Abstract
Cerebral malaria is one of the most severe complications of human infection by the Plasmodium falciparum parasite. In this issue of Cell Host & Microbe, Kessler et al. (2017) provide valuable insights into the diagnosis and pathogenesis of this poorly understood manifestation of malaria.
The World Health Organization (WHO) 2016 World Malaria Report estimates that there were >200 million cases of malaria and >400,000 deaths due to malaria in 2015 (World Health Organization, 2016). Most of these deaths were due to infection by Plasmodium falciparum parasites in children and pregnant women. On a more promising note, the incidence and mortality rates from malaria have decreased 21% and 29%, respectively, in the past 5 years (World Health Organization, 2016). The reasons for these decreases likely include combinations of vector control, intermittent preventive treatment, and widespread use of artemisinin-combination therapy for clinical cases. Nonetheless, morbidity and mortality remain high and demand future study.
Human malaria ranges from subclinical parasitemia to severe malaria with coma and death. While most cases of clinically apparent malaria are uncomplicated and present with fever and malaise, approximately 1% are severe, with mortality rates of 6.1% in children and 35.6% in adults (Bernabeu and Smith, 2017; Dondorp et al., 2008). Cerebral malaria (CM) is of paramount importance, largely because the mortality rate is high and the pathogenesis is poorly understood. Without treatment, CM is nearly 100% fatal. Mortality is decreased by effective anti-malarials but remains unacceptably high, at 15%–20%. CM is defined by the WHO as an unarousable state (Glasgow coma score <11 in adults or a Blantyre coma score <3 in children) persisting >1 hr after any seizure, with evidence of malaria parasites in peripheral blood and exclusion of other causes of encephalopathy. Our basic understanding of CM is complicated by the fact that this “bedside” definition is not specific. In an autopsy study from Malawi, the cause of death for nearly 25% of the patients that died with a clinical diagnosis of CM was due to other (non-malarial) reasons (Taylor et al., 2004). The specificity of diagnosis is improved by evaluating comatose patients for malarial retinopathy, a characteristic grouping of abnormalities including retinal whitening, vessel color changes, and white-centered retinal hemorrhages. However, malarial retinopathy is absent in a third of patients with CM (Taylor and Molyneux, 2015). There is an urgent need to understand the basic pathogenesis of CM so that treatments can be tailored to prevent fatal outcomes.
A major aspect of P. falciparum virulence is the ability of parasites to adhere to the endothelial lining of small blood vessels. This ability, known as sequestration, allows the parasites to resist clearance in the spleen and is predominantly mediated by the surface expression of the highly variable P. falciparum erythrocyte membrane protein 1 (PfEMP1) on infected red blood cells. PfEMP1 is expressed from one of ~60 var genes in the parasite genome in a mutually exclusive manner (Dzikowski et al., 2006; Scherf et al., 1998) for antigen variation, and specific variants variably interact with host proteins and/or carbohydrates on the surface of endothelial cells, including CD36, chondroitin sulfate A, intracellular adhesion molecule 1 (ICAM1), the endothelial protein C receptor (EPCR), and others. The interaction between PfEMP1 and host cell proteins is mediated by extracellular binding domains known as Duffy binding-like (DBL) domains and/or cysteine-rich interdomain regions (CIDRs). Multiple studies have identified groups of var transcripts that are elevated in pediatric patients with severe malaria (reviewed in Bernabeu and Smith, 2017). It is essential to note that although the pathogenesis of CM remains controversial, the presence of parasite sequestration in the brain is a pathologic hallmark for the illness. This sequestration leads to endothelial dysfunction, inflammation, localized coagulopathy, and disruption of normal blood flow.
Recent studies have highlighted the importance of PfEMP1/EPCR binding in severe malaria (reviewed in Bernabeu and Smith, 2017). The normal physiological function of EPCR is to enhance the activation of protein C by the thrombinthrombomodulin complex. Activated protein C then cleaves the protease-activated receptor 1 (PAR1), which promotes anti-inflammatory signals and increases the strength of the endothelial barrier. Activated protein C also cleaves clotting factors to inhibit the coagulation cascade, ultimately decreasing the generation of thrombin. In the absence of activated protein C, increased thrombin levels cleave PAR1 at a different residue and lead to the opposite effects—pro-inflammatory and anti-barrier signals. Thus, when PfEMP1 binds EPCR, blocking and/or displacing activated protein C, there is local inflammation, dysregulated coagulation, and loss of endothelial barrier function (Bernabeu and Smith, 2017).
