Short abstract
Clinical studies show that anxiety and chronic pain are concomitant. The neural basis for the comorbidity is unclear. The prefrontal cortex (PFC) has been recognized as a critical area for affective disorders and chronic pain modulation. In this study, we examined the role of the PFC in the pathogenesis of anxiety associated with chronic pain in a rat model of neuropathic pain with spare nerve injury (SNI). The SNI rats showed apparent anxiety-like behaviors in both open field (OF) test and elevated-plus maze (EPM) test eight weeks after surgery. Thus, the number of entries to the central area in the OF decreased to 45% (±5%, n = 15) of sham control (n = 17), while the overall motor activity (i.e., total distance) was unaffected. In the EPM, the percentage of entries into the open arms significantly (p < 0.001) decreased in SNI rats (SNI: 12.58 ± 2.7%, n = 15; sham: 30.75 ± 2.82%, n = 17), so did the time spent in the open arms (SNI: 4.35 ± 1.45%, n = 15; Sham: 11.65 ± 2.18%, n = 17). To explore the neural basis for the association between anxiety and chronic pain, local field potentials (LFPs) were recorded from the medial PFC (mPFC) and ventral hippocampus. In SNI rats, there were significantly greater increases in both theta-frequency power in the mPFC and theta-frequency synchronization between the mPFC and ventral hippocampus, when animals were displaying elevated anxiety-like behaviors in avoiding anxiogenic regions in EPM and OF chamber. Western blot analyses showed a significant elevation of serotonin transporter expression in the anxious SNI rats. Inhibition of serotonin transporter effectively alleviated anxiety-like behaviors following sub-chronic (15 days) treatment with systemic citalopram (10 mg/kg/day, intraperitoneally). Moreover, the anxiety-like behaviors in the SNI rats were also suppressed by direct mPFC application of serotonin. Taken together, we conclude that the plasticity of serotonin transmission in the mPFC likely contribute to the promotion of anxiety state associated with neuropathic pain.
Keywords: neuropathic pain, anxiety, prefrontal cortex, serotonin transporter, theta-frequency oscillation, plasticity, rats
Introduction
Chronic pain has been found to have a close relationship with emotional disorders, such as anxiety and depression.1–5 In the clinics, nearly half of the patients who suffered from chronic pain were diagnosed with one or more anxiety disorders.6 Various animal models of chronic pain also display anxiety-like behaviors.7–10 However, the neural basis for this comorbidity remains unclear. It might involve plasticity in association with chronic pain in the brain areas which are engaged in both pain processing and affective regulation. Several cortical areas have been suggested to play important roles in the processing of either the sensory or affective-motivational component of pain.11,12 Among these cortical areas, the medial prefrontal cortex has also been demonstrated to be involved in regulation of anxiety13 in addition to its role in pain processing. Human studies reported altered medial prefrontal cortex (mPFC) activity in patients with anxiety disorders.14–18 Deep transcranial magnetic stimulation was found effective in treating post-traumatic stress disorder patients.19,20 The involvement of the mPFC in anxiety-like behaviors was also observed in animal models of anxiety.21,22 Optogenetic inhibition of the prelimbic cortex (PrL), a sub-region of the mPFC, caused anxiogenic effects in naive mice.23 It is evident, therefore, that the mPFC is a critical area for the regulations of both pain and anxiety.
In chronic pain states, the mPFC was found to undergo both structural and functional changes in both human patients and animal pain models.24–34 Thus, both gray matter volume and neural activities in the prefrontal cortex decreased in chronic pain patients.35 Changes in excitability and activity of prefrontal neurons occurred in the conditions of inflammatory and neuropathic pain in animal pain models.30,34,36 These plastic changes would inevitably cause alterations of mPFC functions. Human studies have found that changes in the mPFC activity was associated with the development of chronic pain.35 It may also play a role in causing anxiety in chronic pain conditions. A recent work by Wang et al.23 suggests such an association between changes in the activity of mPFC pyramidal neurons and pain-induced anxiety.
Besides the mPFC, the anterior cingulate cortex (ACC) is the other brain area involved in both pain perception5,11,12 and anxiety.37–41 In chronic pain states, the ACC displayed a series of structural and functional changes as well, which were believed to play an important role in the development of chronic pain.42,43 Moreover, the synaptic plasticity in the ACC has been implicated to be related to pain-induced anxiety.41,44
We thus hypothesize that the plastic changes in the anxiety network, such as the mPFC and ACC, form part of the neural basis for the interaction between chronic pain and anxiety. However, the detailed mechanism of the interaction at molecular, cellular, and circuitry levels is not fully understood. In this study, we examined the role of prefrontal plasticity in the pathogenesis of pain-induced anxiety by utilizing integrative molecular biology, in vivo electrophysiology, and behavioral approaches in a rat model of neuropathic pain with spare nerve injury (SNI) which developed anxiety-like behaviors within two months following nerve injury. We found that, when the SNI rats were displaying elevated anxiety-like behaviors in avoiding anxiogenic regions, there was a significantly greater increase in local field potential (LFP) theta oscillation (4–12 Hz) power in the mPFC, a LFP component being suggested to be highly correlated with anxiety state by previous studies,45 indicating the involvement of the mPFC in pain-related anxiety state. Moreover, we found that serotonin transporter expression in the mPFC was significantly elevated in the anxious SNI rats and local perfusion of serotonin to the mPFC alleviated SNI rat anxiety-like behaviors. These findings suggest that a detraction of serotonin transmission at the mPFC may constitute the mechanism for the promotion of anxiety associated with neuropathic pain.
Materials and methods
Animals
A total of 130 adult male Wistar rats (180–350 g) were used in the study. The animals were housed in groups of three (but individually after electrode implantation) in plastic cages under standard laboratory conditions (12-h light/dark cycle, 20–24°C and humidity 50%) with free access to food and water. The animals were acclimated for one week before experiments. All experimental procedures were approved by the Ethics Committee of Animal Care and Use of East China Normal University and were in accordance with the guidelines of the International Association for the Study of Pain.
Spared nerve injury surgery
All surgical procedures were performed under sodium pentobarbital anesthesia (50 mg/kg, intraperitoneally (i.p.)) with an adequate depth of anesthesia being periodically monitored and confirmed by the lack of nociceptive responses. Rectal temperature was maintained near 37°C by a controlled heating blanket. The SNI surgery is described below. Upon completion of surgery, hemostasis was confirmed and the incisions were closed in layers with sutures. Animals were inspected daily for body weight, food, and water intake, and signs of autotomy and paralysis. In only seven cases, early signs of autotomy (gnawing of claw tips and some surrounding tissue on the injured hindpaw) were observed in operated rats and the animals were promptly euthanized.
Sural-spared sciatic nerve injury was induced in 70 rats by tight ligation of common peroneal and tibial nerves of the sciatic nerve, a modified procedure46 of a previously reported method.47 Following a skin incision made on the lateral surface of the left thigh, the biceps femoris muscle was separated to expose the sciatic nerve and its three terminal branches: the sural, common peroneal, and tibial nerves. The tibal and common peroneal nerves were tightly ligated with 5.0 silk suture, while leaving the sural nerve intact. Great care was taken to avoid damaging of the sural nerve. Muscle and skin were closed in two layers. Sham groups (n = 60) involved exposure of the sciatic nerve and its branches without nerve ligation. After the surgery, the animal was returned to its home cage.
Measurement of mechanical allodynia
To habituate the rats to the test environment, they were placed in transparent plastic boxes (26 cm × 20 cm ×14 cm) on an elevated mesh floor for 1 h daily starting three days before the study and for a minimum of 30 min prior to mechanical sensitivity tests. The mechanical sensitivity tests were performed in all animals one day before and three days after the surgery, followed by a twice per week schedule throughout the entire period of the experiment. Tactile sensitivity was evaluated using a series of calibrated von Frey filaments (0.4–15 g) applied perpendicularly to lateral plantar surface of the ipsilateral hind paws, i.e., the site that corresponds to the dermatome of the sural nerve,47 with sufficient force to bend the filament slightly for 3–5s. An abrupt withdrawal or licking and vigorously shaking of the foot in response to stimulation was considered as pain-like responses. The threshold was determined using the up–down testing paradigm. The 50% paw withdrawal threshold (PWT) was calculated using the nonparametric Dixon test.48 The values of 15 g and 2 g were used as cutoff for pre- and post-surgical conditions, respectively. For SNI rats, only those that developed allodynia with a mechanical threshold less than 6 g by seven days after surgery were included in the behavioral studies (n = 62). All testing was done by the experimenter blind to animal’s surgical conditions.
Behavioral tests
At the fourth or eighth week after SNI surgery, the rats underwent a battery of behavioral tests on anxiety-like behaviors, including open field (OF) test and elevated plus-maze (EPM) test. All behavioral tests were conducted in the morning by the experimenter blind to experimental conditions.
OF test
The locomotor activity and anxiety-like behaviors were evaluated by placing rats in an OF activity test chamber with infrared beam (ITTCINC life science company) in a brightly illuminated (white light) room. The animals were tested for 10 min in an arena (40 cm × 40 cm ×35 cm with transparent walls). The total distance that the rat traveled in the arena was recorded for 10 min as a measure of locomotor activity. The number of entries into the central area (16 × 16 cm2) of the OF was simultaneously counted as a measure of anxiety-like behavior. The test chamber was cleaned with 10% ethanol solution to remove odors after each test.
