Abstract
Aims:
We examined whether the use of a renin-angiotensin-aldosterone system (RAS) inhibitor plays a role in protecting against left atrial appendage thrombus (LAAT) in patients with hypertension complicated by atrial fibrillation (AF).
Methods:
Two observational studies were conducted on patients with diagnoses of hypertension and AF, who were categorized into RAS inhibitor user or nonuser groups. Demographic characteristics, clinical characteristics, echocardiographic parameters and hemostatic markers were examined and the occurrence of LAAT during follow-up were recorded.
Results:
In the first study (n = 131), LA peak systolic strain and LAA emptying flow velocity (LAA eV) were significantly increased in patients on RAS inhibitors compared with the nonuser group (p < 0.05). Lower D-dimer and fibrinogen levels were observed in patients on RAS inhibitors (p < 0.05). In the second study (n = 99), 25.9% (n = 11) of patients on RAS inhibitors developed LAAT, compared with 46.7% (n = 21) in the nonuser group (p < 0.05). After controlling for risk factors related to LAAT, use of RAS inhibitors remained associated with a significantly lower risk of developing LAAT (HR, 0.406; 95% CI, 0.191–0.862; p = 0.019).
Conclusions:
RAS inhibitors use was associated with a significant reduction in the risk of LAAT in patients with hypertension and AF.
Keywords: Atrial fibrillation, hypertension, left atrial appendage thrombus, renin-angiotensin system inhibitor, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers
Introduction
Nonvalvular atrial fibrillation (AF) is the most common sustained cardiac dysrhythmia and the most frequent cause of cardio-embolic stroke.1 Left atrial appendage (LAA) has been recognized as a major source of thromboembolism in patients with AF.2 Many studies have shown the effectiveness of anticoagulation for stroke prevention in AF patients. Unfortunately, the percentage of AF patients on oral anticoagulation therapy in China is extremely low (approximately 2.7%).3 The low rate of anticoagulant therapy for AF patients in China may be due to concerns regarding the possibility of excess bleeding related to the narrow safety window of the vitamin K antagonist warfarin and the need for frequent dose adjustment to maintain optimal international normalized ratio compared to Caucasian populations.4
Hypertension is one of the most prevalent independent risk factors for AF.5 The incidence of stroke is significantly higher among patients with AF complicated by hypertension.6 The renin-angiotensin system (RAS), through the pleiotropic actions of angiotensin II, plays a key role in the pathophysiology of hypertension and AF.7 Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) effectively decrease blood pressure by suppressing the RAS.8 In addition, inhibition of the RAS has been shown to reduce AF development through reverse remodeling.9 Adverse remodeling of the left atrium (LA) is associated with thrombus formation in LAA.10–12 Besides atrial remodeling, a growing body of evidence suggests pro-thrombotic effects of the RAS, through abnormalities in endothelial and platelet function, coagulation and fibrinolysis.13,14 Several studies regarding the antiplatelet, anticoagulant and profibrinolytic effects of losartan have recently been published.15–17 However, whether the inhibition of the RAS directly exerts influence on thrombogenesis or plays a secondary role through the effects on reverse remodeling in AF substrates remains largely unknown. In this study, we tested the hypothesis whether the use of ACEIs or ARBs prevents LAAT formation in patients with hypertension complicated by AF.
Materials and methods
Study population
We conducted two observational studies in parallel by reviewing patients who were admitted to the Second Hospital of Tianjin Medical University with a diagnosis of hypertension and nonvalvular AF from 2010 to 2016. AF was defined as absence of P waves and irregular R-R interval in a 12-lead electrocardiogram or 24-hour Holter recording. The different types of AF were defined according to the European Society of Cardiology guidelines for the management of AF.18
The first study included patients who underwent transthoracic echocardiogram (TTE) during hospitalization. Patients who never used ACEIs and ARBs, or used them less than three months were categorized into the nonuser group. Patients who used ACEIs or ARBs for at least three months were classified into the user group.19 Demographic variables, clinical variables, echocardiographic parameters, and hemostatic markers were recorded. Patients who had been treated with anticoagulation therapy before the visit were excluded.
The second study enrolled 99 consecutive patients who underwent transesophageal echocardiogram (TEE) in our hospital. According to whether patients were using ACEIs and/or ARBs or used them less than three months during follow-up, they were categorized into the users or nonusers group. All included patients had at least two hospital visits; the starting day of follow-up was the first day the patient was diagnosed with both hypertension and AF (first admission). TEE was performed during every visit. Patients with a previous history of LAAT were excluded. Also, patients who had been treated with anticoagulation therapy before the first visit or during the follow-up were excluded. The occurrence of LAAT during the follow-up period, possible risk factors for LAAT on the first admission, as well as the use of RAS inhibitors during the follow-up period were recorded. Patients were followed for at least four months (mean duration = 26.6 months). The primary outcome of this study was the occurrence of LAAT (LAA thrombus and/or sludge).
Additionally, patients with significant valvular disease, previous valve replacement or reconstruction, intracardiac shunting, left ventricular (LV) systolic dysfunction defined as LV ejection fraction (EF) ⩽ 40%, acute myocardial infarction, hyperthyroidism, primary pulmonary hypertension, and respiratory disease were excluded from the study. Patients with inadequate echocardiographic images were also excluded. The local ethics committee approved the study protocol with all study participants providing written informed consent.
