Abstract
Mesenchymal stem cells (MSCs) hold great potential as a regenerative therapy for stroke, leading to increased repair and functional recovery in animal models of cerebral ischaemia. While it was initially hypothesised that cell replacement was an important mechanism of action of MSCs, focus has shifted to their paracrine actions or the so called “bystander” effect. MSCs secrete a wide array of growth factors, chemokines, cytokines and extracellular vesicles, commonly referred to as the MSC secretome. There is evidence suggesting the MSC secretome can promote repair through a number of mechanisms including preventing cell apoptosis, modulating the inflammatory response and promoting endogenous repair mechanisms such as angiogenesis and neurogenesis. In this review, we will discuss the in vitro approaches currently being employed to drive the MSC secretome towards a more anti-inflammatory and regenerative phenotype. We will then examine the role of the secretome in promoting repair and improving recovery in preclinical models of cerebral ischaemia.
Keywords: Cell therapy, mesenchymal stem cell, repair, stroke, secretome
Introduction
Stroke is a major global health problem with limited treatment options which leads to around 6.7 million deaths annually.1 For the 33 million people living with stroke, a significant proportion have some disability.2 Current treatments for acute ischaemic stroke are based on reperfusion through thrombolysis or endovascular therapy. Both approaches are very effective and have led to significant re-organisation of acute stroke services to allow greater access to these treatments. However, due to the narrow therapeutic window for administration of tPA (< 4.5 h of symptom onset), only 5% of patients in the UK receive thrombolysis3 and an estimated 10% would be eligible for endovascular clot retrieval assuming national coverage,4 which is still not the case. Therefore, there is much interest in developing regenerative therapies to alleviate the disability caused by stroke.
One promising candidate being widely investigated as a cell therapy for ischaemic stroke is mesenchymal stem/stromal cells (MSCs), multipotent cells first described by Friedenstein and colleagues in the 1960s and 1970s.5 While initially found in bone marrow, MSCs have since been isolated from most postnatal organs6 including adipose tissue,7 dental pulp,8 lungs, liver, spleen and brain.9,10 MSCs are also present in foetal tissues such as placenta, umbilical cord11 and Wharton’s jelly.12 The International Society for Cellular Therapy (ISCT) has defined the minimum criteria for MSCs as: adherence to tissue culture plastic; multipotency as demonstrated by in vitro differentiation into osteoclasts, adipocytes and chondroblasts; expression of surface markers CD73, CD90 and CD105; and negative for CD34, CD45, CD14 or CD11b, C79α or CD19 and HLA-DR.13
A large number of clinical trials (794 as of January 2018) have been conducted or are ongoing to investigate MSCs as a potential therapy for a wide range of diseases including graft versus host disease, haematological malignancies, diabetes, and neurological diseases such as Alzheimer’s disease and amyotrophic lateral sclerosis.14,15 More specifically, a number of phase I/II clinical trials have suggested MSCs are a safe and feasible therapy for stroke.16–21 MSCs are immune evasive22 and less immunogenic than many other cell types due to low expression of majority histocompatibility complex class I molecules.23 In support of this, a meta-analysis conducted by Lalu et al.14 found no association between acute infusional toxicity and MSC treatment overall and no adverse events in the 13 studies that used allogeneic cells. Thus, allogeneic transplantation without immunosuppressive therapy appears to be safe which has numerous advantages over autologous therapies including decreased cost and time to administration.23
Numerous preclinical studies have demonstrated that treatment with stem cells, including MSCs, promotes functional recovery in rodent models of cerebral ischaemia. Although it was thought initially that the principle mechanism of therapeutic action of stem cells was direct replacement of dead and injured cells, this has been largely disregarded as very few cells reach the site of injury, engraft and survive long term.24,25 Following administration by intravenous (IV) or intra-arterial (IA) injection, the vast majority of MSCs become entrapped in the lungs within 48 h.26,27 Li et al.28 reported that around 4% of cells were present in the ischaemic brain of rats 14 days after tail vein injection. Additionally, only a small percentage (<10%) of transplanted MSCs differentiate and express neuronal markers such as NeuN and MAP-2.29–32 To further disregard the cell replacement hypothesis, MSCs lack expression of the voltage-gated ion channels required for generating action potentials.33 Despite this, MSC treatment leads to significant improvements in functional outcomes and can occur independently of cell migration to the ischaemic brain.28,34 There is growing evidence to support the paracrine actions of MSCs, also known as the bystander effect, in improving outcome in preclinical models of stroke. MSCs secrete a wide range of chemokines, cytokines, growth factors and extracellular vesicles (EVs) collectively termed the secretome.
In this review, we will firstly discuss in vitro approaches to modifying the MSC secretome to enhance a more anti-inflammatory and regenerative phenotype. We will then look at the involvement of the MSC secretome in promoting repair mechanisms, modulating inflammation and improving functional outcomes in preclinical models of cerebral ischaemia.
Approaches to enhancing the MSC secretome
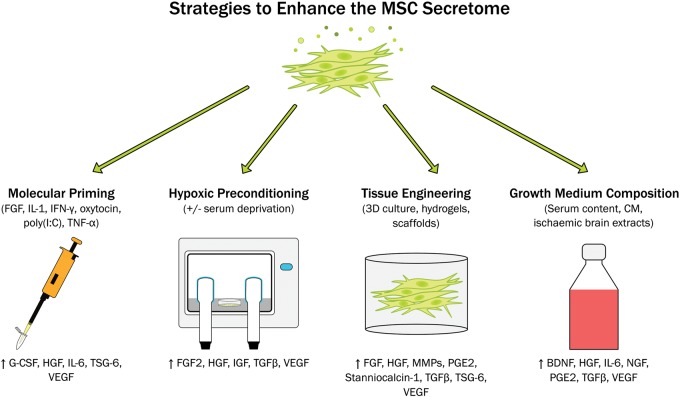
MSCs secrete numerous growth factors, chemokines and cytokines including vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF-1), basic fibroblast growth factor (bFGF), transforming growth factor beta-1 (TGF-β1), nerve growth factor (NGF), placental growth factor (PGF), stromal-derived growth factor (SDF-1/CXCL12), monocyte chemoattractant protein-1 (MCP-1/CCL2), interleukin-6 (IL-6), IL-8, IL-10 and IL-13.35–38 There is some heterogeneity in the secretome of different populations of MSCs. Adipose-derived MSCs were reported to have higher mRNA expression of VEGF-D, IGF-1 and IL-8, while dermal sheath and dermal papilla-derived cells secreted higher concentrations of CCL2 and leptin than other populations.39 Additionally, Du et al.40 found increased expression levels of HGF (hepatocyte growth factor), bFGF, IL-6, IL-8, IL-1α and IL-1β in placenta-derived MSCs and in bone marrow-derived populations, VEGF-A, NGF and angiogenin were higher. Thus, MSCs secrete a number of factors that could promote angiogenesis and neurogenesis, prevent apoptosis and modulate inflammatory responses. The MSC secretome therefore has great potential as a regenerative therapy for stroke and a number of strategies have been employed to further enhance this reparative capacity (Figure 1).
Figure 1.
Summary of in vitro approaches that have been utilised to enhance the therapeutic potential of mesenchymal stem cell secretome. BDNF: brain-derived neurotrophic factor; FGF: fibroblast growth factor; G-CSF: granulocyte-colony stimulating factor; HGF: hepatocyte growth factor; IFN-γ: interferon gamma; IGF: insulin-like growth factor; IL: interleukin; MMPs: matrix metalloproteinases; NGF: nerve growth factor; PGE2: prostaglandin E2; TGF-β: transforming growth factor beta; TNF-α: tissue necrosis factor alpha; TSG-6: TNF-α–stimulated gene 6 protein; VEGF: vascular endothelial growth factor.
