Abstract
Objective:
The purpose of this study was to investigate the characteristics of this unique form of rhinosinusitis.
Methods:
Ninety-one patients with sinus fungus balls were evaluated for clinical characteristics. Nasal tissues obtained from 38 patients with sinus fungus ball, along with 26 controls were used for histopathological, cytokines/chemokines, western blotting, and genetic analyses.
Results:
Patients with fungus balls had significantly more females and their age was older. The presentation of fungus ball was predominantly unilateral (97.8%). Thirty-three patients (36.3%) had risk factors for fungal infection. Macrophage and neutrophil dominated cellular infiltration was found in nasal tissues of fungus ball patients. A tendency of reduced tight junction staining (e-cadherin) and protein expression was found. Interleukin 8 (IL8) and granulocyte colony stimulating factor (G-CSF) significantly increased in sinus fungus ball tissue homogenates when compared with those from controls. Higher prevalence of a single single nucleotide polymorphism (SNP) with E-cadherin was found in the patients with fungus ball.
Conclusions:
We found that patients with sinus fungus ball had robust immune responses, allowing recruitment and activation of macrophages and neutrophils. However, patients with sinus fungus ball could have genetic or acquired weakness in immunity. The fungal hyphae were localized and accumulated within single sinus instead of being eradicated by host.
Keywords: Cytokines, fungal sinusitis, fungus ball, rhinosinusitis, single nucleotide polymorphism, sinus surgery, pathology
Introduction
The prevalence of fungal rhinosinusitis has been on the rise despite its rarity.1–3 The disorder encompasses a variety of manifestations; from being innocuous to rapidly becoming fatal. Fungal rhinosinusitis is broadly divided into two forms: invasive and noninvasive. Its different types are the result of a variety of host-microbial interactions.4 For example, fungus acting as an antigen can cause allergic fungal rhinosinusitis. Fungus can also play a role as an aggressive pathogen able to infect immunocompromised hosts, thus resulting in the invasive fungal rhinosinusitis. Fungus ball (mycetoma) is a noninvasive form of this disorder mostly presented through the accumulation of fungal hyphae in the maxillary sinus. Occasionally, the hyphae accumulation may appear in the sphenoid or other sinuses.5 Sinus fungus balls usually affect immunocompetent hosts and the hyphae do not invade the sinus mucosa, bone or blood vessels. However, with host immunity deteriorating the fungus ball may progress to the invasive form.6
Sinus fungus ball could erode the sinus wall causing facial pain or obstruct the sinus ostium resulting in secondary bacterial infections. Symptomatic fungus ball is an indication that sinus surgery may be required.7–9 Following the complete surgical removal of the fungus ball, anti-fungal medication is not usually indicated.7–9 Patients with asymptomatic sinus fungus ball were occasionally diagnosed through the use of image studies which were performed for other purposes. There are no guidelines for treatment of asymptomatic sinus fungus ball. Nevertheless, removal of the fungus ball for pathologic diagnosis is proper procedure with a low morbidity.1 Based on the low incidence of fungal rhinosinusitis, its detailed pathophysiology has not been fully explored; even though both the genetic background and immune status of the affected hosts have been proposed to be involved in the underlying etiology, along with involvement in anatomical obstruction, environmental exposure, and the toxicity of microbes.10 The purpose of this study was to investigate the clinical presentation, histopathological, inflammatory pattern, and host genetic features of sinus fungus ball.
Methods
Study subjects
The retrospective chart review covered an 8-year period from January 2006 to January 2014. Patients enrolled included those who had undergone sinus surgery in our hospital for the treatment of sinus fungus balls. Their diagnosis was based on radiologic, endoscopic and pathological evidence of fungi contained in a sinus cavity.4,11,12 In brief, radiologic studies showed sinus opacification or calcification. A cheesy or clay-like material was found by use of a sinus endoscopy. In addition, a histopathological examination confirmed the accumulation of dense hyphae in surgical specimens. Patients who demonstrated evidence of an invasive fungal infection, including pathological tissue invasion or associated neurologic or vascular deficits, were excluded. The clinical characteristics (age, gender, and co-morbidities) of the enrolled subjects were analyzed. All subjects with sinus fungus ball underwent computed tomography (CT) scans of the sinuses prior to undergoing surgery. Their CT scans were scored according to the Lund-Mackay system.13
Nasal tissues were obtained from patients who had donated their surgical specimens to the tissue bank of our hospital. Specifically, tissue samples were harvested from the osteomeatal complex from those patients who had maxillary fungus balls, or the sphenoid ostia from those who had sphenoid fungus balls. Control tissues were from the nasal cavity of patients who had undergone septoplasty and partial turbinectomy.
