Short abstract
Background
Population-based estimates of costs of illness and health-related quality of life, by disability levels among people with multiple sclerosis, are lacking.
Objectives
To estimate the annual costs of illness and health-related quality of life, by disability levels, among multiple sclerosis patients, 21–64 years of age.
Methods
Microdata from Swedish nationwide registers were linked to estimate the prevalence-based costs of illness in 2013, including direct costs (prescription drug use and specialised healthcare) and indirect costs (calculated using sick leave and disability pension), and health-related quality of life (estimated from the EQ-5D). Disability level was measured by the Expanded Disability Status Scale (EDSS).
Results
Among 8906 multiple sclerosis patients, EDSS 0.0–3.5 and 7.0–9.5 were associated with mean indirect costs of SEK 117,609 and 461,357, respectively, whereas direct costs were similar between the categories (SEK 117,423 and 102,714, respectively). Prescription drug costs represented 40% of the costs of illness among multiple sclerosis patients with low EDSS, while among patients with high EDSS more than 80% were indirect costs. Among the 1684 individuals who had reported both EQ-5D and EDSS, the lowest health-related quality of life scores were found among those with a high EDSS.
Conclusion
Among people with multiple sclerosis, we confirmed higher costs and lower health-related quality of life in higher disability levels, in particular high indirect costs.
Keywords: Multiple sclerosis, cost of illness, healthcare costs, registries, sick leave, health-related quality of life, disability evaluation, disease progression
Date received: 3 February 2018; Revised received 18 May 2018; accepted: 20 May 2018
Introduction
Due to the early onset and diagnosis, often at 20–40 years of age,1 multiple sclerosis (MS) is the most common degenerative neurological disease in people of working age,2 and its impact on paid work, sickness absence and disability pension is often large.
The total societal costs of illness (COI) for MS in Europe were estimated at EUR 14,500 million in 2010.3 In addition to the direct costs (including healthcare, medication and rehabilitation measures),4 almost one-third of the COI were indirect costs for productivity losses.3 Intangible costs related to pain and suffering, impact on quality of life, etc., should also be considered, in addition to the direct and indirect costs.5 However, important developments have occurred in the treatment of MS since the mid-1990s6 and onwards,7 potentially changing both the costs and health outcomes among people with MS since previous estimates.
With worsening disability, the overall COI among MS patients increases.8–10 Moreover, the distribution of costs have been observed to change over the disease trajectory, with the majority of costs being related to treatment at low disability levels towards a higher proportion of indirect costs and non-medical direct costs at higher disability levels.8,11 Increasing disability has also been associated with decreasing health-related quality of life (HRQoL).12
However, few of the previous studies of MS patients were population based; rather the literature is largely based on questionnaires sent to selected samples of patients from specific healthcare units or patient organisations, and sometimes with low response rates and numbers of participants.8,13 It has been suggested that people with mild MS are underrepresented in surveys, compared to population-based samples,14 thus probably overestimating the mean cost per MS patient. However, patients with mild MS may also report disabilities,15 in which case the total COI for MS could be underestimated from surveys if this patient group is unaccounted for, or its distribution is distorted. To validate survey results in relation to all MS patients in a population, population-based studies examining costs and HRQoL by disability levels among MS patients are warranted.
The aim of the study was to estimate the annual COI and HRQoL, by disability levels, among all people with MS of working age.
Materials and methods
The source population consisted of all individuals 21–64 years old living in Sweden in 2013 who also lived in Sweden in 2009. Microdata were linked between the registers using personal identification numbers. Individuals with MS were identified from the Swedish nationwide clinical MS register (SMSreg),16 administered by the Karolinska University Hospital, covering over 80% of all MS patients in Sweden.16 Individuals 21–64 years old and registered in the SMSreg, in or before 2013, were included as MS patients (N=9183). Sociodemographic characteristics of identified MS patients were obtained from the Longitudinal Integration Database for Health Insurance and Labour Market Studies (LISA), administered by Statistics Sweden.
To evaluate the representativeness of the study population, individuals with MS were also identified using the International Classification of Disease (ICD) code for MS (ICD10 code G35 and ICD9 code 340) registered in the National Patient Register (PAR),17 as individuals with at least one previous MS diagnosis in the inpatient or specialised outpatient care registers (same source population as described above, diagnoses identified since 1987, N=15,330). PAR is administered by the National Board of Health and Welfare.
The Regional Ethical Review Board of Stockholm, Sweden, approved the project (approval numbers: 2007/762-31; 2009/23-32; 2009/1917-32; 2010/466-32; 2011/806-32; 2011/1710-32; 2014/236-32).
Disability in MS patients is typically measured by the Expanded Disability Status Scale (EDSS),18 the most widely accepted and used disability scale for MS patients.19 The EDSS assessments reported in 2013, or the most recent scores in the preceding years, were obtained from the SMSreg.16 EDSS scores were registered for 8906 individuals (97%), of which 5065 individuals (55%) had scores registered during 2013, with 285 individuals also having a registered relapse this year.
Costs for the year 2013 were included, estimated with a societal perspective. Drug costs included both patient out-of-pocket costs and the reimbursement paid by counties, as identified in the Swedish Prescribed Drug Register,20 administered by the National Board of Health and Welfare. In addition, the use of intravenous MS drugs that are not usually dispensed through pharmacies but through the specialised healthcare clinics (in 2013 this included natalizumab and rituximab, hereafter called ‘indented drugs’) were identified through the SMSreg (where most such treatments are registered) for patients on current use, i.e. patients who at 1 July 2013 had such treatment. The unit cost for each indented drug was based on the expected annual cost for each drug: SEK 203,000 for natalizumab and SEK 26,000 for rituximab.21 The most recent registered indented drug was included. Based on the above definition for current use, we added the cost of natalizumab to the drug costs for 1217 patients and of rituximab for 549 patients.