Molecular priming
Priming or preconditioning acts as a sub-lethal event that can trigger an adaptive response to a future injury or damage. Therefore, administration of “trained” cells better able to respond to the ischaemic and inflammatory environment post-stroke may further enhance the efficacy of MSC therapies. MSCs from different sources (mainly bone marrow, adipose, placenta and umbilical cord) and from different species (human, equine, murine) have been preconditioned or primed. Such in vitro preconditioning strategies can be selective and aimed at improving the secretion of certain factors such as anti-inflammatory TNF-α-stimulated gene 6 protein (TSG-6),41 or to increase survival of MSC once transplanted.42 Non-selective approaches aim to modulate the MSC secretome towards a more desirable phenotype by inducing the secretion of immunomodulatory,43 anti-inflammatory44 or pro-angiogenic molecules.45
MSCs are known to be great immune modulators, so they are often used to decrease inflammatory responses. To enhance this characteristic, cells can be primed with inflammatory mediators such as IL-1,43 TNF-α,41,46,47 IFN-γ48,49 or combinations of these.50 In response to these priming stimuli, MSCs secrete higher concentrations of immunomodulatory mediators including prostaglandin E2 (PGE2), IL-6 and granulocyte-colony stimulating factor (G-CSF)43,51 and upregulate adhesion molecule expression.46,50 This leads to increased promotion of endogenous repair mechanisms including angiogenesis46,50 and osteogenesis46 which has been shown to be beneficial in in vivo models of arthritis,48 joint and cartilage injuries47,50 and bone regeneration.46 It has been reported though, that inflammatory priming can lead to an increased immunogenicity.50 As this can be detrimental in future cell therapies, short priming durations with low doses of pro-inflammatory mediators should be used to limit this undesirable effect. For example, our lab demonstrated 5 min of priming with IL-1α drove the MSC secretome towards a more anti-inflammatory phenotype which decreased secretion of TNF-α and IL-6 from inflamed mouse microglia.43 A wide variety of molecules can be used to prime MSCs and modify their secretome. Indeed, the screening of libraries has already become a suitable strategy to detect active molecules.52 As an example, polyinosinic and polycytidylic acid (poly(I:C)) can be used as a toll-like receptor 3 (TLR3) stimulus to induce an increased anti-inflammatory phenotype,44 while oxytocin53 or FGF245 have been used to increase the angiogenic potential of the MSC secretome.
Hypoxia
Another alternative to induce an improved response to ischemic environments is the use of hypoxic or ischaemic preconditioning. This has been shown to induce increased MSC proliferation and migration,54 upregulation of glucose transporters and adhesion molecule expression,55 and drive the secretome towards a pro-angiogenic phenotype.56 More specifically, hypoxic preconditioning of bone marrow-derived MSCs induces increased secretion of FGF2, VEGF, HGF, TGF-β and IGF.57,58 This has also been reported in MSCs derived from other sources including placenta59,60 and adipose tissue.61 Hypoxic preconditioning can enhance the therapeutic potential of MSCs in vivo preventing apoptosis of cardiomyocytes and promoting angiogenesis after myocardial infarction62 as well increasing secretion of VEGF, HGF and FGF in a murine model of critical limb ischaemia.54 Serum deprivation is often used in conjunction with hypoxic preconditioning as it improves the ability of MSCs to induce angiogenesis and endothelial proliferation.63,64
3D culture and biomaterials
The 3D culture of MSCs is another option to achieve a more effective therapy for ischaemic stroke.65–67 Culturing in 3D enhances the angiogenic potential of MSCs by increasing the secretion of molecules including VEGF, HGF and FGF2,65,68,69 and increases anti-inflammatory potential by secreting TSG-6, stanniocalcin-1, PGE2 or TGF-β amongst others.65,70,71 This 3D environment provides more physiological conditions, maintains stemness and increases cell survival and multipotency once transplanted.65,72 Additionally, this increases the ability of MSCs to activate endogenous mechanisms of tissue repair through increased secretion of factors such as matrix metalloproteinases (MMPs) and FGF2.67,73 MSC spheroids have already shown moderate success in promoting bone regeneration74,75 and in inflammatory models such as colitis.76
In some studies, biomaterials including hydrogels, assembling peptides or scaffolds have been utilised to further enhance the anti-inflammatory and pro-trophic phenotype of the MSC secretome. Murphy et al.77 showed that entrapping MSC spheroids in a fibril gel can increase secretion of VEGF and PGE2, increase endothelial cell proliferation and promote angiogenesis in a human 3D skin equivalent wound model. Similarly, conditioned medium (CM) derived from MSCs embedded in collagen and polyethylene glycol hydrogels induced stronger antioxidant and neuroprotective responses in SH-SY5Y cells.78 MSCs cultured with self-assembly peptides induced in vitro outgrowth of axons and neurites from neurons following traumatic brain injury.79 Combined administration of MSCs and biomaterials has been previously shown to promote repair in a number of disease models. For example, embedding MSCs in platelet lysate hydrogels increased engraftment as well as increasing the pro-angiogenic and neo-vascularisation activity of the transplanted cells in a murine model of critical limb ischaemia.80
CM and serum preconditioning
When the molecule intended to trigger a particular effect is not known, or when a specific environment needs to be mimicked, CM or serum is another suitable option for modifying the MSC secretome. MSCs treated with endothelial growth medium show improved viability and endothelial-related functions,81 while priming MSCs with serum from stroke animals increased proliferation and secretion of cytokines, thus improving their therapeutic potential.82 Similarly, when cultured in rat ischaemic brain extracts, MSCs respond by increasing secretion of BDNF, VEGF, NGF and HGF.83 The serum content of growth medium can have a profound effect on the MSC secretome. Zimmerman and McDevit71 showed the secretion of immunomodulatory factors such as PGE2, IL-6 and TGF-β was far increased when MSC spheroids were cultured in growth media containing foetal bovine serum as compared with a specialised MSC serum-free medium.
Role of the MSC secretome in promoting repair in preclinical models of stroke
There is a substantial body of evidence demonstrating MSC transplantation promotes recovery in rodent models of stroke although the mechanisms of action have not been fully elucidated. A number of studies from the early 2000s began to hypothesise that the MSC secretome was involved. Zhao et al.84 suggested that as intracranial (IC) administration of hMSCs one week after middle cerebral artery occlusion (MCAO) in spontaneously hypertensive (SHR) rats was associated with improvements in limb placement but differentiation was limited, recovery might be mediated through secretion of neurotrophic factors from the transplanted cells. Similarly, IV administration of MSCs also improved neurological deficits and the authors proposed neurotrophins from the MSCs decreased apoptosis and promoted endogenous neurogenesis.28 Later work from the same lab also showed that MSC transplantation increased angiogenesis in the ischaemic boundary.85 This was associated with increased endogenous VEGF and VEGF receptor 2 (VEGFR2) expression, which the authors hypothesised were upregulated by secretion of growth factors such as bFGF from the MSCs. In support of this, exogenous IGF-1 from transplanted cells has been detected in the core and ischaemic border zone three days post-MCAO, while expression of endogenous growth factors including VEGF, EGF and bFGF was increased in MSC-treated rats compared with controls.86 Additionally, secretion of a number of other factors from MSCs engrafted in the ischaemic brain has been detected including BDNF, bFGF, CXCL12, platelet-derived growth factor-AA (PDGF-AA) and angiopoietin-2 (Ang-2).87,88
One neurotrophin of particular interest is BDNF which promotes neuronal survival and differentiation through interaction with tyrosine kinase receptors.89 In preclinical models of stroke, IV BDNF administration reduced infarct volume, improved recovery and promoted neurogenesis.90,91 Furthermore, BDNF appears to be an important mediator in the MSC secretome preventing glutamate-induced neuronal death in vitro.92 When transplanted into a stroke model, BDNF secretion from MSCs was associated with increased functional recovery, decreased lesion volume, decreased apoptosis and increased angiogenesis.34 Several studies have shown that overexpression of BDNF in MSCs further enhanced repair and recovery.93–95 However, Koh et al.96 demonstrated that neutralising BDNF did not completely ameliorate the observed improvements in neurological function following human umbilical cord-derived MSC transplantation, suggesting other mediators are important in promoting recovery after stroke.
VEGF has both beneficial and detrimental effects in the post-stroke brain, as reviewed by Greenberg and Jin.97 In brief, VEGF increases neuroprotection, angiogenesis and neurogenesis after focal cerebral ischaemia98 but as a potent inducer of vascular permeability, can also increase blood–brain barrier (BBB) leakage leading to cerebral oedema.99,100 These dual actions appear to be reflected in the literature on the involvement of the MSC secretome in stroke repair. A number of studies have shown overexpression of VEGF in MSCs (VEGF-MSCs) enhanced functional recovery, decreased lesion volume, promoted neurogenesis and decreased neuronal apoptosis in rodent models of cerebral ischaemia.101–103 In contrast, VEGF-MSCs have also been shown to worsen functional outcomes and increase oedema, while Ang-VEGF-MSCs led to improved recovery, decreased lesion volumes and increased angiogenesis.104 In a cardiac arrest-induced model of global cerebral ischaemia, overexpression of both VEGF and BDNF led to decreased apoptosis and increased motor recovery.103 Overexpression of a plethora of other cytokines and growth factors including Ang-1, GDNF, HGF, FGF1 and PIGF were also shown to enhance recovery after cerebral ischaemia.104–109 Interestingly, MSCs transfected with either neurotrophin 3 (NT3) or ciliary neurotrophic factor (CNTF) did not significantly improve functional outcomes.110 Thus, it appears that a combination of mediators are involved in promoting functional recovery in preclinical models of ischaemic stroke as summarised in Table 1.
