Abstract
Worster-Drought syndrome is a congenital, pseudobulbar paresis. There is no identified molecular etiology despite familial cases reported. The authors report a boy who was diagnosed with Worster-Drought syndrome due to longstanding drooling, dysphagia, and impaired tongue movement. Magnetic resonance imaging of the brain was unrevealing. At 14 years old, he remains aphonic with normal facial and extraocular movements. Nonsense mutations in the LINS gene, p.Glu366X and p.Lys393X, were found. Results from neuropsychological testing at 14 years old were consistent with a diagnosis of intellectual disability and revealed nonverbal reasoning skills at a 5-year-old level with relative sparing of his receptive vocabulary and visual attention. Compared to prior testing at 9 years old, his receptive language improved from a 6-year-old to an 8.5-year-old level. The authors report LINS mutations associated with Worster-Drought syndrome. This highlights that despite severe and persistent aphonia, receptive language improvements can be observed within the context of intellectual disability.
Keywords: Worster-Drought syndrome, aphonia, developmental disabilities, LINS, pseudobulbar paresis
Worster-Drought syndrome or congenital pseudobulbar paresis is a neurodevelopmental disorder characterized by an upper motor neuron paralysis of muscles supplied by cranial nerve X, XII and, to a lesser extent, VII. Case series delineating the Worster-Drought syndrome phenotype have been published.1,2 The most common clinical features include expressive language impairment that can be markedly below receptive language skills, drooling, and dysphagia due to impaired tongue movement, learning difficulties, and less severe gross motor delay and pyramidal symptoms. Bulbar dysfunction typically persists and can be associated with comorbid neuropsychiatric diagnoses including: attention deficit hyperactivity disorder, autism, and/or epilepsy.3 Worster-Drought syndrome is differentiated from Foix-Chavany-Marie syndrome or bilateral opercular syndrome by its lack of perisylvian polymicrogyria and/or atrophy.4 Some clinicians have proposed that Worster-Drought syndrome and Foix-Chavany-Marie syndrome exist along a continuum.5,6 A genetic basis for Worster-Drought syndrome has long been suspected for at least a subset of patients since 6% to 20% of patients with Worster-Drought syndrome have family members also affected by Worster-Drought syndrome.1,2,7 In some cases, the inheritance has spanned 3 generations.8
Homozygous mutations in LINS have recently been reported in individuals with severe cognitive impairment and complete aphonia, in siblings of a consanguineous couple of Yemeni ancestry.9 LINS is proposed to play a role in human brain development or functioning based upon research in Drosophilia that shows that LINS is recognized to be a tissue and specific modulator of wingless (Wnt) signaling that functions during a precise stage of development.10
Case Presentation
A 14-year-old boy was diagnosed with Worster-Drought syndrome at 8 years old. He remains aphonic, using sign language and handwriting short phrases to communicate. He shows the classic Worster-Drought syndrome phenotype including drooling, dysphagia, and impaired tongue movement. He underwent an intellectual disability panel for which a paternal sample was also submitted. He was found to have nonsense mutations affecting his LINS alleles: c.1096G>T; p.Glu366X and c.1178T>G; p.Lys393X, the latter being paternally inherited. This case provides an important genetic link for the Worster-Drought syndrome phenotype.
Our patient was born after an uneventful pregnancy via vaginal delivery at 40 weeks’ gestational age. Birth weight was 3,570 g (50th percentile), length 51 cm (50th percentile), and head circumference 34.0 cm (just above 10th percentile). No resuscitation was required, and he was discharged home on the second day of life. Feeding difficulties were noted during infancy. He bottle-fed well but demonstrated slow weight gain in the first 2 years of life. He did not require nasogastric or gastric feeds. His feeding difficulties became clearer as he transitioned to solid food. He drooled excessively. If he drank liquid too quickly, it would run out of his mouth. He had no nasal regurgitation of liquid or solids to suggest velopharyngeal insufficiency. Chewing was difficult and he was unable to extrude his tongue beyond his lower lip and had no side-to-side tongue movement. He needed all food pureed or cut into small pieces. As a result of impaired tongue movements, he would use his fingers to push back a food bolus within his oropharynx permitting swallowing. Electroencephalography revealed a normal awake background, and he had no clinical seizures at any time.
Gross motor delays were evident: He sat independently at 9 months old, walked at 18 months old, and rode a bicycle with training wheels at 7 years old. He was toilet trained at 3 to 4 years old and dry at night by 6 years old. Language regression was noted. Although he had 10 to 12 monosyllabic words at 18 to 24 months old, he lost all expressive language prior to his second birthday. He remains able to verbally communicate with guttural sounds but has not regained any spoken words. Despite complete aphonia, he shows continual progression in both his receptive language and augmentative communication skills. At 14 years old, he can use over 100 sign words and can use a smart tablet to spell words and use word recognition to type grammatically correct sentences. He can print, although his handwriting tends to be quite slow and deliberate. Magnetic resonance imaging brain at 5 years old demonstrated nonspecific changes in the periventricular white matter with no evidence of polymicrogyria or atrophy (Figure 1).
