Abstract
Background:
The management of the furcation areas in multirooted teeth is often challenging due to difficulty in access. Platelet-rich fibrin (PRF), a second-generation platelet concentrate, has shown to accelerate the healing of soft and hard tissues. This study was designed to evaluate the efficacy of autologous PRF as a membrane in treatment of Grade II furcation defects in molars as compared to collagen membrane along with demineralized freeze-dried bone allograft in both the groups.
Materials and Methods:
A split-mouth study was planned with 18 patients having 2 sites of Grade II furcation defects each. Random allocation of the defect site was done for the test and control group. Plaque index, probing depth (PD), relative vertical clinical attachment level (RVCAL), gingival marginal level, and radiographic bone levels were recorded at baseline, 3 months, and 6 months postoperatively.
Results:
Both the groups showed statistically significant outcomes in intragroup comparison from baseline to 3 and 6 months. However, there was no statistical difference between PRF membrane and collagen membrane groups on intergroup comparison.
Conclusion:
There was a significant reduction of PD, improvement in RVCAL, and defect fill with autogenous PRF as membrane. This indicates its role as a regenerative material in treating furcation defects, which can be used as alternative to other expensive membranes.
Key words: Collagen membrane, demineralized freeze-dried bone allograft, furcation defects, platelet rich fibrin, regeneration
INTRODUCTION
Furcation involvement is an invasion of a bifurcation or trifurcation of a multirooted tooth by periodontal disease.[1] The management of the furcation defects, due to its complex anatomy and posterior position in arch, is always a challenge for clinician. These defects have bigger radicular area and potential accessibility to the toxins produced by various bacteria.[2] The success of the furcation therapy depends on the severity of the furcation involvement as well as the treatment regimen. Surgical therapy for furcation provides access for debridement, bone contouring, odontoplasty as well as for regenerative procedures. Various regenerative options such as bone grafts, guided tissue regeneration (GTR), growth factors, and enamel matrix derivatives (EMDs) are now used in regenerative therapy of Grade II furcation involvement. At present, recombinant human platelet-derived growth factor-BB, bone morphogenetic proteins (BMPs), plasma rich in platelet, and plasma rich in growth factors are widely used for periodontal regeneration. Under favorable conditions, use of graft material having osteoconductive and osteoinductive properties can induce up to 60%–70% bone regeneration of the lesion's height or volume, with concomitant improvement in the clinical conditions. Demineralized freeze-dried bone allograft (DFDBA) has been extensively evaluated in the therapy of Grade II furcation involvement. Some of the studies have revealed significant and consistently better gain in bone fill with the use of DFDBA, compared to open flap debridement alone.[3] The available literature suggests that highly cross-linked materials are superior in GTR therapy. Research conducted on Type I collagen has proven its effectiveness for use as a barrier membrane.[4]
Platelet-rich fibrin (PRF) membrane can be used alone or in combination with various graft materials. PRF promotes healing of wound, osseous growth, graft stabilization, and hemostasis. It also helps in improving the handling property of bone grafting material.[5]
Studies have showed positive results of collagen membrane in combination with bone grafts in various periodontal defects. On the other hand, many studies have stated that DFDBA containing BMPs and PRF containing growth factors help in regeneration of periodontal tissue. However, hardly, any study has shown efficacy of PRF as a membrane in association with DFDBA graft for the treatment of Grade II furcation involvement. As PRF membrane is very cost-effective in comparison to collagen membrane, it was decided to evaluate and compare the clinical efficacy of both for treating Grade II furcation. Hence, this study was designed as a split-mouth, randomized controlled study comparing the clinical and radiographic parameters for Grade II furcation defects with DFDBA as a graft and PRF as a membrane in test group and collagen membrane in control group.
MATERIALS AND METHODS
A randomized controlled, clinical, radiographic study was planned after the Ethical Approval from the Institutional Ethics Committee. The patients reporting to the department of periodontology were enrolled for the study. All 18 patients screened for the study signed informed consent after discussing any queries they had with the study investigator.
The criteria for selection of patients were as follows:
Inclusion criteria – (i) Patients with no complicated medical history, (ii) no use of antibiotics or anti-inflammatory drugs in the past 6 months, and (iii) Radiographic evidence of bilateral furcation defects in the molars (buccal, lingual, mesiobuccal, or distobuccal).
