Abstract
High-frequency irreversible electroporation is a nonthermal method of tissue ablation that uses bursts of 0.5- to 2.0-microsecond bipolar electric pulses to permeabilize cell membranes and induce cell death. High-frequency irreversible electroporation has potential advantages for use in neurosurgery, including the ability to deliver pulses without inducing muscle contraction, inherent selectivity against malignant cells, and the capability of simultaneously opening the blood–brain barrier surrounding regions of ablation. Our objective was to determine whether high-frequency irreversible electroporation pulses capable of tumor ablation could be delivered to dogs with intracranial meningiomas. Three dogs with intracranial meningiomas were treated. Patient-specific treatment plans were generated using magnetic resonance imaging-based tissue segmentation, volumetric meshing, and finite element modeling. Following tumor biopsy, high-frequency irreversible electroporation pulses were stereotactically delivered in situ followed by tumor resection and morphologic and volumetric assessments of ablations. Clinical evaluations of treatment included pre- and posttreatment clinical, laboratory, and magnetic resonance imaging examinations and adverse event monitoring for 2 weeks posttreatment. High-frequency irreversible electroporation pulses were administered successfully in all patients. No adverse events directly attributable to high-frequency irreversible electroporation were observed. Individual ablations resulted in volumes of tumor necrosis ranging from 0.25 to 1.29 cm3. In one dog, nonuniform ablations were observed, with viable tumor cells remaining around foci of intratumoral mineralization. In conclusion, high-frequency irreversible electroporation pulses can be delivered to brain tumors, including areas adjacent to critical vasculature, and are capable of producing clinically relevant volumes of tumor ablation. Mineralization may complicate achievement of complete tumor ablation.
Keywords: animal models, brain tumor, dog, neuro-oncology, pulsed electric fields
Introduction
Brain tumors are commonly treated with surgery, radiation therapy, and/or chemotherapeutic regimens.1 Surgical resection, both as a singular modality and in combination with other treatments, is a fundamental component of the management of numerous brain tumors. Operative techniques and attitudes related to the role of and indications for surgical resection in the treatment of various benign and malignant brain tumors have evolved significantly over the past several decades. However, subtotal tumor resection continues to be problematic and associated with increased risk of patient mortality and morbidity. In addition, with some progressive benign tumors, uncertainty exists with respect to the most appropriate rescue therapy to use in the face of surgical failures.2,3 Thus, there remains an unmet clinical need for new, more effective, and less invasive neurosurgical methods.
Given the current limitations of conventional neurosurgery, minimally invasive ablative techniques such as high-intensity focused ultrasound, laser interstitial ablation therapy, microwave ablation, or radiofrequency ablation that have been investigated for use in brain tumor treatment.4-7 Our laboratory focuses on the development of innovative biophysical approaches to cancer treatment that revolve around the use of pulsed electrical fields. Recently, an allied electric field-based technology, termed tumor-treating fields, showed promise in extending survival in people with recurrent and newly diagnosed glioblastoma when used in combination with temozolomide chemotherapy.8,9
Irreversible electroporation (IRE) is a novel ablation method invented by Davalos and colleagues.10 The technique requires placement of minimally invasive electrodes (0.5-2.0 cm apart) into the tumor to deliver monopolar electric pulses (50-100 μs) with an amplitude ranging from 500 to 3000 V. The applied electric field increases the transmembrane potential of cells initially creating nanoscale pores11 that evolve with pulse duration in terms of both pore density12 and pore radius,13 eventually leading to cell death. The nonthermal mechanism of IRE allows for sparing of essential tissue components such as the extracellular matrix and vasculature.14-16 Using spontaneous canine brain tumor and rodent models, it has been demonstrated that IRE can safely ablate malignant gliomas and generate a region of blood–brain barrier (BBB) permeability surrounding the zone of ablation. This peritumoral zone can be exploited to deliver macromolecular drugs to target the infiltrative microscopic tumor burden.15-18
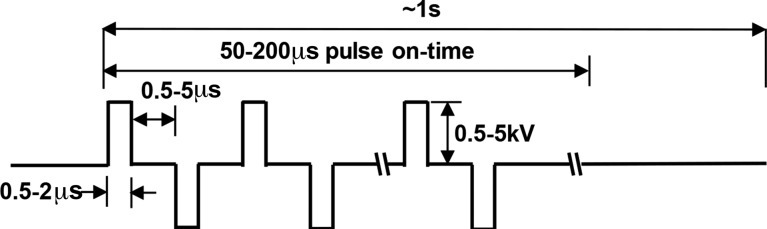
Clinically, IRE pulse delivery requires anesthetic protocols that include neuroparalytic agents to avoid pulse-induced muscle contractions.17-19 This can preclude usage of IRE in “awake” neurosurgical interventions or in severely debilitated patients. Tissue modeling studies indicate that the electric field distribution during IRE may be distorted by heterogeneities in tissue electrical properties such as dense connective tissue or tissue-specific anisotropy.20,21 To overcome these limitations, our group invented a new IRE technology, termed high-frequency IRE (HFIRE), which substitutes the relatively long (50-100 μs) IRE pulses with bursts of short (∼0.5-2 μs) bipolar pulses (Figure 1).21 We have previously shown that HFIRE pulses enable cell-specific ablation in heterogeneous in vitro models of brain cancer, ablate rodent brain tissue in vivo without causing muscular contractions, and induce BBB opening in a penumbra of tissue around the ablation zone.21-24
Figure 1.