Figure 1.
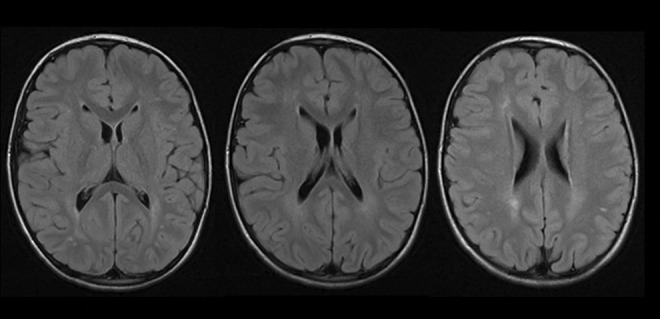
Magnetic resonance imaging of the brain (axial T2-fluid-attenuated inversion recovery sequences) at 5-years-old reveals nonspecific hyperintensities in the subcortical white matter. No cortical dysplasia or atrophy was seen.
He had multiple speech-language and psychological assessments between 6 and 14 years old, with key test scores summarized in Table 1. At 6 years old, his Leiter-R Global IQ was at the 5th percentile, while his Wechsler Preschool and Primary Scale of Intelligence III performance was at the 0.1th percentile. Attention-deficit hyperactivity disorder was diagnosed, which has improved with stimulant treatment. At 9 years old, a speech and language assessment confirmed single-word receptive vocabulary at a 6-year-old level, in contrast with very severe expressive language delay. At 12 years old, psychological testing using the Leiter-R confirmed the diagnosis of moderate intellectual disability. At 14 years old, the authors completed a brief, targeted neuropsychological assessment using select subtests from the Wechsler Intelligence Scale for Children V, the Test of Nonverbal Intelligence 3, and a French adaptation of the Peabody Picture Vocabulary Test–Revised. Results suggested nonverbal reasoning skills at a 5-year-old level, with relative sparing of receptive vocabulary and visual attention. Compared to testing at 9 years old, his receptive vocabulary raw score improved from a 6-year-old level to an 8.5 year-old level (Table 1).
Table 1.
Neuropsychological testing between 6 years old and 14 years old showing percentile score and age-equivalence for select standardized tests. Peabody picture vocabulatory test (echelle de vocabulaire en images peabody) improved from a 6 year old age-equivalence (at 9 years old) to an 8.5 year old age-equivalence (at 14 years old).a
| Age at Testing/Standardized Test Performed | Percentile Score | Age Equivalence |
|---|---|---|
| At 6 years old | ||
| Leiter-R Global IQ | 5% | – |
| Fluid reasoning index | 6% | – |
| Wechsler preschool and primary case of intelligence (3rd ed) | ||
| Performance index | 0.1% | – |
| Processing speed index | <0.1% | – |
| At 9 years old | ||
| EVIPb | 5% | 6 yo |
| At 12 years old | ||
| Leiter-R Global IQ | <0.1% | – |
| Fluid reasoning index | <0.1% | – |
| Spatial visualization index | 0.5% | – |
| At 14 years old | ||
| WISC-V | ||
| Fluid reasoning (figure weights) | 1% | < 6 yo |
| Visuospatial (visual puzzles) | <1% | <6 yo |
| Working memory (picture span) | 1% | 6.2 yo |
| Visual attention/processing speed (cancelation) | 9% | 8.6 yo |
| TONI-3 | 1% | 5.9 yo |
| EVIPb | 1% | 8.5 yo |
Abbreviation: EVIP, Echelle de Vocabulaire en Images Peabody; TONI, test of nonverbal intelligence; WISC, Wechsler Intelligence Scale For Children. yo, year old.
a Performance (percentile score) and age equivalence for select standardized tests. Age equivalences not available for Leiter-R or for Wechsler Preschool and Primary Scale of Intelligence-III Index scores.
b French adaptation of Peabody Picture Vocabulary Test–Revised.
Physical examination at 8 years old noted a head circumference of 53.8 cm (75th percentile). Subtle dysmorphic facial features were noted (Figure 2). He communicated by grunting and pointing with his fingers. He could print his name. Cranial nerve examination revealed normal pupillary constriction, normal fundi, and full and conjugate extraocular movements. Facial movement was full except for the inability to purse his lips when asked. Tongue bulk was slightly reduced, but no fasciculations were apparent. He was unable to move his tongue side-to-side and could not extrude his tongue. His palate was intact and his gag reflex was easily elicited. His sternocleidomastoid and trapezius strength was normal. Muscle power, tone, and deep tendon reflexes were normal. Plantar responses were flexor. He showed no overt motor apraxia and could carry out motor tasks when verbally instructed. General examination was significant only for pes planus.