EPM test
The EPM test was performed to evaluate anxiety-like behaviors on the second day following the OF test. The EPM apparatus consists of two open and two closed arms (15 × 50 cm each arm), both of which were elevated 50 cm from floor. Each rat was placed in the center of the apparatus facing one of the open arms. An entry to an arm was counted when all four paws of the rat were located inside of the arm. The number of entries into either open or enclosed arms as well as the time spent in each arm type were recorded by ANY-maze software during a 5-min test period.49 The frequency of open arm entries was expressed as the percentage of the total number of entries into both open and close arms. The amount of time spent in the open arms was calculated as a percentage of the total time (300 s) spent in the maze. The EPM apparatus was carefully cleaned with 10% ethanol after each test.
Western blot
After the behavioral tests, the animals were anesthetized by sodium pentobarbita (50 mg/kg, i.p.) and then sacrificed by a decapitation. Bilateral PrL (anteroposterior [AP] =+2.5∼+3.5 mm; mediolateral [ML] = 0∼1.0 mm; dorsoventral [DV]=−2.5∼−4.0 mm) and hippocampus were dissected out and then were weighed and homogenized in IP Lysis buffer (50 mM Tris-HCl pH7.4, 250 mM NaCl, 0.5% Triton X100, 10% glycerol) and proteinase inhibitors (1 mM PMSF). The homogenate was then centrifuged in an Eppendorf centrifuge (15,000 g, 5417R, 4°C) for 15 min. The supernatant was collected and centrifuged for 10 min. Following purification of the supernatant, the protein concentration was determined using BCA kit (P0012, Beyotime). Tissue samples were mixed with SDS loading buffer (Lysis buffer), heated to 100°C for 10 min, and stored at −20°C before use. Purified fractions were separated on SDS-PAGE and quantified using Odyssey Licor. Beta-actin was used as loading controls. Equal volume (20 μL) of each tissue sample was loaded on Tris-glycine SDS-polyacrylamide gels (4% upper gel, 10% separate gel), followed by electrophoresis. The samples were transferred onto 0.2 μm PVDF membranes and incubated with primary antibody (rabbit anti-serotonin transporter (SERT), 1:1000, Millipore; mouse anti-beta-actin, 1:5000, Bioworld) at 4°C overnight. The samples were then incubated with secondary antibody (anti-rabbit antibody, anti-mouse antibody 1:15000, Odyssey) for 1 h at room temperature. Band densities were analyzed using Odyssey system (Odyssey). Band densities of SERT in the mPFC and the ventral hippocampus (vHPC) were normalized to beta-actin levels, respectively, to account for expression differences.
Intracranial cannulation
Stereotaxic surgeries were performed in rats anesthetized with a pentobarbital sodium (2%, 30 mg/kg, Merck) and atropine (0.5 ml/100 g). Cannulas were implanted according to the atlas of Paxinos and Watson.50 For PFC microdialysis, bilateral guide cannulae (RWD Life Science) were implanted into the PrL (AP = +3.0 mm; ML = ±0.42 mm; DV = −3.25 mm). Stainless steel dummy cannulas were inserted into each guide to keep cannulas free of debris. The injection cannulas (RWD) extended 0.5 mm beyond the end of the guide cannulas and were connected to a 5-μl Hamilton syringe and driven by a microsyringe pump controller (World Precision instruments). After surgery, all animals were housed individually and allowed a minimum of seven days to recover.
Drug treatment
In our pharmacological experiment, citalopram (CIT; 10 mg/kg, i.p.) was administered to the SNI rats (n = 8) daily for 15 days before behavioral tests. The same volume of saline (0.9%, 2 ml/kg, i.p.) was injected to SNI vehicle control group (n = 9).
Local drug infusions into the bilateral PrL were given in a volume of 0.65 μl/side at the following doses: serotonin (7.7 μg; Sigma) or vehicle (3.77 μg sodium sulfate and 3.48 μg sodium chloride) within 1 min. The injection cannulas were remained in place for 2–3 min to prevent drug backflow. Rats were tested on the EPM after removing the injection cannulas as described before. After each experiment, rats were euthanized by cervical dislocation, and 0.5 μl of black ink was injected through an injection cannula to verify cannula placement. Data from animals with misplaced cannulas were removed from analysis.
In vivo local field potential recording
The rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and placed in a stereotactic apparatus (Kopf Instruments) with the incisor bar set so that bregma and lambda were in the same horizontal plane. Two insulated nicochrome electrodes (33 μm in diameter; California Fine Wire Co.) were placed in the mPFC region (the deep layers of the ventral portion of the prelimbic cortex; AP=+3.3 mm, ML = 0.5 mm, DV =-3.4 mm) and vHPC CA1 region (AP =- 4.8 mm, ML = 4.2 mm, DV = -8.2 mm), respectively, according to the atlas of Paxinos and Watson.50 Three additional holes were drilled for stainless steel anchoring screws, and the ground wire was secured to one of the anchoring screws. Electrodes were cemented to the skull and anchoring screws with dental cement. After the surgery, the animals were housed individually and allowed to recover for at least one week until regaining body weight following electrode implanting. No behavioral test was performed within one week after the surgery.
To acclimatize animals with recording setup, rats were placed to a rectangular box (familiar arena, FA; 54.5 × 42 × 38 cm) in the dark for 30 min daily for at least four days. On the day of recording, rats were placed to the FA for 10 min. After a 1 h resting in their home cages, they were exposed to either the open field or the EPM for 10 min. The interval between the tests was 1 h. The order of presentation of the two environments was counterbalanced across animals. All the recordings were performed under blind conditions.
Local field potentials were filtered (bandpass 1–1000 Hz) and amplified using a four-channel amplifier (Model 440, Brown Lee, Santa Clara, CA). LFPs were digitized by a 16-bit ADC (Micro 1401, CED limited, Cambridge, England) and acquired by Spike 2 software (CED, Cambridge, England).
Upon the completion of recording, animals were deeply anesthetized and electrolytic lesions (50 μA, 15 s) were made to determine the location of the electrode tips. The animals were perfused with 0.9% saline and 4% paraformaldehyde (Sigma). Brain slices were collected by frozen section to visualize and photograph the lesions.
Data analysis
The LFP data were imported into MATLAB (version R2014a) for analysis using custom-written software according to a previously reported method.51 Power spectra were calculated using the Welch method (a 0.4 s moving window, 90% overlap, and 4000 nFFTs). The mean value of power in theta band (4–12 Hz) was taken as the measure of theta power. Fold increases were then calculated by mean theta power in OF or EPM divided by mean theta power in FA. All bar graphs of fold increases shown in the figures were drawn from the data collected while animals were moving at the speed between 7 and 15 cm/s.
To calculate power correlations between two brain areas, LFPs were filtered in theta-band and average theta power was calculated over time through a multitaper spectrogram method with an NW (time-bandwidth product) of 2.5. Then, the average theta power of each animal was plotted as scatter diagram. The linear correlation coefficient for each plot was calculated and averaged across animals for each pair of brain areas.
The phase coherence of the mPFC and the vHPC was determined by calculating the instantaneous theta phases of two signals through the Hilbert transform and then subtracting the phases of the two LFPs from each other. The absolute differences (phase lag) in theta phase between two brain areas were then plotted as histogram, and the width of the plot at half of the peak height was used as a measure of phase coherence of the two signals. If two signals tend to have a constant phase relationship, the phase difference histogram will display a narrow peak. Thus, a narrower width at half of the peak height indicates stronger phase coherence. Power correlation and theta phase coherence was calculated from the data of the entire recording, regardless of the speed of the animal.
Statistical analysis
Statistical analyses of the data were performed using SPSS 16.0 for Windows. All statistical tests were two-tailed, and statistical significance was assigned at p < 0.05. Unpaired t-tests, Mann–Whitney U tests, and one-way analysis of variance (followed by Bonferroni's multiple comparisons test) were used in appropriate conditions to assess the differences. Data are presented as mean± standard error of mean.
Results
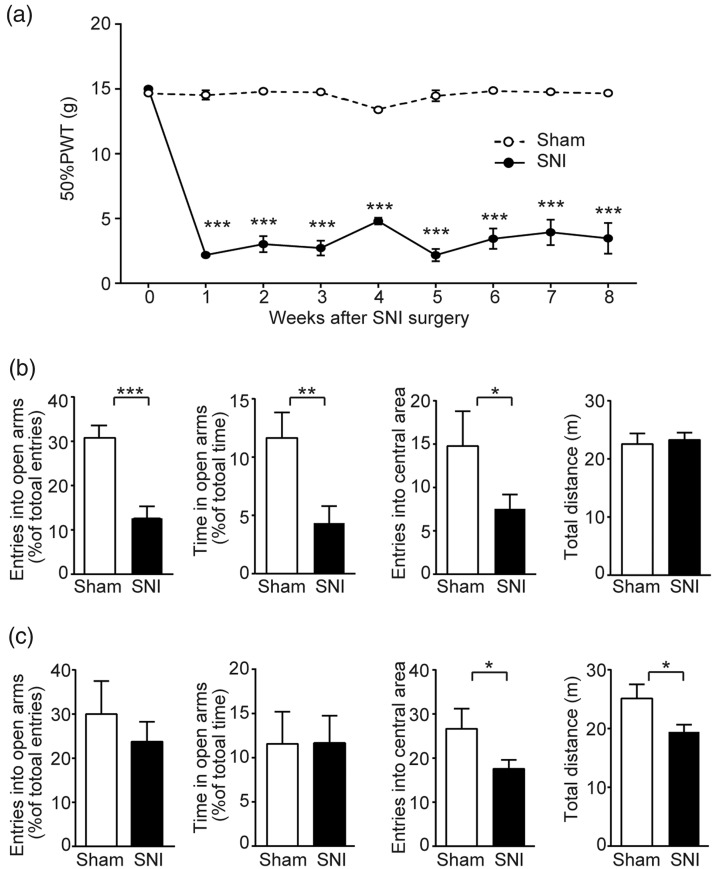
After SNI surgery, the rats developed mechanical allodynia which was characterized by a decreased PWT to tactile stimulation on the ipsilateral (injured) side. On post-operative day 7, mean 50% PWT was significantly (p < 0.001) decreased to 2.18 g (±0.23, n = 15) from a pre-surgical value of 15 g (cut-off value, n = 15). No significant (p > 0.05) change in PWT was seen in sham-operated control animals (n = 17). The neuropathic pain state of the SNI rats remained persistent throughout the entire period (∼8 weeks) of the experiment (Figure 1(a)), providing an appropriate chronic model to study anxiety-like behaviors associated with chronic pain.