Table 1.
Summary of studies investigating the efficacy of MSC therapies in preclinical models of cerebral ischaemia and the proposed involvement of secretome components.
| Publication | MSC therapy | Stroke model | Route | Dose | Timing post-stroke | Results | Potential role of the secretome |
|---|---|---|---|---|---|---|---|
| Chen et al.85 | Human BMSCs | 120 min MCAO, male Wistar rats | IV | 1 × 106 | 24 h | Increased angiogenesis | bFGF |
| Cheng et al.121 | Human UMSCs | 90 min MCAO, male mice | IV | 4 × 106 | 30 min | Improved functional recovery (mNSS), decreased neuroinflammation, decreased infarct volume, decreased oedema | TGF-β |
| Deng et al.102 | Rat BMSCs | Permanent MCAO, male Sprague-Dawley rats | IV | 2 × 106 | 2 or 24 h | Improved functional recovery (mNSS, MWM), decreased apoptosis, increased endogenous neurogenesis | VEGF |
| Ghazavi et al.108 | Rat FGF-ADMSCs | 30 min MCAO, male Wistar rats | IV | 2 × 106 | 30 min | Improved functional recovery (rotarod, Roger’s test), decreased infarct volume, decreased apoptosis | FGF1 |
| Guo et al.72 | Human PMSCs, 3D cultured, dissociated | 120 min MCAO, female Sprague-Dawley rats | IA | 1 × 106 | 24 h | Increased functional recovery (mNSS, adhesive removal), decreased lesion volume, increased angiogenesis | VEGF, bFGF |
| Horita et al.105 | Human GDNF-BMSCs | Permanent MCAO, male Sprague-Dawley rats | IV | 1 × 107 | 3 h | Improved functional recovery (treadmill stress test), decreased infarct volume | GDNF |
| Ishizaka et al.34 | Human MSCs | 75 min MCAO, male Sprague-Dawley rats | IA | 1 × 106 | 1, 4 or 7 days | Improved functional recovery (cylinder test), decreased brain atrophy, increased angiogenesis, decreased activated microglial recruitment | BDNF |
| Jeong et al.95 | Human BDNF-BMSCs | 90 min MCAO, male Sprague-Dawley rats | IC | 5 × 105 | 3 days | Improved functional recovery (adhesive removal, rotarod), decreased infarct volume, decreased apoptosis, increased endogenous neurogenesis | BDNF |
| Koh et al.96 | Human UCMSCs | 120 min MCAO, male Sprague-Dawley rats | IC | 6 × 105 | 2 weeks | Improved functional recovery (NDS), decreased infarct volume, increased endogenous neurogenesis | BDNF |
| Kurozumi et al.93 | Human BDNF-BMSCs | 90 min MCAO, male Wistar rats | IC | 5 × 105 | 24 h | Improved functional recovery (limb placement, treadmill test), decreased infarct volume, decreased apoptosis | BDNF |
| Kurozumi et al.110 | Human BDNF/GDNF/ CNTF/NT3-BMSCs | 90 min MCAO, male Wistar rats | IC | 5 × 105 | 24 h | Improved functional recovery (limb placement, treadmill test), decreased infarct volume | BDNF, GDNF |
| Li et al.28 | Human BMSCs | 120 min MCAO, male Wistar rats | IV | 1 × 106 | 24 h | Improved functional recovery (adhesive removal, mNSS) | Neurotrophins |
| Lin et al.87 | Human UMSCs | 90 min MCAO, male Sprague-Dawley rats | IC | 5 × 105 | 24 h | Improved functional recovery (rotarod), decreased cortical atrophy | BDNF, bFGF, PDGF-AA, Ang-2, CXCL16, neutrophil-activating protein-2 |
| Lin et al.130 | Rat BMSCs | CA global cerebral ischaemia, male Sprague-Dawley rats | IV | 5 × 106 | 2 h | Improved functional recovery (adhesive removal, rotarod), decreased neuroinflammation | TSG-6 |
| Liu et al.109 | Human PGF-BMSCs | Permanent MCAO, male Sprague-Dawley rats | IV | 1 × 107 | 3 h | Improved functional recovery (treadmill stress test, limb placement test), decreased infarct volume, decreased apoptosis, increased angiogenesis | PGF |
| Miki et al.101 | Rat VEGF-BMSCs | 120 min MCAO, male Wistar rats | IC | 1 × 106 | 24 h | Improved functional recovery (mNSS), decreased infarct volume, decreased neuronal apoptosis | VEGF |
| Nakajima et al.125 | Human IL-10-BMSCs | 90 min MCAO, male Sprague-Dawley rats | IV | 1 × 106 | 0 or 3 h | Improved functional recovery (NDS, rotarod), decreased infarct volume, decreased neuroinflammation, decreased neuronal degeneration | IL-10 |
| Nomura et al.94 | Human BDNF-BMSCs | Permanent MCAO, male Sprague-Dawley rats | IV | 1 × 107 | 6 h | Improved functional recovery (treadmill stress test), decreased infarct volume | BDNF |
| Onda et al.107 | Human Ang-VEGF- BMSCs | Permanent MCAO, male Sprague-Dawley rats | IV | 1 × 106 | 6 h | Improved functional recovery (treadmill stress test), decreased infarct volume, increased angiogenesis | Ang-1 |
| Shichinohe et al.92 | Mouse BMSCs | Permanent MCAO, male Balb/c mice | IC | 2 × 105 | 1 week | Improved survival of neurons in per-infarct | BDNF |
| Sheikh et al.128 | Human BMSCs | 90 min MCAO, male Wistar rats | IV | 3 × 106 | 24 h | Decreased microglial activation and proinflammatory gene expression | IL-5, CX3CL1 |
| Toyama et al.104 | Human Ang/VEGF/ Ang-VEGF-BMSCs | Permanent MCAO, male Sprague-Dawley rats | IV | 1 × 106 | 6 h | Improved functional recovery (treadmill stress test), decreased infarct volume, increased angiogenesis | Ang-1, VEGF |
| Toyoshima et al.88 | Rat BMSCs | 90 min MCAO, male Wistar rats | IA | 1 × 106 | 1, 6, 24 or 48 h | Improved functional recovery (mNSS), decreased infarct volume | bFGF, CXCL12 |
| Wakabayashi et al.86 | Human BMSCs | 60 min MCAO, male Wistar rats | IV | 3 × 106 | 24 h | Improved functional recovery (mNSS), decreased infarct volume | IGF-1 |
| Wei et al.131 | Rat BMSCs, hypoxic preconditioning | 90 min MCAO, male Wistar rats | IV | 1 × 106 | 24 h | Increased functional recovery (rotarod), increased angiogenesis, decreased microglial activation | HIF-1α, BDNF, GDNF, VEGF, CXCL12 |
| Yoo et al.122 | Human BMSCs | 120 min MCAO, male Sprague-Dawley rats | IC | 5 × 105 | 3 days | Improved functional recovery (adhesive removal, rotarod), decreased neuroinflammation, decreased infarct volume | TGF-β |
| Zacharek et al.132 | Rat BMSCs isolated post-MCAO | 120 min MCAO, male Wistar rats | IV | 1 × 106 | 24 h | Increased functional recovery (mNSS, foot fault), increased angiogenesis | Ang1, bFGF, GDNF, VEGF |
| Zhao et al.84 | Human BMSCs | Permanent MCAO, male SHR rats | IC | 1 × 106 | 1 week | Improved functional recovery (limb placement) | Neurotrophins |
| Zhao et al.106 | Rat HGF-BMSCs | 120 min MCAO, male Wistar rats | IV | 1 × 106 | 24 h | Improved functional recovery (mNSS), decreased infarct volume, decreased neuronal apoptosis | HGF |
| Zhou et al.103 | Rat BDNF-VEGF- BMSCs | CA global cerebral ischaemia, male Sprague-Dawley rats | IV | 3 × 106 | 2 h | Improved functional recovery (NDS), decreased apoptosis, increased angiogenesis | BDNF, VEGF |
BMSCs: bone marrow-derived mesenchymal stem cells; CA: cardiac arrest; IN: intranasal: MCAO: middle cerebral artery occlusion; MWM: Morris water maze; mNSS: modified neurological severity score; NDS: neurological deficit score; PMSCs: placenta-derived mesenchymal stem cells; UMSCs: umbilical cord-derived mesenchymal stem cells.