Typical high-frequency irreversible electroporation (HFIRE) waveform cycles consist of a series of 0.5 to 2 μs pulses of alternating polarity separated by 0.5 to 5 μs of no energy delivery. Cycles are repeatedly delivered (10-100 cycles) to form bursts which are delivered at a ∼1 Hz frequency. Amplitude of voltage delivery ranges from 0.25 to 5.0 kV.
We hypothesized that HFIRE pulse parameters derived from patient-specific computational therapeutic plans could be delivered to dogs with spontaneous brain tumors safely and without inducing muscular contractions and that these pulses would result in tumor ablation. We evaluated these objectives in a cohort of 3 dogs with intracranial meningiomas using a treat and resect paradigm.
Materials and Methods
This was a prospective, single-center, pilot study designed according to Idea, Development, Exploration, Assessment, Long-Term Study (IDEAL) stages 1/2a of surgical innovation to evaluate the feasibility of ablating brain tumors with HFIRE.25 Client-owned dogs with naturally occurring intracranial meningiomas were recruited through the treatment center’s referral network and by registry of the trial on a publicly accessible, national veterinary clinical trials database.26 To be eligible for the trial, dogs had to have clinical signs of brain disease, a diagnostic brain magnetic resonance imaging (MRI) scan demonstrating a solitary mass lesion >1 cm in diameter with imaging characteristics compatible with a meningioma, Karnofsky Performance Score (KPS) ≥60, and be free of significant concurrent cardiopulmonary, renal, and hepatic disease or other malignancies. When applicable, dogs with structural epilepsy had to have seizures that were controlled on anticonvulsant medications.16 Exclusion criteria included any type of prior brain radiotherapy or treatment with a cytotoxic chemotherapy drug within 6 weeks of trial enrollment. Cases were enrolled passively following an investigator panel review of clinical data from each candidate, and a single neurosurgeon performed all treatments. Dog owners provided written-informed consent to enroll their dogs into the study. All study procedures complied with the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (protocol #16-017).
Pulses were delivered through a custom-built HFIRE-waveform generator (VoltMed Inc, Blacksburg, Virginia) coupled with 2 or more blunt-tip electrodes (Ø = 1.2 mm; 200-104 302; Angiodynamics, Inc, Queensbury, New York). The electrodes have an overall length of 15 cm and were connected to the pulse generator via 1.8 m insulated cables. The active exposure length of the electrode tips can be adjusted in 5 mm increments over a range of 40 mm.
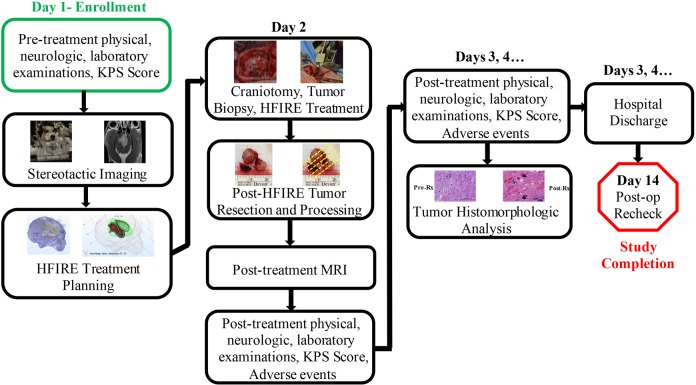
The procedural workflow is summarized in Figure 2. On the day of admission (day 1), dogs underwent pretreatment KPS scoring, and complete physical, neurological, and laboratory examinations. They were anesthetized using a complete intravenous protocol consisting of premedication with methadone and midazolam, induction with propofol, and maintenance with propofol and remifentanil constant rate infusions. Anesthetized dogs were instrumented in an MRI-compatible, small animal stereotactic headframe (Dynatech; Dynatech Machining, Union City, California). Magnetic resonance imaging images of the brain were obtained for therapeutic planning (see Supplemental Methods—MRI Protocol) as reported previously.16,27 Parasagittal meningiomas were classified using the Sindou schema after acquisition of MRI venograms.28 After stereotactic images were obtained, dogs were recovered from anesthesia.
Figure 2.
Workflow for high-frequency irreversible electroporation (HFIRE) for canine meningioma treatment.
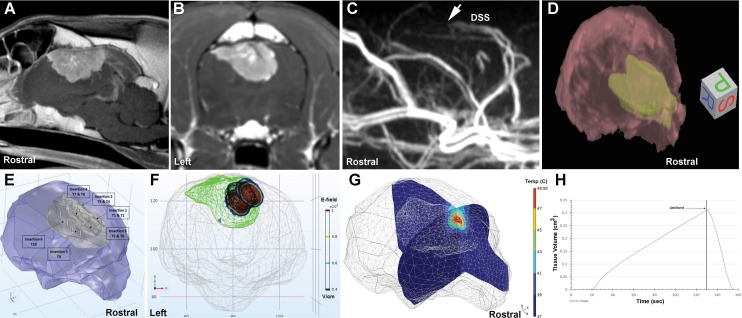
Patient-specific HFIRE treatment plans were developed using MRI-based tissue segmentation, volumetric meshing, and finite element modeling (Figure 3) according to previously described methods (see Supplemental Methods—HFIRE Treatment Planning).16,29 The therapeutic planning procedure was customizable and generated 3-dimensional patient- and tumor-specific outputs (Figures 3E-H). These outputs depicted the expected electric field distribution and Joule heating, given the electrode approach and configuration for each electrode pair being used in the treatment.
Figure 3.