Histologic characteristics and immunohistochemistry of sinus fungus balls
Tissues were fixed overnight in a 10% neutral buffered formalin. Following fixation, tissues were embedded in paraffin, cut into 4-μm-thick sections, and stained with hematoxylin and eosin (H&E). Immunohistochemistry (IHC) was performed to determine tissue cellular infiltration, changes in tight junction, and mucus production. Paraffin sections were de-paraffinized and repeatedly rinsed before being incubated with primary antibodies for 20-80 minutes at 37°C. An UltraView Universal DAB Detection Kit (Ventana Medical Systems, Inc., Tucson, AZ, USA) was used for chromogenic detection. Some sections were further counterstained with haematoxyline (Sigma-Aldrich, Bornem, Belgium). CD68 (Agilent Technologies, Inc., Santa Clara, CA, USA; 1:200 dilution), and MPO (Agilent Technologies, Inc., Santa Clara, CA, USA; 1:10,000 dilution) were used as the primary antibodies for the evaluation of cellular infiltrations (macrophages and neutrophils). Zo-1 (Heidelberg Engineering Inc., Franklin, MA, USA; 1:200 dilution) and E-cadherin (Thermo Fisher Scientific Inc., Fremont, CA, USA; 1:100 dilution) were used as the primary antibodies for evaluating the epithelium inter-cellular junction. Periodic acid–Schiff (PAS) staining (Muto Pure Chemicals CO., LTD., Tokyo, Japan) was used to visualize fungal hyphae and to evaluate mucus production. Histological slides were ultimately examined using a bright-field microscope, with the digital images captured for analyses. Tissue cellular infiltration was determined by the amount of inflammatory cells (eosinophil, neutrophil, or macrophage) found in the sub-epithelial area. Loss of epithelial integrity was defined as epithelium staining of Zo-1 and E-cadherin. Slides were evaluated independently by two coauthors (KL Liang and WC Wang). For each slide, the quantity of staining was assessed using a semi-quantitative approach according to an arbitrary 5-point visual scale outlined as follows: 0 absent, 1 weak, 2 moderate, 3 intense, and 4 very intense.14
Western blotting
Nasal tissues were lysed with a T-per tissue protein extraction buffer (Thermo Scientific, Waltham, MA, USA). Protein concentrations were measured with BAC Protein Assay (Pierce Chemical Co., Rockford, IL, USA). Equal amounts of proteins from samples were subjected to electrophoretic separation in either 8% or 12% polyacrylamide gel prior to being transferred to a polyvinylidene difluoride membrane. They were then healed in blocking buffer for 1 hour at room temperature. The membrane was incubated with primary antibodies of Zo-1 (Thermo Scientific, Waltham, MA, USA), E-cadherin (BD Pharmingen, San Diego, CA, USA), Claudin-1, Catenin-β (both supplied by Elabscience Biotechnology, Houston, USA), and glyceraldehyde phosphate dehydrogenase (GAPDH; Proteintech, Rosemont, USA) overnight at 4°C. On the following day, the membranes were subsequently incubated with an horseradish peroxidase (HRP)-conjugated secondary antibody after repeated washing and reacted at room temperature for 1 hour, followed by electrochemiluminescent detection (Millipore Billerica, MA, USA). The density of each protein band was scanned using ImageJ Software, version 1.46r (National Institutes of Health, Bethesda, MD) and compared by densitometry.
Inflammatory cytokines/chemokines expression of nasal tissues
Cytokines/chemokines in nasal tissue homogenates were determined using Bio-Plex® (Bio-Rad, Hercules, CA, USA) including interferon-gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), IL1β, IL4, IL6, IL8, IL10, IL13, IL17A, granulocyte colony stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), Eotaxin and RANTES. The tests were performed according to the manufacturer’s recommendations.
Detecting clinically relevant single nucleotide polymorphisms in patients with sinus fungus ball
Human genomic DNA was isolated from both the nasal tissues of the fungus balls and controls using the QIAmp DNA miniKit (Qiagen Biosciences, Germantown, MD) according to standard procedures. Genotyping assays were performed using the commercially available Taqman® pre-developed assay reagents for allelic discrimination (Applied Biosystems, Foster City, CA, USA) in 20 μL reactions containing approximately 1 μL of DNA input (20 ng), nuclease free water, genotype Master Mix, and probe/primer assay mix. Reactions were run on the StepOnePlus Real-Time polymerase chain reaction (PCR) system according to standard procedures for 40 cycles. The names and details of single nucleotide polymorphism (SNP) used are listed in Table 1.
Table 1.
Selected single nucleotide polymorphisms in this study.
| SNP name | Substitution | SNP ID | TaqMann® Assay ID (Applied Biosystems, Foster City, CA, USA) |
|---|---|---|---|
| IL815 | A/T | rs4073 | C_11748116_10 |
| IL1016,17 | T/G | rs1800872 | C_1747363_10 |
| IL1016,17 | T/C | rs1800896 | C_1747360_10 |
| IL1518 | T/C | rs12508866 | C_1752409_10 |
| IL1518 | G/T | rs6842735 | C_29318124_10 |
| IL1518 | A/G | rs1519551 | C_8865650_10 |
| TNFα15 | G/A | rs1800629 | C_7514879_10 |
| INFγ19 | G/A | rs2069705 | C_15944115_20 |
| CR115 | A/G | rs17047660 | C_25598593_10 |
| CR115 | A/G | rs17047661 | C_25473166_10 |
| IL1RN18 | C/T | rs4252041 | C_27884744_10 |
| TLR417,20 | A/G | rs4986790 | C_11722238_20 |
| TLR921 | A/G | rs5743836 | C_32645383_10 |
| CH122 | A/G | rs16948383 | C_32810010_10 |
| CH122 | C/T | rs2276330 | C_15881320_10 |
| CH122 | A/C | rs3785078 | C_27481925_20 |
| CH122 | C/G | rs7203904 | C_29032982_30 |
Ethical consideration
Enrolled subjects had all signed informed consents prior to enrollment. The Institutional Review Board of the study hospital approved the study.
Statistical analyses
Clinical data were represented in terms of frequencies and means (with standard error, SD). Statistical comparisons were performed using GrapPad Prism software version 6 (GraphPad Software, Inc., La Jolla, California, USA). Both Mann–Whitney and Pearson chi-square tests were applied whenever appropriate.