Immunomodulation
While the consensus in the literature is that the MSC secretome promotes recovery after stroke through mechanisms including neuroprotection, neurogenesis and angiogenesis after stroke, its role in immunomodulation is not clear. MSCs exert numerous immunomodulatory effects on immune cell populations including inhibition of proliferation of natural killer (NK) cells,111 inhibition of both B and T cell proliferation112–114 and suppression of dendritic cell (DC) differentiation and migration.115,116 Additionally, co-culture of MSCs drives the secretome of DCs, T cells, macrophages and NK cells towards anti-inflammatory phenotypes.117,118 A number of molecules secreted by MSCs including PGE2, TSG-6, TGF-β, HGF and IL-10 have been implicated in mediating these immunosuppressive effects.119,120 For example, Di Nicola et al.113 showed TGF-β and HGF secretion was involved in MSC suppression of T-lymphocyte proliferation.113 Following on from this, TGF-β secretion from transplanted MSCs improved the systemic inflammatory response after stroke decreasing Th17 cells and increasing regulatory T cells in the peripheral immune system.121 This was associated with decreased infarct volume and improved functional recovery. Furthermore, transplantation of TGF-β silenced MSCs did not decrease CD68+ cell infiltration or prevent microglial cell death as demonstrated in non-modified cells.122
IL-10, often referred to as an anti-inflammatory cytokine, is an inducer of immune tolerance and has previously been shown to have neuroprotective effects and decrease pro-inflammatory signalling in preclinical models of cerebral ischaemia.123,124 Transplantation of MSCs overexpressing IL-10 led to decreased microglial activation and pro-inflammatory cytokine (IL-6, TNF-α and IL-1β) concentrations in the brain after stroke compared with non-modified MSCs and vehicle.125 Administration of IL-10-MSCs was also neuroprotective leading to decreased neuronal degeneration and improved functional recovery. CX3CL1 (fractalkine) may also have a role in immunomodulation after cerebral ischaemia. Its receptor CX3CR1 is expressed by microglia and CX3CL1-CX3CR1 signalling supresses neurotoxic microglia activity.126 Secretion of CXC3CL1 from MSCs has previously been shown to shift microglia towards a neuroprotective phenotype.127 Sheikh et al.128 suggested CX3CL1 and IL-5 were involved in decreasing microglial activation and inhibiting expression of pro-inflammatory gene expression, namely COX-2 and iNOS, in the core and ischaemic border zone.
TSG-6 secretion from MSCs has previously been shown to decrease inflammation in peritonitis and corneal injury models.41,129 MSC administration in a cardiac arrest-induced global cerebral ischaemia rat model led to decreased serum pro-inflammatory cytokines and S100B concentrations and decreased expression of neutrophil elastase in the cerebral cortex.130 While TSG-6 expression in the cerebral cortex was upregulated, it was not possible to determine whether this was due to secretion from the MSCs or endogenous cells.
Secretome modification
As discussed earlier, a number of in vitro strategies have been utilised to enhance the MSC secretome but few have investigated whether these lead to enhanced recovery of function in preclinical models of cerebral ischaemia. Transplantation of hypoxic preconditioned MSCs was superior to normoxic-treated cells leading to larger improvements in functional recovery, increased angiogenesis and decreased microglial activation.131 The authors proposed this was mediated by enhanced secretion of trophic factors and reported upregulated expression of BDNF, VEGF, GDNF, and CXCL12 in hypoxic cells. Similarly, Zacharek et al.132 demonstrated that MSCs isolated from rats after MCAO provided a better allogeneic stroke therapy compared with cells from naïve animals and was associated with increased Ang1, bFGF, GDNF and VEGF expression. The 3D culture of MSCs has also been shown to enhance recovery. MSCs cultured as spheroids and then dissociated prior to IA administration led to improved functional outcomes, increased angiogenesis and decreased lesion volume.72
MSC CM treatment
In further support of the important role of the MSC secretome, CM has also been shown to promote recovery in rodent models of cerebral ischaemia. Egashira et al.133 reported that adipose-derived hMSC CM administered by intracerebroventricular (ICV) injection 1 h prior to MCAO in inbred DDY mice led to decreased lesion volume and neurological deficits at 24 h post-stroke. Additionally, delayed administration of CM from spheroid cultured cells beginning at day 8 post-stroke led to decreased microglial apoptosis, increased endothelial cell proliferation and improved rotarod performance at day 15.134 IV135 and intranasal136 administration of CM has also been reported to improve recovery.
MSC-derived EVs
In very recent years, preclinical studies have begun demonstrating the role of mesenchymal stem cell-derived EVs in stroke repair (summarised in Table 2). MSCs secrete a number of EVs including exosomes which are characteristically 30–100 nm in diameter and contain micro RNAs, messenger RNAs and proteins.137 Microvesicles (MVs), also known as shedding vesicles, ectosomes or microparticles, ranging from 60 nm to 1 µm in diameter are also secreted.138 Systemic administration of EVs derived from MSCs has been shown to promote functional recovery in rodent models of cerebral ischaemia and this was associated with mechanisms including neuroprotection, white matter repair, neurogenesis and angiogenesis.139–142 In a transient global ischaemia model, exosome therapy also ameliorated impairments in memory and hippocampal synaptic transmission.143 Furthermore, MSC-derived exosomes have been shown to be equally effective as MSCs in improving functional outcomes, further supporting the importance of the secretome in promoting stroke repair.140 Lee et al.141 showed that MVs derived from MSCs preconditioned with either normal or ischaemic brain extracts further enhanced recovery compared with MVs from untreated cells. There are a limited number of studies postulating on the role of specific EVs in stroke repair. Overexpression of miR-133b144,145 and miR-17-92 cluster146 was associated with increased functional recovery and repair. In a diabetic mouse model, miR-126 was shown to promote functional recovery, angiogenesis, white matter remodelling and decrease BBB permeability.147
Table 2.
Preclinical studies on the effect of MSC-derived exosomes on repair and recovery after ischaemic stroke.
| Publication | Exosome therapy | Stroke model | Route | Dose | Timing post-stroke | Results |
|---|---|---|---|---|---|---|
| Chen et al.147 | Human UMSCs/UMSCs MiR-126−/− | Distal MCAO, male db/db mice | IV | 1 × 106 cells | 3 days | Increased functional recovery (adhesive removal, food pellet catching), decreased haemorrhagic transformation, decreased BBB permeability, increased vascular and white matter remodelling |
| Deng et al.143 | Mouse BMSC-derived EVs | Transient global cerebral ischaemia, male C57Bl/6 mice | IC | 200 µg | 0 h | Improved cognitive impairment (MWM), improved synaptic transmission and long-term potentiation |
| Doeppner et al.140 | Human BMSC-derived exosomes | 30 min MCAO, male C57Bl/6 mice | IV | From 2 × 106 cells | 1, 3 and 5 days | Increased functional recovery (rotarod, tightrope, corner turn), neuroprotection, angiogenesis, neurogenesis, decreased immunosuppression |
| Otero-Ortega et al.142 | Rat AMSC-derived exosomes | Endothelin-1 SCI, male Sprague-Dawley rats | IV | 100 µg | 24 h | Increased functional recovery (beam walking, Rogers test, rotarod), reduced lesion volume, increased axonal sprouting, oligodendrogenesis, remyelination and fibre tract integrity |
| Lee et al.141 | Human MSC-derived MVs, treated with normal/ischaemic brain extract | Permanent MCAO, male Sprague-Dawley rats | IA | 0.2 mg/kg | 48 h | Increased functional recovery (torso twisting test, open field, balance beam, prehensile traction score, mNSS), decreased inflammation, decreased lesion volume, increased neurogenesis, angiogenesis |
| Xin et al.139 | Rat BMSC-derived exosomes | 120 min MCAO, male Wistar rats | IV | 100 µg | 24 h | Increased functional recovery (mNSS, foot fault), neurite remodelling, neurogenesis and angiogenesis |
| Xin et al.144 | Rat BMSCs wt/MiR-133b−/ BMSCs MiR-133b+ | 120 min MCAO, male Wistar rats | IV | 3 × 106 cells | 24 h | Increased functional recovery (adhesive removal, foot fault), increased axonal plasticity and neurite remodelling |
| Xin et al.145 | Rat BMSC-derived, wt/MiR-133b−/MiR-133b+ | 120 min MCAO, male Wistar rats | IA | 3 × 1011 particles | 24 h | Increased functional recovery (mNSS, foot fault), increased neurite remodelling |
| Xin et al.146 | Rat BMSC-derived, overexpressing MiR-17-92 cluster | 120 min MCAO, male Wistar rats | IV | 100 µg | 24 h | Increased functional recovery (mNSS, foot fault), neurite remodelling, neurogenesis and oligodendrogenesis |
AMSCs: adipose-derived mesenchymal stem cells BMSCs: bone marrow-derived mesenchymal stem cells; EVs: extracellular vesicles; MCAO: middle cerebral artery occlusion; mNSS: modified neurological severity score; MWM: Morris water maze; MVs: microvesicles; SCI: subcortical infarct; UMSCs: umbilical cord-derived mesenchymal stem cells.