High-frequency irreversible electroporation (HFIRE) therapeutic planning for Sindou Type VI parasagittal meningioma, dog 2. Pretreatment sagittal (A) and transverse (B) postcontrast magnetic resonance imaging (MRI) appearance of the tumor. C, MRI venogram demonstrating abrupt filling defect (arrow) in the dorsal sagittal sinus due to tumor infiltration. D, Three-dimensional MRI rendering of brain (red) and tumor (green) that is imported into finite element analysis software for segmentation and treatment planning. E, Segmented brain (purple) and tumor (gray) demonstrating trajectories of 6 separate electrode insertions for the 10 individual ablative treatments (T) planned for dog 2. Treatments T1, T3, T5, and T7 were performed first and then the respective electrode pairs withdrawn 5 to 6 mm along the same trajectories to execute ablations T2, T4, T6, and T8. F, Frontal view of electric field distribution for treatments T1, T3, T5, and T7. G, Representative joule heating for treatment T1, with volumetric tissue temperature exposure as a function of treatment time (H).
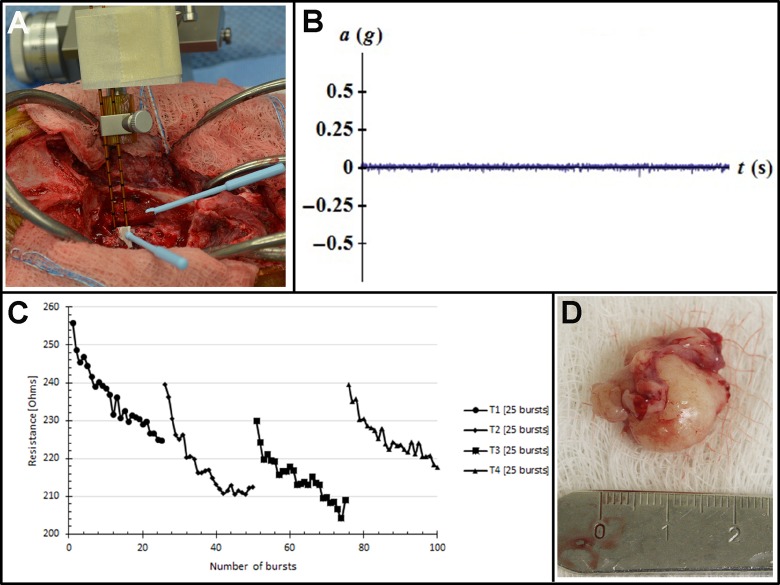
On day 2, dogs were placed under general anesthesia, instrumented in the stereotactic headframe, and aseptically prepared for surgery. To monitor for muscle contractions, a 3-axis accelerometer breakout board (ADXL335; Adafruit Industries, New York, New York) with a sensing range of ±3 g was sutured to the skin of each dog in the dorsal cervical region at the level of the second cervical vertebra (Figure 4). In the operating theater, each dog underwent a craniectomy approach of sufficient size to expose the tumor for HFIRE treatment and subsequent tumor resection. Following completion of the tumor exposure, biopsies of the tumor were obtained using 16-gauge Sedan side cutting needles. The trajectories chosen for biopsy were identical to those used for electrode placement. The HFIRE treatments were then delivered stereotactically according to pretreatment plans by mounting and advancing the electrodes to the target region using micromanipulator arms of the headframe (Figure 4). The biopsy/electrode entry locations on the surface of the tumor for each ablation were marked with surgical inks (MarginMarker; Vector Surgical, Waukesha, Wisconsin) to facilitate morphological evaluations of ablations. Pulse delivery was synchronized with the electrocardiogram (Ivy Cardiac Trigger Monitor 3000, Branford, Connecticut), and tissue resistance was monitored during pulse delivery. The electrodes were removed from the brain, and 1 hour was allowed to elapse to allow for evolution of the ablations. Next, each patient underwent tumor resection using standard techniques (Figure 4). When the time necessary to complete all ablations and resect the tumors was accounted for, this approach allowed for tumors to be left in situ for 2 to 4 hours following pulse delivery. Following resection, surgical wounds were closed routinely, and then immediate posttreatment brain MRI examinations performed. All dogs received perioperative antibiotics (cefazolin, 22 mg/kg, (intravenous)IV, q 8 hours) and buprenorphine (0.02 mg/kg, IV or SC, q 6-8 hours) for at least 24 hours following recovery from the HFIRE treatment. Following anesthetic recovery on day 2, and on each subsequent day of hospitalization until discharge, each dog underwent posttreatment KPS scoring; complete physical, neurological, and laboratory examinations; and adverse event (AE) monitoring. The study ended after each dog completed a 14-day posttreatment recheck clinical examination, KPS score, and AE assessment.
Figure 4.
Intraoperative high-frequency irreversible electroporation (HFIRE) treatment of meningioma in dog 2. A, Stereotactic electrode placement in situ for treatment T1. B, No displacement of the accelerometer is recorded during treatment T1. C, Plot of tumor resistance changes during treatments T1 to T4, indicating occurrence of electroporation. D, Resected tumor for morphologic ablation analysis.