Exclusion criteria – (i) Patients with previous systemic conditions and pregnant females, (ii) patients allergic to local anesthetics, chlorhexidine, antibiotics, and analgesics, (iii) previous history of allergy to collagen membrane and DFDBA, and (iv) patients who had a habit of smoking and tobacco chewing.
The initial therapy was performed with full-mouth scaling and root planing followed by oral hygiene instructions and selective occlusal grinding wherever indicated. The baseline parameters were recorded after initial therapy, within 14 days before surgery. The treatment for the defects was randomly assigned by a coin toss method to receive either DFDBA + PRF (test group) or DFDBA + collagen (control group), and the results of the randomization were sealed in an opaque envelope for allocation. An acrylic stent was fabricated for each patient and was used for recording all the clinical parameters.
All clinical parameters such as clinical attachment level (CAL), probing depth (PD), and gingival marginal level (GML) were recorded at baseline [Figures 1 and 2] and after 3- and 6-month interval following periodontal surgery.
Figure 1.
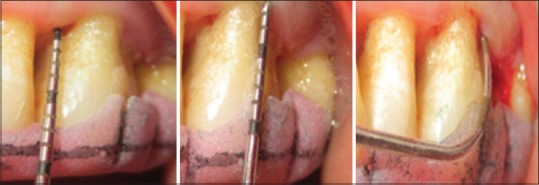
Preoperative measurement at test site
Figure 2.
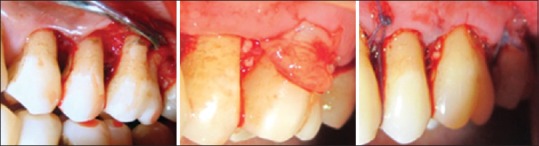
Flap elevation, demineralized freeze-dried bone allograft, and platelet-rich fibrin membrane placement, sutures at test site
The clinical and radiographic records were obtained before local anesthesia. A full-thickness mucoperiosteal flap was reflected attempting to retain all soft tissues at treated sites. The furcation defect was thoroughly debrided with a combination of mini five curettes (Hu Friedy) and ultrasonic instrumentation. The defects on control site were grafted with DFDBA and collagen membrane (CollaGuide™) while test site with DFDBA and PRF membrane [Figures 3 and 4].
Figure 3.
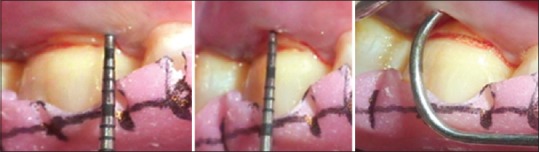
Preoperative measurement at control site
Figure 4.
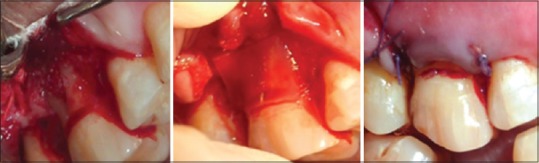
Demineralized freeze-dried bone allograft placed and covered by collagen membrane and sutures
PRF was prepared according to the procedure described by Choukroun et al.[6] PRF was prepared by collecting patient's blood from the median cubital vein using a 10 ml syringe and centrifuged at 3000 rpm for 10 min. Blood centrifugation leads to the formation of a fibrin clot that was immediately converted into membrane with the use of PRF box.
Following grafting procedure, the flap was repositioned and sutured back. It was secured with direct loop interrupted Vicryl 4-0 suture to obtain primary closure. Periodontal pack was placed and postoperative instructions were given to the patients. Radiographic evaluation was done at the end of 6 months by taking an intraoral periapical radiograph with grid and parallel cone technique [Figures 5 and 6].
Figure 5.
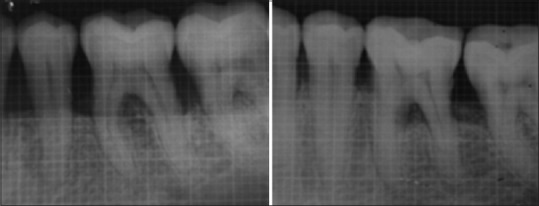
Test site X-ray baseline and 6 months
Figure 6.