Echocardiographic measurements
Exams were carried out using a commercially available ultrasound system (IE 33, Philips Healthcare Inc). TEE examination was performed using a three-dimensional matrix array probe (X7-2t, carrier frequency 2–7 MHz); TTE methods were used on a 1–5 MHz phased S5-1 probe. All images were digitally stored and analyzed using off-line post-processing with QLAB Software packages.
The following parameters were assessed using standard views and techniques:20 left atrial dimension (LAD), left ventricular end-diastolic diameter (LVEDd), LVEF, and the ratio of the early transmitral flow velocity and the early mitral annular velocity (E:e’), as measured by TTE. Tissue Doppler velocities were measured at the septal and lateral annuli using spectral Doppler tissue imaging. LA strain was estimated as the average of longitudinal strain data from the apical four-chamber, two-chamber and apical long-axis views. A total of 13 LA segments were analyzed. The LA peak systolic strain during ventricular systole was calculated by taking the mean for all 13 segments. LAA emptying flow velocity (LAA eV) was obtained on parasternal short-axis view.
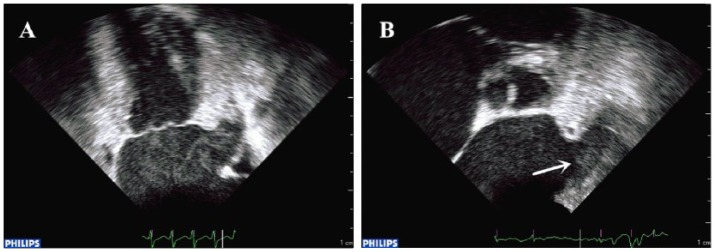
Because it is generally difficult to observe the entire LAA by TTE, TEE has been thought to be a sensitive and specific method to assess LAA function and detect thrombus formation.21 All TEE images were reviewed to determine the presence or absence of LAAT (LAAT (+) and LAAT (–)), spontaneous echo contrast (SEC), LAA ejection fraction (LAAEF), LAA filling flow velocity (LAA fV) and depressed LAA eV (<40 cm/sec) by pulsed-wave Doppler.22 LAAT was defined as a circumscribed and uniformly echodense intracavitary mass distinct from the underlying LA or LAA endocardium and the pectinate muscles, and present in more than one imaging plane.23 SEC was defined as dynamic “smoke-like” echoes with the characteristic swirling motion with optimal gain setting during the entire cardiac cycle.24 It was graded according to the classification (1 to 4+) proposed by Fatkin et al.25 When dense SEC (grade 3+ or 4+) was present and organized into a dynamic and gelatinous, but not solid or well-formed, echodensity present throughout the cardiac cycle, sludge was reported.26 Sludge within the LAA has been independently associated with subsequent thromboembolic events in patients with AF.27 Therefore, LAA sludge was categorized as LAAT. Representative figures of patients with preserved LAA sludge and LAA thrombus are shown in Figure 1(a) and (b), respectively.
Figure 1.
Transesophageal echocardiogram showing two representative examples of (a) left atrial appendage sludge and (b) left atrial appendage thrombus (arrow).
In patients with AF, echocardiographic parameters such as LA strain were calculated as mean values from five cardiac cycles. We carefully measured parameters only in those cycles in which the preceding and measured cardiac cycles were nearly equivalent.
Statistical analysis
Continuous variables were expressed as a mean ± standard deviation (SD), while categorical variables were expressed as a number or percentage. Chi square test was used for nominal variables and Student t test was used for comparison of continuous variables, where appropriate; the Levene test was used to check the homogeneity of variance; equivalent nonparametric tests were used when Kolmogorov-Smirnov was in favor of absence of normal distribution. The Kaplan-Meier method was used to estimate time to LAAT for the RAS inhibitor user group and nonuser group. The log-rank test was used to compare differences in time to LAAT between the RAS inhibitor user group and nonuser group. Univariate and multivariable analyses were performed using Cox proportional hazards regression. Variables that showed a p value less than 0.1 by univariate logistic regression analysis were entered into the multivariate analysis. All p values were two sided with a level of 0.05 for statistical significance. All statistical analysis was performed using SPSS (version 23.0, SPSS, Chicago, IL, USA).
Results
TTE study
A total of 131 patients who underwent TTE during hospitalization were included. The mean age of the study population was 62.1 years, 56.5% were male. Among these patients, 58.8% (n = 77) patients were using RAS inhibitors. The demographic and clinical characteristics, echocardiographic parameters and hemostatic markers were compared between the RAS inhibitor user and nonuser groups (Table 1).
Table 1.