Figure 1.
Behavioral changes of SNI rats after surgery. (a) Time course of development and maintenance of tactile allodynia following SNI surgery. Mechanical threshold was expressed by 50% PWT measured by a series of von Frey filaments using the up–down method at the surgery side after surgery. (b) Apparent anxiety-like behaviors of SNI rats at the eighth week after surgery. SNI rats displayed a decrease in open arm entries and open arm time in EPM, as well as the number of entries into the central area of the open field. No difference in the total distance travelled in the open field between SNI and sham group. The number of entries into open arms was calculated as the percentage of total entries into both open and close arms in EPM. The time in open arms was expressed as the percentage of total time in EPM. The number of entries into the central area and the total distance were counted for an entire test period (10 min). (c) Lack of anxiety-like behaviors of the SNI rats at the fourth week after surgery. No difference was seen in either entries into or time in open arms in EPM. SNI rats showed decreases in both total distance and number of entries into central area. Data are represented as mean ± s.e.m. Statistical significance is assessed between Sham and SNI groups: *p < 0.05, **p < 0.01, ***p < 0.001 (unpaired t-test). SNI group (n = 15), Sham group (n = 17); for time in open arms at the fourth week after surgery: SNI group (n = 12), Sham group (n = 10).
SNI: spare nerve injury; PWT: paw withdrawal threshold.
Anxiety-like behaviors associated with chronic pain in SNI rats
The SNI rats exhibited anxiety-like behaviors in both EPM and OF tests at the eighth post-operative week. In the EPM test, the SNI group showed a significant (p < 0.01) decrease in both open arm entries (Sham: 30.75 ± 2.82%, n = 17; SNI: 12.58 ± 2.72%, n = 15; Figure 1(b)) and time spent in open arms (Sham: 4.35 ± 1.45%, n = 17; SNI: 11.65 ± 2.18%, n = 15; Figure 1(b)). In the OF test, the SNI rats displayed significantly (p < 0.05) fewer entries (7.53 ± 1.65, n = 15) into central area compared with Sham group (14.77 ± 4.01, n = 17; Figure 1(b)). On the other hand, there was no significant difference (p > 0.05) in the total distance (Figure 1(b)), indicating that the locomotor ability of the SNI animals was unaffected.
The anxiety-like behaviors of SNI rats were not developed at the fourth post-operative week in our model. In the EPM, compared with the sham group, the SNI group showed no significant reduction either in open arm entries (Sham: 30.00 ± 7.50%, n = 17; SNI: 23.75 ± 4.50%, n = 15; Figure 1(c)) or in open arm time (Sham: 11.60 ± 3.64%, n = 10; SNI: 11.70 ± 3.08%, n = 12; Figure 1(c)). Although the SNI rats displayed significantly (p < 0.05) fewer entries into central area (17.54 ± 2.02, n = 15) in the OFT than the Sham group (26.64 ± 4.55, n = 17; Figure 1(c)), the difference may result from a temporary trauma of locomotor ability following surgery in the SNI animals (p < 0.05, Figure 1(c)).
Enhancement of theta oscillation in medial prefrontal cortex of SNI rats
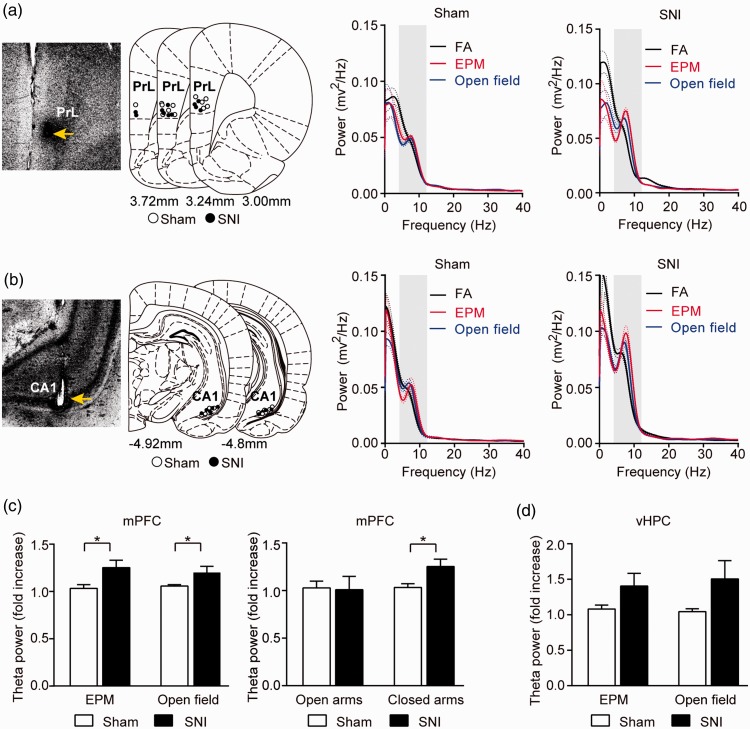
Previous studies have suggested that medial prefrontal cortex and vHPC are involved in the modulation of anxiety.52–55 The changes of theta-frequency (4–12 Hz) oscillation in the mPFC and the vHPC and the theta-frequency synchronization between these two areas were found to be highly correlated with anxiety state.51,56 To understand the neural basis for chronic pain-induced anxiety, we examined the changes in the theta-frequency oscillations of the LFPs recorded from the mPFC and the vHPC (Figure 2(a) and (b)) in both the SNI and Sham rats.
Figure 2.
Enhancement of LFP theta-band power in the mPFC of the SNI rats. (a) Power spectra of the mPFC LFPs recorded when animals were in the FA (black), EPM (red), and open field (blue). Dotted lines represent the s.e.m. Shaded area indicates theta-band (4–12 Hz). Left: recording sites in the prelimbic cortex, a sub-region of the mPFC, marked by electrical lesion. (b) Power spectra of the vHPC LFPs recorded when animals were in the FA (black), EPM (red), and open field (blue). Left: recording sites in the vHPC marked by electrical lesion. (c) Enhanced theta-band power increase in the mPFC of SNI rats. Left: augmented theta-band power of mPFC LFPs in EPM and open field. Increase of theta-power was expressed as fold increase relative to FA. Right: augmentation of mPFC theta power confined in the closed arms of EPM. The components of theta-band power in open and closed arms were dissected and individually compared to that in the FA. (d) Theta power of the vHPC LFPs in the EPM and open field. Data represented as mean ± s.e.m. Statistical significance is assessed between Sham and SNI groups: *p < 0.05 (unpaired t test). Sham group: n = 12, SNI group: n = 11.
SNI: spare nerve injury; EPM: elevated-plus maze; FA: familiar arena; PrL: prelimbic cortex; mPFC: medial PFC; vHPC: ventral hippocampus.
As previously described, the power spectra of LFPs was found to be affected by moving speed of animals.51 To obtain a reliable comparison across conditions, the analysis of theta power was performed using the data collected when the animals were moving at a similar speed range (7–15 cm/s) to avoid data contamination by the speed. We compared the fold increases of theta power in EPM and OFT relative to FA between groups. In the mPFC, the SNI rats (n = 11) displayed significantly (p < 0.05) greater fold increases of theta power in both EPM and OFT (Figure 2(a) and (c)) compared with sham group (n = 12). Interestingly, when the mPFC theta power in each compartment of the EPM was dissected and individually compared with that in the FA, the increase in the mPFC theta power of SNI group was found to be present only when the rats were in the closed arms of the EPM, but not in the open arms (Figure 2(c)). In the vHPC, no statistically significant difference in the fold increase of theta power was observed between SNI and Sham groups in either the EPM or the OFT (Figure 2(b) and (d)).
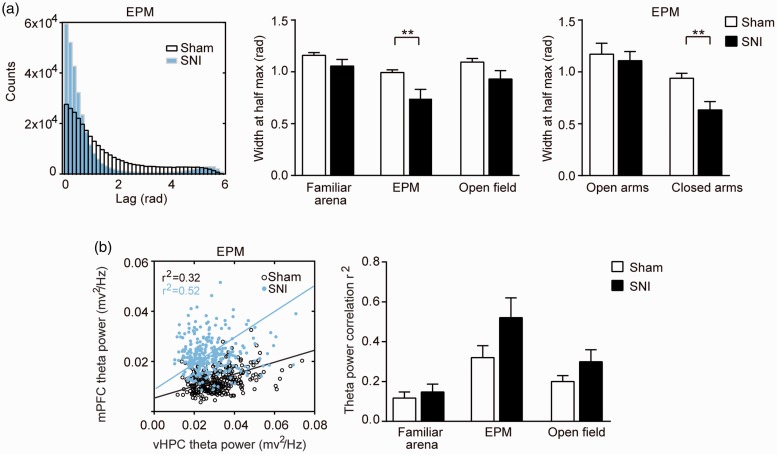
Exposure to anxiogenic environment has been found to increase theta-frequency synchrony between the mPFC and the vHPC.51 We further analyzed the theta phase coherence and theta power correlation between the mPFC and the vHPC in our neuropathic pain animals. We found that, when animals were in EPM, the theta phase coherence between the mPFC and the vHPC was significantly (p < 0.01) stronger in the SNI group, as demonstrated by the narrower peaks in the phase difference histograms (Figure 3(a)) and the smaller values of the widths at half peaks (Figure 3(a)). Similar to the increase of mPFC theta power (Figure 2(c)), this increase of theta phase coherence only existed in the closed arms of EPM (Figure 3(a)). On the other hand, the power correlation of theta-frequency between the mPFC and the vHPC was not significantly different between SNI and Sham groups in any environment (Figure 3(b)).