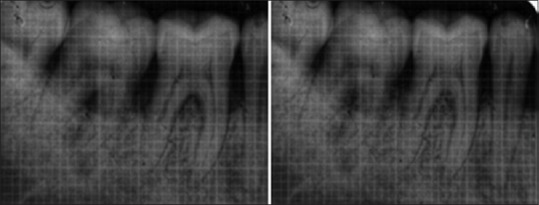
Control site X-ray baseline and 6 months
Descriptive and inferential statistical analyses were done in the present study. Level of significance was fixed at P = 0.05 and any value < 0.05 was considered to be statistically significant. Student's t-tests (two-tailed, unpaired) were used to find the significance of study parameters on continuous scale between two groups. Analysis of variance (ANOVA) was used to find the significance of study parameters between three or more groups followed by post hoc analysis if the ANOVA values were significant. Mann–Whitney U-test was used to find the significance of study parameters on a continuous scale between two groups. Kruskal–Wallis test was used to find the significance of study parameters between three or more groups [Flowchart 1].
Flowchart 1.
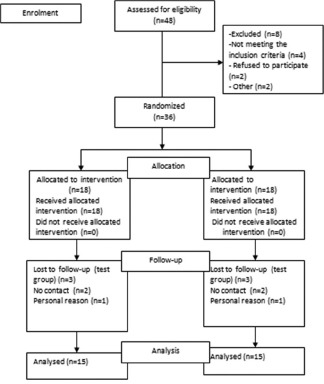
Consort flowchart for patient recruitment
RESULTS
A total of 18 patients with 36 sites were treated for the clinically controlled, split-mouth study, of which 3 patients were lost during follow-up. A total of 30 sites were examined from baseline to 3 months and 6 months. Wound healing was uneventful for all treated sites. No significant difference in healing was noted between the two groups. The results showed no significant difference in the baseline values of plaque index (PI) scores, PD, GML, and CAL in terms of mean ± standard deviation (SD) between both the groups using unpaired t-test [Table 1]. The comparison of the PI scores, PD, and CAL in terms of mean ± SD at different time intervals from baseline, 3 months, and 6 months in the PRF group was done using ANOVA test [Table 2]. There was high statistical difference noted from baseline to 3 months and 3 months to 6 months for PI score that was 0.98 ± 0.2, 0.58 ± 0.1, and 0.52 ± 0.1, respectively. PD at baseline, 3 months, and 6 months was 5.5 ± 0.7, 3.35 ± 0.5, and 3.06 ± 0.3, respectively. High statistical difference was noted with P < 0.001 for baseline to 3 months and 6 months. CAL at baseline, 3 months, and 6 months was 11.02 ± 0.7, 8.62 ± 0.5, and 8.22 ± 0.5, respectively. High statistical difference was noted with P < 0.001 for baseline to 3 months and 6 months in terms of CAL. The comparison of the GML in terms of mean rank at different time intervals from baseline, 3 months, and 6 months in the PRF group was performed using Kruskal–Wallis test [Table 3]. Statistically, there was no difference from baseline to 3 months and 6 months (P = 0.229). The comparison of the PI scores, PD, and CAL in terms of mean ± SD at different time intervals from baseline, 3 months, and 6 months in the collagen group was done using ANOVA test [Table 4]. There was high statistical difference noted from baseline to 3 months and 3 months to 6 months for PI score which was 5.24 ± 0.7, 3.17 ± 0.5, and 2.40 ± 0.4, respectively. High statistical difference was noted with P < 0.001 for baseline to 3 months and 6 months. CAL at baseline, 3 months, and 6 months was 10.75 ± 1.1, 8.60 ± 0.6, and 7.75 ± 0.7, respectively. High statistical difference was noted with P < 0.001 for baseline to 3 months and 6 months in terms of CAL. Table 5 shows comparison of the GML in terms of mean rank at different time intervals from baseline, 3 months, and 6 months in the PRF group using Kruskal–Wallis test. Statistically, there was no difference from baseline to 3 months and 6 months (P = 0.907). The comparison of the mean difference (baseline – 6 months) of PI scores, PD, and CAL in terms of mean ± SD between both the groups was done using unpaired t-test. Statistically, there was no difference from baseline to 6 months. Table 6 shows comparison of the mean difference (baseline – 6 months) of GML in terms of mean rank between both the groups using Mann–Whitney test. Statistically, there was no difference from baseline to 6 months. Table 7 shows the comparison of the radiographic evaluation in terms of mean ± SD among both the groups using unpaired t-test which showed mean percentage of bone fill which was 53.13 ± 5.7 for test group and 52.73 ± 3.4 for control group, no statically significant difference was noted.
