
| AT102202 | Hepatocytes | Increases expression of HMGCR | unknown interactions | [36] | |
| Lnc-HC | Hepatocytes | Improves glucose tolerance and reduces cholesterol and triglyceride levels in rats |
Degrades Cyp7a1 and Abca1 mRNA to promote cholesterol accumulation in hepatocytes |
lncRNA / protein Interacts with hnRNPA2/B1 to target mRNAs for degradation |
[39] |
| HULC | Hepatocytes | Decreases PPARA and ACSL1 mRNA in HepG2 cells |
Increases ACSL1 to enhance cell triglyceride & cholesterol content Enhances lipogenesis in tumors |
lncRNA / DNA Methylates CpG islands in promoter of miR-9 |
[42] |
| LncLSTR | Hepatocytes | Reduces plasma triglycerides through enhanced triglyceride clearance |
lncRNA / protein Interacts with TDP-43 to prevent its binding to Cyp8b1 promoter |
[46] | |
| TRIBAL | Hepatocytes | Decreases TRIB1 levels | No effect on TRIB1 | unknown interactions | [49] |
| RP5.883A20.1 | Macrophages | Promotes uptake of oxLDL Decreases cholesterol efflux Increases TNFα, IL-1β |
lncRNA / RNA Upregulates miR-382–5p resulting in down regulation of NFIA |
[50] | |
| H19 | Macrophages | Decreases foam cell formation Decreases cytokine levels |
Unknown interactions | [51] | |
| LincRNA-DYNLRB2–2 | Macrophages | Increases cholesterol efflux Reduces cholesterol content |
Unknown interactions Upregulates GPR119 and ABCA1 |
[52] | |
| MeXis | Macrophages | Increases macrophage foam cell formation and accelerates atherosclerosis |
Increases Abca1 expression and cholesterol efflux to apoA1 in macrophages |
lncRNA / protein Alters chromosomal architecture of the Abca1 locus through DDX17 |
[53■■] |
KEY POINTS.
Long noncoding RNAs are an understudied subclass of noncoding RNA that have mutifaceted functions in epigenetic regulation, transcriptional activation or repression, remodeling of chromatin architecture, posttranscriptional regulation of mRNAs, and modulation of protein activity.
Long noncoding RNAs are able to bind proteins, DNA, RNA or a combination thereof, to function as guides, scaffolds or decoys to execute their biological roles.
To date, long noncoding RNAs have been described to play roles in lipid metabolism through their effects on the SREBP transcription factors, apolipoproteins, triglyceride metabolism and macrophage cholesterol uptake and efflux.
Acknowledgements
None.
Financial support and sponsorship
This work was supported by grants from the NIH [R01HL119047 (K.J.M.), R35HL135799 (K.J.M.) and T32HL098129 (C.v.S.)] and the American Heart Association [17PRE32970002 (K.R.S.) and 14POST2018 0018 (C.v.S.)].
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 2014; 157:77–94. [DOI] [PubMed] [Google Scholar]
- 2.Rayner KJ, Moore KJ. MicroRNA control of high-density lipoprotein metabolism and function. Circ Res 2014; 114:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Z Progress and prospects of long noncoding RNAs in lipid homeostasis. Mol Metab 2016; 5:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perkel JM. Visiting ‘noncodarnia’. Biotechniques 2013; 54:303–304. [DOI] [PubMed] [Google Scholar]
- 5.Kapranov P, Cheng J, Dike S, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 2007; 316: 1484–1488. [DOI] [PubMed] [Google Scholar]
- 6.Hon CC, Ramilowski JA, Harshbarger J, et al. An atlas of human long noncoding RNAs with accurate 5’ ends. Nature 2017; 543:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palazzo AF, Lee ES. Noncoding RNA: what is functional and what is junk? Front Genet 2015; 6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitz SU, Grote P, Herrmann BG. Mechanisms of long noncoding RNA function in development and disease. Cell Mol Life Sci 2016; 73:2491–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma L, Bajic VB, Zhang Z. On the classification of long noncoding RNAs. RNA Biol 2013; 10:925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell 2018; 172:393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ An excellent overview ofthe recent insights into lncRNA classification and function, and discussion of future strategies to advance lncRNA research.