Healthcare costs were calculated from diagnosis-related group (DRG) codes in PAR, and were transformed to costs using DRG weights22 and the national average cost per 1.0 DRG (SEK 50,229).23 The inclusion of hospitalisation costs was based on the date of discharge, including a maximum duration of 365 hospital days per patient. Patient out-of-pocket costs for healthcare use are not registered, and were not included in the analysis. In 2013, patient out-of-pocket costs covered 1.2% of all costs to the county councils.24 The national annual ceiling for healthcare out-of-pocket costs was SEK 1100 for healthcare use in a 12-month period.25
Indirect costs were calculated from productivity losses based on the number of net days on sick leave or disability pension compensated by the Social Insurance Agency (from LISA). Sweden has a universal public sickness absence insurance system covering all individuals with income from work or unemployment benefits, if they due to disease or injury have temporarily or permanently reduced work capacity or permanent work incapacity. The indirect costs were calculated by the human capital approach,4 using the age-adjusted mean wage and the social security contributions. The first 14 days of a sick leave spell, paid by the employer, were not included.
HRQoL measured by the EuroQol Group’s five-dimension (i.e. mobility, self-care, usual activities, pain/discomfort and anxiety/depression) health state questionnaire with three levels of severity (i.e. no, moderate, or extreme problems), EQ-5D, was obtained from SMSreg. Each respondents’ result was transformed using both the Swedish experience-based value set26,27 and the UK general population-based value set,28 respectively, to estimates of the respondents’ HRQoL. The EQ-5D responses were complemented with results from the EuroQol Group’s visual analogue scale (EQ-VAS): from ‘worst imaginable health state’ (=0) to ‘best imaginable health state’ (=100). EQ-5D responses reported in 2013 were used, or the most recent response given in preceding years. Responses were available from 1955 (21%) of included individuals, of which 1732 (86%) of the responses were from 2013.
Analyses
Characteristics, mean COI (summarised and by cost components) and HRQoL were reported by disability-level categories (EDSS ≤3.5, 4–5.5, 6–6.5, and ≥7, respectively). The costs and HRQoL were analysed, by disability levels, using two-tailed t-tests or analysis of variance (ANOVA) (to account for unequal variances, ANOVA results were simulated with 5000 repetitions) and accounting for unequal variances. A P value below 0.05 was used for statistical significance. Due to skewness, all 95% confidence intervals (CIs) were calculated using bootstrap with 1000 repetitions. Costs were compared for patients identified in the SMSreg to those found in PAR. Accumulation of costs by components (types of costs), were described graphically by HRQoL and disability level. Furthermore, mean COI, direct and indirect costs were converted to Euros using the 2013 exchange rate (EUR 1 = SEK 8.6494).
Analyses were conducted using Stata version 14.1.
Sensitivity analyses
Sensitivity analyses were conducted of the cost estimates to account for the time elapsed since reporting EDSS and HRQoL (including only those individuals with responses reported in 2013, all responses during the last 2 years and all individuals with responses during the last 5 years), as more recent estimates may be more representative for the current health state of each MS patient.
Results
Men, older patients and those with a lower educational level were more likely to be in the higher EDSS categories, compared to other MS patients in the SMSreg (Table 1).
Table 1.
Characteristics of MS patients alive in 2013 and identified from the SMSreg and PAR, respectively, and by disability levels/categories for those identified in the SMSreg.
|
MS patients in SMSreg, by level of disability measured as EDSS |
All MS patients identified in SMSreg | All individuals with previous MS diagnosis in PAR | ||||
|---|---|---|---|---|---|---|
| 0–3.5 | 4–5.5 | 6–6.5 | 7–9.5 | |||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Sex | ||||||
| Men | 1629 (27) | 275 (29) | 302 (31) | 303 (34) | 2588 (28) | 4548 (30)* |
| Women | 4481 (73) | 673 (71) | 659 (69) | 584 (66) | 6595 (72)* | 10,782 (70) |
| Age groups | ||||||
| 21–24 years old | 162 (3) | 6 (1) | 3 (0.3) | 1 (0.1) | 189 (2) | 326 (2) |
| 25–29 years old | 412 (7) | 16 (2) | 7 (1) | 4 (0.5) | 464 (5) | 731 (5) |
| 30–34 years old | 699 (11) | 33 (3) | 19 (2) | 14 (2) | 803 (9)* | 1226 (8) |
| 35–39 years old | 934 (15) | 70 (7) | 48 (5) | 28 (3) | 1121 (12)*** | 1640 (11) |
| 40–44 years old | 1003 (16) | 114 (12) | 75 (8) | 78 (9) | 1326 (14)** | 2020 (13) |
| 45–49 years old | 994 (16) | 187 (20) | 152 (16) | 123 (14) | 1498 (16) | 2421 (16) |
| 50–54 years old | 836 (14) | 169 (18) | 186 (19) | 169 (19) | 1388 (15) | 2334 (15) |
| 55–59 years old | 623 (10) | 176 (19) | 222 (23) | 222 (25) | 1266 (14) | 2336 (15)** |
| 60–64 years old | 447 (7) | 177 (19) | 249 (26) | 248 (28) | 1128 (12) | 2296 (15)*** |
| Education | ||||||
| ≤9 yearsa | 479 (8) | 145 (15) | 150 (16) | 156 (18) | 960 (10) | 1908 (12)*** |
| 10–12 years | 2774 (45) | 464 (49) | 503 (52) | 446 (50) | 4337 (47) | 7376 (48) |
| ≥13 years | 2857 (47) | 339 (36) | 308 (32) | 285 (32) | 3886 (42)*** | 6046 (39) |
| Country of birth | ||||||
| Sweden | 5522 (90) | 843 (89) | 859 (89) | 775 (87) | 8246 (90) | 13,655 (89) |
| Other than Sweden | 588 (10) | 105 (11) | 102 (11) | 112 (13) | 937 (10) | 1675 (11) |
| Type of living areab | ||||||
| Larger cities | 2692 (44) | 446 (47) | 369 (38) | 407 (46) | 3990 (43)*** | 5817 (38) |
| Medium-sized municipalities | 1914 (31) | 281 (30) | 320 (33) | 269 (30) | 2888 (31) | 5307 (35)*** |
| Smaller municipalities | 1504 (25) | 221 (23) | 272 (28) | 211 (24) | 2305 (25) | 4206 (27)*** |
| Geographical regionc | ||||||
| East Sweden | 2596 (42) | 426 (45) | 331 (34) | 379 (43) | 3801 (41)*** | 5872 (38) |
| South Sweden | 2247 (37) | 319 (34) | 414 (43) | 329 (37) | 3487 (38) | 6572 (43)*** |
| North Sweden | 1267 (21) | 203 (21) | 216 (22) | 179 (20) | 1895 (21)*** | 2886 (19) |
Results from two-tailed z-tests for all individuals identified from PAR versus SMSreg: *P≤0.05, **P≤0.01, ***P≤0.001. Bold indicates higher proportion among patients within a specific EDSS category, and italics indicate lower proportion in the EDSS category, as compared to all individuals identified from SMSreg, at P≤0.05, using two-tailed z-tests.