Comparison of clinical variables, echocardiographic parameters and hemostatic markers between ACEI/ARB users and nonusers in the first study.
| Variables | ACEI/ARB user (n = 77) |
ACEI/ARB nonuser (n = 54) |
p value |
|---|---|---|---|
| Age (years) | 62.493±8.184 | 61.611±8.924 | 0.211 |
| Age ⩾65 years, n (%) | 33 (42.9%) | 24 (44.4%) | 0.857 |
| Male gender, n (%) | 40 (51.9%) | 34 (63.0%) | 0.278 |
| SBP (mmHg) | 140.779±21.145 | 145.944±18.573 | 0.151 |
| DBP (mmHg) | 93.948±13.941 | 96.277±14.034 | 0.350 |
| BMI | 26.575±4.038 | 26.025±3.080 | 0.401 |
| AF type, n (%) | 0.079 | ||
| Paroxysmal AF | 63 (81.8%) | 35 (64.8%) | 0.027 |
| Persistent AF | 12 (15.6%) | 15 (27.8%) | 0.089 |
| Long-standing persistent | 2 (2.6%) | 4 (7.4%) | 0.383 |
| OMI, n (%) | 4 (5.2%) | 1 (1.9%) | 0.603 |
| Vascular disease, n (%) | 3 (3.9%) | 4 (7.4%) | 0.628 |
| CHD, n (%) | 59 (76.6%) | 35 (64.8%) | 0.139 |
| Hyperlipidemia, n (%) | 51 (66.2%) | 35 (64.8%) | 0.866 |
| Diabetes mellitus, n (%) | 23 (29.9%) | 12 (22.2%) | 0.330 |
| Congestive heart failure, n (%) | 4 (5.2%) | 3 (5.6%) | 1 |
| Prior stroke or TIA, n (%) | 9 (11.7%) | 8 (14.8%) | 0.600 |
| CHA2DS2–VASc score | 2.610±1.319 | 2.537±1.462 | 0.765 |
| Smoking history, n (%) | 30 (39.0%) | 22 (40.7%) | 0.838 |
| Drinking history, n (%) | 18 (23.4%) | 11 (20.4%) | 0.683 |
| Antiplatelet drugs, n (%) | 46 (59.7%) | 23 (42.6%) | 0.053 |
| Statins, n (%) | 40 (51.9%) | 24 (44.4%) | 0.398 |
| Echocardiography | |||
| LAD (mm) | 39.229±3.978 | 41.489±6.153 | 0.020 |
| LVEDd (mm) | 49.329±6.548 | 47.924±+4.346 | 0.142 |
| LVEF (%) | 57.689±6.557 | 59.094±6.081 | 0.216 |
| E:e’ ratio | 13.280±4.459 | 15.296±6.014 | 0.039 |
| LAA eV (cm/s) | 41.906±17.963 | 34.264±21.963 | 0.037 |
| LA peak systolic strain (%) | 34.002±13.222 | 26.813±11.599 | 0.002 |
| Hemostatic markers | |||
| D-dimer (ug/l) | 278.181±62.607 | 320.148±91.486 | 0.004 |
| Fibrinogen (g/l) | 3.262±0.821 | 3.660±0.994 | 0.018 |
ACEI/ARB: angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; AF: atrial fibrillation; OMI: old myocardial infarction; TIA: transient ischemic attack; CHD: coronary heart disease; LAD: left atrial dimension; LVEDd: left ventricular end-diastolic dimension; E:e’ ratio: the ratio of the early transmitral flow velocity and the early mitral annular velocity; LAA eV: LAA emptying flow velocity; LVEF: left ventricular ejection fraction.
LA peak systolic strain, LAA emptying velocity, E:e’ ratio and LAD were significantly lower in ACEI/ARB nonusers compared with those who used an ACEI/ARB (p < 0.05). No significant differences in LVEDd and LVEF between the ACEI/ARB user and nonuser groups were observed. Higher levels of D-dimer and fibrinogen were observed in ACEI/ARB nonusers compared to users (p < 0.05, Table1).
TEE study
Patients with a diagnosis of AF and hypertension who had at least two hospital visits and underwent TEE at each admission were identified from 2010 to 2016. Patients who had been treated with anticoagulation therapy before or after the first visit were excluded. In addition, patients with a previous history of LAAT were excluded. A total of 99 patients were included. The mean age was 61.6 years, 44.4% of the patients were male. Among these patients, 54.5% (n = 54) were using RAS inhibitors. Demographic, clinical variables and echocardiographic parameters on the first admission were compared between the ACEI/ARB user and nonuser groups (Table 2). The demographic and clinical characteristics did not differ significantly between these groups. ACEI/ARB users had higher LAA fV compared with nonusers. No significant differences in LAA eV and LAAEF were observed between these two groups.
Table 2.
Comparison of clinical variables and echocardiographic parameters on the first admission between ACEI/ARB users and nonusers in the second study.