Conclusions and future directions
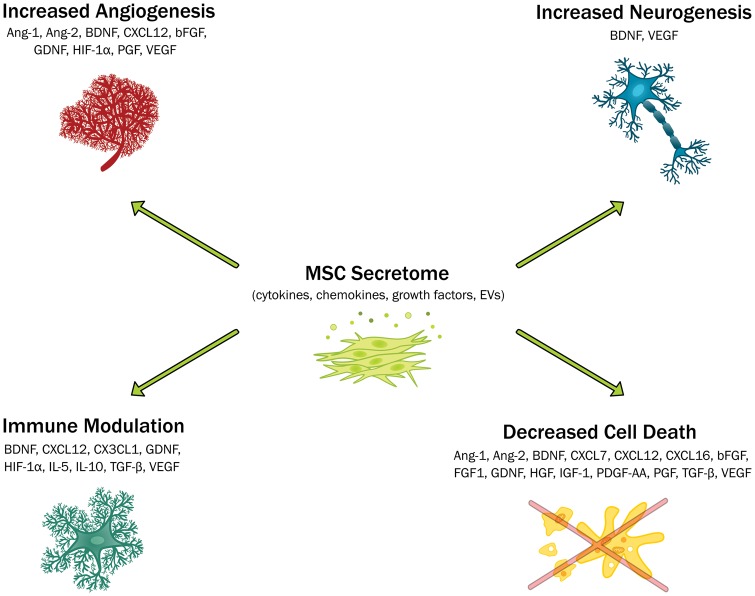
There is a growing body of evidence demonstrating the role of the MSC secretome in promoting recovery in rodent models of cerebral ischaemia. This has been proposed to occur through a number of mechanisms including decreased neuroinflammation, neuroprotection, increased angiogenesis and neurogenesis (Figure 2). However, there is currently no consensus in the literature on what mediators in the MSC secretome are important in promoting repair and functional recovery after stroke. While a strong case can be made for BDNF in particular with multiple citations supporting its role, neutralising BDNF did not completely abolish post-stroke recovery. It is therefore likely that a combination of mediators is important in promoting recovery and repair after stroke. In support of this, meta-analysis has demonstrated that G-CSF does not improve outcomes in stroke patients.148 A number of in vitro strategies have been used to drive the secretome towards a more desirable anti-inflammatory and pro-trophic phenotype including priming with pro-inflammatory cytokines, hypoxic preconditioning, biomaterials and 3D culture (Figure 1). However, the efficacy of these approaches has not been extensively assessed in preclinical models.
Figure 2.
The role of the mesenchymal stem cell secretome in promoting repair and recovery after ischaemic stroke. The main mechanisms of action are highlighted along with the proposed mediators. Ang: angiopoietin; BDNF: brain-derived neurotrophic factor; CXCL: chemokine C-X-C motif ligand; CX3CR1: CX3C chemokine receptor 1; bFGF: basic fibroblast growth factor; GDNF: glial cell line-derived neurotrophic factor; HGF: hepatocyte growth factor; HIF-1α: Hypoxia-inducible factor 1-alpha; IGF-1: insulin-like growth factor 1; IL: interleukin; PDGF-AA: platelet-derived growth factor AA; PGF: placental growth factor; TGF-β: transforming growth factor beta: VEGF: vascular endothelial growth factor.
There are several challenges to be overcome in translating the MSC secretome into a safe and effective therapy for ischaemic stroke such as the optimal timing of administration. The majority of preclinical studies elected to administer MSCs, CM and exosomes during the acute phase of stroke (≤ 48 h) where secondary damage is mediated by reactive oxygen species, migration of immune cells to the ischaemic brain and production of pro-inflammatory cytokines such as IL-1.149 As a number of studies have demonstrated immunomodulatory and neuroprotective effects of the MSC secretome, such a time point may hold therapeutic potential. In contrast, one study reported that administration of MSCs to rats at 1 month post-stroke also led to functional recovery. This was associated with decreased glial scarring and increased proliferating cells in the subventricular zone, suggesting MSC treatment may have promoted neurogenesis.150 As MSCs secrete multiple growth factors which can activate endogenous repair mechanisms, administration at delayed time points should be investigated further.151 Determining the optimal timing of administration may prove to be a difficult balancing act and repeated dosing should be considered. For example, VEGF induces vascular permeability so if administered at acute time points may increase BBB breakdown leading to increased cerebral oedema and exacerbate injury. Another challenge will be determining the best therapy. While MSCs are generally immune evasive and have been shown to be well tolerated in clinical trials in stroke, the increasing number of preclinical studies demonstrating the efficacy of MSC-derived CM and EVs could mitigate the need to administer cells. This may prove more translatable as these cell-free alternatives can be cryopreserved without any concerns over cell viability so could be stored for long periods of time and shipped worldwide. Another challenge will be determining the route of administration. Preclinical studies and clinical trials have employed both systemic routes such as IV and IV and direct routes such as IC. As improvements in recovery can occur independently of MSC engraftment or even migration to the ischaemic brain, perhaps systemic routes which are simpler, less invasive and less likely to cause adverse events should be adopted.
Looking forward, the biggest challenge to preclinical scientists is that there is currently no clear consensus on the optimum culture conditions and preconditioning strategy to maximise the regenerative potential of the MSC secretome. Future work should focus on assessing the efficacy of more approaches to modifying secretome in rodent models of cerebral ischaemia and increasing our understanding of the mediators involved in promoting repair. There is growing interest in cell-free approaches such as exosomes or CM and these should also be more fully investigated. In summary, while there are a number of hurdles to overcome on road to translation, the MSC secretome holds much potential as a regenerative therapy for ischaemic stroke.