aIncludes 50 persons with missing educational level in the LISA register.
bBased on population density according to the H-region classification scheme: larger cities (H1–H2), medium-sized municipalities (H3–H4), or smaller municipalities (H5–H6), Statistics Sweden. Report no. MIS 2003:1.
cBased on Eurostat’s Nomenclature of Territorial Units for Statistics classification (NUTS1): East Sweden (SE1), South Sweden (SE2), or North Sweden (SE3), European Union amendment No 105/2007 to Regulation (EC) No 1059/2003.
EDSS: Expanded Disability Status Scale; MS: multiple sclerosis; n: number of people; PAR: the national patient register; SMSreg: the Swedish MS register.
The mean COI of MS patients was SEK 313,915 (95% CI SEK 309,066–318,765) in 2013 (Table 2). This corresponds to a mean COI of EUR 36,293, of which the estimated mean direct costs were EUR 13,242 and mean indirect costs were EUR 23,051. The mean COI corresponded to EUR 27,173 among patients with EDSS of 3.5 or less, EUR 47,431 among EDSS 4–5.5, EUR 56,235 among EDSS 6–6.5, and EUR 65,215 among EDSS of 7 and greater.
Table 2.
Mean direct and indirect costs in 2013 (summarised and by cost components) among individuals with MS, presented by disability levels.
|
MS patients in SMSreg, by level of disability measured as EDSS |
All MS patients identified in SMSreg | All individuals with a previous MS diagnosis in PAR | ||||
|---|---|---|---|---|---|---|
| 0–3.5 | 4–5.5 | 6–6.5 | 7–9.5 | |||
| Cost components | SEK (95%CI) n=6110 | SEK (95%CI) n=948 | SEK (95%CI) n=961 | SEK (95%CI) n=887 | SEK (95%CI) n=9183 | SEK (95%CI) n=15,330 |
| Prescription drug use | 90,589 (88,599–92,579) | 68,159 (63,230–73,088) | 50,029 (45,639–54,419) | 26,437 (23,318–29,557) | 79,258* (77,638–80,877) | 60,415a (59,298–61,533) |
| Outpatient specialised healthcare use | 14,147 (13,760–14,534) | 16,065 (15,042–17,088) | 17,386 (15,792–18,980) | 18,752 (16,938–20,566) | 15,248* (14,865–15,630) | 14,079 (13,781–14,378) |
| Inpatient healthcare use | 12,687 (11,208–14,166 | 20,702 (15,577–25,826) | 31,060 (26,122–35,998) | 57,525 (46,838–68,212) | 20,033 (18,449–21,618) | 19,612 (18,338–20,886) |
| Direct costs | 117,423 (114,844–120,003) | 104,926 (97,442–112,411) | 98,475 (91,423–105,528) | 102,714 (91,035–114,394) | 114,539* (112,274–116,803) | 94,107a (92,276–95,937) |
| Sick leaveb | 32,107 (29,904–34,310) | 47,969 (40,933–55,005) | 32,226 (26,538–37,913) | 10,622 (7,253–13,992) | 32,183 (30,315–34,050) | 31,286 (29,825–32,748) |
| disability pension | 85,502 (81,602–89,403) | 257,357 (243,483–271,232) | 355,698 (343,903–367,493) | 450,735 (442,482–458,987) | 167,194 (162,879–171,510) | 168,653 (165,171–172,236) |
| indirect costs | 117,609 (113,507–121,712) | 305,326 (292,865–317,787) | 387,924 (377,485–398,362) | 461,357 (454,384–468,331) | 199,377 (195,119–203,635) | 199,940 (196,539–203,340) |
| COI | 235,032 (230,153–239,912) | 410,252 (395,550–424,955) | 486,399 (473,829–498,969) | 564,071 (550,150–577,993) | 313,915* (309,066–318,765) | 294,046a (290,033–298,060) |
Results from two-tailed t-tests for all individuals identified from PAR versus SMSreg: *P≤0.0001. Any non-overlapping 95% CIs, between EDSS categories, are indicated in the table: higher 95% CIs as bold text and lower 95% CIs as italics.
aThe cost of prescription drugs is underestimated among patients not in the SMSreg, since indented injection/infusion drugs are not reported in the Swedish Prescribed Drug Register.