| Variables | ACEI/ARB user (n = 54) |
ACEI/ARB nonuser (n = 45) |
p value |
|---|---|---|---|
| Age (years) | 61.963±8.520 | 61.200±9.004 | 0.666 |
| Age ⩾65 years, n (%) | 20 (37.0%) | 17 (37.8%) | 0.940 |
| Male gender, n (%) | 25 (46.3%) | 19 (42.2%) | 0.685 |
| SBP (mmHg) | 139.870±19.357 | 146.400±20.239 | 0.105 |
| DBP (mmHg) | 93.666±12.536 | 98.844±14.602 | 0.061 |
| BMI | 26.470±4.228 | 26.129±3.218 | 0.658 |
| AF type, n (%) | 0.459 | ||
| Paroxysmal AF | 44 (81.5%) | 32 (71.1%) | 0.224 |
| Persistent AF | 8 (14.8%) | 11 (24.4%) | 0.226 |
| Long-standing persistent | 2 (3.7%) | 2 (4.4%) | 1 |
| OMI, n (%) | 1 (1.9%) | 2 (4.4%) | 0.872 |
| Vascular disease, n (%) | 1 (1.9%) | 3 (6.7%) | 0.485 |
| CHD, n (%) | 39 (72.2%) | 29 (64.4%) | 0.406 |
| Hyperlipidemia, n (%) | 31 (57.4%) | 30 (66.7%) | 0.346 |
| Diabetes mellitus, n (%) | 18 (33.3%) | 11 (24.4%) | 0.333 |
| Congestive heart failure, n (%) | 2 (3.7%) | 1 (2.2%) | 1 |
| Prior stroke or TIA, n (%) | 4 (7.4%) | 3 (6.7%) | 0.744 |
| Smoking history, n (%) | 17 (31.5%) | 15 (33.3%) | 0.844 |
| Drinking history, n (%) | 9 (16.7%) | 6 (13.3%) | 0.645 |
| Antiplatelet drugs, n (%) | 35 (64.8%) | 24 (53.3%) | 0.246 |
| Statins, n (%) | 26 (48.1%) | 20 (44.4%) | 0.713 |
| LAA eV (cm/s) | 43.511±15.750 | 37.988±17.007 | 0.097 |
| LAA fV (cm/s) | 50.011±16.201 | 41.393±17.213 | 0.012 |
| LAAEF (%) | 52.361±13.188 | 47.502±13.821 | 0.077 |
SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; AF: atrial fibrillation; OMI: old myocardial infarction; CHD: coronary heart disease; TIA: transient ischemic attack; LAA eV: LAA emptying flow velocity; LAA fV: LAA filling flow velocity; LAAEF: LAA ejection fraction.
The occurrence of LAAT, SEC and echocardiographic parameters during the follow-up period between ACEI/ARB users and nonusers are shown in Table 3. The overall incidence of LAAT was 35.4% (n = 35). Among patients on RAS inhibitors, 25.9% (n = 11) developed an LAAT, compared with 46.7% (n = 21) in the nonuser group (p < 0.05, Table 3). Lower flow velocities in LAA, lower LAAEF and a higher prevalence of SEC were observed in the ACEI/ARB nonuser group compared with the ACEI/ARB user group (Table 3).
Table 3.
Comparison of the occurrence of LAAT, SEC and echocardiographic parameters during the follow-up period between ACEI/ARB users and nonusers in the second study.
| Variables | ACEI/ARB user (n = 54) |
ACEI/ARB nonuser (n = 45) |
p value |
|---|---|---|---|
| LAAT, n (%) | 14 (25.9%) | 21 (46.7%) | 0.032 |
| SEC, n (%) | 25 (46.3%) | 31 (68.9%) | 0.024 |
| LAA eV (cm/s) | 39.042±15.975 | 30.188±14.449 | 0.005 |
| LAA fV (cm/s) | 46.550±16.164 | 35.848±14.382 | 0.001 |
| LAAEF (%) | 47.098±14.174 | 37.091±12.467 | 0.000 |
LAAT: left atrial appendage thrombus; SEC: spontaneous echocardiographic contrast; LAA eV: LAA emptying flow velocity; LAA fV: LAA filling flow velocity; LAAEF: LAA ejection fraction.
Subgroup analyses showed that LAAT incidence was significantly reduced in the ARB (n = 32) group when compared with the nonuser group (p = 0.026). However, there was no significant difference between the ACEI group (n = 22) and nonuser group in LAAT incidence (p = 0.247), while LAAT incidence tended to be reduced in the ACEI group compared with the nonuser group (31.8% vs. 46.7%). Furthermore, no significant difference was observed in incidence of LAAT between patients on ACEIs and those on ARBs (p = 0.413).
Kaplan-Meier LAAT free survival analysis displayed a reduced risk of LAAT in RAS inhibitor users compared with nonusers (log-rank test p = 0.032, Figure 2). Cox proportional hazards regression analysis was performed to identify the risk factors for LAAT (Table 4). Univariate analysis showed that age ⩾ 65 years, SBP, AF type, diabetes mellitus and prior stroke or TIA were positively associated with LAAT. The use of antiplatelet drugs and RAS inhibitors was negatively associated with LAAT (Table 4). After controlling for other factors related to LAAT (age ⩾ 65 years, SBP, AF type, diabetes mellitus, prior stroke or TIA, antiplatelet drugs) the use of RAS inhibitors remained significantly associated with lower risk of developing LAAT (hazard ratio (HR), 0.368; 95% confidence interval (CI), 0.170–0.797; p = 0.011) (Table 4).
Figure 2.