Results
A total of 91 patients with sinus fungus ball were enrolled for the evaluation of their clinical data. Thirty-eight sinus tissues from fungus ball patients harvested during functional endoscopic sinus surgery (FESS) and 26 nasal tissues harvested during septoplasty and turbinectomy were used for histopathological, cytokines/chemokines, western blotting, and genetic analyses. Tissue samples were randomly assigned for the aforementioned studies.
Clinical characteristics of study subjects
The clinical characteristics of the 91 patients with sinus fungus ball are listed in Table 2. We discovered that the subjects with sinus fungus ball were more likely to be females and at an older age. A great majority of sinus fungus ball was presented unilaterally (97.8%). Only two patients had bilateral sinus mycetoma, with one experiencing post-irradiated nasopharyngeal carcinoma and the other chronic leukemia. Thirty-three patients (36.3%) had known risk factors in the etiology of fungal infections, including diabetes, liver cirrhosis, hematological malignancy, medical immune depressant, and had been receiving chemotherapy. Those who had known risk factors for fungal infections were found to be older than those who were without risk factors (P < .001). Except for that circumstance, all other clinical characteristics were similar.
Table 2.
Clinical characteristics of study subjects.
| Group | Fungus ball (N = 91) |
|---|---|
| Mean age (range) | 57.51 (23-82) |
| Female (N, %) | 66 (72.5%) |
| Unilateral (N, %) | 89 (97.8%) |
| Side (Right/left/bilateral) | (56/33/2) |
| Maxillary sinus (N, %) | 79 (86.8%) |
| Sphenoid sinus (N, %) | 12 (13.2%) |
| Smokera (N, %) | 11 (12.1%) |
| Allergic rhinitis (N, %) | 9 (9.9%) |
| Asthma (N, %) | 1 (1.1%) |
| Symptoms (N, %) | |
| Nasal obstruction | 36 (39.6%) |
| Rhinorrhea | 33 (36.3%) |
| Nasal bleed | 17 (18.7%) |
| Foul odor | 10 (11%) |
| Post nasal drip | 35 (38.5%) |
| Headache | 11 (12.1%) |
| Facial pain | 13 (14.3%) |
| Hyposmia | 3 (3.3%) |
| Facial swelling | 2 (2.2%) |
| Asymptomatic | 3 (3.3%) |
| Pathogen (identified by morphology) | |
| Aspergillus | 86 (94.5%) |
| Undetermined | 5 (5.5%) |
| Computed tomography findings | |
| Mean Lund-Mackay CT score (SD) | 4.74 (2.53) |
| Calcified density (N, %) | 66 (72.5%) |
| Sclerotic changes (N, %) | 57 (62.6%) |
| Bony erosion (N, %) | 59 (64.8%) |
| Deviated nasal septum (N, %) | 5 (5.5%) |
| Concha Bullosa (N, %) | 8 (8.8%) |
| Risk factors of fungal infection (N, %) | 33 (36.3%) |