The primary end point was to evaluate the clinical feasibility of HFIRE for the treatment of brain tumors. For the purpose of this study, clinical feasibility was defined as the successful delivery of HFIRE pulses to the brains of canine patients without inducing severe toxicity within 14 days of the procedure. Severe toxicity was clinically defined by a ≥20-point decline in the KPS from pretreatment values or development of grades 3, 4, or 5 AE, as classified according to the National Cancer Institute’s Cancer Therapy Evaluation Program’s Common Terminology Criteria for Adverse Events, as reported previously.16
Secondary end points included direct neurotoxicity evaluations determined from posttreatment imaging studies and morphologic evaluation of tumor ablations. Following resection, each tumor was immersion fixed en bloc in 10% neutral-buffered formalin for 48 hours. After fixation, the tumor was mounted in matrix slicer (Zivic Instruments, Pittsburgh, Pennsylvania), photographed, and then serially sectioned in the transverse plane at 2-mm intervals. Tumor specimens were oriented such that sectioning occurred parallel to the long axis of biopsy and electrode insertion tracts. Sections were stained routinely with hematoxylin and eosin (H&E; Sakura Finetek, Torrance, California). Light microscopy was used to type and grade tumors according to World Health Organization criteria and to perform qualitative morphometric analyses. Ablation volumes were obtained using commercial image analysis software with a Cavalieri estimator (Stereo Investigator, MBF Biosciences, Williston, Vermont).
Results
Three dogs with intracranial meningiomas (Table 1) enrolled in and completed the study between March and July 2016. The pulse parameters delivered to each patient and resulting ablation volumes can be found in Table 2. No evidence of muscle or nerve excitation or cardiac arrhythmia during any pulse delivery was observed in any dog. Additionally, no displacement was detected by the accelerometers for any of the delivered pulses (Figure 4B) nor were any significant posttreatment laboratory abnormalities attributable to HFIRE detected in any dog.
Table 1.
Canine Intracranial Meningioma Patient Clinicopathological Data.
| Dog Number | Signalment | Body Weight, kg | Clinical Signs | Tumor Location, Type, and WHO Grade | Tumor Volume, cm3 | Pretreatment KPS Score | Prior Therapy |
|---|---|---|---|---|---|---|---|
| 1 | Mixed breed, MC, 6 years | 6.5 | Seizures | Cerebral convexity, Atypical, Grade II | 2.28 | 90 | Surgery; Prednisone; Phenobarbital; Bromide; Levetiracetam |
| 2 | Siberian Husky, MC, 7 years | 31 | Seizures, Circling, Hemiparesis | Parasagittal, Sindou Type VI, Grade I | 8.26 | 70 | Prednisone; Phenobarbital; Levetiracetam |
| 3 | Staffordshire Terrier, F, 11 years | 27 | Seizures, SE | Parasagittal, Sindou Type IV, Grade I | 1.86 | 90 | Prednisone; Phenobarbital |
Abbreviations: F, female; KPS, Karnofsky Performance Score; MC, male, castrated; SE, status epilepticus; WHO, World Health Organization.
Table 2.
HFIRE Treatment Parameters and Quantitative Outputs.
| Dog Number | Treatment Parameters | Quantitative Outputs | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment Number | Voltage, V | Electrode Gap, mm | Pulse Shape, µsa | Number of Bursts | Planned Ablation Volume, cm3 | Maximum Voltage, V | Maximum Current, I | Measured Ablation Volume, cm3 | |
| 1 | 1 | 1000 | 5 | 2-5-2 | 4 × 25 | 0.39 | 1029.82 | 4.92 | 0.43 |
| 2 | 1000 | 5 | 2-5-2 | 4 × 25 | 0.49 | 1028.15 | 4.83 | 0.46 | |
| 2 | 1 | 1000 | 5 | 2-5-2 | 4 × 25 | 0.64 | 1046.66 | 1.73 | NP |
| 2 | 1000 | 5 | 2-5-2 | 4 × 25 | 0.64 | 1028.95 | 2.09 | NP | |
| 3 | 1000 | 5 | 2-5-2 | 4 × 25 | 0.62 | 1044.48 | 1.73 | NP | |
| 4 | 1000 | 5 | 2-5-2 | 4 × 25 | 0.62 | 1033.48 | 1.99 | NP | |
| 5 | 1000 | 5 | 2-5-2 | 4 × 25 | 0.61 | 1029.68 | 2.94 | NP | |
| 6 | 1000 | 5 | 2-5-2 | 4 × 25 | 0.61 | 1036.58 | 3.21 | NP | |
| 7 | 1000 | 5 | 2-5-2 | 4 × 25 | 0.67 | 1023.17 | 2.44 | NP | |
| 8 | 1000 | 5 | 2-5-2 | 4 × 25 | 0.67 | 1052.79 | 2.99 | NP | |
| 9 | 1414 | 7.07 | 2-5-2 | 4 × 25 | 1.21 | 1431.72 | 5.12 | 1.29 | |
| 10 | 1414 | 7.07 | 2-5-2 | 4 × 25 | 1.29 | 1444.82 | 2.75 | 1.21 | |
| 3 | 1 | 750 | 5 | 2-5-2 | 4 × 25 | 0.20 | 779.08 | 1.24 | 0.25b |
Abbreviations: HFIRE, high-frequency irreversible electroporation; NP, not performed.
a All patients received 2 µs HFIRE pulses (cycle = 2 µs +ON, 5 µs no energy, 2 µs—ON) with a total ON time of 100 µs per burst (see Figure 1, Supplemental Digital Content 1). Time of energy delivery for all ablations was ≤3 minutes. Planned ablation volume estimated by volume of tissue exposed to 500 V/cm or higher.
b Nonhomogeneous ablation achieved.
No intra- or postoperative AE were observed in dogs 1 and 3, and these dogs were discharged from the hospital with static clinical examinations 24 hours after the HFIRE procedure (Table 3; Supplemental Material—Patient Videos). Intraoperatively, dog 2 experienced intracranial hemorrhage and subsequent hypotension following disruption of a collateral vein during tumor resection. Hemorrhage was controlled with topical hemostatic agents, temporary venous hemoclipping, and blood patches. Postoperatively, dog 2 developed a depressed level of consciousness, an exacerbation of preexisting hemiparesis, and 10-point postoperative decline in KPS score from baseline. Due to intraoperative AE, immediate postoperative imaging was not performed in dog 2. The hypotension resolved upon anesthetic recovery. Dog 2 was discharged from the hospital 7 days after the HFIRE procedure, and its neurological status returned to pretreatment value by the day-14 recheck (Table 3).