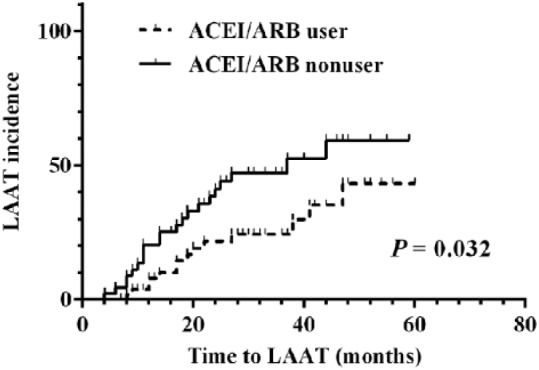
Kaplan-Meier LAAT free survival curve between ACEI/ARB users and ACEI/ARB nonusers of the second study. ACEI/ARB: angiotensin-converting enzyme inhibitors/angiotensin receptor blocker; LAAT: left atrial appendage thrombus.
Table 4.
Association between LAAT and clinical variables in the second study.
| Variables | HR | 95% CI | p value |
|---|---|---|---|
| Univariate analysis | |||
| Age ⩾65 years | 2.854 | 1.436–5.672 | 0.003 |
| gender | 0.924 | 0.474–1.798 | 0.815 |
| SBP | 1.017 | 0.999–1.036 | 0.063 |
| DBP | 1.018 | 0.991–1.046 | 0.184 |
| BMI | 1.066 | 0.977–1.163 | 0.152 |
| AF type | 2.523 | 1.491–4.269 | 0.001 |
| OMI | 0.699 | 0.095–5.124 | 0.725 |
| Vascular disease | 0.046 | 0.000–38.803 | 0.370 |
| CHD | 1.222 | 0.593–2.515 | 0.587 |
| Hyperlipidemia | 0.878 | 0.444–1.735 | 0.708 |
| Diabetes mellitus | 1.761 | 0.901–3.443 | 0.098 |
| Congestive heart failure | 1.930 | 0.460–8.099 | 0.369 |
| Prior stroke or TIA | 3.781 | 1.647–8.683 | 0.002 |
| Smoking history | 0.907 | 0.444–1.853 | 0.790 |
| Drinking history | 1.359 | 0.592–3.119 | 0.469 |
| ACEI/ARB | 0.487 | 0.247–0.958 | 0.037 |
| Antiplatelet drugs | 0.545 | 0.279–1.061 | 0.074 |
| Statins | 0.919 | 0.471–1.791 | 0.803 |
| Multivariate analysis | |||
| Age ⩾65 years | 2.266 | 1.066–4.817 | 0.033 |
| SBP | 1.023 | 1.003–1.044 | 0.026 |
| AF type | 1.727 | 0.949–3.143 | 0.074 |
| Diabetes mellitus | 2.250 | 1.076–4.702 | 0.031 |
| Prior stroke or TIA | 2.630 | 0.952–7.269 | 0.062 |
| ACEI/ARB | 0.368 | 0.170–0.797 | 0.011 |
| Antiplatelet drugs | 0.818 | 0.391–1.710 | 0.594 |
LAAT: left atrial appendage thrombus; SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; AF: atrial fibrillation; OMI: old myocardial infarction; CHD: coronary heart disease; TIA: transient ischemic attack; ACEI/ARB: angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; HR: hazard ratio; CI: confidence interval.
Discussion
AF is associated with a higher risk of thromboembolic events and stroke.18 The pathophysiological mechanisms underlying thrombogenesis in AF are complex. Thrombus formation takes place in LA or LAA, and embolization can occur through the systemic circulation to the brain or other distant sites. Hypertension is one of the most prevalent independent risk factors for AF.5 The RAS, through the pleiotropic actions of angiotensin II, plays a key role in the pathophysiology both of hypertension and AF.7 Besides increases in blood pressure, the prothrombotic state in hypertension is also induced by activation of the RAS, leading to abnormalities in endothelial and platelet function, coagulation and fibrinolysis.13,14 Hypertension leads to increases in LA pressure, atrial dilation and alterations in wall stress.28 Based on this modulation of the RAS cascade, RAS inhibition may be a potential therapeutic target for reducing thrombosis and stroke. In this retrospective cohort study, we found that the absolute risk reduction in LAAT for RAS inhibitor users was 20.8%. Our results thus indicate that the use of ACEIs or ARBs may prevent the occurrence of LAAT in patients with hypertension complicated by AF. To the best our knowledge, this is the first study to assess the prevention of LAAT formation by RAS inhibitors in patients with hypertension and AF.