EDSS: Expanded Disability Status Scale; MS: multiple sclerosis; PAR: the national patient register; SEK: Swedish Krona; SMSreg: the Swedish MS register; 95% CI: 95% confidence interval.
Among all individuals with at least one previous MS diagnosis in PAR, the mean COI was SEK 294,046 (SEK 290,033–298,060) (Table 2).
Prescription drug costs represented 40% of the COI among MS patients with EDSS of 3.5 or less. In the higher EDSS categories the COI increase was driven by higher indirect costs (representing 82% of the COI in patients with EDSS ≥7). The mean cost for inpatient care was more than four times higher among patients with EDSS of 7 or greater compared to EDSS of 3.5 or less. The highest mean indirect cost resulting from sick leave was estimated among those with EDSS 4–5.5.
Those with low EDSS had on average higher estimated HRQoL, while the lowest HRQoL estimates were found among those with EDSS of 7 or greater (Table 3). The largest accumulation of costs occurred among patients with high HRQoL (steep slope in both Figures 1 and 2, using the Swedish and UK value sets, respectively); however, this was mostly explained by the large proportion of patients with high HRQoL. The same was found when examining accumulated costs by EDSS (Figure 3), but it appears that the accumulation of prescription drug costs was larger among those with EDSS in the lowest category (≤3.5), than the accumulation of the population (steeper slope for the drug costs). Similarly, the accumulation of productivity losses due to disability pension was largest in those with higher EDSS (≥6).
Table 3.
HRQoL among 1955 individuals with MS in 2013, presented by disability levels.
|
MS patients with EQ-5D responses, by level of disability measured by EDSS |
All MS patients with EQ-5D responses | ||||
|---|---|---|---|---|---|
| 0–3.5 | 4–5.5 | 6–6.5 | 7–9.5 | ||
| Health dimensions | Median (Q1–Q3) n=1323 | Median (Q1–Q3) n=191 | Median (Q1–Q3) n=122 | Median (Q1–Q3) n=48 | Median (Q1–Q3) n=1684 |
| Mobility | 1 (1–1) | 2 (2–2) | 2 (2–2) | 2 (2–3) | 1 (1–2) |
| Self-care | 1 (1–1) | 1 (1–1) | 1 (1–2) | 2 (1–3) | 1 (1–1) |
| Usual activities | 1 (1–2) | 2 (1–2) | 2 (1–2) | 2 (2–3) | 1 (1–2) |
| Pain/discomfort | 2 (1–2) | 2 (2–2) | 2 (2–2) | 2 (2–3) | 2 (1–2) |
| Anxiety/depression | 1 (1–2) | 2 (1–2) | 2 (1–2) | 2 (1–2) | 1 (1–2) |
| Swedish experience-based index values, mean (95% CI) | 0.867 (0.860–0.873) | 0.752 (0.735–0.768) | 0.724 (0.704–0.745) | 0.626 (0.584–0.668) | 0.836 (0.830–0.842) |
| UK general population-based index values, mean (95% CI) | 0.766 (0.753–0.778) | 0.579 (0.541–0.616) | 0.526 (0.476–0.576) | 0.141 (0.019–0.264) | 0.709 (0.697–0.722) |
| EQ-VAS scale | 71 (70–73) | 57 (54–60) | 54 (49–58) | 45 (38–52) | 68 (67–69) |
Any non-overlapping 95% CIs, between EDSS categories, are indicated in the table: higher 95% CIs as bold text and lower 95% CIs as italics.
EDSS: Expanded Disability Status Scale; EQ-5D: EuroQol Group’s five-dimension health state questionnaire with three levels of severity; HRQoL: health-related quality of life; MS: multiple sclerosis; Q1–Q3: interquartile range, 1st to 3rd quartile.
Figure 1.
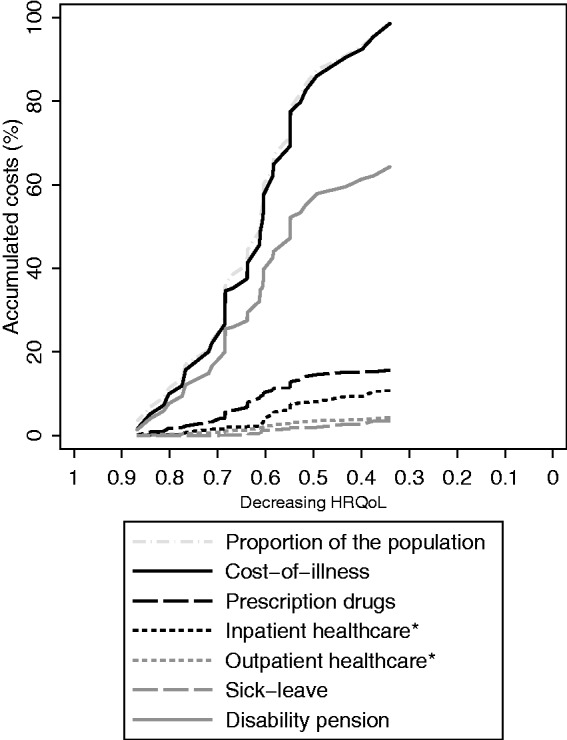
Accumulation of costs by components (types of costs), described graphically among MS patients, sorted from high to low HRQoL (translated from EQ-5D using the Swedish experience-based value set). HRQoL: health-related quality of life; EQ-5D: EuroQol Group’s five-dimension health state questionnaire with three levels of severity; MS: multiple sclerosis.
Figure 2.