Figure 3.
Theta-frequency synchrony between the mPFC and vHPC. (a) Increased theta-phase coherence between mPFC and vHPC in SNI rats. Left: representative histograms of theta phase differences in the EPM. Instantaneous theta phase of LFPs from mPFC and vHPC were subtracted from each other and the absolute differences in theta phase (Lag) were plotted as histograms. A narrower peak in the histogram indicates a more consistent phase relationship. Middle: Width of theta phase difference histogram at half of peak counts for sham and SNI rats in familiar arena, EPM, and open field. SNI rats showed higher phase coherence of theta oscillation in EPM, indicated by smaller values of the widths at half peaks of theta phase difference histogram. Right: The increase of theta phase coherence is confined in the closed arms of EPM. (b) No significant difference in theta power correlation between sham and SNI rats. Left: Representative scatter plots of theta power of mPFC and vHPC in the EPM of sham and SNI rats. The linear correlation coefficient (r2) was calculated by linear regression to measure the power correlation between mPFC and vHPC. Right: averaged linear correlation coefficient of sham and SNI group in each of the three environments. Data represented as mean ± s.e.m. Statistical significance is assessed between Sham and SNI groups: **p < 0.01 (unpaired t-test). Sham group: n = 12, SNI group: n = 11.
SNI: spare nerve injury; EPM: elevated-plus maze; mPFC: medial PFC; vHPC: ventral hippocampus.
Elevation of expression of prefrontal serotonin reuptake transporter in SNI rats
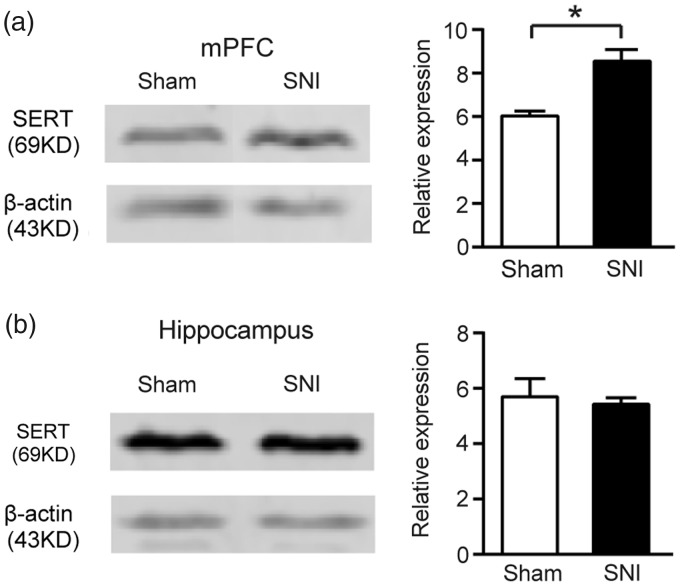
The SERT is a key regulator of brain serotonergic activity, which modulates serotonin concentration in the extracellular space via reuptake mechanisms. Changes in SERT expression were found to be associated with mood disorders in humans.57–59 To test whether SERT is involved in the pain-related anxiety-like behaviors, we examined SERT protein levels in the medial prefrontal cortex and hippocampus.
The Western blot analysis revealed that in the medial prefrontal cortex, the expression of SERT was significantly (p < 0.05) increased in the SNI group compared with sham group (SNI: 8.54 ± 0.53, n = 4; Sham: 6.03 ± 0.23, n = 5; Figure 4(a)). In contrast, the hippocampus showed no significant change of SERT expression in the SNI rats (SNI: 5.43 ± 0.23; n = 5 Sham: 5.70 ± 0.65, n = 5; Figure 4(b)).
Figure 4.
Elevation of SERT expression in the mPFC in SNI rats. (a) Elevated SERT expression in the mPFC of SNI rats at the eighth week following nerve injury. Left: Representative Western blot analysis; Right: Bar graph of relative protein expression of SERT in the mPFC of sham (n = 4) and SNI (n = 5) rats. (b) No significant change in SERT expression in hippocampus. Left: Representative Western blot analysis; Right: Bar graph of relative protein expression of SERT in hippocampus of sham (n = 5) and SNI (n = 5) rats. Data are represented as mean ± s.e.m. *p < 0.05, Sham versus SNI, by Mann–whitney U-test.
SNI: spare nerve injury; SERT: serotonin transporter; mPFC: medial PFC.
Alleviation of pain-associated enhancement of anxiety-like behaviors by systemic treatment of a selective serotonin reuptake inhibitor and local infusion of 5HT into the mPFC in SNI rats
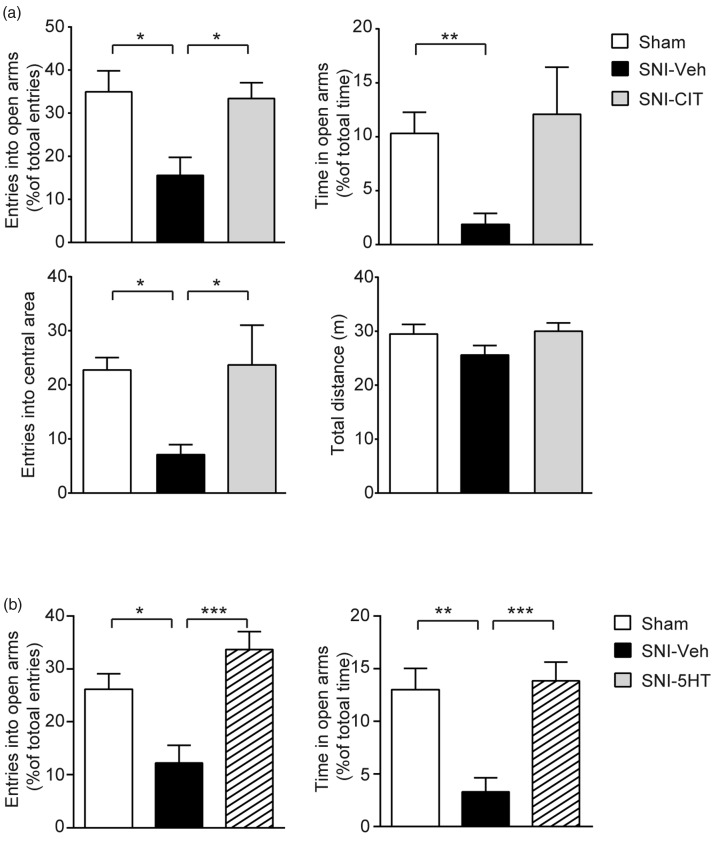
To confirm the involvement of the SERT in mediating the anxiety-like behaviors associated with neuropathic pain, we first examined the effect of pharmacological inhibition of SERT using a selective serotonin reuptake inhibitor (SSRI), CIT. The drug was administered sub-chronically (10 mg/kg, i.p., q.d.) to the SNI rats. After 15 days treatment, compared with the vehicle-treated SNI rats (SNI-Veh), the SNI rats treated with citalopram (SNI-CIT) showed significantly higher frequency of open arm entries (SNI-CIT: 33.41 ± 3.67%, n = 8; SNI-Veh: 15.56 ± 4.19%, n = 9; p < 0.05) and longer time spent in open arms (SNI-CIT: 12.09 ± 4.36%; SNI-Veh: 1.88 ± 1.02%) in the EPM, as well as more entries into the central area in the OFT (SNI-CIT: 23.67 ± 7.37; SNI-Veh: 7.11 ± 1.85; p < 0.05), without affecting the motor ability (Figure 5(a)). Notably, performances of the CIT-treated SNI rats in both the EPM and OFT were comparable to those of the sham control animals (Figure 5(a)).
Figure 5.
Anxiolytic effects of pharmacological modulation of serotonergic activity in anxious SNI rats. (a) Citalopram treatment alleviated anxiety-like behaviors of SNI animals. Sub-chronic administration of Citalopram (10 mg/kg, i.p., q.d., for 15 days) effectively reversed the reductions of open arm entries (top left) and open arm time (top right) in EPM in SNI rats, as well as the number of entries into the central area in open field (bottom left). No difference in the total distance travelled in open field among the three groups (bottom right). Sham group: n = 8, SNI-Veh group: n = 9, SNI-CIT group: n = 8. (b) Intra mPFC perfusion of serotonin (7.7 μg in 0.65 μL) reversed anxiety-like behaviors of SNI animals. SNI rats received serotonin infusion which significantly increased open arm entries (left) and open arm time (right) in EPM, both values being comparable with those of sham group. Sham group: n = 10, SNI-Veh group: n = 9, SNI-5HT group: n = 10. Data are represented as mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, Sham versus SNI-Veh, SNI-Veh versus SNI-CIT or SNI-5HT, by one-way ANOVA followed by Bonferroni's multiple comparisons test.
CIT: citalopram; Veh: vehicle; 5HT: serotonin; SNI: spare nerve injury.