| Diabetes | 16 (17.6%) |
| Hematological malignancy | 1 (1.1%) |
| Non-hematological malignancy§ | 6 (6.6%) |
| Medical immunosuppressant | 7 (7.7%) |
| Liver cirrhosis | 2 (2.2) |
| Chronic renal disease | 1 (1.1%) |
Current or ex-smoker.
Head neck cancers and a gastric cancer.
Histologic characteristics and IHC of sinus fungal balls
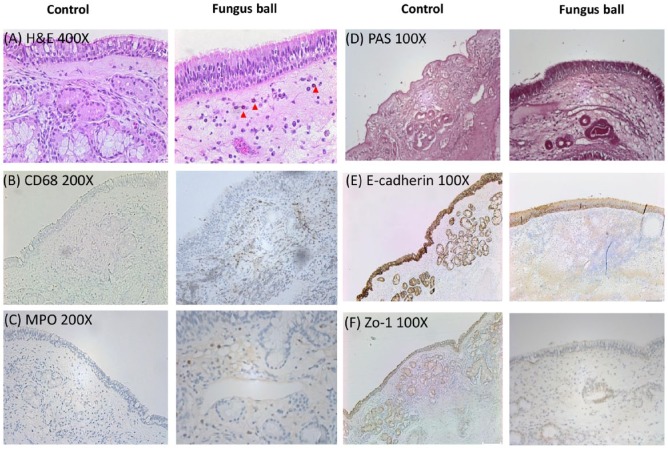
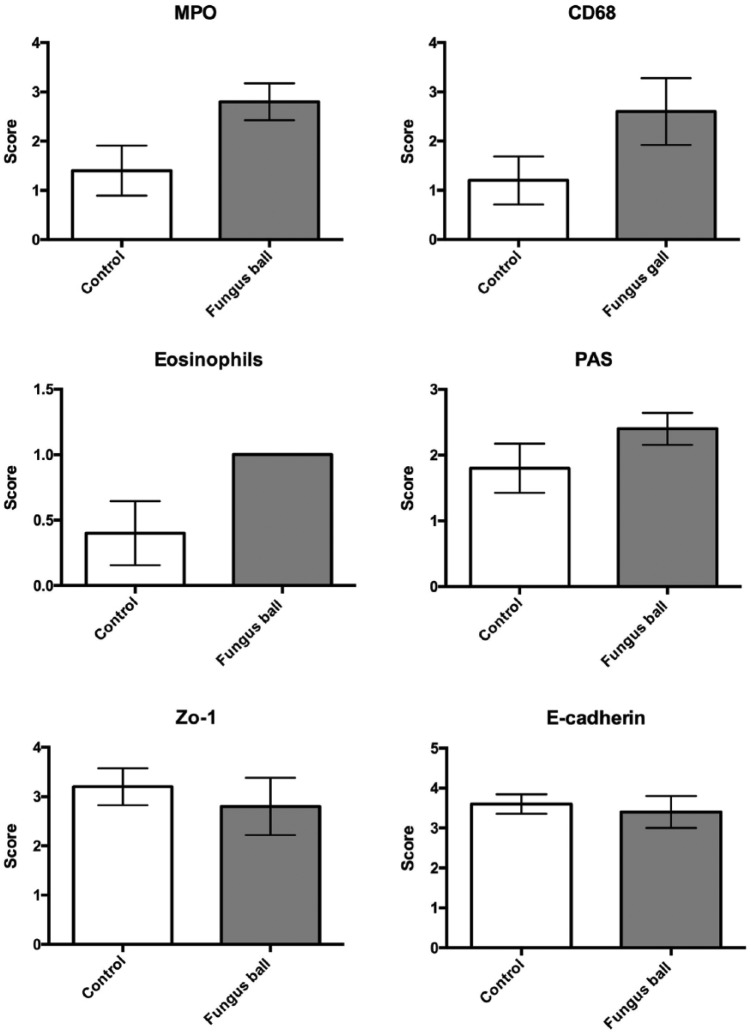
Ten nasal samples were obtained from the tissue bank (5 fungal balls and 5 controls) for the purpose of IHC studies. Representative photomicrographs are shown in Figure 1. The summary for abundance of staining regarding the H&E and IHC staining scores are shown in Figure 2. Mild eosinophils infiltration was identified in the H&E staining of sinus fungus ball tissues. Predominantly, macrophages and neutrophils infiltration were found in nasal tissues from patients with fungus ball. There was mildly decreased staining of tight junction staining (Zo-1 and E-cadherin). However, there were no significant changes in mucosal integrity between the controls and the patients with fungus ball in mucosal integrity.
Figure 1.
Representative microphotographs of H&E staining and immunohistochemistry (IHC) staining showing morphological and inflammatory changes of nasal mucosa in the controls and fungus ball. H&E staining 400X (A), eosinophils marked with red arrow head; CD68 200X (B); MPO 200X (C); PAS staining 100X (D); E-cadherin 100X (E); Zo-1 100X (F).
Figure 2.
Compare the abundance of immunohistochemistry staining between tissue sections of the controls and fungus ball. The abundance of staining was assessed with a semi-quantitative approach according to an arbitrary 5-point visual scale described as follows: 0 absent, 1 weak, 2 moderate, 3 intense, and 4 very intense.
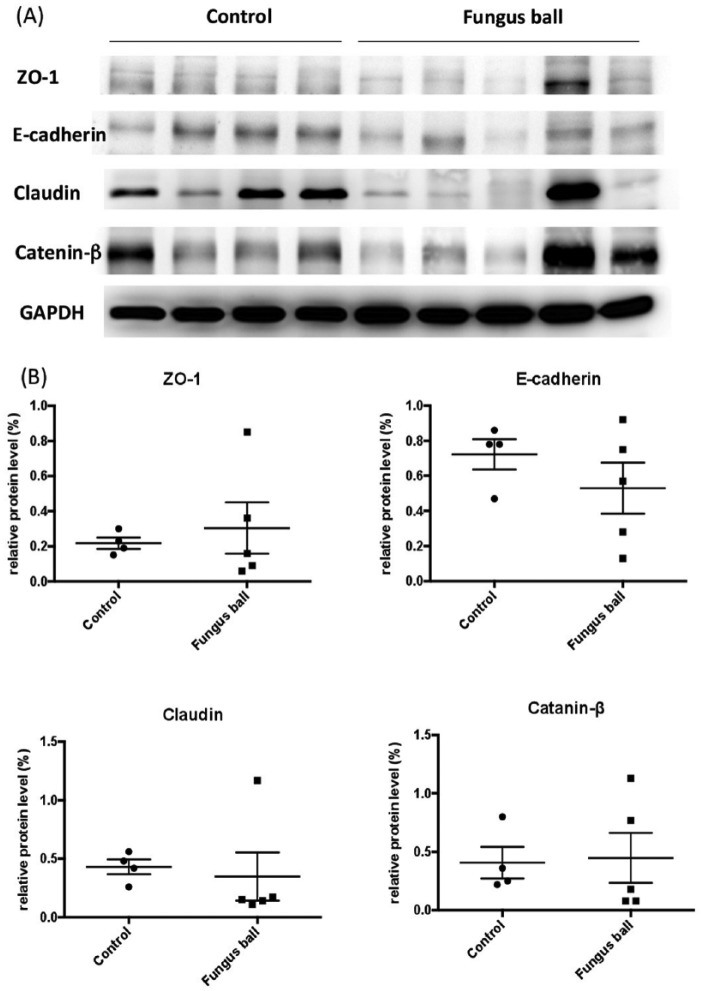
Western blotting of nasal tissue
Nine nasal samples were used for analysis of tight/adherens junction protein expression. The results are summarized in Figure 3. There were no significant differences in tight junction protein expression between the nasal samples of the controls and fungus ball patients (P = .873, .381, .191, and .5 for zo-1, E-cadherin, claudin, and catenin-β, respectively). Nevertheless, there was a tendency for decreased E-cadherin protein expression in nasal tissues from fungus ball patients.
Figure 3.
Western blot analyses of tight junction protein expression obtained in 9 study subjects (A). The relative expressions to house keeping protein are shown in (B).