Table 1.
Comparison of the baseline values of plaque index scores, probing depth, gingival marginal levels, and clinical attachment level in terms of mean±standard deviation
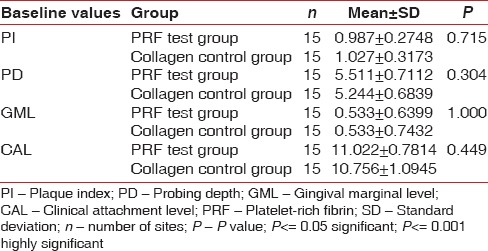
Table 2.
Comparison of the plaque index scores, probing depth, and clinical attachment level in terms of mean±standard deviation at different time intervals in the platelet-rich fibrin group using analysis of variance test
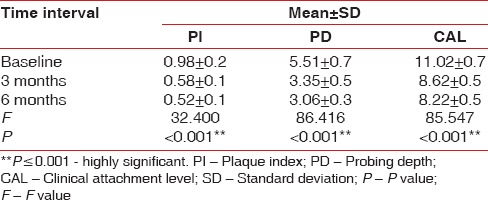
Table 3.
Comparison of the gingival marginal level in terms of mean rank at different time intervals in the platelet-rich fibrin group using Kruskal-Wallis test

Table 4.
Comparison of the plaque index scores, probing depth, and clinical attachment level in terms of mean±standard deviation at different time intervals in the collagen group using analysis of variance
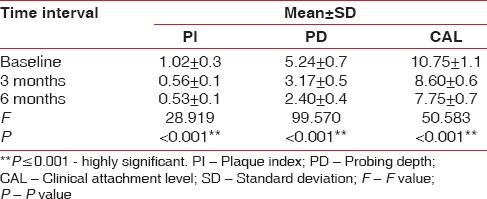
Table 5.
Comparison of the gingival marginal level in terms of mean rank at different time intervals in the collagen group using Kruskal-Wallis test

Table 6.
Comparison of the mean difference baseline - 6 months of gingival marginal level in terms of mean rank among both the groups using Mann-Whitney test

Table 7.
Comparison of the radiographic evaluation in terms of mean±standard deviation among both the groups using unpaired t-test

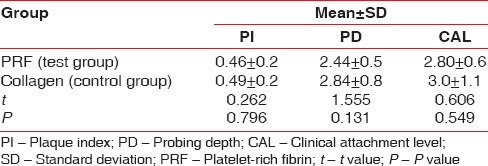
A statistically significant reduction in the PI was observed in both the test and control sites at 3 months and 6 month, postoperatively. However, the difference between the test and control sites was statistically insignificant [Table 8]. None of the treated defects progressed to Grade III. The clinical and radiographic parameters at baseline 3 and 6 months and intragroup comparison shows greater PD reduction from baseline to 3 months and 6 months with no significant difference between both the groups. At 6 months postoperatively, no statistical significant difference was noted between the test group and control group in all the parameters. Test sites presented with a greater vertical defect fill 53.13 ± 5.7 than the control sites 52.7 ± 3.4 at 6 months [Table 7], but there was no statistical significant difference between both the values.
Table 8.
Comparison of the mean difference baseline - 6 months of plaque index scores, probing depth, and clinical attachment level in terms of mean±standard deviation among both the groups using unpaired t-test
DISCUSSION
This study evaluates the clinical efficacy of autologous PRF as a membrane with DFDBA, comparing it with collagen membrane with DFDBA in the treatment of Grade II furcation involvement in humans. The split-mouth design was selected to minimize the bias due to interpatient variability. In a study done by Eto et al.,[7] favorable results were obtained using bovine-derived hydroxyapatite in Grade II furcation involvement, but no difference was noted compared to open flap debridement. Khanna et al.[8] concluded that bone graft combined with membrane showed better results in clinical parameters as compared to open flap debridement alone. Surgical considerations such as stability of wound and maintenance of space are significant aspects of GTR procedures. Second-generation membranes are bioabsorbable, thus eliminating the need for second surgery for membrane removal. They reduce the chances of loss of regenerated attachment, and increase acceptance by patients. Also, the integration with host tissues is good, enhancing tissue coverage, reducing barrier exposure and resisting or preventing microbial colonization.