Figure 2.
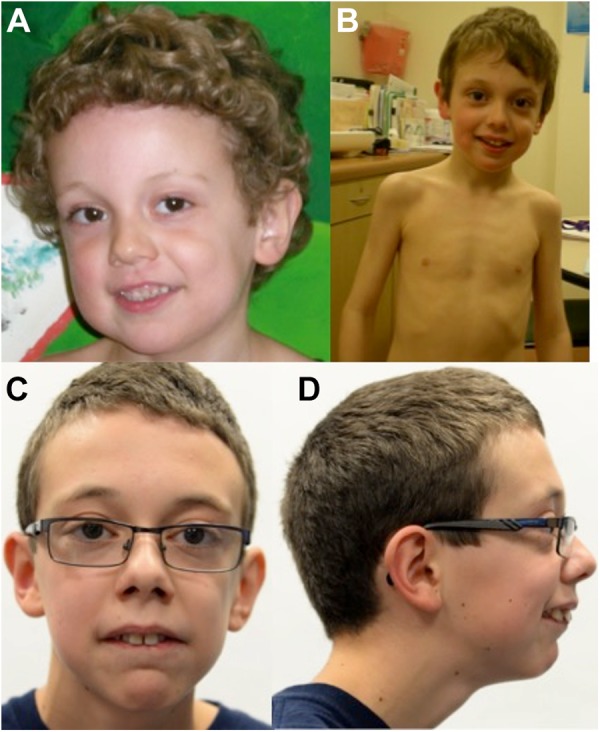
The patient at (A) 4 years 11 months old had a tall forehead, large cheeks, and a small pointed chin; at (B) 7 years old showed a long face with large cheeks, pointed chin, and thin muscle mass; at (C and D), 14 years of age he had a long face, overbite and required corrective lenses for mild myopia.
He developed a mild S-shaped scoliosis (at 14 years old) of up to 18°. He has had normal echocardiogram, nerve conduction studies, and normal chromosomal microarray, Fragile X testing, and biochemical testing (serum creatine kinase, lactic acid, plasma amino acids, and acylcarnitine profile). Clinical molecular testing using an expanded intellectual disability panel of approximately 2000 genes with submission of a paternal sample (GeneDx, Gaithersburg, Maryland) at 14 years old identified him to 2 nonsense mutations affecting LINS: c. 1096G>T; p.Glu366X and c.1178T>G; p.Lys393X. Only the latter mutation was paternally inherited, while a maternal sample was not available at the time.
Discussion
LINS mutations have previously been reported in 2 families. The first family consists of 2 siblings of Yemeni descent who demonstrated homozygous mutations (c.1219_1222 + 1delAAAGG) altering splicing and thereby predicted to result in loss of function of LINS. The children demonstrated severe cognitive impairment, with the eldest demonstrating complete aphonia at 9 years old.9 One of the 2 children was normocephalic with a head circumference of 51 cm at 8 years old (17th percentile for a male), while the other showed head circumference at the 3rd percentile at 3 years old. Homozygous LINS mutations causing frameshift mutations have also been reported in a syndromic patient of Iranian descent who had testing performed for intellectual disability.11 The latter case was described as part of a large cohort of patients who were studied for known and novel genetic causes of intellectual disability with little detail provided about the patient’s phenotype.
LINS is the human homologue of Drosophilia segment-polarity gene lin. LINS expression has been confirmed in human fetal brain.12 Although the precise role of LINS has not been elucidated, it has been linked to the Wnt pathway, a complex cascade of 85 genes that is essential for normal human brain development and function.9,13 Wnt signaling has been linked to cell proliferation, apoptosis, differentiation, migration, polarization, and other cellular processes.13 Mutations in various other Wnt pathway genes have been implicated in human neurodevelopmental diseases including ASPM (autosomal recessive primary microcephaly),14 CCDC88C (nonsyndromic hydrocephalus),13 and potentially EXM2 (schizencephaly).15 Like other children with LINS mutations, our patient demonstrates global developmental delay and intellectual disability. However, his phenotype is consistent with classic Worster-Drought syndrome which has not been previously reported to be associated with LINS. The children reported by Akawi et al9 also demonstrate complete aphonia, although they do not report the same level of oromotor dyspraxia that was observed in our patient.