Inflammatory cytokines/chemokines concentration in nasal tissue
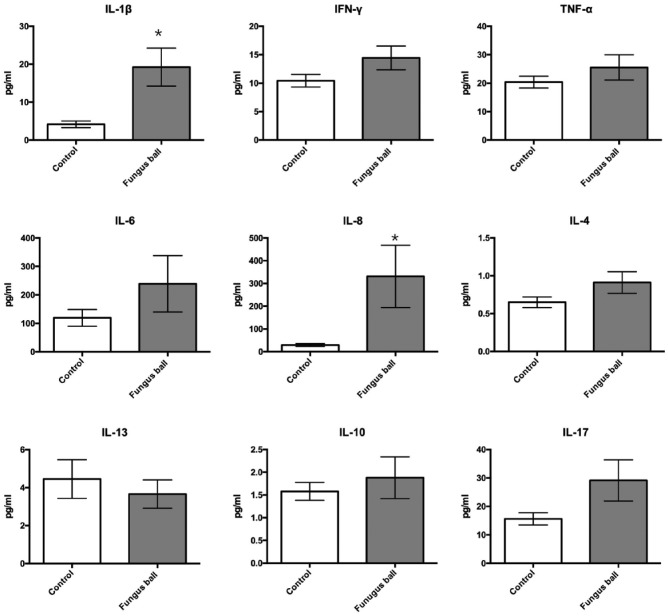
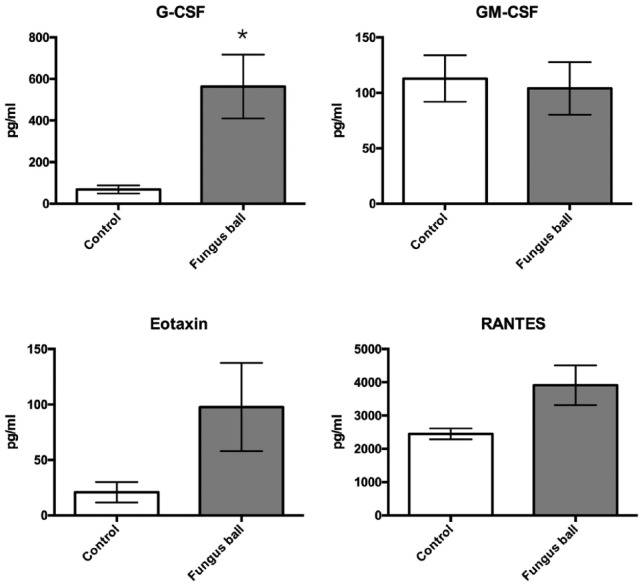
Twenty-six nasal samples were obtained for cytokines/chemokines analyses (14 fungus balls and 12 controls). The results of cytokine and chemokine levels are shown in Figures 4 and 5. IL1β and IL8 significantly increased in tissues from patients with fungus ball (P = .007 and 0.0008, respectively). There were no significant differences in TH2, Treg, and T17 cytokines including IL-4, IL-13, IL10, and IL17 between tissues from the controls and fungus ball patients (P = .163, 0.716, > 0.99, and 0.134, respectively). Among chemokines, only G-CSF significantly increased in tissues from fungus ball patients than those of the controls (P = .002).
Figure 4.
Comparison of cytokine level in tissue homogenates between sinus fungus ball and controls.
Figure 5.
Comparison of chemokine level in tissue homogenates between sinus fungus ball and controls.
Association of genotype with sinus fungal ball susceptibility
Nineteen nasal samples (5 controls and 14 fungus balls) were used for genotype analyses. The genotype and allele distribution associated with fungus infection in lungs are listed in Table 3. We found no differences in genotype and allele distribution among SNPs that are known to be associated with invasive pulmonary fungal infection and fungus ball in the lung. However, a significant difference in prevalence was found with a SNP (rs7203904) among patients with fungus balls (Table 4). This SNP has been reported to be associated with epithelial E-cadherin expression in the asthma patients.
Table 3.
Genotype and allele frequencies of the selected SNPs associated with fungal infection of the lung.
| SNPs | Genotype | Fungus ball (N = 14) | Control (N = 5) | P a | P b |
|---|---|---|---|---|---|
| IL8 | AA | 1 | 1 | .575 | |
| rs4073(A > T) | AT | 9 | 2 | ||
| TT | 4 | 2 | |||
| A allele | 11 | 4 | .968 | ||
| T allele | 17 | 6 | |||
| IL10 | TT | 6 | 2 | .190 | |
| rs1800872 (T > G) | TG | 7 | 1 | ||
| GG | 1 | 2 | |||
| T allele | 19 | 5 | .315 | ||
| G allele | 9 | 5 | |||
| IL10 | CC | 14 | 5 | - | |
| rs100896 (T > C) | - | ||||
| IL15 | TT | 9 | 3 | .981 | |
| rs12508866 (T > C) | CT | 5 | 2 | ||
| T allele | 23 | 8 | .986 | ||
| C allele | 5 | 2 | |||
| IL15 | GG | 9 | 3 | .951 | |
| rs6842735 (G > T) | GT | 5 | 2 | ||
| G allele | 23 | 8 | .963 | ||
| T allele | 5 | 2 | |||
| IL15 | AG | 1 | 1 | .674 | |
| rs1519551 (G > A) | AA | 13 | 4 | ||
| G allele | 1 | 1 | .687 | ||
| A allele | 27 | 9 | |||
| TNFα | GG | 11 | 4 | .814 | |
| rs1800629 (G > A) | AG | 2 | 1 | ||
| AA | 1 | 0 | |||
| G allele | 24 | 9 | .923 | ||
| A allele | 4 | 1 | |||
| IFNγ | GG | 6 | 2 | .577 | |
| rs2069705 (G > A) | AG | 6 | 3 | ||
| AA | 2 | 0 | |||
| G allele | 18 | 7 | .781 | ||
| A allele | 10 | 3 | |||
| CR1 | AA | 14 | 5 | - | |
| rs17047660 (A > G) | - | ||||
| CR1 | AA | 14 | 5 | - | |
| rs17047661 (A > G) | - | ||||
| IL1RN | CC | 14 | 5 | - | |
| rs4252041 (C > T) | - | ||||
| TLR4 | AA | 14 | 5 | - | |
| rs4986790 (A > G) | - | ||||
| TLR9 | AA | 14 | 5 | - | |
| rs5743836 (A > G) | - |
Chi square test for differences in frequency distribution of genotypes between cases and control.