The rationale for using collagen membrane in this study is based on the following observations:First, it is a hemostatic agent. Wikesjö et al.[9] and Haney et al.[10] have stated that, for regeneration to occur, the clot to form and adhere to the surface of the root, thereby facilitating complete wound maturation. Second, the vascular channels once infiltrate membrane; it can act as a lattice for migrating periodontal ligament fibroblasts. Third, collagen has chemotactic property for fibroblasts in vitro, which further enhances cell migration. In addition, it is possible to form collagen into different shapes, facilitating easy manipulation and adaptation on the surface of the root. Furthermore, being a weak immunogen, which is bioabsorbable, no second surgery is required to remove it.
The present study evaluates clinical and radiographic parameters to study the effect of PRF on periodontal tissue and also compare it with the collagen membrane, which is widely used for GTR techniques. PRF contains a fibrin matrix with tetramolecular structure; in which platelets, cytokines, leukocytes, and circulating stem cells are incorporated. Due to slow polymerization of fibrin during processing of PRF, there is incorporation of platelet cytokines and glycan chains in the fibrin meshes. As a result, PRF, unlike the other platelet concentrates, progressively releases cytokines during remodeling of fibrin matrix. This process explains the healing properties of PRF observed clinically. In addition, PRF slows the blood activation process, which can induce an elevated leukocyte degranulation and cytokine from pro-inflammatory mediators, such as interleukin (IL)-1b, IL-6, and tumor necrosis factor-A, to anti-inflammatory cytokines, such as IL-4. It is also found that PRF organizes as a dense fibrin scaffold with a high number of leukocytes concentrated in one part of the clot, with a specific slow release of growth factors (e.g., transforming growth factor-1b, platelet-derived growth factor-AB, and vascular endothelial growth factor) and glycoproteins (e.g., thrombospondin-1) during 7 days. Leukocytes have a powerful influence on growth factor release immune regulation, anti-infectious activities, and remodeling of matrix during healing. To the best of our knowledge, there were no studies comparing the use of PRF as membrane and that of collagen membrane in the treatment of furcation defects. When comparing change in clinical and radiologic parameters with other regenerative materials, PRF shows a greater effect in soft- and hard-tissue regeneration,[5] especially considering first-generation platelet concentrate (PRP). All the clinical and radiographic parameters showed better results for the PRF group in this study. This was almost same to results obtained with the collagen membrane.
A mean PD reduction from baseline 5.51 ± 0.7 to 3 months 3.35 ± 0.5 and 6 months 3.06 ± 0.3 was noted to the test sites compared to baseline 5.24 ± 0.7, 3 months 3.17 ± 0.5 mm to 6 months in the control sites was observed which was statically not significant postoperatively in the present study. Thus, the test sites showed similar PD reduction to control site. These findings are similar to the results of the studies by Lekovic et al.,[11] but the study conducted by Tsao et al.[12] showed that there was a greater vertical bone fill in the group which was treated with graft alone as compared to the group in which combination of graft and collagen membrane was used. The positive changes in VPD could be due to gingival recession or an improvement in CAL. No significant apical shift in the gingival margin was observed in both groups at 3 and 6 months.
The gain in clinical attachment was recorded both in the test group and control group that is from baseline 11.02 mm to 3 months 8.62 mm and at 6 months 8.22 mm for test group and the control group baseline 10.75 mm to 3 months 8.60 mm and at 6 months 7.75 mm postoperatively. Between the groups, there was no significant difference either at 3 months or at 6 months postoperatively. The results are in agreement with the study results of Wang et al.[13] suggesting an improved CAL in sites treated using the combination technique.
In some of the images, it was not possible to detect radiographic changes. This shortcoming could have arisen due to the two-dimensional representation of a three-dimensional structure and as Grade II furcations need not necessarily show radiographic change. Some of the test and control sites showed bone fill at 6 months, which could be compatible with new bone growth, but it is difficult to ascertain if regeneration or new bone growth has occurred, since this would require surgical reentry or histological evaluation. Due to the inherent limitations in assessing bone fill using these techniques, bone fill was considered only as an additional parameter. Studies have shown that radiographs, even those taken with standardized methods, are less reliable than clinical probing techniques. Here, in this study, test sites presented with a greater vertical defect fill 53.13 ± 5.7 than the control sites 52.73 ± 3.4 at 6 months Table 7, but they were not statistically significant.