An important observation for our patient was that the degree of expressive language impairment, at least partly attributable to his oromotor apraxia and aphonia, appeared much more severe than the degree of both receptive language impairment and cognitive impairment. Such an association has been described in children with Worster-Drought syndrome.3 It is worth noting that expressive language is very difficult to assess given the oromotor apraxia and that based on qualitative observations, our patient continues to develop his augmentative communication skills. Our patient showed a demonstrable improvement in his receptive language scores between ages 9 and 14 years old. Although his receptive language skills remained several years below what would be expected for his age, they had nevertheless improved from a 6-year-old level to an 8.5-year-old level with repeat testing. As such, the importance of adequate educational supports designed by experienced speech-language and/or occupational therapists is critical to ensuring that patients optimize their learning potential.
The mutations predicted to result in loss of function of LINS are an important observation as loss of function of the LINS gene could be responsible for Worster-Drought syndrome in some other children. Further clinical research will be necessary within this population to elucidate the role of LINS in the development of oromotor pathways.
Acknowledgments
The authors would like to thank the patient and his parents for their permission to report these findings.
Footnotes
Author Contribution: HJM drafted the manuscript, contributed to conception and design of the case report, contributed to acquisition, analysis, and interpretation of clinical data, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. AH contributed to acquisition, analysis, and interpretation of all neuropsychological test results, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. JR contributed to acquisition, analysis and interpretation of clinical data and all genetic test results, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: The patient's parent provided consent for the publication of this case including written consent for the use of photographs.
References
- 1. Worster-Drought C. Congenital suprabulbar paresis. Dev Med Child Neurol. 1974;16(30 suppl 30):1–33. [PubMed] [Google Scholar]
- 2. Clark M, Carr L, Reilly S, Neville BG. Worster-Drought syndrome, a mild tetraplegic perisylvian cerebral palsy. Brain. 2000;123(pt 10):2160–2170. [DOI] [PubMed] [Google Scholar]
- 3. Clark M, Harris R, Jolleff N, Price K, Neville BG. Worster-Drought syndrome: poorly recognized despite severe and persistent difficulties with feeding and speech. Dev Med Child Neurol. 2010;52(1):27–32. [DOI] [PubMed] [Google Scholar]
- 4. Christen HJ, Hanefield F, Kruse E, Imhauser S, Ernst JP, Finkenstaedt M. Foix-Chavany-Marie (anterior operculum) syndrome in childhood: a reappraisal of Worster-Drought syndrome. Dev Med Child Neurol. 2000;42(2):122–132. [DOI] [PubMed] [Google Scholar]
- 5. Nevo Y, Segev Y, Gelman Y, Rieder-Grosswasser I, Harel S. Worster-Drought and congenital perisylvian syndromes—a continuum? Pediatr Neurol. 2001;24(2):153–155. [DOI] [PubMed] [Google Scholar]
- 6. Clark M, Chong WK, Cox T, Neville BG. Congenital perisylvian dysfunction—is it a spectrum? Dev Med Child Neurol. 2010;52(1):33–39. [DOI] [PubMed] [Google Scholar]
- 7. Clark M, Neville BG. Familial and genetic associations in Worster-Drought syndrome and perisylvian disorders. Am J Med Genet A. 2008;146A(1):35–42. [DOI] [PubMed] [Google Scholar]
- 8. Patton MA, Baraitser M, Brett EM. A family with congenital suprabulbar paresis (Worster-Drought syndrome). Clin Genet. 1986;29(2):147–150. [DOI] [PubMed] [Google Scholar]
- 9. Akawi NA, Al-Jasmi F, Al-Shamsi AM, Ali BR, Al-Gazali L. LINS, a modulator of the WNT signaling pathway, is involved in human cognition. Orph J Rare Dis. 2013;8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hatini V, Bokor P, Goto-Mandeville R, DiNardo S. Tissue and stage-specific modulation of Wingless signalling by the segment polarity gene lines. Genes Dev. 2000;14(11):1364–1376. [PMC free article] [PubMed] [Google Scholar]
- 11. Najmabadi H, Hu H, Garshasbi M, et al. Deep sequencing of 50 novel genes for recessive cognitive disorders. Nature. 2011;478(7367):57–63. [DOI] [PubMed] [Google Scholar]
- 12. Katoh M. Molecular clonic and characterization of human WINS1 and mouse Wins2, homologous to Drosophilia segment polarity gene Lines (Lin). Int J Mol Med. 2002;10(2):155–159. [PubMed] [Google Scholar]
- 13. Ekici AB, Hilfinger D, Jatzwauk M, et al. Disturbed Wnt signalling due to a mutation in CCDC88C causes autosomal recessive non-syndromic hydrocephalus with medial diverticulum. Mol Syndromol. 2010:1(3):99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buchman JJ, Durak O, Tsai LH. ASPM regulates Wnt signaling pathway activity in the developing brain. Genes Dev. 2011;25(18):1909–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bouchet C, Gonzales M, Vuillaumier-Barrot S, et al. Molecular heterogeneity in fetals forms of type II lissencephaly. Hum Mutat. 2007;28(10):1020–1027. [DOI] [PubMed] [Google Scholar]