Chi square test for differences in frequency distribution of alleles between cases and controls.
Table 4.
Genotype and allele frequencies of the selected SNPs associated with E-cadherin.
| SNPs | Genotype | Fungus ball (N = 14) | Control (N = 5) | P a |
|---|---|---|---|---|
| CH1 | AA | 1 | 0 | .209 |
| rs16958383 (A > G) | AG | 5 | 0 | |
| GG | 8 | 5 | ||
| A allele | 7 | 0 | .08 | |
| G allele | 21 | 10 | ||
| CH1 | CT | 1b | 0 | .523 |
| rs2276330 (C > T) | TT | 12 | 5 | |
| C allele | 1b | 0 | .529 | |
| T allele | 25 | 10 | ||
| CH1 | AA | 12 | 3 | .226 |
| rs3785078 (A > C) | AC | 2 | 2 | |
| A allele | 26 | 8 | .255 | |
| C allele | 2 | 2 | ||
| CH1 | CC | 1 | 0 | .085 |
| rs7203904 (C > G) | CG | 7 | 0 | |
| GG | 6 | 5 | ||
| C allele | 9 | 0 | .040c | |
| G allele | 19 | 10 |
Chi square test for differences in frequency distribution of genotypes or alleles between cases and controls.
One sample failed to determine the genotype.
P < .05.
Discussion
Sinus fungus balls mostly occurring in females and older individuals with a predominantly unilateral presentation are findings that are consistent with previous studies.23,24 Our results also demonstrated that fungus ball mostly occurs in the maxillary sinus. CT scans of sinus fungus ball were characterized with calcified density, sclerotic, or erosion changes in the sinus wall.2 Otherwise, symptoms from sinus fungus ball were not specific and were difficult to differentiate from non-fungal rhinosinusitis. The etiology of fungus ball has not been well investigated. Tai et al25 reported that fungus ball is not associated with osteomeatal complex obstruction but with another as-yet-unexplained mechanism. The host’s immunological status is presumably the main determinant for fungal rhinosinusitis. Fungus balls more often occur in immunocompetent hosts or persons with a very subtle immunodeficiency.4,6,12 However, a substantial proportion (36.3%) of these types had known risk factors associated with fungal infection in our study. These included those with diabetes under treatment, those who had received chemotherapy or immunosuppressant regents, had a history of hematological malignancy, or experienced chronic renal diseases. It has been reported that those patients who developed an immunocompromised status could experience their fungus balls progressing into invasive fungal sinusitis.6 It is therefore advisable to carefully follow-up on such patients with immunodeficiency in order to minimize their risk of developing invasive fungal rhinosinusitis.
The main diagnostic tool for detecting fungal infection is the histopathological examination of affected tissues. Previous studies have reported that only a minority of fungus balls (10%-30%) exhibited positive findings in their tissue cultures.8,26,27 A possible explanation for this is that most fungi in fungus balls are localized outside the mucosa, while also being non-viable for culture. The procedure involving a fugal culture is typically time-consuming and carries a very low diagnostic sensitivity, whereas histopathology usually provides a rapid presumptive diagnosis. In certain circumstances, histopathology is the only means of locating evidence related to the absence of culture findings. Identifying the morphological characteristics of fungal elements can help toward the classification of the infecting fungus. Finally, the host’s reactions to fungal infection (e.g. inflammation, necrosis, or hemorrhage), and the extent of fungal infection revealed by histopathology provides valuable information for physicians.28 PAS staining is the standard method for microscopic visualization of fungal hyphae.29 All patients diagnosed with fungus balls in this study had visualized conglomerated fungal hyphae in their nasal specimens. In addition, PAS staining remains an important tool for the purpose of evaluating mucus production. Increased epithelial mucus production and granular hypertrophy were observed in patients with fungus balls.
IHC and inflammatory cytokines/chemokines analyses in this study provided important information on the disease mechanism of sinus fungus ball. Park et al30 reported that mucosal cellular infiltration increases with sinus fungus ball. We discovered that there was mild eosinophils infiltration in patients with sinus fungus balls. Nevertheless, neutrophils and macrophages were the predominant inflammatory cells in the mucosa of fungus balls. Specific inflammatory cytokine/chemokine changes were found in the tissue homogenates of sinus fungus ball patients. IL-1β, which was produced by activated macrophage, significantly elevated in fungus ball. TH2, Treg, and T17 cytokines in nasal tissues from fungus ball patients were not significantly different to those of the controls. The IL-17A level of fungus ball tissue slightly increased compared to that of the controls. Werner et al31 had reported that IL-17A provided clearance of Aspergillus fumigatus in their mice experiments. Chemotactic factors (IL8, Eotaxin, and G-CSF) elevated in sinus fungus ball tissues, indicating that there was activation and recruitment of macrophage, eosinophils and neutrophils. Immune responses to Aspergillus in humans involved both macrophages and neutrophils, which can ingest and kill the fungus.16 Indeed, we did discover that cytokines associated with the function of macrophage and neutrophil were specifically high. The significant rise of chemokines and cytokines indicated a robust innate immunity with activation and recruitment of innate cells in patients with sinus fungus ball.