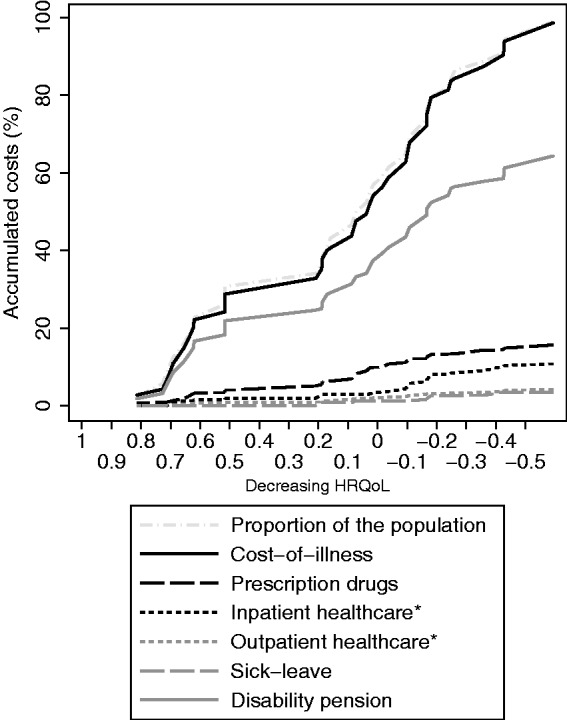
Accumulation of costs by components (types of costs), described graphically among MS patients, sorted from high to low HRQoL (translated from EQ-5D using the UK general population-based value set). HRQoL: health-related quality of life; EQ-5D: EuroQol Group’s five-dimension health state questionnaire with three levels of severity; MS: multiple sclerosis.
Figure 3.
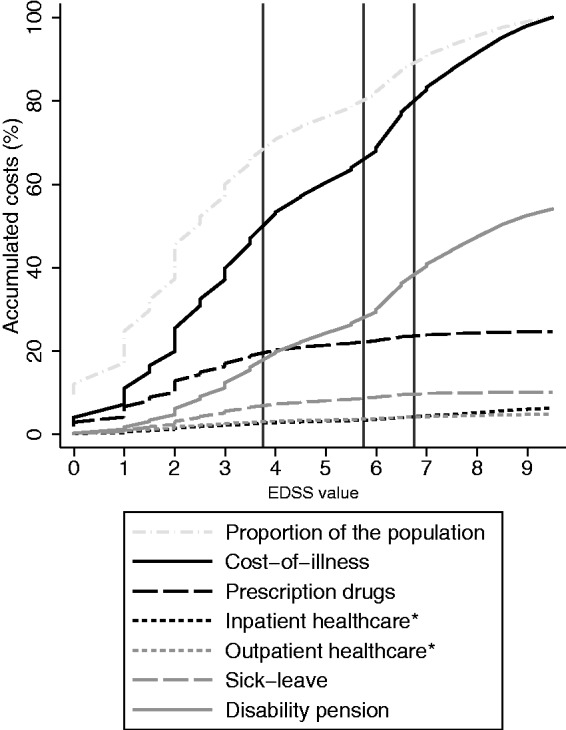
Accumulation of costs by components (types of costs), described graphically among MS patients, sorted by disability level (measured by EDSS). The vertical lines indicates the EDSS categories presented in the tables. EDSS: Expanded Disability Status Scale; MS: multiple sclerosis.
The sensitivity analysis accounting for time elapsed since reporting EDSS showed that the mean COI was very similar among those with EDSS responses during 2013 compared to that among all MS patients (Table 4), but with a different distribution between direct and indirect costs. Moreover, patients with relapses had both higher mean direct costs and COI than other patients reporting EDSS during 2013.
Table 4.
Sensitivity analysis by years since reporting of EDSS and EQ-5D, respectively.
| Included MS patients | N | Direct costs | Indirect costs | Total COI |
|---|---|---|---|---|
| 95% CIa (SEK) | 95% CIa (SEK) | 95% CIa (SEK) | ||
| All registered in SMSreg | 9183 | 112,216–116,862 | 195,070–203,684 | 309,012–318,818 |
| All with registered EDSS | 8906 | 110,225–114,942 | 196,611–205378 | 308,590–318,566 |
| EDSS registered 2008–2013 | 8350 | 114,547–119,409 | 193,415–202,394 | 309,786–319,979 |
| … and relapse | 1753 | 103,929–116,303 | 196,988–216,595 | 305,201–328,614 |
| EDSS registered 2011–2013 | 7362 | 119,971–125,195 | 186,980–196,423 | 308,882–319,686 |
| … and relapse | 1656 | 105,844–118,752 | 195,322–215,468 | 305,597–329,789 |
| EDSS registered 2013 | 5065 | 130,399–136,334 | 174,950–186,068 | 307,537–320,214 |
| … and relapse | 285 | 143,233–168,798 | 161,768–204,351 | 311,904–366,246 |
| All with registered EQ-5D | 1955 | 169,936–178,563 | 146,070–162,787 | 318,851–338,505 |
| EQ-5D registered 2008–2013 | 1979 | 169,866–178,439 | 146,936–163,598 | 319,647–339,192 |
| … and relapse | 489 | 166,705–184,605 | 144,622–178,042 | 317,460–356,515 |
| EQ-5D registered 2011–2013 | 1978 | 169,522–178,522 | 147,012–163,679 | 319,809–339,355 |
| … and relapse | 489 | 166,705–184,605 | 144,622–178,042 | 317,460–356,515 |
| EQ-5D registered 2013 | 1732 | 170,981–179,960 | 142,492–160,103 | 316,422–337,114 |
| … and relapse | 384 | 168,134–187,450 | 132,056–168,402 | 306,544–349,497 |
aConfidence intervals are not bootstrapped for this analysis.
EDSS: Expanded Disability Status Scale; EQ-5D: EuroQol Group’s five-dimension health state questionnaire with three levels of severity; HRQoL: health-related quality of life; MS: multiple sclerosis; SEK: Swedish Krona; SMSreg: the Swedish MS register; 95% CI: 95% confidence interval.