Table 3.
HFIRE Clinical End Point Summary.
| Dog | Day 1 KPS | Day 2 KPS | Day 3 KPS | Day 14 KPS | Adverse Events (AE) | Posttreatment Imaging | Post-HFIRE Survival,=days | |||
|---|---|---|---|---|---|---|---|---|---|---|
| SOC | Grade | AE | HFIRE Attribution | |||||||
| 1 | 90 | 90 | 90 | 90 | None | NA | NA | NA | Gross total resection, No AE | Alive >445 |
| 2 | 70 | 60 | 60 | 70 | Procedural complication | 2 | Intraoperative venous injury | Unrelated | Not performed | >188 |
| Procedural complication | 3 | Intraoperative hemorrhage | Unrelated | |||||||
| Blood | 1 | Anemia | Unrelated | |||||||
| Vascular | 3 | Hypotension | Unrelated | |||||||
| Nervous | 2 | Depressed level of consciousness | Unlikely | |||||||
| Nervous, other | 2 | Hemiparesis | Unlikely | |||||||
| 3 | 90 | 90 | 90 | 90 | None | NA | NA | NA | Gross total resection, No AE | 76 |
Abbreviations: HFIRE, high-frequency irreversible electroporation; KPS, Karnofsky Performance Score; NA, not applicable.
No imaging evidence of direct neurotoxicity or collateral damage to brain tissues outside HFIRE treatment zones was observed in dogs 1 and 3 on immediate posttreatment MRI examinations or in dogs 1 and 2 within 6 months of treatment (Figure 5). In dogs 1 and 2, ablations completely disrupted the cytoarchitecture of the tumors (Figure 6A-D), resulting in homogeneous regions of tumor necrosis clearly delineated from adjacent untreated areas. In dog 3 (Figure 6E and F), ablations resulted in nonuniform treatment regions characterized by patchy necrosis and, in areas surrounding psammoma bodies, a marked neutrophilic and lymphocytic infiltrate surrounding islands of edematous but viable tumor cells.
Figure 5.
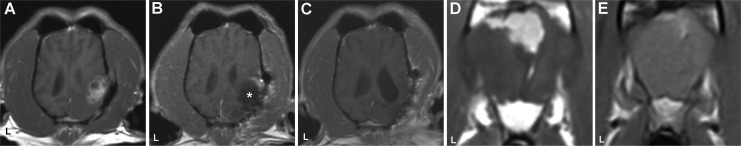
Pre- and post-high-frequency irreversible electroporation (HFIRE) magnetic resonance imaging (MRI) of dogs 1 (A-C) and 2 (D and E). Pretreatment dorsal planar MRI demonstrating recurrent meningioma (A) in occipital lobe in dog 1. Immediate post-HFIRE treatment (B), MRI with peripheral contrast enhancement of the rostral aspect of the resection cavity (asterisk). Six-month posttreatment (C) MRI with remodeling of treated region and no evidence of tumor. Pretreatment transverse MRI (D) demonstrating bilateral parasagittal meningioma in the frontal lobe, dog 2. E, Transverse MRI demonstrating suspected tumor focus along the superficial meninges and falx cerebri in the frontal lobe 5 months post-HFIRE treatment. L indicates patient’s left in all panels, and all panels are postcontrast T1W images.
Figure 6.
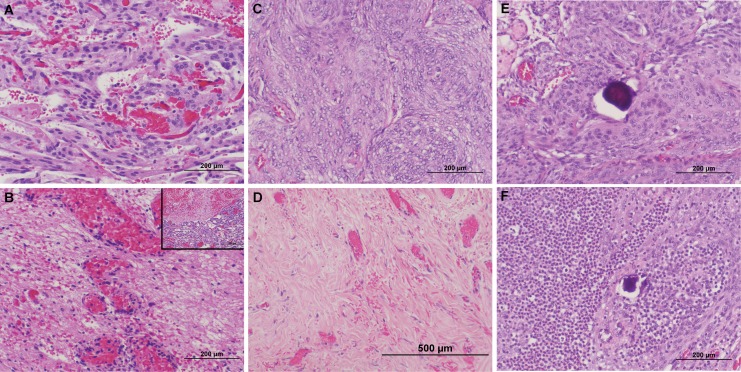
Histopathological appearances of high-frequency irreversible electroporation (HFIRE)-treated canine meningiomas. In dogs 1 (A and B) and 2 (C and D), comparison of pretreatment tumor biopsies (top panels) to posttreatment samples (bottom panels) reveals uniform HFIRE-induced tumor necrosis (B and D), with a sharp line of demarcation apparent between ablated and nontreated regions (B; inset). E, Pretreatment biopsy of psammomatous meningioma in dog 3 (C). Following HFIRE pulse delivery (F), foci of viable tumor cells remain surrounding psammoma bodies, and there is a marked neutrophilic and lymphocytic intratumoral infiltrates. Pre- and posttreatment images from all dogs obtained along identical biopsy/electrode insertion tracts. All panels stained with hematoxylin and eosin.