If the increase in the SERT expression contributed to the observed anxiety-like behaviors by decreasing serotonin concentration in the extracellular space, then a direct application of serotonin to the mPFC could overturn the impact thereby exerting an anxiolytic effect. To test this, we performed bilateral local infusion of 5HT into the mPFC of the SNI rats through dual guide cannulae while measuring anxiety-related behaviors using EPM. Following intra mPFC perfusion of 5HT (7.7 μg in 0.65 μL), the anxiety-like behaviors of the SNI rats were suppressed. Thus, the treated SNI rats showed significant increases in the frequency of open arm entries (SNI-5HT: 33.7 ± 3.37, n = 10; SNI-Veh: 12.2 ± 3.34, n = 9; p < 0.001), as well as the time spent in the open arms of EPM (SNI-5HT: 13.8 ± 1.79; SNI-Veh: 3.3 ± 1.34; p < 0.001), compared with those received vehicle infusion (Figure 5(b)). The behaviors of SNI-5HT group in the EPM were comparable to those of sham group (p > 0.05). These results indicate that the alteration of serotonergic activity in the mPFC plays a critical role in chronic pain-related anxiety-like behaviors. On the other hand, the performance of the sham rats in the EPM was not significantly affected by the same amount of 5-HT applied to the mPFC (Supplemental Figure 1).
The mixed serotonin/noradrenaline reuptake inhibitors, but not SSRIs, have been shown to produce analgesic actions in animal models of neuropathic pain.60–63 To confirm that the anxiolytic effects of citalopram and 5-HT observed in the present study were not due to their analgesic effects which might consequently diminish the associated anxiety behaviors, we measured the mechanical threshold (50% PWT) in the SNI rats after 15 days systemic citalopram (10 mg/kg, i.p., q.d.) or vehicle treatment, as well as after local mPFC infusion of 5-HT (7.7 μg in 0.65 μL) or vehicle. The results showed no significant difference either between citalopram and vehicle (Supplemental Figure 2(a)) or between 5-HT and vehicle groups (Supplemental Figure 2(b)), indicating that neither systemic citalopram nor local mPFC serotonin affected the mechanical allodynia in the SNI rats. Our finding is consistent with previous observations that citalopram was ineffective on mechanical allodynia in neuropathic rats.64 It is also in line with several other studies63,65,66 which indicated that the analgesic efficacies of SSRIs were poor and analgesic actions of the mixed serotonin/noradrenaline reuptake inhibitors were not predominately dependent on their actions on blocking 5-HT reuptake.
Discussion
The present study demonstrates the involvement of the mPFC in the pathogenesis of anxiety associated with chronic pain in an animal model of neuropathic pain. We found that the mPFC underwent plastic changes in chronic pain conditions, as indicated by an elevation of SERT expression, as well as a greater enhancement of its LFP theta-frequency activity when the SNI rats were displaying elevated anxiety-like behaviors in avoiding anxiogenic environment. The anxiety-like behaviors of the SNI rats were effectively suppressed by local application of serotonin to the mPFC. Thus, we propose that the modulation of serotonin transmission and the consequent alteration of neural activities in the local circuitry of the mPFC likely constitute the mechanism by which chronic pain promotes anxiety state in the neuropathic rats. These findings provide important information for the understating of the neural basis of the association between chronic pain and anxiety.
In the clinic, anxiety has been recognized as an important comorbidity in patients with chronic pain. It is reported that a considerable number of chronic pain patients suffer from depression or anxiety disorders as a result of severe pain.1–5 In preclinical studies, anxiety-like behaviors were also observed in various animal models of chronic pain, such as nerve injury-induced neuropathy,7,67–74 virally induced neuropathy,75 drug-induced toxic neuropathy,75,76 diabetic neuropathy,77 and inflammatory pain models.7,8,10,78 However, a number of studies reported disparate findings. Some groups failed to show anxiety-like behavior associated with neuropathic pain.79–81 Others reported different behavioral profiles in the same anxiety tests.7,10 A number of factors might be attributed to the incongruency. The time of measurement seems to be critical for detecting anxiety-like behaviors. Most studies reporting positive results were conducted at least four weeks after induction of chronic pain,7,67,70–74 which is in accordance with the temporal frame for the emergence of anxiety-like manifestations.67 However, the study which failed to observe anxiety-like behaviors79 was conducted within two weeks of pain onset, a time point at which pain chronicity may not yet be established. In addition, the difference in the pain models used in the studies appears as another important factor for inconsistent anxiety behaviors.69 Beyond time or model difference, variations in animal strains and genetic background, or differences in experimental conditions (e.g., surgical procedures, handling, single or repeated measures per animal), or environmental conditions (e.g., noise, temperature, odors, housing condition, diet) might also affect behavioral outcomes to cause some of the variance across studies.
In the present study, we used the SNI rats which have been consistently reported to display anxiety-like behaviors by previous studies.70,71,73,74 The SNI rats displayed persistent mechanical allodynia, a characteristic sign of neuropathic pain, which lasted over eight weeks. The long-lasting pain behavior in this model allowed the development of anxiety associated with chronic pain state. When the SNI rats were tested eight weeks following nerve injury, they exhibited apparent anxiety-like behaviors in both EPM and OF tests, two classical anxiety tests in rodents.82 These behaviors were more likely associated with chronic than acute pain state, as only a trend of anxiety was observed at earlier stage, i.e., the fourth post-operative week. These SNI rats exhibited decreases in the activities in the open arms and the central areas in the EPM and the OF test, respectively. We consider these behavioral changes as anxiety-like behaviors, rather than surgery-induced motor impairment, as indicated by the unchanged total distance that the SNI rats traveled in the OF. Thus, the SNI rats manifest a deterioration of affective function and thus provide an appropriate animal model to study chronic pain-related anxiety-like behaviors.
Although the close correlation between chronic pain and anxiety is well recognized, the neural mechanism underlying their interaction remains unclear. The mPFC is known to play an important role in the modulation of anxiety52–54,83,84 through regulating activities of amygdala as well as other parts of the anxiety network, such as hypothalamus and brainstem.84–87 Studies in both human patients and laboratory animals have demonstrated that, in the chronic pain conditions, the mPFC undergoes both structural and functional changes.27,30,33,34,88 These plastic changes may cause the alternation of mPFC function which consequently promotes pain-related anxiety state.23 In our study, we found that the mPFC activity at theta-frequency (4–12 Hz) displayed a significantly greater increase in the neuropathic rats which concurrently displayed elevated anxiety-like behaviors in avoiding anxiogenic regions in the EPM and OF test chamber. Given that the theta-frequency activity in the mPFC has been found to be positively correlated with anxiety-like behaviors,51,89 this finding strongly suggests the involvement of the mPFC in the pain-related anxiety state. Interestingly, the enhancement of mPFC theta power increase only occurred when the animals were in the closed (safe) arms of EPM, suggesting its engagement particularly in the inhibition of exploratory behavior and avoidance of aversive environment. This observation is consistent with previous reports from studies of innate anxiety in normal rats.51,87,90
With regard to the cause of the enhancement of mPFC theta-frequency activity, the manifestation could arise from pain-induced plastic changes in the local circuitry within the mPFC and/or in other regions in the anxiety network which provide synaptic inputs to the mPFC. In our study, we found that the expression of SERT in the mPFC was elevated in the anxious SNI rats. We postulate that the observed elevation of SERT and the resultant detraction of serotonin transmission at the mPFC possibly mediated the enhancement of mPFC theta-frequency activity and the promotion of anxiety-like behaviors in the SNI rats. This hypothesis is supported by multiple lines of evidence.
First, abnormality in brain serotonin system has been implicated in anxiety and depression. The SERT is a key regulator of brain serotonergic activity by modulating extracellular concentration of serotonin via reuptake mechanisms. Changes in expression of SERT are associated with mood disorders in humans.57–59 Pharmacological inhibition of SERT via selective serotonin reuptake inhibitors, such as citalopram,91,92 enhances serotonergic transmission and decreases symptoms of depression and certain types of anxiety (for review, see Wallace et al.93). Moreover, serotonergic transmission is known to play an important role in regulating prefrontal functions.45,94 In our study, we found that the expression of SERT in the mPFC was elevated, suggesting its involvement in the pathogenesis of pain-induced anxiety in chronic pain animals.
Second, since the elevation of the prefrontal SERT expression would cause a decrease of serotonin concentration in the mPFC, conversely, a direct application of serotonin to the mPFC could overturn the impact thereby exerting an anxiolytic effect. In our study, local intra mPFC injection of 5-HT in vivo was able to effectively reverse anxiety-like behaviors in the SNI rats (Figure 5(b)), confirming that the reduction of prefrontal 5-HT, resulted from the overexpression of SERT, contributed to the promotion of anxiety-like behaviors of the SNI rats. When the same amount of 5-HT was applied to the mPFC in the sham rats, it did not produce significant effect on animal’s performance in the EPM. We speculate that the difference in the effectiveness of locally applied 5-HT between normal and neuropathic rats was because the amount of the applied 5-HT did not alter the prefrontal synaptic concentration in the normal rats, but it caused an elevation in the SNI rats so as to bring the lowered serotonin concentration back to normal level. This seems to be a reasonable explanation since our data indicated that the same amount of 5-HT was able to reverse the behavior of the SNI rats to the level comparable to that of sham rats. Therefore, the absence of an effect of 5-HT on the sham rats at a dose which was effective on the SNI animals further supports the anxiogenic role of detraction of prefrontal serotonin transmission in chronic pain conditions. However, future experiments with increased doses of 5-HT is needed to support this stipulation.