Acknowledgements
The authors would like to thank Raymond Wong for his valuable feedback on the manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by an EPSRC and MRC Centre for Doctoral Training in Regenerative Medicine studentship grant EP/L014904/1.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.World Health Organisation. Global status report on noncommunicable diseases. Report, Switzerland: World Health Organisation, 2014. [Google Scholar]
- 2.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2014; 383: 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee S, Williamson DA, Habib N, et al. The potential benefit of stem cell therapy after stroke: an update. Vasc Health Risk Manag 2012; 8: 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMeekin P, White P, James MA, et al. Estimating the number of UK stroke patients eligible for endovascular thrombectomy. Eur Stroke J 2017; 2: 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2008; 2: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meirelles L da S, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 2006; 119(Pt 11): 2204–2213. [DOI] [PubMed] [Google Scholar]
- 7.Zuk PAPA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002; 13: 4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. J Dent Res 2002; 81: 531–535. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Y, Vaessen B, Lenvik T, et al. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol 2002; 30: 896–904. [DOI] [PubMed] [Google Scholar]
- 10.in ’t Anker PS, Noort WA, Scherjon SA, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica 2003; 88: 845–852. [PubMed] [Google Scholar]
- 11.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells 2003; 21: 105–110. [DOI] [PubMed] [Google Scholar]
- 12.Wang H-S, Hung S-C, Peng S-T, et al. mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 2004; 22: 1330–1337. [DOI] [PubMed] [Google Scholar]
- 13.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. the International Society for Cellular Therapy position statement. Cytotherapy 2006; 8: 315–317. [DOI] [PubMed] [Google Scholar]
- 14.Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One 2012; 7: e47559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant 2016; 25: 829–848. [DOI] [PubMed] [Google Scholar]
- 16.Bang OY, Lee JS, Lee PH, et al. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 2005; 57: 874–882. [DOI] [PubMed] [Google Scholar]
- 17.Lee JS, Hong JM, Moon GJ, et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010; 28: 1099–1106. [DOI] [PubMed] [Google Scholar]
- 18.Bhasin A, Srivastava MVP, Kumaran SS, et al. Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc Dis Extra 2011; 1: 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honmou O, Houkin K, Matsunaga T, et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain 2011; 134: 1790–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Y, Zhu W, Zhu J, et al. Feasibility of delivering mesenchymal stem cells via catheter to the proximal end of the lesion artery in patients with stroke in the territory of the middle cerebral artery. Cell Transplant 2013; 22: 2291–2298. [DOI] [PubMed] [Google Scholar]
- 21.Steinberg GK, Kondziolka D, Wechsler LR, et al. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke 2016; 47: 1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ankrum J, Ong J, Karp J. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol 2014; 32: 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Huang X, Wang H, et al. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res Ther 2015; 6: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh S-H, Choi C, Chang D-J, et al. Early neuroprotective effect with lack of long-term cell replacement effect on experimental stroke after intra-arterial transplantation of adipose-derived mesenchymal stromal cells. Cytotherapy 2015; 17: 1090–1103. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal H, Shang H, Sattah AP, et al. Human adipose-derived stromal/stem cells demonstrate short-lived persistence after implantation in both an immunocompetent and an immunocompromised murine model. Stem Cell Res Ther 2014; 5: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao J, Dennis JE, Muzic RF, et al. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs 2001; 169: 12–20. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Guo L, Ge J, et al. Excess integrins cause lung entrapment of mesenchymal stem cells. Stem Cells 2015; 33: 3315–3326. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Chen J, Chen XG, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology 2002; 59: 514–523. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Sanberg PR, Li Y, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 2001; 32: 2682–2688. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci 2001; 189: 49–57. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 2001; 32: 1005–1011. [DOI] [PubMed] [Google Scholar]
- 32.Ding D-C, Shyu W-C, Chiang M-F, et al. Enhancement of neuroplasticity through upregulation of beta1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol Dis 2007; 27: 339–353. [DOI] [PubMed] [Google Scholar]
- 33.Hofstetter CP, Schwarz EJ, Hess D, et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A 2002; 99: 2199–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishizaka S, Horie N, Satoh K, et al. Intra-arterial cell transplantation provides timing-dependent cell distribution and functional recovery after stroke. Stroke 2013; 44: 720–726. [DOI] [PubMed] [Google Scholar]
- 35.Schinköthe T, Bloch W, Schmidt A. In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev 2008; 17: 199–206. [DOI] [PubMed] [Google Scholar]
- 36.Park CW, Kim K-S, Bae S, et al. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int J Stem Cells 2009; 2: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakano N, Nakai Y, Seo T-B, et al. Characterization of conditioned medium of cultured bone marrow stromal cells. Neurosci Lett 2010; 483: 57–61. [DOI] [PubMed] [Google Scholar]
- 38.Kupcova Skalnikova H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie 2013; 95: 2196–2211. [DOI] [PubMed] [Google Scholar]
- 39.Hsiao ST-F, Asgari A, Lokmic Z, et al. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev 2012; 21: 2189–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du WJ, Chi Y, Yang ZX, et al. Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res Ther 2016; 7: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi H, Lee RH, Bazhanov N, et al. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF- B signaling in resident macrophages. Blood 2011; 118: 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Afzal MR, Haider HK, Idris NM, et al. Preconditioning promotes survival and angiomyogenic potential of mesenchymal stem cells in the infarcted heart via NF-kappaB signaling. Antioxid Redox Signal 2010; 12: 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Redondo-Castro E, Cunningham C, Miller J, et al. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther 2017; 8: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saether EE, Chamberlain CS, Aktas E, et al. primed mesenchymal stem cells alter and improve rat medial collateral ligament healing. Stem Cell Rev Rep 2016; 12: 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorin C, Rochefort GY, Bascetin R, et al. Priming dental pulp stem cells with fibroblast growth factor-2 increases angiogenesis of implanted tissue-engineered constructs through hepatocyte growth factor and vascular endothelial growth factor secretion. Stem Cells Transl Med 2016; 5: 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu Z, Chen Y, Dunstan C, et al. Priming adipose stem cells with tumor necrosis factor-alpha preconditioning potentiates their exosome efficacy for bone regeneration. Tissue Eng Part A 2017; 23: 1212–1220. [DOI] [PubMed] [Google Scholar]
- 47.Aktas E, Chamberlain CS, Saether EE, et al. Immune modulation with primed mesenchymal stem cells delivered via biodegradable scaffold to repair an Achilles tendon segmental defect. J Orthop Res 2017; 35: 269–280. [DOI] [PubMed] [Google Scholar]
- 48.Maumus M, Manferdini C, Toupet K, et al. Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. Stem Cell Res 2013; 11: 834–844. [DOI] [PubMed] [Google Scholar]
- 49.Sivanathan KN, Gronthos S, Rojas-Canales D, et al. Interferon-gamma modification of mesenchymal stem cells: implications of autologous and allogeneic mesenchymal stem cell therapy in allotransplantation. Stem Cell Rev Reports 2014; 10: 351–375. [DOI] [PubMed] [Google Scholar]
- 50.Barrachina L, Remacha AR, Romero A, et al. Priming equine bone marrow-derived mesenchymal stem cells with proinflammatory cytokines: implications in immunomodulation-immunogenicity balance, cell viability, and differentiation potential. Stem Cells Dev 2017; 26: 15–24. [DOI] [PubMed] [Google Scholar]
- 51.Prasanna SJ, Gopalakrishnan D, Shankar SR, et al. Pro-inflammatory cytokines, IFNγ and TNFα, influence immune properties of human bone marrow and wharton jelly mesenchymal stem cells differentially. PLoS One 2010; 5: e9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang Z, Concannon J, Ng KS, et al. Tetrandrine identified in a small molecule screen to activate mesenchymal stem cells for enhanced immunomodulation. Sci Rep 2016; 6: 30263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim Y, Kwon J, Hong M, et al. Restoration of angiogenic capacity of diabetes-insulted mesenchymal stem cells by oxytocin. BMC Cell Biol 2013; 14: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee J, Yoon Y, Lee S. Hypoxic preconditioning promotes the bioactivities of mesenchymal stem cells via the HIF-1α-GRP78-Akt axis. Int J Mol Sci 2017; 18: 1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathew SA, Chandravanshi B, Bhonde R. Hypoxia primed placental mesenchymal stem cells for wound healing. Life Sci 2017; 182: 85–92. [DOI] [PubMed] [Google Scholar]