- 11.St Laurent G, Wahlestedt C, Kapranov P. The landscape of long noncoding RNA classification. Trends Genet 2015; 31:239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem 2012; 81:145–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J, Sun BK, Erwin JA, et al. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 2008; 322:750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grote P, Wittler L, Hendrix D, et al. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell 2013; 24:206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotake Y, Nakagawa T, Kitagawa K, et al. Long noncoding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene 2011; 30:1956–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tripathi V, Ellis JD, Shen Z, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell 2010; 39:925–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cesana M, Cacchiarelli D, Legnini I, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 2011; 147:358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang K, Long B, Zhou LY, et al. CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation. Nat Commun 2014; 5:3596. [DOI] [PubMed] [Google Scholar]
- 19.Redfern AD, Colley SM, Beveridge DJ, et al. RNA-induced silencing complex (RISC) Proteins PACT, TRBP, and Dicer are SRA binding nuclear receptor coregulators. Proc Natl Acad Sci U S A 2013; 110:6536–6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trimarchi T, Bilal E, Ntziachristos P, et al. Genome-wide mapping and characterization of Notch-regulated long noncoding RNAs in acute leukemia. Cell 2014; 158:593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan C, Chen J, Chen N. Long noncoding RNA MALAT1 promotes hepatic steatosis and insulin resistance by increasing nuclear SREBP-1c protein stability. Sci Rep 2016; 6:22640. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ This study reports new roles for the lincRNA MALAT1 in lipid homeostasis through its actions on SREBP-1c protein.
- 22.Wei Y, Niu B. Role of MALAT1 as a prognostic factor for survival in various cancers: a systematic review of the literature with meta-analysis. Dis Markers 2015; 2015:164635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan B, Tao ZF, Li XM, et al. Aberrant expression of long noncoding RNAs in early diabetic retinopathy. Invest Ophthalmol Vis Sci 2014; 55:941–951. [DOI] [PubMed] [Google Scholar]
- 24.Liu JY, Yao J, Li XM, et al. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis 2014; 5:e1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biddinger SB, Almind K, Miyazaki M, et al. Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes 2005; 54:1314–1323. [DOI] [PubMed] [Google Scholar]
- 26.Liu C, Yang Z, Wu J, et al. lncRNA H19 interacts with polypyrimidine tract-binding protein 1 to reprogram hepatic lipid homeostasis. Hepatology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ This study describes a dual function of lncRNA H19 in stabilizing hepatic SREBP-1c mRNA as well as SREBP-1c protein levels through binding of PTBP1.
- 27.Giovarelli M, Bucci G, Ramos A, et al. H19 long noncoding RNA controls the mRNA decay promoting function of KSRP. Proc Natl Acad Sci U S A 2014; 111:E5023–E5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang WC, Fu WM, Wang YB, et al. H19 activates Wnt signaling and promotes osteoblast differentiation by functioning as a competing endogenous RNA. Sci Rep 2016; 6:20121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li D, Cheng M, Niu Y, et al. Identification of a novel human long noncoding RNAthat regulates hepatic lipid metabolism by inhibiting SREBP-1c. Int J Biol Sci 2017; 13:349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Auboeuf D, Dowhan DH, Li X, et al. CoAA, a nuclear receptor coactivator protein at the interface of transcriptional coactivation and RNA splicing. Mol Cell Biol 2004; 24:442–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sallam T, Jones MC, Gilliland T, et al. Feedback modulation of cholesterol metabolism by the lipid-responsive noncoding RNA LeXis. Nature 2016; 534:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ This excellent describes a regulatory role fora LXR-responsive lncRNA, LeXis, as a decoy for a DNA-interacting protein. LeXis binds Raly to block its interaction at the promoters of genes regulating cholesterol biosynthesis and alters cholesterol levels in vivo.
- 32.Tontonoz P, Wu X, Jones M, et al. Long noncoding RNA facilitated gene therapy reduces atherosclerosis in a murine model offamilial hypercholesterolemia. Circulation 2017; 136:776–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halley P, Kadakkuzha BM, Faghihi MA, et al. Regulation ofthe apolipoprotein gene cluster by a long noncoding RNA. Cell Rep 2014; 6:222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elshourbagy NA, Boguski MS, Liao WS, et al. Expression of rat apolipoprotein A-IV and A-I genes: mRNA induction during development and in response to glucocorticoids and insulin. Proc Natl Acad Sci U S A 1985; 82:8242–8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin W, Li X, Xie L, et al. A long noncoding RNA, APOA4-AS, regulates APOA4 expression depending on HuR in mice. Nucleic Acids Res 2016; 44:6423–6433. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ This study describes the cis regulatiory mechanisms of an antisense lncRNA, APOA4AS, on the stability, expression and synthesis of its adjacent mRNA APOA4.