E-cadherin and Zo-1 are tight junction proteins important in both the control of tissue architecture and the maintenance of tissue integrity. Consistent with this, Man et al32 found that manipulation of the E-cadherin function alters epithelial integrity, resulting in airway infections. Another study reported that E-cadherin CDH1 polymorphism was associated with airway remodeling and lung function in patients with asthma.22 Our findings are in line with that study, as we too saw a decrease in epithelial tight junction protein expression in patients with fungus balls. These findings are consistent with the role of tight/adherens junction protein in structural abnormality of the airway, as related to the defective clearance of inhaled fungal spores that may subsequently develop into hyphae.22
It remains unclear why most individuals remain asymptomatic after inhalation of this fungus while only a small minority experience fungal infections. It is becoming apparent that Single Nucleotide Polymorphism (SNP) in key immunity genes can increase susceptibility to certain infections.21,33 It is plausible that such genetic variations in the immune system may lead to an imbalance between the pro- and anti-inflammatory responses, resulting in a predisposition to fungal infections.21 Several gene polymorphisms are candidates for prognostic biomarkers regarding invasive aspergillosis in the lung.17,19–21 Few studies with small patient numbers have investigated the genetic association of pulmonary aspergilloma, a disorder similar to sinus fungus ball. These limited studies have shown that polymorphisms in several genes involved in innate immunity and cytokines predispose a patient’s susceptibility to mycetoma.15,17,18,20,21 No studies have yet investigated the role of SNP in sinus fungus ball. In our study, no increased prevalence was found with the several SNPs known to be associated with fungal infections. One possible explanation for this discrepancy with other reports is the differences in geographical location and the ethnic population of patients. In addition, our case number was small. Furthermore, the lack of SNP variations associated with invasive Aspergillosis may partly explain our findings that those fungus ball patients with immunodeficiency did not progress to experiencing invasive fungal infections. Despite that, we found an increase in SNP variations was related to E-cadherin in our patients with fungal balls.
Conclusions
There are unique characteristics surrounding sinus fungus ball, including its occurrence in female patients, older individuals, patients with subtle immune deficiency, and with those with a predominantly unilateral presentation. We performed immunochemistry staining, western blotting, chemokines and cytokines analyses, and discovered the prevalence of several known SNPs associated with fungal infection or respiratory diseases. We found that patients with sinus fungus ball experienced robust immune responses, allowing for the recruitment and activation of both macrophages and neutrophils. However, patients with sinus fungus ball could possibly have a genetic or acquired weakness in their immunity or barrier function. The fungal hyphae were localized and accumulated within a single sinus, rather than being eradicated by its host.
Acknowledgments
The assistance from the Center for Translational Medicine of the Hospital is greatly appreciated.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by grants from Taichung Veterans General Hospital (TCVGH-1047004 C and TCVGH-1057003 C) and Yen Tjing Ling Medical foundation (CI-105-17 and CI-106-21).
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: RSJ: data collection, manuscript preparation; WCH: data collection; KLL: manuscript preparation.
References
- 1. Kim JS, So SS, Kwon SH. The increasing incidence of paranasal sinus fungus ball: a retrospective cohort study in two hundred forty-five patients for fifteen years. Clin Otolaryngol. 2017;42:175–179. [DOI] [PubMed] [Google Scholar]
- 2. Yoon YH, Xu J, Park SK, Heo JH, Kim YM, Rha KS. A retrospective analysis of 538 sinonasal fungus ball cases treated at a single tertiary medical center in Korea (1996-2015). Int Forum Allergy Rhinol. 2017;7:1070–1075. [DOI] [PubMed] [Google Scholar]
- 3. Lee JS, Shin SY, Lee KH, Kim SW, Cho JS. Change of prevalence and clinical aspects of fungal ball according to temporal difference. Eur Arch Otorhinolaryngol. 2013;270:1673–1677. [DOI] [PubMed] [Google Scholar]
- 4. deShazo RD, Chapin K, Swain RE. Fungal sinusitis. N Engl J Med. 1997;337:254–259. [DOI] [PubMed] [Google Scholar]
- 5. deShazo RD, O’Brien M, Chapin K, et al. Criteria for the diagnosis of sinus mycetoma. J Allergy Clin Immunol. 1997;99:475–485. [DOI] [PubMed] [Google Scholar]
- 6. Ferguson BJ. Fungus balls of the paranasal sinuses. Otolaryngol Clin North Am. 2000;33:389–398. [DOI] [PubMed] [Google Scholar]
- 7. Ledderose GJ, Braun T, Betz CS, Stelter K, Leunig A. Functional endoscopic surgery of paranasal fungus ball: clinical outcome, patient benefit and health-related quality of life. Eur Arch Otorhinolaryngol. 2012;269:2203–2208. [DOI] [PubMed] [Google Scholar]