Dogs were followed off protocol for 6 months or until death (Table 3). No dog received other treatment in the 6 months following HFIRE ablation. Dog 1 was alive, seizure free, and had no evidence of tumor 6 months after HFIRE treatment (Figure 5C). Dog 2 was alive 6 months post-HFIRE treatment but required escalation of anticonvulsant drug therapy for persistent posttreatment seizure activity and had suspected residual or recurrent tumor identified on MRI examination performed 5 months after treatment (Figure 5D and E). Dog 3 died 76 days after treatment due to complications arising from recurrent status epilepticus.
Discussion
In this study, we introduce HFIRE as a novel ablative technique for the treatment of brain tumors. We have previously shown that IRE is capable of safely ablating defined focal areas of normal canine and rodent brains as well as spontaneous glioma.15-18 Given the varying electrical properties that exist between different tissue types as well as the inherent biophysical differences between IRE and HFIRE pulses, we believed this early-stage investigation was required to comply with IDEAL recommendations as a first step for the organ- and indication-specific evaluation of HFIRE.15-18,21-23 Our results indicate that delivery of HFIRE pulses to brain tumors is feasible using standard equipment and techniques available in contemporary neurosurgical practice. We have also demonstrated that in dogs with naturally occurring meningiomas, which are a faithful model of human disease, the HFIRE pulse parameters employed were administered without inducing muscle contractions and were capable of producing clinically relevant volumes of tumor ablation.30 Although the treat and resect study design used precluded a specific evaluation of the safety of HFIRE, treatments were administered to this small cohort of dogs with acceptable clinical morbidity.
Treatment planning is fundamental to safe and effective pulse delivery as well as the continued neurosurgical evolution of HFIRE and IRE. However, the complexity of the therapeutic planning procedure remains an obstacle to widespread clinical implementation of HFIRE and IRE for brain cancer treatments.17 A comprehensive solution that combines all of the necessary components of the HFIRE workflow in a user-friendly platform that can be incorporated into contemporary neurosurgical theaters is currently being developed and validated in canine patients with brain tumors. The software allows for anatomically accurate tissue-specific segmentation, determination of tumor dimensions, and formulation of virtual electrode insertion approaches that can be used in surgery.31 These volumetric representations are then used to computationally simulate the electric field distribution surrounding the active electrodes during pulse delivery to determine tumor coverage and cell kill probabilities and to avoid thermal damage.32 An additional, previously recognized limitation regarding the feasibility of HFIRE or IRE includes the difficulty in intraoperative confirmation of tumor ablation in some organs, such as the pancreas, or in deep-seated tumors. In these cases, it may not be possible to visualize changes in tumors or the visible tumor may fail to demonstrably change in appearance following treatment. Distinctive alterations in the gross appearance of the tumors were not observed in this study following pulse delivery, despite histopathologic evidence of successful tumor ablation. However, we demonstrated that the evaluation of successful electroporation can be achieved in real time by monitoring of changes in tumor resistance during treatment, a technique whose clinical utility has also been shown in pancreatic carcinoma.33
Although a limitation of this study is the small sample size, patient-specific HFIRE treatments were delivered successfully to all dogs, with no adverse effects directly attributable to the HFIRE procedure observed. This pilot study contributes to the growing body of evidence demonstrating the potential utility of IRE in a variety of organs including the brain, liver, pancreas, kidney, and prostate.15-18,34-38 Transient intra- and postoperative AEs were observed in dog 2, which had a Sindou type VI meningioma. These AEs were attributed to disruption of a collateral vein during tumor resection rather than the HFIRE treatment. The ideal approach to the surgical management of invasive parasagittal meningiomas is debatable. Risk to venous structures is a recognized complication, and venous disruption can be associated with postoperative neurological deterioration as occurred in dog 2.39 As HFIRE pulses were delivered in immediate proximity to collateral and bridging veins without thrombotic complications, the vascular disruption observed during resection also illustrates the potential vascular sparing advantages of HFIRE. Notably, unlike other thermal ablation methods, HFIRE/IRE is unaffected by the heat-sink effect, which can result in incomplete tumor ablation near large vessels as a result of heat loss due to blood flow.40
Morphological evaluations of resected tumors revealed that HFIRE induced rapid tumor necrosis, well-delineated ablation zones, and treated volumes that approximated the planned volumes. The pathological effects of HFIRE in meningiomas are similar to what have been observed in other HFIRE/IRE studies, including normal and neoplastic brain tissues.15-18,40 Although HFIRE produced homogeneous ablations in 2 of 3 dogs, our results in dog 3 reaffirm that while HFIRE performed well around some tissue heterogeneities, intratumoral mineralization may distort the electrical field distribution and preclude complete ablation.20,24,41 To account for regional tissue heterogeneities or anisotropy, the use of multiple electrode configurations or shorter HFIRE pulse durations could be considered to facilitate complete ablation.42 As this study was intentionally limited by peracute tumor resection following HFIRE treatment, the long-term effects of the inflammatory response observed in the incompletely ablated tumor of dog 3 are unknown. Immediate tumor resection was performed in this study for ethical reasons, and the pathology results provide evidence that observed HFIRE-induced tumor ablations are not completely dependent on the induction of acute inflammation, nor were they a product of chronic tissue remodeling.
The brain presents some challenges to the clinical application of ablative technologies for the treatment of cancer, as it is often not feasible or desirable to extend lethal energy delivery to a wide margin of normal tissue surrounding tumors to maximize local disease control. However, HFIRE offers advantages that may be beneficial for extending the margins when treating brain tumors. In vitro investigations have suggested that the enlarged nuclear-to-cytoplasm ratio characteristic of many cancerous cells, including malignant glioma and glioma stem cells, results in a significant enhancement in their susceptibility to destruction by HFIRE pulses.22 In engineered coculture tumor models containing glioma cells and normal glia, the lethal energy threshold required for HFIRE ablation of the malignant cells is significantly less than that required for normal astrocytes.22 Although further mechanistic studies are required to characterize and further demonstrate the in vivo selectivity of HFIRE against malignant cells, this biophysical tumor-targeting effect has the potential to allow for enhancing the margins of effectively treated tissue.