Third, a recent study reported that local intra-mPFC application of a 5-HT receptor agonist decreased prefrontal theta oscillations and at the same time suppressed anxiety-related avoidance behaviors.45 These findings provide direct evidence that prefrontal serotonin plays an important role in the regulation of animal innate anxiety-like behaviors by affecting prefrontal theta oscillations. This assumption is in line with previous findings that activation of 5-HT receptors suppressed theta oscillation,95,96 while inhibition of serotonergic neurons led to increase of theta wave activity.97,98 The association of serotonin system and modulation of mPFC theta-frequency activity is further supported by a study in which 5-HT1A receptor knockout mice, a genetic model of increased anxiety, had larger mPFC theta power increases than wild-type mice.51
Taken together, all these lines of evidence collectively suggest that the change of SERT activity occurred locally within the mPFC contributed to the pathogenesis of pain-induced anxiety in the anxious SNI rats, presumably via increasing the theta activity originated locally in the mPFC and/or the theta input from hippocampus through a presynaptic mechanism.45 Nevertheless, the change of serotonin neurotransmission in the mPFC may constitute an important part of the mechanism underlying the role of the mPFC in promotion of pain-related anxiety. Other local players might be also engaged, as implicated by a recent study showing that activation of cyclin-dependent kinase 5 enhanced anxiety-like behaviors in an animal model of inflammatory pain.23
In spite of this local mechanism, the involvement of other regions in the anxiety network84 should not be neglected. It is well known that the mPFC receives direct projections from vHPC,99,100 which were thought to regulate anxiety-related behaviors by transmitting theta-frequency activity.51,52,90,101 An increase of vHPC activity thus could have also contributed to drive the enhancement of mPFC theta-frequency activity, a mechanism employed in normal animals for promoting anxiety-like behaviors to avoid exposure to anxiogenic environment. However, our data do not seem to support the hippocampal contribution to the enhancement of mPFC theta-band activity in the SNI rats. We found that the power of theta-band activity in the vHPC was not significantly altered in the SNI rats, and furthermore, the theta power correlation between the two areas was not significantly changed. Although the theta-frequency coherence between the vHPC and the mPFC was increased in the SNI rats, this was most likely due to an increase in hippocampal input to the mPFC which took place at the mPFC through a disinhibition of synaptic transmission at presynaptic site as a consequence of a reduction in mPFC serotonin. This assumption is in line with the finding by a recent study45 that local mPFC infusion of serotonin inhibited hippocampal inputs to the mPFC via activation of presynaptic 5-HT1B receptors, which consequently suppressed prefrontal theta oscillations and elicited anxiolytic effects. Therefore, the vHPC was unlikely involved in the elevation of mPFC theta activity in pain conditions. Besides, other brain areas in the neural network, such as amygdala, could also provide regulatory inputs to the mPFC,84,102 the involvement of which in regulating the mPFC activity was not examined in the present study.
Nevertheless, our finding that the plastic change of the mPFC mediates pain-induced anxiety is in accordance with a recent study showing that anxiety-like behaviors were promoted by inflammatory pain-induced deactivation of excitatory neurons in the prelimbic cortex.23 Thus, the change of serotonin neurotransmission identified in our study, along with other local players, such as cyclin-dependent kinase 5,23 may constitute an important part of the mechanism underlying the role of the mPFC in promotion of pain-related anxiety.
The mechanism for the association between chronic pain and anxiety states could be multifaceted. Besides the role of the prefrontal cortex, other brain areas might also contribute to the pathogenesis of the pain-induced anxiety. Changes in the opioidergic function in the amygdala were found to be associated with pain-induced anxiety.7 Furthermore, a recent study indicated that ACC was involved in the interactions between chronic pain and anxiety states by two forms of LTP.44 It is, therefore, reasonable to postulate that the comorbidity of anxiety and chronic pain is the consequence of the plastic changes occurred in the affective network in chronic pain conditions. In support of this, studies in both human patients and laboratory animals have demonstrated that chronic pain causes molecular, structural, and functional changes in the network, including the mPFC,27,103,104 amygdala,105,106 anterior cingulate cortex,107 and hippocampus.108 Nonetheless, the mPFC is clearly a key component of such a neuronal network involved in linking chronic pain and anxiety, while other brain regions have also been implicated.
Anxiety is usually an adaptive state of increased apprehension about possible future danger. However, inappropriate expression of anxiety is maladaptive and, in humans, can lead to anxiety disorders. It appears that, in the chronic pain conditions, the activity in the neural circuits that support normal anxiety is modulated, which may have contributed to the potentiation of anxiety state in neuropathic animals.
In conclusion, our study identifies the role of prefrontal cortex in the association between anxiety and chronic pain. Our findings not only provides an important information for the understating of neural basis of the pathogenesis of pain-induced anxiety but also offer an insight into the development of more effective, non-drug based therapeutical interventions, such as music and meditation, through modulating prefrontal activities109–111 to suppress or reverse chronic pain-induced neuroplasticity.
Supplemental Material
Supplemental material, Supplementary Figures for Plastic change of prefrontal cortex mediates anxiety-like behaviors associated with chronic pain in neuropathic rats by Kangning Sang, Chaofei Bao, Yushi Xin, Shunan Hu, Xian Gao, Yongsheng Wang, Mark Bodner, Yong-Di Zhou and Xiao-Wei Dong in Molecular Pain
Author Contributions
X-WD, KS, and CB designed the study; KS, CB, and YX performed the surgery and the in vivo recording experiments; KS, CB, SH, and YX performed the behavioral experiments; YX, XG, and YW conducted the Western blotting; KS, CB, X-WD, Y-DZ, and MB analyzed the data; X-WD, KS, and CB wrote the paper.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a research fund from the M.I.N.D. Research Institute, California to X-W Dong. This work was also supported by the Key Specialist Projects of Shanghai Municipal Commission of Health and Family Planning (ZK2015B01) and the Programs Foundation of Shanghai Municipal Commission of Health and Family Planning (201540114).
Supplemental Material
Supplementary material is available for this article online.
References
- 1.McCracken LM, Zayfert C, Gross RT. The Pain Anxiety Symptoms Scale: development and validation of a scale to measure fear of pain. Pain 1992; 50: 67–73. [DOI] [PubMed] [Google Scholar]
- 2.McCracken LM, Gross RT. Does anxiety affect coping with chronic pain? Clin J Pain 1993; 9: 253–259. [DOI] [PubMed] [Google Scholar]
- 3.Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain 1997; 13: 116–137. [DOI] [PubMed] [Google Scholar]
- 4.McCracken LM, Spertus IL, Janeck AS, Sinclair D, Wetzel FT. Behavioral dimensions of adjustment in persons with chronic pain: pain-related anxiety and acceptance. Pain 1999; 80: 283–289. [DOI] [PubMed] [Google Scholar]
- 5.Bushnell MC, Čeko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013; 14: 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroenke K, Outcalt S, Krebs E, Bair MJ, Wu J, Chumbler N, Yu Z. Association between anxiety, health-related quality of life and functional impairment in primary care patients with chronic pain. Gen Hosp Psychiatry 2013; 35: 359–365. [DOI] [PubMed] [Google Scholar]
- 7.Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Nanjo K, Matsuzawa K, Yamazaki M, Suzuki T. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology 2006; 31: 739–750. [DOI] [PubMed] [Google Scholar]
- 8.Ji G, Fu Y, Ruppert KA, Neugebauer V. Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Mol Pain 2007; 3: 13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace VC, Segerdahl AR, Blackbeard J, Pheby T, Rice AS. Anxiety-like behaviour is attenuated by gabapentin, morphine and diazepam in a rodent model of HIV anti-retroviral-associated neuropathic pain. Neurosci Lett 2008; 448: 153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parent A, Beaudet N, Beaudry H, Bergeron J, Bérubé P, Drolet G, Sarret P, Gendron L. Increased anxiety-like behaviors in rats experiencing chronic inflammatory pain. Behav Brain Res 2012; 229: 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005; 9: 463–484. [DOI] [PubMed] [Google Scholar]
- 12.Tracey I. Nociceptive processing in the human brain. Curr Opin Neurobiol 2005; 15: 478–487. [DOI] [PubMed] [Google Scholar]
- 13.Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res 2011; 223: 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson JR, Jr., Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc Natl Acad Sci U S A 2001; 98: 688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci 2004; 7: 184–188. [DOI] [PubMed] [Google Scholar]
- 16.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 2005; 62: 273–281. [DOI] [PubMed] [Google Scholar]
- 17.Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 2012; 36: 2130–2142. [DOI] [PubMed] [Google Scholar]