- 56.Liu L, Gao J, Yuan Y, et al. Hypoxia preconditioned human adipose derived mesenchymal stem cells enhance angiogenic potential via secretion of increased VEGF and bFGF. Cell Biol Int 2013; 37: 551–560. [DOI] [PubMed] [Google Scholar]
- 57.Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res 2004; 94: 678–685. [DOI] [PubMed] [Google Scholar]
- 58.Crisostomo PR, Wang Y, Markel TA, et al. Human mesenchymal stem cells stimulated by TNF- , LPS, or hypoxia produce growth factors by an NF B- but not JNK-dependent mechanism. AJP Cell Physiol 2008; 294: C675–C682. [DOI] [PubMed] [Google Scholar]
- 59.Yust-Katz S, Fisher-Shoval Y, Barhum Y, et al. Placental mesenchymal stromal cells induced into neurotrophic factor-producing cells protect neuronal cells from hypoxia and oxidative stress. Cytotherapy 2012; 14: 45–55. [DOI] [PubMed] [Google Scholar]
- 60.Du L, Yu Y, Ma H, et al. Hypoxia enhances protective effect of placental-derived mesenchymal stem cells on damaged intestinal epithelial cells by promoting secretion of insulin-like growth factor-1. Int J Mol Sci 2014; 15: 1983–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rasmussen JG, Frøbert O, Pilgaard L, et al. Prolonged hypoxic culture and trypsinization increase the pro-angiogenic potential of human adipose tissue-derived stem cells. Cytotherapy 2011; 13: 318–328. [DOI] [PubMed] [Google Scholar]
- 62.Hu X, Yu SP, Fraser JL, et al. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg 2008; 135: 799–808. [DOI] [PubMed] [Google Scholar]
- 63.Wei X, Liu C, Wang H, et al. Surface phosphatidylserine is responsible for the internalization on microvesicles derived from hypoxia-induced human bone marrow mesenchymal stem cells into human endothelial cells. PLoS One 2016; 11: e0147360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oskowitz A, McFerrin H, Gutschow M, et al. Serum-deprived human multipotent mesenchymal stromal cells (MSCs) are highly angiogenic. Stem Cell Res 2011; 6: 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cesarz Z, Tamama K. Spheroid culture of mesenchymal stem cells. Stem Cells Int 2016; 2016: 9176357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods 2010; 16: 735–749. [DOI] [PubMed] [Google Scholar]
- 67.Santos JM, Camões SP, Filipe E, et al. 3D spheroid cell culture of umbilical cord tissue-derived MSCs (UCX®) leads to enhanced paracrine induction of wound healing. Stem Cell Res Ther 2015; 6: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laschke MW, Schank TE, Scheuer C, et al. Three-dimensional spheroids of adipose-derived mesenchymal stem cells are potent initiators of blood v. Acta Biomater 2013; 9: 6876–6884. [DOI] [PubMed] [Google Scholar]
- 69.Laranjeira P, Pedrosa M, Pedreiro S, et al. Effect of human bone marrow mesenchymal stromal cells on cytokine production by peripheral blood naive, memory and effector T cells. Stem Cell Res Ther 2015; 6: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bartosh TJ, Ylöstalo JH, Mohammadipoor A, et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiin fl ammatory properties. PNAS 2010; 107: 13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zimmermann JA, Mcdevitt TC. Pre-conditioning mesenchymal stromal cell spheroids for immunomodulatory paracrine factor secretion. Cytotherapy 2014; 16: 331–345. [DOI] [PubMed] [Google Scholar]
- 72.Guo L, Ge J, Zhou Y, et al. Three-dimensional spheroid-cultured mesenchymal stem cells devoid of embolism attenuate brain stroke injury after intra-arterial injection. Stem Cells Dev 2014; 23: 978–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhang SH, Lee S, Shin J-Y, et al. Transplantation of Cord Blood Mesenchymal Stem Cells as Spheroids Enhances Vascularization. Tissue Eng Part A 2012; 18: 2138–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suenaga H, Furukawa KS, Suzuki Y, et al. Bone regeneration in calvarial defects in a rat model by implantation of human bone marrow-derived mesenchymal stromal cell spheroids. J Mater Sci Mater Med 2015; 26: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamaguchi Y, Ohno J, Sato A, et al. Mesenchymal stem cell spheroids exhibit enhanced in-vitro and in-vivo osteoregenerative potential. BMC Biotechnol 2014; 14: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Molendijk I, Barnhoorn MC, de Jonge-Muller ESM, et al. Intraluminal injection of mesenchymal stromal cells in spheroids attenuates experimental colitis. J Crohns Colitis 2016; 10: 953–964. [DOI] [PubMed] [Google Scholar]
- 77.Murphy KC, Whitehead J, Zhou D, et al. Engineering fibrin hydrogels to promote the wound healing potential of mesenchymal stem cell spheroids. Acta Biomater 2017; 64: 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chierchia A, Chirico N, Boeri L, et al. Secretome released from hydrogel-embedded adipose mesenchymal stem cells protects against the Parkinson’s disease related toxin 6-hydroxydopamine. Eur J Pharm Biopharm 2017; 121: 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi W, Huang CJ, Xu XD, et al. Transplantation of RADA16-BDNF peptide scaffold with human umbilical cord mesenchymal stem cells forced with CXCR4 and activated astrocytes for repair of traumatic brain injury. Acta Biomater 2016; 45: 247–261. [DOI] [PubMed] [Google Scholar]
- 80.Robinson ST, Douglas AM, Chadid T, et al. A novel platelet lysate hydrogel for endothelial cell and mesenchymal stem cell-directed neovascularization. Acta Biomater 2016; 36: 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Oliveira LF, Almeida TR, Ribeiro Machado MP, et al. Priming mesenchymal stem cells with endothelial growth medium boosts stem cell therapy for systemic arterial hypertension. Stem Cells Int 2015; 2015: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim EH, Kim DH, Kim HR, et al. Stroke serum priming modulates characteristics of mesenchymal stromal cells by controlling the expression miRNA-20a. Cell Transplant 2016; 25: 1489–1499. [DOI] [PubMed] [Google Scholar]
- 83.Chen X, Li Y, Wang L, et al. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology 2002; 22: 275–279. [DOI] [PubMed] [Google Scholar]
- 84.Zhao L-R, Duan W-M, Reyes M, et al. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol 2002; 174: 11–20. [DOI] [PubMed] [Google Scholar]
- 85.Chen J, Zhang ZG, Li Y, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res 2003; 92: 692–699. [DOI] [PubMed] [Google Scholar]
- 86.Wakabayashi K, Nagai A, Sheikh AM, et al. Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res 2010; 88: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 87.Lin Y-C, Ko T-L, Shih Y-H, et al. Human umbilical mesenchymal stem cells promote recovery after ischemic stroke. Stroke 2011; 42: 2045–2053. [DOI] [PubMed] [Google Scholar]
- 88.Toyoshima A, Yasuhara T, Kameda M, et al. Intra-arterial transplantation of allogeneic mesenchymal stem cells mounts neuroprotective effects in a transient ischemic stroke model in rats: analyses of therapeutic time window and its mechanisms. PLoS One 2015; 10: e0127302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hyman C, Hofer M. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature 1991; 50: 230–232. [DOI] [PubMed] [Google Scholar]
- 90.Schäbitz WR, Sommer C, Zoder W, et al. Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke 2000; 31: 2212–2217. [DOI] [PubMed] [Google Scholar]
- 91.Schabitz W-R, Steigleder T, Cooper-Kuhn CM, et al. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke 2007; 38: 2165–2172. [DOI] [PubMed] [Google Scholar]
- 92.Shichinohe H, Ishihara T, Takahashi K, et al. Bone marrow stromal cells rescue ischemic brain by trophic effects and phenotypic change toward neural cells. Neurorehabil Neural Repair 2015; 29: 80–89. [DOI] [PubMed] [Google Scholar]
- 93.Kurozumi K, Nakamura K, Tamiya T, et al. BDNF Gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther 2004; 9: 189–197. [DOI] [PubMed] [Google Scholar]
- 94.Nomura T, Honmou O, Harada K, et al. I.V. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience 2005; 136: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jeong CH, Kim SM, Lim JY, et al. Mesenchymal stem cells expressing brain-derived neurotrophic factor enhance endogenous neurogenesis in an ischemic stroke model. Biomed Res Int 2014; 2014: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koh S-H, Kim KS, Choi MR, et al. Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res 2008; 1229: 233–248. [DOI] [PubMed] [Google Scholar]
- 97.Greenberg DA, Jin K. Vascular endothelial growth factors (VEGFs) and stroke. Cell Mol Life Sci 2013; 70: 1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun Y, Jin K, Xie L, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest 2003; 111: 1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest 2000; 106: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Bruggen N, Thibodeaux H, Palmer JT, et al. VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J Clin Invest 1999; 104: 1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miki Y, Nonoguchi N, Ikeda N, et al. Vascular endothelial growth factor gene-transferred bone marrow stromal cells engineered with a herpes simplex virus type 1 vector can improve neurological deficits and reduce infarction volume in rat brain ischemia. Neurosurgery 2007; 61: 586–595. [DOI] [PubMed] [Google Scholar]