Patients with HRQoL assessments had higher mean direct costs and lower mean indirect costs than other patients in the SMSreg, while the mean COI was similar regardless of years since reporting (Table 4). Moreover, the sensitivity analysis found that among patients with HRQoL reported in 2013, there was a trend towards higher costs in 2013 if the EDSS was reported a few years earlier, rather than in 2013 (Figure 4).
Figure 4.
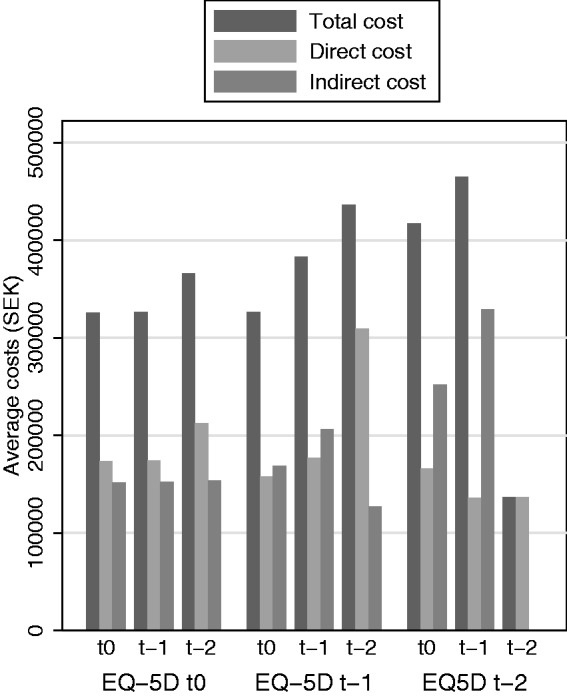
Total, direct and indirect costs, respectively, by time since last reporting of EDSS and EQ-5D during the last 2 years (excluding one person with relapse during EQ-5D response). EDSS: Expanded Disability Status Scale; EQ-5D: EuroQol Group’s five-dimension health state questionnaire with three levels of severity; MS: multiple sclerosis.
Discussion
In this population-based study of MS patients we observed higher mean COI and lower mean HRQoL among those with more severe disability, and the main driver of the observed higher COI was the larger productivity losses due to disability pension. Moreover, patients with less disability had higher costs for prescription drugs and those with intermediate disability levels had larger productivity losses from sick leave.
A major strength of this study is the representative and large study group. The availability of personal identification numbers enabled the use of registers containing administrative data with nationwide coverage to examine the external validity of our results. Although there were some statistically significant differences in the characteristics of the respective MS patients, identified in SMSreg and PAR, the absolute differences were small. This indicates that our study sample was overall representative, and that the generalisability of the findings was high. In addition, 97% of patients in the SMSreg had a registered EDSS score, thus providing the opportunity to explore disability in a representative sample of the MS population. The difference in costs between patients identified in SMSreg and PAR can to a large extent be explained by the availability of data on indented drugs, which cannot be solved by adding overall sales of the indented drugs to PAR data, because not all such drugs are prescribed only for MS (moreover, overall sales figures could not be analysed at the individual level).
However, there are limitations in what information is captured in these data sources. MS patients also have high non-medical costs (personal assistance, etc.), informal care and intangibles;5 however, such information is not available in the Swedish registers. According to a recent study, personal assistance and other community services represented a large proportion of the COI among MS patients with high EDSS scores, in particular in Sweden.29 Moreover, DRG-based healthcare costs do not reflect the actual resource use of the specific healthcare encounters, but are based on an expected resource burden estimated from a combination of information (diagnosis, type of encounter and some sociodemographic characteristics).30 However, DRGs are commonly used for national scale costing of healthcare resource use.23 Measuring indirect costs using the human capital approach has been criticised for overestimating the total cost of productivity losses.31 However, it is also possible to use the method when friction periods are unknown, and it has been argued to be valid for measuring indirect costs.32 There are some documented weaknesses in measuring disability by EDSS regarding reliability and sensitivity to change; nevertheless, EDSS is deemed suitable for monitoring disease progression.19 Furthermore, the costs estimated in this study are not costs induced by MS disease only, but rather the COI among MS patients, and the results thus need to be interpreted with this in mind.
The SMSreg has high and improving coverage.16 We found a lower registration rate among working-age adults in the SMSreg (60%) compared to the estimated rate for the whole MS population (>80%).16 This may be the result of having used data from one year earlier than in the previous study.16 However, it appears, from the comparison with individuals identified through PAR, as if our study population was representative for all individuals with any previous MS diagnosis, with regard to patient characteristics, healthcare resource use and productivity losses. It has, moreover, been suggested that not all individuals with an ICD code indicating MS in PAR are in fact diagnosed with MS (due to miscoding of, for example, examinations of suspected MS cases that are later found to have another diagnosis); thus the population identified from the SMSreg will probably represent a larger proportion of all working-age MS patients.
Our results from the sensitivity analysis, that COI were higher if EDSS was reported during 2013, indicate that EDSS assessments are reported to a slightly higher degree when patients are worse in their disease, or possibly among those who currently have a more expensive treatment (higher direct costs) and during relapses. In contrast, EQ-5D was not always reported at the same time as EDSS. It can be speculated that MS patients were more likely to report EDSS during worsening of the disease, while EQ-5D appears to have been used very infrequently before 2013. It is known that some variables in the SMSreg have primarily been used for patients initiating some of the newer treatments, e.g. as part of the IMSE study,16 thus potentially biasing the analysis of HRQoL towards pertaining to newly diagnosed MS patients.