- 18.Ball TM, Ramsawh HJ, Campbell-Sills L, Paulus MP, Stein MB. Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorders. Psychol Med 2013; 43: 1475–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isserles M, Shalev AY, Roth Y, Peri T, Kutz I, Zlotnick E, Zangen A. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder – a pilot study. Brain Stimul 2013; 6: 377–383. [DOI] [PubMed] [Google Scholar]
- 20.Tendler A, Roth Y, Barnea-Ygael N, Zangen A. How to use the H1 deep transcranial magnetic stimulation coil for conditions other than depression. J Vis Exp 2017; 119: 55100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bi LL, Wang J, Luo ZY, Chen SP, Geng F, Chen YH, Li SJ, Yuan CH, Lin S, Gao TM. Enhanced excitability in the infralimbic cortex produces anxiety-like behaviors. Neuropharmacology 2013; 72: 148–156. [DOI] [PubMed] [Google Scholar]
- 22.Vialou V, Bagot RC, Cahill ME, Ferguson D, Robison AJ, Dietz DM, Fallon B, Mazei-Robison M, Ku SM, Harrigan E, Winstanley CA, Joshi T, Feng J, Berton O, Nestler EJ. Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of DeltaFosB. J Neurosci 2014; 34: 3878–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang GQ, Cen C, Li C, Cao S, Wang N, Zhou Z, Liu XM, Xu Y, Tian NX, Zhang Y, Wang J, Wang LP, Wang Y. Deactivation of excitatory neurons in the prelimbic cortex via Cdk5 promotes pain sensation and anxiety. Nat Commun 2015; 6: 7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 2004; 24: 10410–10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stern J, Jeanmonod D, Sarnthein J. Persistent EEG overactivation in the cortical pain matrix of neurogenic pain patients. NeuroImage 2006; 31: 721–731. [DOI] [PubMed] [Google Scholar]
- 26.Zhuo M. Cortical excitation and chronic pain. Trends Neurosci 2008; 31: 199–207. [DOI] [PubMed] [Google Scholar]
- 27.Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci U S A 2009; 106: 2423–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci 2009; 29: 13746–13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seifert F, Maihofner C. Central mechanisms of experimental and chronic neuropathic pain: findings from functional imaging studies. Cell Mol Life Sci 2009; 66: 375–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji G, Neugebauer V. Pain-related deactivation of medial prefrontal cortical neurons involves mGluR1 and GABA(A) receptors. J Neurophysiol 2011; 106: 2642–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruscheweyh R, Deppe M, Lohmann H, Stehling C, Floel A, Ringelstein EB, Knecht S. Pain is associated with regional grey matter reduction in the general population. Pain 2011; 152: 904–911. [DOI] [PubMed] [Google Scholar]
- 32.Obermann M, Rodriguez-Raecke R, Naegel S, Holle D, Mueller D, Yoon MS, Theysohn N, Blex S, Diener HC, Katsarava Z. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. NeuroImage 2013; 74: 352–358. [DOI] [PubMed] [Google Scholar]
- 33.Cauda F, Palermo S, Costa T, Torta R, Duca S, Vercelli U, Geminiani G, Torta DM. Gray matter alterations in chronic pain: a network-oriented meta-analytic approach. NeuroImage Clin 2014; 4: 676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu XB, Liang B, Gao YJ. The increase of intrinsic excitability of layer V pyramidal cells in the prelimbic medial prefrontal cortex of adult mice after peripheral inflammation. Neurosci Lett 2016; 611: 40–45. [DOI] [PubMed] [Google Scholar]
- 35.Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 2012; 15: 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cardoso-Cruz H, Lima D, Galhardo V. Impaired spatial memory performance in a rat model of neuropathic pain is associated with reduced hippocampus-prefrontal cortex connectivity. J Neurosci 2013; 33: 2465–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hay P, Sachdev P, Cumming S, Smith JS, Lee T, Kitchener P, Matheson J. Treatment of obsessive-compulsive disorder by psychosurgery. Acta Psychiatr Scand 1993; 87: 197–207. [DOI] [PubMed] [Google Scholar]
- 38.Osuch EA, Ketter TA, Kimbrell TA, George MS, Benson BE, Willis MW, Herscovitch P, Post RM. Regional cerebral metabolism associated with anxiety symptoms in affective disorder patients. Biol Psychiatry 2000; 48: 1020–1023. [DOI] [PubMed] [Google Scholar]
- 39.Gross C, Hen R. The developmental origins of anxiety. Nat Rev Neurosci 2004; 5: 545–552. [DOI] [PubMed] [Google Scholar]
- 40.Kim SS, Wang H, Li XY, Chen T, Mercaldo V, Descalzi G, Wu LJ, Zhuo M. Neurabin in the anterior cingulate cortex regulates anxiety-like behavior in adult mice. Mol Brain 2011; 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhuo M. Neural mechanisms underlying anxiety-chronic pain interactions. Trends Neurosci 2016; 39: 136–145. [DOI] [PubMed] [Google Scholar]
- 42.Vartiainen N, Kallio-Laine K, Hlushchuk Y, Kirveskari E, Seppanen M, Autti H, Jousmaki V, Forss N, Kalso E, Hari R. Changes in brain function and morphology in patients with recurring herpes simplex virus infections and chronic pain. Pain 2009; 144: 200–208. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Cao DY, Remeniuk B, Krimmel S, Seminowicz DA, Zhang M. Altered brain structure and function associated with sensory and affective components of classic trigeminal neuralgia. Pain 2017; 158: 1561–1570. [DOI] [PubMed] [Google Scholar]
- 44.Koga K, Descalzi G, Chen T, Ko HG, Lu J, Li S, Son J, Kim T, Kwak C, Huganir RL, Zhao MG, Kaang BK, Collingridge GL, Zhuo M. Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron 2015; 85: 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kjaerby C, Athilingam J, Robinson SE, Iafrati J, Sohal VS. Serotonin 1B receptors regulate prefrontal function by gating callosal and hippocampal inputs. Cell Rep 2016; 17: 2882–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong XW, Jia Y, Lu SX, Zhou X, Cohen-Williams M, Hodgson R, Li H, Priestley T. The antipsychotic drug, fluphenazine, effectively reverses mechanical allodynia in rat models of neuropathic pain. Psychopharmacology 2007; 195: 559–568. [DOI] [PubMed] [Google Scholar]
- 47.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 2000; 87: 149–158. [DOI] [PubMed] [Google Scholar]
- 48.Dixon W. The up-and-down method for small samples. J Am Stat Assoc 1965; 60: 967–978. [Google Scholar]
- 49.Gonçalves L, Silva R, Pinto-Ribeiro F, Pêgo JM, Bessa JM, Pertovaara A, Sousa N, Almeida A. Neuropathic pain is associated with depressive behaviour and induces neuroplasticity in the amygdala of the rat. Exp Neurol 2008; 213: 48–56. [DOI] [PubMed] [Google Scholar]
- 50.Paxinos G, Watson C, Carrive P, Kirkcaldie M, Ashwell K. Chemoarchitectonic atlas of the rat brain. UK: Academic Press, 2009. [Google Scholar]
- 51.Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron 2010; 65: 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adhikari A, Topiwala MA, Gordon JA. Single units in the medial prefrontal cortex with anxiety-related firing patterns are preferentially influenced by ventral hippocampal activity. Neuron 2011; 71: 898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adhikari A. Distributed circuits underlying anxiety. Front Behav Neurosci 2014; 8: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schoenfeld TJ, Kloth AD, Hsueh B, Runkle MB, Kane GA, Wang SS-H, Gould E. Gap junctions in the ventral hippocampal-medial prefrontal pathway are involved in anxiety regulation. J Neurosci 2014; 34: 15679–15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS, Ordek G, Zhou P, Ong SK, Wright MA, Zweifel L, Paninski L, Hen R, Kheirbek MA. Anxiety cells in a hippocampal-hypothalamic circuit. Neuron 2018; 97: 670–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gordon JA, Lacefield CO, Kentros CG, Hen R. State-dependent alterations in hippocampal oscillations in serotonin 1A receptor-deficient mice. J Neurosci 2005; 25: 6509–6519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Furlong RA, Ho L, Walsh C, Rubinsztein JS, Jain S, Paykel ES, Easton DF, Rubinsztein DC. Analysis and meta-analysis of two serotonin transporter gene polymorphisms in biopolar and unipolar affective disorders. Am J Med Genet 1998; 81: 58–63. [PubMed] [Google Scholar]
- 58.Gutiérrez B, Pintor L, Gastó C, Rosa A, Bertranpetit J, Vieta E, Fañanás L. Variability in the serotonin transporter gene and increased risk for major depression with melancholia. Hum Genet 1998; 103: 319–322. [DOI] [PubMed] [Google Scholar]
- 59.Neumeister A, Konstantinidis A, Stastny J, Schwarz MJ, Vitouch O, Willeit M, Praschak-Rieder N, Zach J, de Zwaan M, Bondy B, Ackenheil M, Kasper S. Association between serotonin transporter gene promoter polymorphism (5HTTLPR) and behavioral responses to tryptophan depletion in healthy women with and without family history of depression. Arch Gen Psychiatry 2002; 59: 613–620. [DOI] [PubMed] [Google Scholar]
- 60.Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain 2002; 100: 1–6. [DOI] [PubMed] [Google Scholar]
- 61.Obata H, Conklin D, Eisenach JC. Spinal noradrenaline transporter inhibition by reboxetine and Xen2174 reduces tactile hypersensitivity after surgery in rats. Pain 2005; 113: 271–276. [DOI] [PubMed] [Google Scholar]
- 62.Jefferies K. Treatment of neuropathic pain. Semin Neurol 2010; 30: 425–432. [DOI] [PubMed] [Google Scholar]
- 63.Nakajima K, Obata H, Iriuchijima N, Saito S. An increase in spinal cord noradrenaline is a major contributor to the antihyperalgesic effect of antidepressants after peripheral nerve injury in the rat. Pain 2012; 153: 990–997. [DOI] [PubMed] [Google Scholar]
- 64.Bomholt SF, Mikkelsen JD, Blackburn-Munro G. Antinociceptive effects of the antidepressants amitriptyline, duloxetine, mirtazapine and citalopram in animal models of acute, persistent and neuropathic pain. Neuropharmacology 2005; 48: 252–263. [DOI] [PubMed] [Google Scholar]