There are several pathophysiological mechanisms that could explain our findings. First, the RAS participates in structural remodeling and electrophysiological abnormalities in the cardiac myocardium.29 which can increase the susceptibility to arrhythmia and the development of AF.30–33 Electrical, contractile and structural remodeling are dominant factors for AF genesis, and fibrosis plays a key role in structural remodeling in the heart.34–36 Many clinical studies have shown that LA mechanical remodeling could result in thrombus formation in the LAA.10–12 These findings are physiologically plausible, as atrial emptying tends to be attenuated with impaired LA function and increased LA fibrosis, causing atrial blood stasis and thrombus formation.37 Upstream therapy for AF involving RAS inhibitors, such as ACEIs or ARBs, can reduce atrial stretch and fibrosis, which can potentially decrease the likelihood of developing AF.38,39
It is known that there are three types of LA mechanical function: reservoir, conduit and booster pump function.40 The burden of LA fibrosis, analyzed by magnetic resonance imaging, shows an inverse correlation with LA strain evaluated by two-dimensional speckle tracking.41 Moreover, LA systolic strain was suggested to be an independent predictor of LA reverse remodeling.42 These results indicated that LA peak systolic strain is correlated with mechanical and structural remodeling of the LA and is helpful to assess LA reservoir function.43 One study showed that LA peak systolic strain was significantly correlated with LAA eV in patients with AF. LA peak systolic strain decreased with progressive LA enlargement and increasing age. LA peak systolic strain thus appears to be a reliable marker for LAA dysfunction and thrombus formation in patients with AF.44 Several studies suggested that the normal range of LA peak systolic strain is 42.2 ± 6.1%.45
In our study, patients who used ACEI/ARB inhibitors had significantly greater LA peak systolic strain and LAA eV compared with those who did not use ACEI/ARB inhibitors. Inhibition of the RAS by ACEIs or ARBs appears to effectively improve LA function by reducing fibrosis and reversing remodeling. Moreover, an increasing body of evidence suggests a prothrombotic state following RAS activation.13,14 An extensive cross-talk connects coagulation and inflammation, and angiotensin II-induced vascular inflammation is a possible mechanism underlying the prothrombotic state. Also, RAS blockade has been proven to show anti-inflammatory effects.19
AF is involved in systemic inflammation. Therefore, patients with hypertension complicated by AF are at greater predisposition to greater platelet activity and activation of coagulation cascades. Thus, the prothrombotic state can be synergistically promoted, and inhibition of the activated RAS by ACEIs or ARBs might be more effective in patients with AF complicated by hypertension than in patients without hypertension. Evidence for the protective role of some RAS inhibitors against athero-thrombotic cardiovascular disease has been well demonstrated.14 For example, the Losartan Intervention For End Point Reduction in Hypertension (LIFE) study showed that hypertension patients with left-ventricular hypertrophy and AF benefited more from losartan compared with atenolol, a β-blocker, in preventing cardiovascular morbidity and mortality, stroke and cardiovascular death, even though equivalent blood pressure reductions were provided by both agents.46 Several studies reported the antiplatelet, anticoagulant and profibrinolytic effects of losartan, which may explain the better prognosis of losartan in the LIFE study.15–17 Another study showed that losartan’s effects on inhibiting platelet activation were as high as aspirin.47 Regarding coagulation activation and fibrinolytic function, our study investigated the association between two hemostatic markers (plasma D-dimer and fibrinogen) and RAS inhibitors. Higher levels of D-dimer and fibrinogen were observed in patients who did not receive ACEI/ARB inhibitor treatment compared to those who did.
Antithrombotic agents are routinely used for the prevention of thromboembolism in patients with nonvalvular AF. These include anticoagulant drugs, such as unfractionated heparin, low molecular weight heparin, warfarin, and direct thrombin and factor Xa inhibitors, as well as antiplatelet drugs such as aspirin and clopidogrel.18 In our study, patients who were treated with anticoagulation therapy were excluded. Platelet inhibitors, alone or in combination, are less effective than warfarin, but are better tolerated by some patients, and are associated with a lower risk of intracerebral hemorrhage. Our study also showed that antiplatelet drug use was negatively associated with LAAT occurrence on univariate analysis. However, multivariate analysis by controlling for other risk factors related to LAAT showed the use of antiplatelet drugs was not significantly associated with LAAT (HR, 0.818; 95% CI, 0.391–1.710; p = 0.594).
Limitations
Our findings should be interpreted in light of the following limitations. First, this study population had a relatively small sample size with a limited number of events. Second, patients with prior antiplatelet therapy were not excluded. Third, because of the study’s retrospective design, duration of ACEI/ARB use, the use of other antihypertensive drugs as well as antiarrhythmic therapy could not be assessed accurately. Fourth, it was not possible to assess the dose-response relationship in our study because many patients had frequent dosage adjustments. Finally, baseline characteristics show ACEI/ARB nonusers in the TTE group to have more nonparoxysmal AF and larger LA diameter, which could have influenced the study findings.
Conclusions
RAS inhibitors decreased the levels of D-dimer and fibrinogen and reversed LA remodeling in patients with hypertension complicated by AF. These in turn were associated with a reduction in the risk of LAAT. Larger-scale prospective studies are needed to explore the therapeutic effect of RAS inhibitors and the intra-class differences between ACEIs and ARBs on the thrombogenic process in patients with AF.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants (81570298, 30900618, 81270245 to T.L.) from the National Natural Science Foundation of China, and the Tianjin Natural Science Foundation (16JCZDJC34900 to T.L.); and the Science and Technology Foundation of Tianjin Sanitary Bureau (2015KZ105 to H.F.) and the Tianjin Natural Science Foundation (16JCYBJC25000 to H.F.).