Our results are in line with previous finding that drug costs were the main cost driver among those with low EDSS and productivity losses among those with high EDSS.8 Moreover, an approximate proportionality (1:2:3) was previously found of the COI between EDSS categories, with EDSS roughly categorised as less than 3, 4–6, over 6.8 Similar proportionalities in costs have also been reported in previous reviews of the literature.9,10 Nevertheless, this approximate proportionality was not seen in our study, in which costs were more similar (1.7–2.4 times higher than costs in the lowest category). This may be the result of our register-based method excluding the costs associated with informal care and non-medical costs. It is also possible that our age criteria excluding MS patients over 64 years of age resulted in the higher EDSS category in particular being biased towards healthier groups of patients and thus reducing the mean cost. However, that is contradicted by the high average COI in our study among patients in the highest EDSS category, compared to the results by Kobelt and colleagues.29 Moreover, we found the mean direct cost per patient was very similar across the EDSS categories. This is in contrast to results by, for example, Tyas et al.,33 which showed increasing medical costs for each increase in EDSS except for the very lowest levels. This highlights the importance and need for comparable data and categorisation between studies, and comprehensive reporting of cost components.
Compared to previous studies, we also found a lower mean EDSS and higher HRQoL.5 This can be explained by our population-based sample (and by the set age limits), as most previous studies have recruited patients from specific clinics or patient organisations, and consequently have often resulted in underestimating patients with milder disease.8,14 Moreover, the finding of lower HRQoL among patients with higher EDSS is in line with previous research.14,34
Comparing our results further with those in the recent publication by Kobelt and colleagues29 can improve how we quantify the economic impact of MS in society. Their study was based on questionnaire responses from MS patients in 16 European countries, including 1864 patients from Sweden,29 (representing ≈10% of all MS patients in Sweden).16,35 In the lowest EDSS category, they identified a mean COI of approximately EUR purchasing power parities (PPPs) 20,000 in 2015,29 which is slightly lower than our estimated EUR 27,000 in 2013 values. (PPPs is a way of translating costs between currencies by purchasing power (available from the Organisation for Economic Co-operation and Development, OECD) instead of the exchange rate.) In the highest EDSS category, however, they found a mean COI of EUR PPPs 75,000.29 That figure included approximately EUR PPPs 50,000 for community services and informal care;29 resource uses unavailable in registers. The remaining EUR PPPs 25,00029 should, thus, be compared to our estimated EUR 57,600, potentially indicating a healthy responder effect within each EDSS category in the Kobelt study, which is also strengthened by their reported HRQoL estimates within each EDSS category.36 In addition, the study by Kobelt and colleagues calculates costs based on resource use reported by participants to be caused by their MS,37 which differs from the all-cause costs reported here. Diagnosis-specific costs can seldom be derived from register data, but we have previously reported that MS was the main condition for resource use corresponding to approximately 50% of all direct and indirect costs of MS patients.38 In particular, in that study, MS as a main condition was associated with indirect costs, the cost category that is driving costs among those with worse disability, and which is also the cost that causes the large difference in results between our study and the study by Kobelt and colleagues.36
Conclusion
We confirmed higher costs and lower HRQoL among people with MS who have higher levels of disability. This was particularly so for indirect costs, although the effect was less pronounced than in previous studies when examining differences between categories of EDSS already equating to high disability.
Conflict of Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Hanna Gyllensten, Emilie Friberg and Petter Tinghög were funded by an unrestricted research grant from Biogen. Furthermore, Hanna Gyllensten is employed part-time by Statfinn & EPID (part of IQVIA) Research, which is a contract research organisation that performs commissioned pharmacoepidemiological studies and thus its employees have been and currently are working in collaboration with several pharmaceutical companies. Andrius Kavaliunas declares that there is no conflict of interest. Kristina Alexanderson has received unrestricted research grants from Biogen and from the Swedish Research Council for Working Life, Health and Welfare. Jan Hillert has received honoraria for serving on advisory boards for Biogen and Novartis and speaker’s fees from Biogen, Merck-Serono, Bayer-Schering, Teva and Sanofi-Aventis. He has served as principal investigator for projects sponsored by, or received unrestricted research support from, Biogen, Merck-Serono, TEVA, Novartis and Bayer-Schering. His MS research is funded by the Swedish Research Council.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Biogen and the Swedish Research Council for Health, Working Life and Welfare (2007-1762).
References
- 1.Stüve O, Oksenberg J. Multiple Sclerosis Overview In: Pagon RA, Adam MP, Ardinger HH, et al. (eds) Gene Reviews (R). Seattle: University of Washington, 1993. [Google Scholar]
- 2.Gustavsson A, Svensson M, Jacobi F, et al. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 2011; 21: 718–779. [DOI] [PubMed] [Google Scholar]
- 3.Olesen J, Gustavsson A, Svensson M, et al. The economic cost of brain disorders in Europe. Eur J Neurol 2012; 19: 155–162. [DOI] [PubMed] [Google Scholar]
- 4.Segel JE. Cost-of-Illness Studies – A Primer RTI-UNC Center of Excellence in Health Promotion Economics/RTI International, Research Triangle Park, NC, USA, January 2006.
- 5.Trisolini M, Honeycutt A, Wiener J, et al. Global economic impact of multiple sclerosis RTI International, May 2010.Research Triangle Park, NC, USA.