- 65.Sindrup SH, Otto M, Finnerup NB, Jensen TS. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol 2005; 96: 399–409. [DOI] [PubMed] [Google Scholar]
- 66.Hache G, Guiard BP, Nguyen TH, Quesseveur G, Gardier AM, Peters D, Munro G, Coudore F. Antinociceptive activity of the new triple reuptake inhibitor NS18283 in a mouse model of chemotherapy-induced neuropathic pain. Eur J Pain 2015; 19: 322–333. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki T, Amata M, Sakaue G, Nishimura S, Inoue T, Shibata M, Mashimo T. Experimental neuropathy in mice is associated with delayed behavioral changes related to anxiety and depression. Anesth Analg 2007; 104: 1570–1577. [DOI] [PubMed] [Google Scholar]
- 68.Matsuzawa-Yanagida K, Narita M, Nakajima M, Kuzumaki N, Niikura K, Nozaki H, Takagi T, Tamai E, Hareyama N, Terada M, Yamazaki M, Suzuki T. Usefulness of antidepressants for improving the neuropathic pain-like state and pain-induced anxiety through actions at different brain sites. Neuropsychopharmacology 2008; 33: 1952–1965. [DOI] [PubMed] [Google Scholar]
- 69.Roeska K, Doods H, Arndt K, Treede RD, Ceci A. Anxiety-like behaviour in rats with mononeuropathy is reduced by the analgesic drugs morphine and gabapentin. Pain 2008; 139: 349–357. [DOI] [PubMed] [Google Scholar]
- 70.Leite-Almeida H, Almeida-Torres L, Mesquita AR, Pertovaara A, Sousa N, Cerqueira JJ, Almeida A. The impact of age on emotional and cognitive behaviours triggered by experimental neuropathy in rats. Pain 2009; 144: 57–65. [DOI] [PubMed] [Google Scholar]
- 71.Seminowicz DA, Laferriere AL, Millecamps M, Yu JS, Coderre TJ, Bushnell MC. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. NeuroImage 2009; 47: 1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Radulovic J, Martina M, Miller RJ, Apkarian AV. Abnormalities in hippocampal functioning with persistent pain. J Neurosci 2012; 32: 5747–5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leite-Almeida H, Cerqueira JJ, Wei H, Ribeiro-Costa N, Anjos-Martins H, Sousa N, Pertovaara A, Almeida A. Differential effects of left/right neuropathy on rats' anxiety and cognitive behavior. Pain 2012; 153: 2218–2225. [DOI] [PubMed] [Google Scholar]
- 74.Low LA, Millecamps M, Seminowicz DA, Naso L, Thompson SJ, Stone LS, Bushnell MC. Nerve injury causes long-term attentional deficits in rats. Neurosci Lett 2012; 529: 103–107. [DOI] [PubMed] [Google Scholar]
- 75.Wallace VC, Blackbeard J, Segerdahl AR, Hasnie F, Pheby T, McMahon SB, Rice AS. Characterization of rodent models of HIV-gp120 and anti-retroviral-associated neuropathic pain. Brain 2007; 130: 2688–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wallace VC, Segerdahl AR, Blackbeard J, Pheby T, Rice AS. Anxiety-like behaviour is attenuated by gabapentin, morphine and diazepam in a rodent model of HIV anti-retroviral-associated neuropathic pain. Neurosci Lett 2008; 448: 153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramanathan M, Jaiswal AK, Bhattacharya SK. Differential effects of diazepam on anxiety in streptozotocin induced diabetic and non-diabetic rats. Psychopharmacology 1998; 135: 361–367. [DOI] [PubMed] [Google Scholar]
- 78.Jiménez-Velázquez G, López-Muñoz FJ, Fernández-Guasti A. Parallel anxiolytic-like and antinociceptive actions of diazepam in the anterior basolateral amygdala and dorsal periaqueductal gray. Brain Res 2010; 1349: 11–20. [DOI] [PubMed] [Google Scholar]
- 79.Kontinen VK, Kauppila T, Paananen S, Pertovaara A, Kalso E. Behavioural measures of depression and anxiety in rats with spinal nerve ligation-induced neuropathy. Pain 1999; 80: 341–346. [DOI] [PubMed] [Google Scholar]
- 80.Hasnie FS, Wallace VC, Hefner K, Holmes A, Rice AS. Mechanical and cold hypersensitivity in nerve-injured C57BL/6J mice is not associated with fear-avoidance- and depression-related behaviour. Br J Anaesth 2007; 98: 816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Urban R, Scherrer G, Goulding EH, Tecott LH, Basbaum AI. Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain 2011; 152: 990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mesquita A, Tavares H, Silva R, Sousa N. Febrile convulsions in developing rats induce a hyperanxious phenotype later in life. Epilepsy Behav 2006; 9: 401–406. [DOI] [PubMed] [Google Scholar]
- 83.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol 2012; 63: 129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nat Neurosci 2015; 18: 1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 2004; 51: 32–58. [DOI] [PubMed] [Google Scholar]
- 86.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol 2006; 73: 61–71. [DOI] [PubMed] [Google Scholar]
- 87.Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci 2014; 17: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luongo L, de Novellis V, Gatta L, Palazzo E, Vita D, Guida F, Giordano C, Siniscalco D, Marabese I, De Chiaro M, Boccella S, Rossi F, Maione S. Role of metabotropic glutamate receptor 1 in the basolateral amygdala-driven prefrontal cortical deactivation in inflammatory pain in the rat. Neuropharmacology 2013; 66: 317–329. [DOI] [PubMed] [Google Scholar]
- 89.Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, Abdi A, Baufreton J, Bienvenu TC, Herry C. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature 2014; 505: 92–96. [DOI] [PubMed] [Google Scholar]
- 90.Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, Spellman TJ, Gordon JA. Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron 2016; 89: 857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sánchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Psychopharmacology 1997; 129: 197–205. [DOI] [PubMed] [Google Scholar]
- 92.Zohar J, Westenberg H. Anxiety disorders: a review of tricyclic antidepressants and selective serotonin reuptake inhibitors. Acta Psychiatr Scand 2000; 101: 39–49. [DOI] [PubMed] [Google Scholar]
- 93.Mandrioli R, Mercolini L, A Saracino M, A Raggi M. Selective serotonin reuptake inhibitors (SSRIs): therapeutic drug monitoring and pharmacological interactions. Curr Med Chem 2012; 19: 1846–1863. [DOI] [PubMed] [Google Scholar]
- 94.Puig MV, Gulledge AT. Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol Neurobiol 2011; 44: 449–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hirose A, Tsuji R, Shimizu H, Tatsuno T, Tanaka H, Kumasaka Y, Nakamura M. Inhibition by 8-hydroxy-2-(di-n-propylamino) tetralin and SM-3997, a novel anxiolytic drug, of the hippocampal rhythmical slow activity mediated by 5-hydroxytryptamine1A receptors. Naunyn Schmiedeberg's Arch Pharmacol 1990; 341: 8–13. [DOI] [PubMed] [Google Scholar]
- 96.Hajos M, Hoffmann WE, Weaver RJ. Regulation of septo-hippocampal activity by 5-hydroxytryptamine(2C) receptors. J Pharmacol Exp Ther 2003; 306: 605–615. [DOI] [PubMed] [Google Scholar]
- 97.Staubli U, Xu FB. Effects of 5-HT3 receptor antagonism on hippocampal theta rhythm, memory, and LTP induction in the freely moving rat. J Neurosci 1995; 15: 2445–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nitz DA, McNaughton BL. . Hippocampal EEG and unit activity responses to modulation of serotonergic median raphe neurons in the freely behaving rat. Learn Mem 1999; 6: 153–167. [PMC free article] [PubMed] [Google Scholar]
- 99.Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol 1991; 313: 574–586. [DOI] [PubMed] [Google Scholar]
- 100.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 2007; 212: 149–179. [DOI] [PubMed] [Google Scholar]
- 101.Ciocchi S, Passecker J, Malagon-Vina H, Mikus N, Klausberger T. Brain computation. Selective information routing by ventral hippocampal CA1 projection neurons. Science 2015; 348: 560–563. [DOI] [PubMed] [Google Scholar]
- 102.Groenewegen HJ, Wright CI, Uylings HB. The anatomical relationships of the prefrontal cortex with limbic structures and the basal ganglia. J Psychopharmacol 1997; 11: 99–106. [DOI] [PubMed] [Google Scholar]
- 103.Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci 2006; 26: 12165–12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci 2008; 28: 1398–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Han JS, Neugebauer V. mGluR1 and mGluR5 antagonists in the amygdala inhibit different components of audible and ultrasonic vocalizations in a model of arthritic pain. Pain 2005; 113: 211–222. [DOI] [PubMed] [Google Scholar]
- 106.Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci 2010; 30: 5451–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, Shim J, Lee K, Collingridge GL, Kaang BK, Zhuo M. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science 2010; 330: 1400–1404. [DOI] [PubMed] [Google Scholar]
- 108.Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Radulovic J, Martina M, Miller RJ, Apkarian AV. Abnormalities in hippocampal functioning with persistent pain. J Neurosci 2012; 32: 5747–5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yamamoto S, Kitamura Y, Yamada N, Nakashima Y, Kuroda S. Medial profrontal cortex and anterior cingulate cortex in the generation of alpha activity induced by transcendental meditation: a magnetoencephalographic study. Acta Med Okayama 2006; 60: 51–58. [DOI] [PubMed] [Google Scholar]
- 110.Janata P. The neural architecture of music-evoked autobiographical memories. Cereb Cortex 2009; 19: 2579–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Carlson E, Saarikallio S, Toiviainen P, Bogert B, Kliuchko M, Brattico E. Maladaptive and adaptive emotion regulation through music: a behavioral and neuroimaging study of males and females. Front Hum Neurosci 2015; 9: 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary Figures for Plastic change of prefrontal cortex mediates anxiety-like behaviors associated with chronic pain in neuropathic rats by Kangning Sang, Chaofei Bao, Yushi Xin, Shunan Hu, Xian Gao, Yongsheng Wang, Mark Bodner, Yong-Di Zhou and Xiao-Wei Dong in Molecular Pain