- 8. Nicolai P, Lombardi D, Tomenzoli D, et al. Fungus ball of the paranasal sinuses: experience in 160 patients treated with endoscopic surgery. Laryngoscope. 2009;119:2275–2279. [DOI] [PubMed] [Google Scholar]
- 9. Klossek JM, Serrano E, Peloquin L, Percodani J, Fontanel JP, Pessey JJ. Functional endoscopic sinus surgery and 109 mycetomas of paranasal sinuses. Laryngoscope. 1997;107:112–117. [DOI] [PubMed] [Google Scholar]
- 10. Montone KT. Pathology of fungal rhinosinusitis: a review. Head Neck Pathol. 2016;10:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. deShazo RD, O’Brien M, Chapin K, Soto-Aguilar M, Gardner L, Swain R. A new classification and diagnostic criteria for invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 1997;123:1181–1188. [DOI] [PubMed] [Google Scholar]
- 12. deShazo RD. Fungal sinusitis. Am J Med Sci. 1998;316:39–45. [DOI] [PubMed] [Google Scholar]
- 13. Lund VJ, Kennedy DW. Quantification for staging sinusitis. The Staging and Therapy Group. Ann Otol Rhinol Laryngol Suppl. 1995;167:17–21. [PubMed] [Google Scholar]
- 14. Meng J, Zhou P, Liu Y, et al. The development of nasal polyp disease involves early nasal mucosal inflammation and remodelling. PLoS ONE. 2013;8: e82373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van de Sande WW, Fahal A, Verbrugh H, van Belkum A. Polymorphisms in genes involved in innate immunity predispose toward mycetoma susceptibility. J Immunol. 2007;179:3065–3074. [DOI] [PubMed] [Google Scholar]
- 16. Sambatakou H, Pravica V, Hutchinson IV, Denning DW. Cytokine profiling of pulmonary aspergillosis. Int J Immunogenet. 2006;33:297–302. [DOI] [PubMed] [Google Scholar]
- 17. de Boer MG, Jolink H, Halkes CJ, et al. Influence of polymorphisms in innate immunity genes on susceptibility to invasive aspergillosis after stem cell transplantation. PLoS ONE. 2011;6: e18403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith NL, Hankinson J, Simpson A, Bowyer P, Denning DW. A prominent role for the IL1 pathway and IL15 in susceptibility to chronic cavitary pulmonary aspergillosis. Clin Microbiol Infect. 2014;20: O480–O488. [DOI] [PubMed] [Google Scholar]
- 19. Lupianez CB, Canet LM, Carvalho A, et al. Polymorphisms in host immunity-modulating genes and risk of invasive aspergillosis: results from the AspBIOmics consortium. Infect Immun. 2015;84:643–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carvalho A, Pasqualotto AC, Pitzurra L, Romani L, Denning DW, Rodrigues F. Polymorphisms in toll-like receptor genes and susceptibility to pulmonary aspergillosis. J Infect Dis. 2008;197:618–621. [DOI] [PubMed] [Google Scholar]
- 21. Pana ZD, Farmaki E, Roilides E. Host genetics and opportunistic fungal infections. Clin Microbiol Infect. 2014;20:1254–1264. [DOI] [PubMed] [Google Scholar]
- 22. Ierodiakonou D, Postma DS, Koppelman GH, et al. E-cadherin gene polymorphisms in asthma patients using inhaled corticosteroids. Eur Respir J. 2011;38:1044–1052. [DOI] [PubMed] [Google Scholar]
- 23. Lee KC. Clinical features of the paranasal sinus fungus ball. J Otolaryngol. 2007;36:270–273. [DOI] [PubMed] [Google Scholar]
- 24. Nomura K, Asaka D, Nakayama T, et al. Sinus fungus ball in the Japanese population: clinical and imaging characteristics of 104 cases. Int J Otolaryngol. 2013;2013:731640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsai TL, Guo YC, Ho CY, Lin CZ. The role of ostiomeatal complex obstruction in maxillary fungus ball. Otolaryngol Head Neck Surg. 2006;134:494–498. [DOI] [PubMed] [Google Scholar]
- 26. Dufour X, Kauffmann-Lacroix C, Ferrie JC, Goujon JM, Rodier MH, Klossek JM. Paranasal sinus fungus ball: epidemiology, clinical features and diagnosis. A retrospective analysis of 173 cases from a single medical center in France, 1989–2002. Med Mycol. 2006;44:61–67. [DOI] [PubMed] [Google Scholar]
- 27. Legent F, Billet J, Beauvillain C, Bonnet J, Miegeville M. The role of dental canal fillings in the development of Aspergillus sinusitis. A report of 85 cases. Arch Otorhinolaryngol. 1989;246:318–320. [DOI] [PubMed] [Google Scholar]
- 28. Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pagella F, Matti E, De Bernardi F, et al. Paranasal sinus fungus ball: diagnosis and management. Mycoses. 2007;50:451–456. [DOI] [PubMed] [Google Scholar]
- 30. Park HJ, Seoh JY, Han KH, Moon KR, Lee SS. The role of mucosal immunity in fungus ball of the paranasal sinuses. Acta Otolaryngol 2012;132: S58–S62. [DOI] [PubMed] [Google Scholar]
- 31. Werner JL, Gessner MA, Lilly LM, et al. Neutrophils produce interleukin 17A (IL-17A) in a dectin-1- and IL-23-dependent manner during invasive fungal infection. Infect Immun. 2011;79:3966–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Man Y, Hart VJ, Ring CJ, Sanjar S, West MR. Loss of epithelial integrity resulting from E-cadherin dysfunction predisposes airway epithelial cells to adenoviral infection. Am J Respir Cell Mol Biol. 2000;23:610–617. [DOI] [PubMed] [Google Scholar]
- 33. Harrison TS, Levitz SM. Immunology. In: Anaissie E, McGinnis M, Pfaller M. (eds) Clinical mycology. 2nd ed. London: Churchill Livingstone; 2009:33–53. [Google Scholar]