Conclusion
This study provides the first evidence of organ- and indication-specific feasibility of HFIRE in the brain for tumor ablation. Delivery of HFIRE pulses derived from patient-specific therapeutic plans resulted in the rapid ablation of intracranial meningiomas without causing muscular contraction or other AEs. The results provide the technical and descriptive foundations for larger and future investigations into the efficacy of HFIRE for the treatment of brain tumors, and possibly other focal neurological disorders that may benefit from nonthermal ablation.
Supplementary Material
Acknowledgments
The authors would like to thank Timothy O’Brien and Drs Jamie King, Jeffrey Ruth, and Kevin Lahmers for assistance with canine patient management, and Mindy Quigley for client relations and study coordination.
Abbreviations
- AE
adverse event
- BBB
blood–brain barrier
- HFIRE
high-frequency irreversible electroporation
- IRE
Irreversible electroporation
- KPS
Karnofsky Performance Score
- MRI
magnetic resonance imaging
Authors’ Note: This work was presented in part in abstract form at the 2nd Word Congress on Electroporation and Pulsed Electric Fields in Biology, Medicine and Food & Environmental Technologies; September 25, 2017; Norfolk, VA.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Mr Latouche and Drs Arena and Garcia are Voltmed Inc. employees and stockholders. Drs Davalos and Rossmeisl are on the Voltmed Inc. scientific advisory board and holders of Voltmed Inc. stock. Mr Latouche and Drs Arena, Garcia, Davalos, and Rossmeisl hold patents and have patents pending in the use of irreversible electroporation for cancer treatment.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Virginia Biosciences Health Research Corporation, “The Catalyst.”
ORCID iD: John H. Rossmeisl, DVM  http://orcid.org/0000-0003-1655-7076
http://orcid.org/0000-0003-1655-7076
Supplemental Material: Supplemental material for this article is available online
References
- 1. DeAngelis L. Brain tumors. N Eng J Med. 2001;344(2):114–123. [DOI] [PubMed] [Google Scholar]
- 2. Brown TJ, Brennan MC, Li M, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2016;2(11):1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rogers L, Barani I, Chamberlain M, et al. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122(1):4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coluccia D, Fandino J, Schwyzer L, et al. First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound. J Ther Ultrasound. 2014;2:17 doi:10.1186/2050-5736-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi Y, Mao Y. Magnetic resonance thermometry guided laser interstitial thermal therapy in neurosurgery, a promising tool for dural-based lesions? World Neurosurg. 2017;98:836–838. [DOI] [PubMed] [Google Scholar]
- 6. Chrastina J, Novak Z, Feitova V, Riha I. Experience with radiofrequency thermoablation of brain tumors. Rozhl Chir. 2008;87(7):338–343. [PubMed] [Google Scholar]
- 7. Habash RW, Bansal R, Krewski D, Alhafid HT. Thermal therapy, Part III: ablation techniques. Crit Rev Biomed Eng. 2007;35(1-2):37–121. [DOI] [PubMed] [Google Scholar]
- 8. Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48(14):2192–2202. [DOI] [PubMed] [Google Scholar]
- 9. Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–2543. [DOI] [PubMed] [Google Scholar]
- 10. Edd JF, Horowitz L, Davalos RV, Mir LM, Rubinsky B. In Vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans Biomed Eng. 2006;53(5):1409–1415. [DOI] [PubMed] [Google Scholar]
- 11. Tieleman DP, Leontiadou H, Mark AE, et al. Simulation of pore formation in lipid bilayers by mechanical stress and electric field. J Am Chem Soc. 2003;125(21):6382–6383. [DOI] [PubMed] [Google Scholar]
- 12. Gowrishankar TR, Esser AT, Vasilkoski Z, et al. Microdosimetry for conventional and supra-electroporaiton in cells with organelles. Biochem Biophys Res Comm. 2006;341(4):1266–1276. [DOI] [PubMed] [Google Scholar]
- 13. Smith KC, Neu JC, Krassowska W. Model of creation and evolution of stable electropores for DNA delivery. Biophys J. 2004;86(5):2813–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Onik G, Mikus P, Rubinsky B. Irreversible electroporation: implications for prostate ablation. Technol Cancer Res Treat 2007;6(4):295–300. [DOI] [PubMed] [Google Scholar]
- 15. Rossmeisl JH, Jr, Garcia PA, Roberston JL, Ellis TL, Davalos RV. Pathology of non-thermal irreversible electroporation (N-TIRE)-induced ablation of the canine brain. J Vet Sci 2013;14(4):433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rossmeisl JH, Jr, Garcia PA, Pancotto TE, et al. Safety and feasibility of the Nanoknife system for irreversible electroporation ablative treatment of spontaneous canine intracranial glioma. J Neurosurg. 2015;123(4):1008–1025. [DOI] [PubMed] [Google Scholar]
- 17. Garcia PA, Pancotto T, Rossmeisl JH, et al. Non-thermal irreversible electroporation (N-TIRE) and adjuvant fractionated radiotherapeutic multimodal therapy for intracranial malignant glioma in a canine patient. Technol Cancer Res Treat. 2011;10(1):73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sharabi S, Kos B, Last D, et al. A statistical model describing combined irreversible electroporation and electroporation-induced blood-brain barrier disruption. Radiol Oncol 2016;50(1):28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nielsen K, Scheffer HJ, Vieveen JM, et al. Anesthetic management during open and percutaneous irreversible electroporation. Br J Anaesth. 2014;113(6):985–992. [DOI] [PubMed] [Google Scholar]