- 36.Liu G, Zheng X, Xu Y, et al. Long noncoding RNAs expression profile in HepG2 cells reveals the potential role of long noncoding RNAs in the cholesterol metabolism. Chin Med J (Engl) 2015; 128:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng XX, Xu YL, Li SH, et al. Green tea intake lowers fasting serum total and LDL cholesterol in adults: a meta-analysis of 14 randomized controlled trials. Am J Clin Nutr 2011; 94:601–610. [DOI] [PubMed] [Google Scholar]
- 38.Kuriyama S, Shimazu T, Ohmori K, et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA 2006; 296:1255–1265. [DOI] [PubMed] [Google Scholar]
- 39.Lan X, Yan J, Ren J, et al. A novel long noncoding RNA Lnc-HC binds hnRNPA2B1 to regulate expressions of Cyp7a1 and Abca1 in hepatocytic cholesterol metabolism. Hepatology 2016; 64:58–72. [DOI] [PubMed] [Google Scholar]
- 40.Panzitt K, Tschernatsch MM, Guelly C, et al. Characterization of HULC, a novel gene with striking up-regulation in hepatocellular carcinoma, as noncoding RNA. Gastroenterology 2007; 132:330–342. [DOI] [PubMed] [Google Scholar]
- 41.Yu X, Zheng H, Chan MT, Wu WK. HULC: an oncogenic long noncoding RNA in human cancer. J Cell Mol Med 2017; 21:410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui M, Xiao Z, Wang Y, et al. Long noncoding RNA HULC modulates abnormal lipid metabolism in hepatoma cells through an miR-9-mediated RXRA signaling pathway. Cancer Res 2015; 75:846–857. [DOI] [PubMed] [Google Scholar]
- 43.Hegele RA. Plasma lipoproteins: genetic influences and clinical implications. Nat Rev Genet 2009; 10:109–121. [DOI] [PubMed] [Google Scholar]
- 44.Lee EB, Lee VM, Trojanowski JQ. Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nat Rev Neurosci 2011; 13:38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab 2013; 17:657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li P, Ruan X, Yang L, et al. A liver-enriched long noncoding RNA, lncLSTR, regulates systemic lipid metabolism in mice. Cell Metab 2015; 21:455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 2010; 466:707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chasman DI, Pare G, Mora S, et al. Forty-three loci associated with plasma lipoprotein size, concentration, and cholesterol content in genome-wide analysis. PLoS Genet 2009; 5:e1000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Douvris A, Soubeyrand S, Naing T, et al. Functional analysis of the TRIB1 associated locus linked to plasma triglycerides and coronary artery disease. J Am Heart Assoc 2014; 3:e000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu YW, Zhao JY, Li SF, et al. RP5-833A20.1/miR-382-5p/NFIA-dependent signal transduction pathway contributes to the regulation of cholesterol homeostasis and inflammatory reaction. Arterioscler Thromb Vasc Biol 2015; 35:87–101. [DOI] [PubMed] [Google Scholar]
- 51.Han Y, Ma J, Wang J, Wang L L. Silencing of H19 inhibitsthe adipogenesis and inflammation response in ox-LDL-treated Raw264.7 cells by up-regulating miR-130b. Mol Immunol 2018; 93:107–114. [DOI] [PubMed] [Google Scholar]
- 52.Hu YW, Yang JY, Ma X, et al. A lincRNA-DYNLRB2-2/GPR119/GLP-1R/ABCA1-dependent signal transduction pathway is essential for the regulation of cholesterol homeostasis. J Lipid Res 2014; 55:681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sallam T, Jones M, Thomas BJ, et al. Transcriptional regulation of macrophage cholesterol efflux and atherogenesis by a long noncoding RNA. Nat Med 2018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ This report describes the function the LXR-responsive lncRNA MeXis on expression of the nearby Abca1 locus. In macrophages, MeXis alters the chromosomal architecture around the Abca1 locus, thereby increasing its expression. Gain-of-function and loss-of-function of MeXis alters Abca1 expression and function in macrophages, and its expression protects from atherosclerosis in mice.
- 54.Blackwood EM, Kadonaga JT. Going the distance: a current view of enhancer action. Science 1998; 281:60–63. [DOI] [PubMed] [Google Scholar]
- 55.De Santa F, Barozzi I, Mietton F, et al. A large fraction of extragenic RNA pol II transcription sites overlap enhancers. PLoS Biol 2010; 8:e1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim TK, Hemberg M, Gray JM, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature 2010; 465:182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaikkonen MU, Spann NJ, Heinz S, et al. Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol Cell 2013; 51:310–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ilott NE, Heward JA, Roux B, et al. Long noncoding RNAs and enhancer RNAs regulate the lipopolysaccharide-induced inflammatory response in human monocytes. Nat Commun 2014; 5:3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kartha RV, Subramanian S. Competing endogenous RNAs (ceRNAs): new entrants to the intricacies of gene regulation. Front Genet 2014; 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014; 505:344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jens M, Rajewsky N. Competition between target sites of regulators shapes posttranscriptional gene regulation. Nat Rev Genet 2015; 16:113–126. [DOI] [PubMed] [Google Scholar]
- 62.Yamamura S, Imai-Sumida M, Tanaka Y, Dahiya R. Interaction and cross-talk between noncoding RNAs. Cell Mol Life Sci 2018; 75:467–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stein EA, Dufour R, Gagne C, et al. Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: results of a randomized, double-blind, placebo-controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulation 2012; 126:2283–229. [DOI] [PubMed] [Google Scholar]
- 64.Ajufo E, Rader DJ. New therapeutic approaches for familial hypercholesterolemia. Annu Rev Med 2018; 69:113–131. [DOI] [PubMed] [Google Scholar]