ORCID iD: Tong Liu  https://orcid.org/0000-0003-0482-0738
https://orcid.org/0000-0003-0482-0738
References
- 1. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS [article in Polish]. Kardiol Pol 2016; 74: 1359–1469. [DOI] [PubMed] [Google Scholar]
- 2. García-Fernández MA, Torrecilla EG, San Román D, et al. Left atrial appendage Doppler flow patterns: Implications on thrombus formation. Am Heart J 1992; 124: 955–961. [DOI] [PubMed] [Google Scholar]
- 3. Zhou ZQ, Hu DY, Chen J, et al. An epidemiological survey of atrial fibrillation in China [article in Chinese]. Zhonghua Nei Ke Za Zhi 2004; 43: 491–494. [PubMed] [Google Scholar]
- 4. Hu D, Sun Y. Epidemiology, risk factors for stroke, and management of atrial fibrillation in China. J Am Coll Cardiol 2008; 52: 865–868. [DOI] [PubMed] [Google Scholar]
- 5. Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: Population-based estimates. Am J Cardiol 1998; 82: 2N-9N. [DOI] [PubMed] [Google Scholar]
- 6. Manolis AJ, Rosei EA, Coca A, et al. Hypertension and atrial fibrillation: Diagnostic approach, prevention and treatment. Position paper of the Working Group ‘Hypertension Arrhythmias and Thrombosis’ of the European Society of Hypertension. J Hypertens 2012; 30: 239–252. [DOI] [PubMed] [Google Scholar]
- 7. Seccia TM, Caroccia B, Muiesan ML, et al. Atrial fibrillation and arterial hypertension: A common duet with dangerous consequences where the renin angiotensin-aldosterone system plays an important role. Int J Cardiol 2016; 206: 71–76. [DOI] [PubMed] [Google Scholar]
- 8. Robles NR, Cerezo I, Hernandez-Gallego R. Renin-angiotensin system blocking drugs. J Cardiovasc Pharmacol Ther 2014; 19: 14–33. [DOI] [PubMed] [Google Scholar]
- 9. Ducharme A, Schiffrin EL. Reviewing the future of renin-angiotensin system blockade: The role of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in the prevention of atrial fibrillation. Can J Cardiol 2010; 26 (Suppl E): 21E-23E. [DOI] [PubMed] [Google Scholar]
- 10. Verhorst PM, Kamp O, Visser CA, et al. Left atrial appendage flow velocity assessment using transesophageal echocardiography in nonrheumatic atrial fibrillation and systemic embolism. Am J Cardiol 1993; 71: 192–196. [DOI] [PubMed] [Google Scholar]
- 11. Kamp O, Verhorst PM, Welling RC, et al. Importance of left atrial appendage flow as a predictor of thromboembolic events in patients with atrial fibrillation. Eur Heart J 1999; 20: 979–985. [DOI] [PubMed] [Google Scholar]
- 12. Sparks PB, Mond HG, Vohra JK, et al. Mechanical remodeling of the left atrium after loss of atrioventricular synchrony. A long-term study in humans. Circulation 1999; 100: 1714–1721. [DOI] [PubMed] [Google Scholar]
- 13. Remkova A, Remko M. The role of renin-angiotensin system in prothrombotic state in essential hypertension. Physiol Res 2010; 59: 13–23. [DOI] [PubMed] [Google Scholar]
- 14. Brown NJ, Vaughan DE. Prothrombotic effects of angiotensin. Adv Intern Med 2000; 45: 419–429. [PubMed] [Google Scholar]
- 15. Guerra-Cuesta JI, Montón M, Rodríguez-Feo JA, et al. Effect of losartan on human platelet activation. J Hypertens 1999; 17: 447–452. [DOI] [PubMed] [Google Scholar]
- 16. Li-Saw-Hee FL, Beevers DG, Lip GY. Effect of antihypertensive therapy using enalapril or losartan on haemostatic markers in essential hypertension: A pilot prospective randomised double-blind parallel group trial. Int J Cardiol 2001; 78: 241–246. [DOI] [PubMed] [Google Scholar]
- 17. Soejima H, Ogawa H, Suefuji H, et al. Comparison of effects of losartan versus enalapril on fibrinolysis and coagulation in patients with acute myocardial infarction. Am J Cardiol 2001; 87: 1408–1411. [DOI] [PubMed] [Google Scholar]
- 18. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016; 37: 2893–2962. [DOI] [PubMed] [Google Scholar]
- 19. Sakamoto T, Kudoh T, Sakamoto K, et al. Antithrombotic effects of losartan in patients with hypertension complicated by atrial fibrillation: 4A (Angiotensin II Antagonist of platelet Aggregation in patients with Atrial fibrillation), a pilot study. Hypertens Res 2014; 37: 513–518. [DOI] [PubMed] [Google Scholar]
- 20. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18: 1440–1463. [DOI] [PubMed] [Google Scholar]
- 21. Yamashita E, Takamatsu H, Tada H, et al. Transesophageal echocardiography for thrombus screening prior to left atrial catheter ablation. Circ J 2010; 74: 1081–1086. [DOI] [PubMed] [Google Scholar]
- 22. Beigel R, Wunderlich NC, Ho SY, et al. The left atrial appendage: Anatomy, function, and noninvasive evaluation. JACC Cardiovasc Imaging 2014; 7: 1251–1265. [DOI] [PubMed] [Google Scholar]
- 23. Aschenberg W, Schlüter M, Kremer P, et al. Transesophageal two-dimensional echocardiography for the detection of left atrial appendage thrombus. J Am Coll Cardiol 1986; 7: 163–166. [DOI] [PubMed] [Google Scholar]