- 20. Kotnik T, Mir LM, Flisar K, Puc M, Miklavcic D. Cell membrane electropermeabilization by symmetrical bipolar rectangular pulses. Part I: increased efficiency of permeabilization. Bioelectrochemistry. 2001;54(2):83–90. [DOI] [PubMed] [Google Scholar]
- 21. Arena CB, Sano MB, Rylander MN, Davalos RV. Theoretical considerations of tissue electroporation with high-frequency bipolar pulses. IEEE Trans Biomed Eng 2011;58(5):1474–1482. [DOI] [PubMed] [Google Scholar]
- 22. Ivey JW, Latouche EL, Sano MB, Rossmeisl JH, Davalos RV, Verbridge SS. Targeted cellular ablation based on the morphology of malignant cells. Sci Rep. 2015;5:17157 doi:10.1038/srep17157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arena CB, Sano MB, Rossmeisl JH, et al. High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction. Biomed Eng Online. 2011;10:102 doi:10.1186/1475-925X-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arena CB, Garcia PA, Sano MB, et al. Focal blood-brain-barrier disruption with high-frequency pulsed electrical fields. Technology. 2014;2(3):1–8. [Google Scholar]
- 25. McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374(9695):1105–1112. [DOI] [PubMed] [Google Scholar]
- 26. American Veterinary Medical Association. Animal Health Studies Database. 2016, https://ebusiness.avma.org/aahsd/study_search.aspx. Accessed July 6, 2018.
- 27. Rossmeisl JH, Andriani RT, Cecere T, et al. Frame-based stereotactic biopsy of canine brain masses: technique and clinical results in 26 cases. Front Vet Sci. 2015;2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sindou M. Meningiomas invading the sagittal or transverse sinuses, resection with venous reconstruction. J Clin Neurosci. 2001;8(suppl 1):8–11. [DOI] [PubMed] [Google Scholar]
- 29. Garcia P, Rossmeisl J, Neal R, Ellis TL, et al. Intracranial nonthermal irreversible electroporation: In vivo analysis. J Membr Biol. 2010;236(1):127–136. [DOI] [PubMed] [Google Scholar]
- 30. LeBlanc AK, Mazcko C, Brown DE, et al. Creation of an NCI comparative brain tumor consortium: informing the translation of new knowledge from canine to human brain tumor patients. Neuro Oncol. 2016;18(9):1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marčan M, Pavliha D, Kos B, Forjanič T, Miklavčič D. Web-based tool for visualization of electric field distribution in deep-seated body structures and planning of electroporation-based treatments. Biomed Eng Online. 2016;14(suppl 3):S4–S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garcia PA, Kos B, Rossmeisl JH, Jr, Pavliha D, Miklavčič D, Davalos RV. Predictive therapeutic planning for irreversible electroporation treatment of spontaneous malignant glioma. Med Phys. 2017;44(9):4968–4980. [DOI] [PubMed] [Google Scholar]
- 33. Dunki-Jacobs EM, Philips P, Martin RC. Evaluation of resistance as a measure of successful tumor ablation during irreversible electroporation of the pancreas. J Am Coll Surg. 2014;218(2):179–187. [DOI] [PubMed] [Google Scholar]
- 34. Pech M, Janitzky A, Wendler JJ, et al. Irreversible electroporation of renal cell carcinoma: a first-in-man phase I clinical study. Cardiovasc Intervent Radiol. 2011;34(1):132–138. [DOI] [PubMed] [Google Scholar]
- 35. Thomson KR, Cheung W, Ellis SJ, et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol. 2011;22(5):611–621. [DOI] [PubMed] [Google Scholar]
- 36. Cheung W, Kavnoudias H, Roberts S, Szkandera B, Kemp W, Thomson KR. Irreversible electroporation for unresectable hepatocellular carcinoma: initial experience and review of safety and outcomes. Technol Cancer Res Treat. 2013;12(3):233–241 [DOI] [PubMed] [Google Scholar]
- 37. Martin RC, McFarland K, Ellis S, Velanovich V. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J Am Coll Surg. 2012;215(3):361–369. [DOI] [PubMed] [Google Scholar]
- 38. Valerio M, Stricker PD, Ahmed HU, et al. Initial assessment of safety and clinical feasibility of irreversible electroporation in the focal treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2014;17(4):343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ricci A, Di Vitantonio H, De Paulis M, et al. Parasagittal meningiomas: our experience and the reconstruction of the superior sagittal sinus. Surg Neurol Int. 2017;19(8):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Golberg A, Yarmush ML. Nonthermal irreversible electroporation: fundamentals, applications, and challenges. IEEE Trans Biomed Eng. 2013;60(3):707–714. [DOI] [PubMed] [Google Scholar]
- 41. van den Bos W, de Bruin DM, Jurhil RR, et al. The correlation between the electrode configuration and histopathology of irreversible electroporation ablations in prostate cancer patients. World J Urol. 2016;34(5):657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bhonsle SP, Arena CB, Sweeney DC, Davalos RV. Mitigation of impedence changes due to electroporation therapy using bursts of high-frequency bipolar pulses. Biomed Eng Online. 2015;14(3):1 doi:10.1186/1475-925X-14-S3-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.