CONCLUSION
Management of furcation has always been a challenge to treat. Both the PRF as a membrane and collagen membrane in combination with DFDBA has shown promising results in the management of furcation. The results of this study showed a significant closure of the furcation defects clinically as well as radiographically in both the groups. Looking at the clinical outcomes and economics involved PRF could prove to be better option in management of Grade II furcation defects. Further longitudinal studies with long-term follow-up are still required to assess the benefit of PRF in the management of periodontal osseous defects in general and furcation in particular.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Goldman MJ, Ross IF, Goteiner D. Effect of periodontal therapy on patients maintained for 15 years or longer. A retrospective study. J Periodontol. 1986;57:347–53. doi: 10.1902/jop.1986.57.6.347. [DOI] [PubMed] [Google Scholar]
- 2.Hirschfeld L, Wasserman B. A long-term survey of tooth loss in 600 treated periodontal patients. J Periodontol. 1978;49:225–37. doi: 10.1902/jop.1978.49.5.225. [DOI] [PubMed] [Google Scholar]
- 3.Anderegg CR, Martin SJ, Gray JL, Mellonig JT, Gher ME. Clinical evaluation of the use of decalcified freeze-dried bone allograft with guided tissue regeneration in the treatment of molar furcation invasions. J Periodontol. 1991;62:264–8. doi: 10.1902/jop.1991.62.4.264. [DOI] [PubMed] [Google Scholar]
- 4.Quteish D, Singrao S, Dolby AE. Light and electron microscopic evaluation of biocompatibility, resorption and penetration characteristics of human collagen graft material. J Clin Periodontol. 1991;18:305–11. doi: 10.1111/j.1600-051x.1991.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 5.Mehta D, Deshpande N, Dave D, Modi B, Bharwani A. Platelet rich fibrin: New treatment modality in Grade II furcation defects. J Oral Dis Marker. 2016;1:1–5. [Google Scholar]
- 6.Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e56–60. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Eto AL, Joly JC, Jeffcoat M, de Araújo NS, de Araújo VC, Cury PR. The use of anorganic bovine derived hydroxyapatite matrix/cell derived peptide (P-15) in the treatment of class II furcation defects: A clinical and radiographic study in humans. J Periodontol. 2007;78:2277–83. doi: 10.1902/jop.2007.070234. [DOI] [PubMed] [Google Scholar]
- 8.Khanna D, Malhotra S, Naidu DV. Treatment of grade II furcation involvement using resorbable guided tissue regeneration membrane: A six-month study. J Indian Soc Periodontol. 2012;16:404–10. doi: 10.4103/0972-124X.100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wikesjö UM, Nilvéus RE, Selvig KA. Significance of early healing events on periodontal repair: A review. J Periodontol. 1992;63:158–65. doi: 10.1902/jop.1992.63.3.158. [DOI] [PubMed] [Google Scholar]
- 10.Haney JM, Nilvéus RE, McMillan PJ, Wikesjö UM. Periodontal repair in dogs: Expanded polytetrafluoroethylene barrier membranes support wound stabilization and enhance bone regeneration. J Periodontol. 1993;64:883–90. doi: 10.1902/jop.1993.64.9.883. [DOI] [PubMed] [Google Scholar]
- 11.Lekovic V, Camargo PM, Weinlaender M, Vasilic N, Aleksic Z, Kenney EB, et al. Effectiveness of a combination of platelet-rich plasma, bovine porous bone mineral and guided tissue regeneration in the treatment of mandibular grade II molar furcations in humans. J Clin Periodontol. 2003;30:746–51. doi: 10.1034/j.1600-051x.2003.00368.x. [DOI] [PubMed] [Google Scholar]
- 12.Tsao YP, Neiva R, Al-Shammari K, Oh TJ, Wang HL. Factors influencing treatment outcomes in mandibular class II furcation defects. J Periodontol. 2006;77:641–6. doi: 10.1902/jop.2006.050133. [DOI] [PubMed] [Google Scholar]
- 13.Wang HL, O'Neal RB, Thomas CL, Shyr Y, MacNeil RL. Evaluation of an absorbable collagen membrane in treating class II furcation defects. J Periodontol. 1994;65:1029–36. doi: 10.1902/jop.1994.65.11.1029. [DOI] [PubMed] [Google Scholar]