- 24. Fatkin D, Herbert E, Feneley MP. Hematologic correlates of spontaneous echo contrast in patients with atrial fibrillation and implications for thromboembolic risk. Am J Cardiol 1994; 73: 672–676. [DOI] [PubMed] [Google Scholar]
- 25. Fatkin D, Kelly RP, Feneley MP. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J Am Coll Cardiol 1994; 23: 961–969. [DOI] [PubMed] [Google Scholar]
- 26. Troughton RW, Asher CR, Klein AL. The role of echocardiography in atrial fibrillation and cardioversion. Heart 2003; 89: 1447–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lowe BS, Kusunose K, Motoki H, et al. Prognostic significance of left atrial appendage “sludge” in patients with atrial fibrillation: A new transesophageal echocardiographic thromboembolic risk factor. J Am Soc Echocardiogr 2014; 27: 1176–1183. [DOI] [PubMed] [Google Scholar]
- 28. Barbier P, Alioto G, Guazzi MD. Left atrial function and ventricular filling in hypertensive patients with paroxysmal atrial fibrillation. J Am Coll Cardiol 1994; 24: 165–170. [DOI] [PubMed] [Google Scholar]
- 29. Borghi C; SIIA Task Force, Rossi F, et al. Role of the renin-angiotensin-aldosterone system and its pharmacological inhibitors in cardiovascular diseases: Complex and critical issues. High Blood Press Cardiovasc Prev 2015; 22: 429–444. [DOI] [PubMed] [Google Scholar]
- 30. Freestone B, Beevers DG, Lip GY. The renin-angiotensin-aldosterone system in atrial fibrillation: A new therapeutic target? J Hum Hypertens 2004; 18: 461–465. [DOI] [PubMed] [Google Scholar]
- 31. Schotten U, Verheule S, Kirchhof P, et al. Pathophysiological mechanisms of atrial fibrillation: A translational appraisal. Physiol Rev 2011; 91: 265–325. [DOI] [PubMed] [Google Scholar]
- 32. Choy L, Yeo JM, Tse V, et al. Cardiac disease and arrhythmogenesis: Mechanistic insights from mouse models. Int J Cardiol Heart Vasc 2016; 12: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kallergis EM, Goudis CA, Vardas PE. Atrial fibrillation: A progressive atrial myopathy or a distinct disease? Int J Cardiol 2014; 171: 126–133. [DOI] [PubMed] [Google Scholar]
- 34. Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 2002; 54: 230–246. [DOI] [PubMed] [Google Scholar]
- 35. Burstein B, Nattel S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol 2008; 51: 802–809. [DOI] [PubMed] [Google Scholar]
- 36. Tse G, Yeo JM. Conduction abnormalities and ventricular arrhythmogenesis: The roles of sodium channels and gap junctions. Int J Cardiol Heart Vasc 2015; 9: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Doukky R, Garcia-Sayan E, Gage H, et al. The value of diastolic function parameters in the prediction of left atrial appendage thrombus in patients with nonvalvular atrial fibrillation. Cardiovasc Ultrasound 2014; 12: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakashima H, Kumagai K. Reverse-remodeling effects of angiotensin II type 1 receptor blocker in a canine atrial fibrillation model. Circ J 2007; 71: 1977–1982. [DOI] [PubMed] [Google Scholar]
- 39. Kumagai K, Nakashima H, Urata H, et al. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J Am Coll Cardiol 2003; 41: 2197–2204. [DOI] [PubMed] [Google Scholar]
- 40. Todaro MC, Choudhuri I, Belohlavek M, et al. New echocardiographic techniques for evaluation of left atrial mechanics. Eur Heart J Cardiovasc Imaging 2012; 13: 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuppahally SS, Akoum N, Burgon NS, et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: Relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging 2010; 3: 231–239. [DOI] [PubMed] [Google Scholar]
- 42. Machino-Ohtsuka T, Seo Y, Ishizu T, et al. Significant improvement of left atrial and left atrial appendage function after catheter ablation for persistent atrial fibrillation. Circ J 2013; 77: 1695–1704. [DOI] [PubMed] [Google Scholar]
- 43. Kokubu N, Yuda S, Tsuchihashi K, et al. Noninvasive assessment of left atrial function by strain rate imaging in patients with hypertension: A possible beneficial effect of renin-angiotensin system inhibition on left atrial function. Hypertens Res 2007; 30: 13–21. [DOI] [PubMed] [Google Scholar]
- 44. Morris DA, Takeuchi M, Krisper M, et al. Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: Multicentre study. Eur Heart J Cardiovasc Imaging 2015; 16: 364–372. [DOI] [PubMed] [Google Scholar]
- 45. Cameli M, Caputo M, Mondillo S, et al. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc Ultrasound 2009; 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wachtell K, Hornestam B, Lehto M, et al. Cardiovascular morbidity and mortality in hypertensive patients with a history of atrial fibrillation: The Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. J Am Coll Cardiol 2005; 45: 705–711. [DOI] [PubMed] [Google Scholar]
- 47. Montón M, Jiménez A, Núñez A, et al. Comparative effects of angiotensin II AT-1-type receptor antagonists in vitro on human platelet activation. J Cardiovasc Pharmacol 2000; 35: 906–913. [DOI] [PubMed] [Google Scholar]