- 102.Deng Y Bin, Ye WB, Hu ZZ, et al. Intravenously administered BMSCs reduce neuronal apoptosis and promote neuronal proliferation through the release of VEGF after stroke in rats. Neurol Res 2010; 32: 148–156. [DOI] [PubMed] [Google Scholar]
- 103.Zhou L, Lin Q, Wang P, et al. Enhanced neuroprotective efficacy of bone marrow mesenchymal stem cells co-overexpressing BDNF and VEGF in a rat model of cardiac arrest-induced global cerebral ischemia. Cell Death Dis 2017; 8: e2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Toyama K, Honmou O, Harada K, et al. Therapeutic benefits of angiogenetic gene-modified human mesenchymal stem cells after cerebral ischemia. Exp Neurol 2009; 216: 47–55. [DOI] [PubMed] [Google Scholar]
- 105.Horita Y, Honmou O, Harada K, et al. Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res 2006; 84: 1495–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao M-Z, Nonoguchi N, Ikeda N, et al. Novel therapeutic strategy for stroke in rats by bone marrow stromal cells and ex vivo HGF gene transfer with HSV-1 vector. J Cereb Blood Flow Metab 2006; 26: 1176–1188. [DOI] [PubMed] [Google Scholar]
- 107.Onda T, Honmou O, Harada K, et al. Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J Cereb Blood Flow Metab 2008; 28: 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ghazavi H, Hoseini SJ, Ebrahimzadeh-Bideskan A, et al. Fibroblast growth factor type 1 (FGF1)-overexpressed adipose-derived mesenchaymal stem cells (AD-MSCFGF1) induce neuroprotection and functional recovery in a rat stroke model. Stem Cell Rev Rep 2017; 3: 670–685. [DOI] [PubMed] [Google Scholar]
- 109.Liu H, Honmou O, Harada K, et al. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain 2006; 129: 2734–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kurozumi K, Nakamura K, Tamiya T, et al. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther 2005; 11: 96–104. [DOI] [PubMed] [Google Scholar]
- 111.Spaggiari GM, Capobianco A, Abdelrazik H, et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 2007; 111: 1327–1333. [DOI] [PubMed] [Google Scholar]
- 112.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002; 30: 42–48. [DOI] [PubMed] [Google Scholar]
- 113.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002; 99: 3838–3843. [DOI] [PubMed] [Google Scholar]
- 114.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006; 107: 367–372. [DOI] [PubMed] [Google Scholar]
- 115.Ivanova-Todorova E, Bochev I, Mourdjeva M, et al. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett 2009; 126: 37–42. [DOI] [PubMed] [Google Scholar]
- 116.Jung Y-J, Ju S-Y, Yoo E-S, et al. MSC–DC interactions: MSC inhibit maturation and migration of BM-derived DC. Cytotherapy 2007; 9: 451–458. [DOI] [PubMed] [Google Scholar]
- 117.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815–1822. [DOI] [PubMed] [Google Scholar]
- 118.Maggini J, Mirkin G, Bognanni I, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One 2010; 5: e9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paul G, Anisimov S V. The secretome of mesenchymal stem cells: potential implications for neuroregeneration. Biochimie 2013; 95: 2246–2256. [DOI] [PubMed] [Google Scholar]
- 120.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 2013; 13: 392–402. [DOI] [PubMed] [Google Scholar]
- 121.Cheng Q, Zhang Z, Zhang S, et al. Human umbilical cord mesenchymal stem cells protect against ischemic brain injury in mouse by regulating peripheral immunoinflammation. Brain Res 2015; 1594: 293–304. [DOI] [PubMed] [Google Scholar]
- 122.Yoo S-W, Chang D-Y, Lee H-S, et al. Immune following suppression mesenchymal stem cell transplantation in the ischemic brain is mediated by TGF-β. Neurobiol Dis 2013; 58: 249–257. [DOI] [PubMed] [Google Scholar]
- 123.Ooboshi H, Ibayashi S, Shichita T, et al. Postischemic gene transfer of interleukin-10 protects against both focal and global brain ischemia. Circulation 2005; 111: 913–919. [DOI] [PubMed] [Google Scholar]
- 124.Liesz A, Bauer A, Hoheisel JD, et al. Intracerebral interleukin-10 injection modulates post-ischemic neuroinflammation: an experimental microarray study. Neurosci Lett 2014; 579: 18–23. [DOI] [PubMed] [Google Scholar]
- 125.Nakajima M, Nito C, Sowa K, et al. Mesenchymal stem cells overexpressing interleukin-10 promote neuroprotection in experimental acute ischemic stroke. Mol Ther Methods Clin Dev 2017; 6: 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Biber K, Neumann H, Inoue K, et al. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci 2007; 30: 596–602. [DOI] [PubMed] [Google Scholar]
- 127.Giunti D, Parodi B, Usai C, et al. Mesenchymal stem cells shape microglia effector functions through the release of CX3CL1. Stem Cells 2012; 30: 2044–2053. [DOI] [PubMed] [Google Scholar]
- 128.Sheikh AM, Nagai A, Wakabayashi K, et al. Mesenchymal stem cell transplantation modulates neuroinflammation in focal cerebral ischemia: contribution of fractalkine and IL-5. Neurobiol Dis 2011; 41: 717–724. [DOI] [PubMed] [Google Scholar]
- 129.Roddy GW, Oh JY, Lee RH, et al. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-α stimulated gene/protein 6. Stem Cells 2011; 29: 1572–1579. [DOI] [PubMed] [Google Scholar]
- 130.Lin Q, Zhao S, Zhou L, et al. Mesenchymal stem cells transplantation suppresses inflammatory responses in global cerebral ischemia: contribution of TNF-α-induced protein 6. Acta Pharmacol Sin 2013; 34: 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wei L, Fraser JL, Lu Z-Y, et al. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis 2012; 46: 635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zacharek A, Shehadah A, Chen J, et al. Comparison of Bone marrow stromal cells derived from stroke and normal rats for stroke treatment. Stroke 2010; 41: 524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Egashira Y, Sugitani S, Suzuki Y, et al. The conditioned medium of murine and human adipose-derived stem cells exerts neuroprotective effects against experimental stroke model. Brain Res 2012; 1461: 87–95. [DOI] [PubMed] [Google Scholar]
- 134.Cho YJ, Song HS, Bhang S, et al. Therapeutic effects of human adipose stem cell-conditioned medium on stroke. J Neurosci Res 2012; 90: 1794–1802. [DOI] [PubMed] [Google Scholar]
- 135.Tsai M-J, Tsai S-K, Hu B-R, et al. Recovery of neurological function of ischemic stroke by application of conditioned medium of bone marrow mesenchymal stem cells derived from normal and cerebral ischemia rats. J Biomed Sci 2014; 21: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhao Q, Hu J, Xiang J, et al. Intranasal administration of human umbilical cord mesenchymal stem cells-conditioned medium enhances vascular remodeling after stroke. Brain Res 2015; 1624: 489–496. [DOI] [PubMed] [Google Scholar]
- 137.Zhang ZG, Chopp M. Exosomes in stroke pathogenesis and therapy. J Clin Invest 2016; 126: 1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Crivelli B, Chlapanidas T, Perteghella S, et al. Mesenchymal stem/stromal cell extracellular vesicles: from active principle to next generation drug delivery system. J Control Release 2017; 262: 104–117. [DOI] [PubMed] [Google Scholar]
- 139.Xin H, Li Y, Cui Y, et al. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab 2013; 33: 1711–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Doeppner TR, Herz J, Görgens A, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med 2015; 4: 1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee JY, Kim E, Choi S-M, et al. Microvesicles from brain-extract-treated mesenchymal stem cells improve neurological functions in a rat model of ischemic stroke. Sci Rep 2016; 6: 33038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Otero-Ortega L, Laso-García F, Gómez-de Frutos M, del C, et al. White matter repair after extracellular vesicles administration in an experimental animal model of subcortical stroke. Sci Rep 2017; 7: 44433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Deng M, Xiao H, Zhang H, et al. Mesenchymal stem cell-derived extracellular vesicles ameliorates hippocampal synaptic impairment after transient global ischemia. Front Cell Neurosci 2017; 11: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xin H, Li Y, Liu Z, et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 2013; 31: 2737–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xin H, Wang F, Li Y, et al. Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from MicroRNA 133b-overexpressing multipotent mesenchymal stromal cells. Cell Transplant 2017; 26: 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xin H, Katakowski M, Wang F, et al. MicroRNA-17–92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke 2017; 48: 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen J, Ning R, Zacharek A, et al. MiR-126 contributes to human umbilical cord blood cell-induced neurorestorative effects after stroke in type-2 diabetic Mice. Stem Cells 2016; 34: 102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.England TJ, Sprigg N, Alasheev AM, et al. Granulocyte-colony stimulating factor (G-CSF) for stroke: an individual patient data meta-analysis. Sci Rep 2016; 6: 36567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tobin MK, Bonds JA, Minshall RD, et al. Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here. J Cereb Blood Flow Metab 2014; 34: 1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shen LH, Li Y, Chen J, et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab 2007; 27: 6–13. [DOI] [PubMed] [Google Scholar]
- 151.Sinden JD, Vishnubhatla I, Muir KW. Prospects for stem cell-derived therapy in stroke. Prog Brain Res 2012; 201: 119–167. [DOI] [PubMed] [Google Scholar]