- 6.Kremer D, Kury P, Dutta R. Promoting remyelination in multiple sclerosis: current drugs and future prospects. Mult Scler 2015; 21: 541–549. [DOI] [PubMed] [Google Scholar]
- 7.Kim W, Zandona ME, Kim SH, et al. Oral disease-modifying therapies for multiple sclerosis. J Clin Neurol 2015; 11: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernstsson O, Gyllensten H, Alexanderson K, et al. Cost of illness of multiple sclerosis – a systematic review. PloSOne 2016; 11: e0159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orlewska E. Economic burden of multiple sclerosis: what can we learn from cost-of-illness studies? Expert Rev Pharmacoecon Outcomes Res 2006; 6: 145–154. [DOI] [PubMed] [Google Scholar]
- 10.Patwardhan MB, Matchar DB, Samsa GP, et al. Cost of multiple sclerosis by level of disability: a review of literature. Mult Scler 2005; 11: 232–239. [DOI] [PubMed] [Google Scholar]
- 11.Naci H, Fleurence R, Birt J, et al. Economic burden of multiple sclerosis: a systematic review of the literature. Pharmacoeconomics 2010; 28: 363–379. [DOI] [PubMed] [Google Scholar]
- 12.Naci H, Fleurence R, Birt J, et al. The impact of increasing neurological disability of multiple sclerosis on health utilities: a systematic review of the literature. J Med Econ 2010; 13: 78–89. [DOI] [PubMed] [Google Scholar]
- 13.Marrie RA, Cohen J, Stuve O, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult Scler 2015; 21: 263–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruutiainen J, Viita AM, Hahl J, et al. Burden of illness in multiple sclerosis (DEFENSE) study: the costs and quality-of-life of Finnish patients with multiple sclerosis. J Med Econ 2016; 19: 21–33. [DOI] [PubMed] [Google Scholar]
- 15.Johansson S, Ytterberg C, Claesson IM, et al. High concurrent presence of disability in multiple sclerosis. Associations with perceived health. J Neurol 2007; 254: 767–773. [DOI] [PubMed] [Google Scholar]
- 16.Hillert J, Stawiarz L. The Swedish MS registry – clinical support tool and scientific resource. Acta Neurol Scand 2015; 132: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiberg M, Friberg E, Stenbeck M, et al. Sources and level of income among individuals with multiple sclerosis compared to the general population: a nationwide population-based study. Mult Scler 2015; 21: 1730–1741. [DOI] [PubMed] [Google Scholar]
- 18.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 19.Meyer-Moock S, Feng YS, Maeurer M, et al. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol 2014; 14: 58-2377-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register –opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007; 16: 726–735. [DOI] [PubMed] [Google Scholar]
- 21.The Dental and Pharmaceutical Benefits Agency. TLV, database of drug prices https://www.tlv.se/in-english/medicines.html (2012, accessed 19 June 2018).
- 22.The National Board of Health and Welfare. National DRG weights www.socialstyrelsen.se/klassificeringochkoder/norddrg/vikter (2014, accessed 1 June 2015).
- 23.Karlsson Å, Serdén L. Vårdkostnader 2013 för NordDRG: en sammanställning av material från den nationella kostnadsdatabasen. The National Board of Health and Welfare; The Swedish Association of Local Authorities and Regions, 2015.
- 24.Statistik om hälso- och sjukvård samt regional utveckling 2013. Swedish Association of Local Authorities and Regions, August 2014.
- 25.Knutsson H. Patientavgifter fr.o.m. den 1 januari 2013 [Patient fees from 1 Jan 2013]. The Swedish Association of Local Authorities and Regions, 01/11 2013.
- 26.Burström K, Sun S, Gerdtham UG, et al. Swedish experience-based value sets for EQ-5D health states. Qual Life Res 2014; 23: 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aronsson M, Husberg M, Kalkan A, et al. Differences between hypothetical and experience-based value sets for EQ-5D used in Sweden: Implications for decision makers. Scand J Public Health 2015; 43: 848–854. [DOI] [PubMed] [Google Scholar]
- 28.Dolan P, Roberts J. Modelling valuations for EQ-5D health states: an alternative model using differences in valuations. Med Care 2002; 40: 442–446. [DOI] [PubMed] [Google Scholar]
- 29.Kobelt G, Thompson A, Berg J, et al. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler 2017; 23: 1123–1136. DOI:10.1177/1352458517694432 [DOI] [PMC free article] [PubMed]
- 30.NordDRG Users’ Manual Version 2012 SWE PR1b. http://www.norddrg.net/norddrgmanual/NordDRG_2012_SWE/index.htm (2012, accessed 24 March 2016).
- 31.Koopmanschap MA, Rutten FF, van Ineveld BM, et al. The friction cost method for measuring indirect costs of disease. J Health Econ 1995; 14: 171–189. [DOI] [PubMed] [Google Scholar]
- 32.Johannesson M, Karlsson G. The friction cost method: a comment. J Health Econ 1997; 16: 249–259. [DOI] [PubMed] [Google Scholar]
- 33.Tyas D, Kerrigan J, Russell N, et al. The distribution of the cost of multiple sclerosis in the UK: how do costs vary by illness severity? Value Health 2007; 10: 386–389. [DOI] [PubMed] [Google Scholar]
- 34.Kwiatkowski A, Marissal JP, Pouyfaucon M, et al. Social participation in patients with multiple sclerosis: correlations between disability and economic burden. BMC Neurol 2014; 14: 115-2377-14-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahlgren C, Odén A, Lycke J. High nationwide prevalence of multiple sclerosis in Sweden. Mult Scler 2011; 17: 901–908. [DOI] [PubMed] [Google Scholar]
- 36.Brundin L, Kobelt G, Berg J, et al. New insights into the burden and costs of multiple sclerosis in Europe: results for Sweden. Mult Scler 2017; 23: 179–191. [DOI] [PubMed] [Google Scholar]
- 37.Kobelt G, Eriksson J, Phillips G, et al. The burden of multiple sclerosis 2015: methods of data collection, assessment and analysis of costs, quality of life and symptoms. Mult Scler 2017; 23: 4–16. [DOI] [PubMed] [Google Scholar]
- 38.Gyllensten H, Wiberg M, Tinghög P, et al. Costs of illness of multiple sclerosis in Sweden: a population-based register study of people of working age. Eur J Health Econ 2018; 19: 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]