The study in this issue by Kessler et al. (2017) directly evaluates the pathogenesis of pediatric CM and provides insights into this poorly understood illness. Patients were recruited at a single center in Malawi by a team with extensive experience in identifying CM cases. The enrolled patients included 75 with CM (57 with and 18 without evidence of malaria retinopathy) and 38 with uncomplicated malaria. Of the 75 CM patients, 67 had sufficient materials obtained for their downstream analysis, including measurement of multiple clinical laboratory values and quantification of var gene expression patterns. Although the study addresses some of the differences between retinopathy-positive and retinopathy-negative cases, a powerful aspect is their evaluation of brain swelling. Of the CM patients, 67 underwent MRI evaluation for direct evidence of brain swelling. The value of the MRI evaluations of CM patients cannot be overstated, particularly because this was done in a resource-limited setting. This allowed an objective measurement of brain swelling and a sub-categorization of the CM patients into groups with absent, mild/moderate, and severe swelling. Eight patients with CM died, seven of whom were in the retinopathy-positive category and one of whom was in the retinopathy-negative category. As a result, the investigators could evaluate a sub-category of fatal cases, all of whom were in the severe brain swelling group. Consistent with previous studies (Dondorp et al., 2005), the investigators found that higher parasite biomass, as measured by serum levels of the parasite HRP2 protein, was correlated with severe disease. Similarly, lower platelet counts in peripheral blood were associated with severe malaria. By evaluating these lab values relative to the amount of brain swelling, the investigators show that the increase of parasite biomass and the decrease in platelet count were “proportional” to the level of brain pathology. In other words, the group of fatal cases had higher parasite biomass than that of cases that were non-fatal, severe, and with brain swelling, who in turn had more than the mild brain swelling group (the opposite was true for platelet count).
In addition, the investigators used multiple methods to measure var expression and found that transcripts encoding EPCR-binding domains were correlated with CM. These data were incorporated into a machine-learning algorithm that provided statistical robustness to their findings. In a final series of experiments, the investigators found that transcripts encoding for the CIDRα.7 domain, which is predicted to bind both ICAM1 and EPCR, were “prominent” in most of the patients with severe brain swelling and the “dominant” transcripts in six severe swelling cases. When this region was recombinantly expressed, it bound tightly to EPCR-expressing tissue culture cells. Furthermore, the authors demonstrated that CIDRa1.7 binding to EPCR diminished the ability of activated protein C to protect against thrombin-induced disruption of permeability in a human brain endothelial cell tissue culture model. These experiments demonstrated a causal link between EPCR binding by parasite proteins and a disruption of endothelial monolayer permeability. Thus, the study suggests a model linking parasite sequestration to endothelial dysfunction, inflammation, and local coagulopathy.
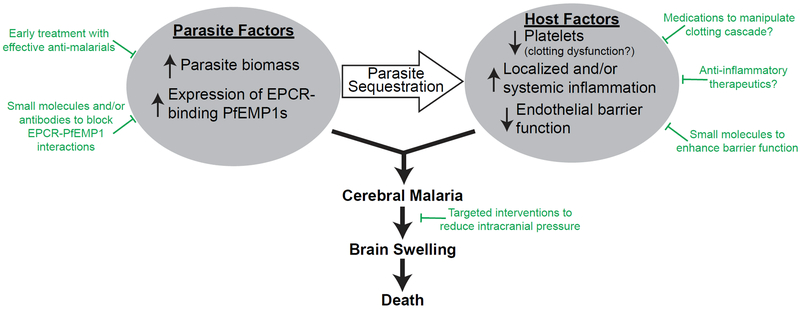
The findings of this study have multiple potential translational implications (Figure 1). If testing could be performed in a clinical setting to measure levels of HRP2 and platelets, as well as the frequency of EPCR-binding transcripts, this information could be used to identify patients at high risk of developing CM and to see if the findings are applicable more globally. Measurement of peripheral platelet count is already easily performed. An assay to measure total parasite biomass by HRP2 is needed but seems relatively straightforward by rapid diagnostic tests that already exist. For example, a semiquantitative measurement could be obtained by testing serial dilutions of patient blood (Sinha et al., 2015). One could imagine a rapid PCR-based diagnostic test to measure levels of EPCR-binding transcripts from a peripheral blood sample. The availability of mechanical ventilators is extremely limited in malaria-endemic regions. However, it may be possible to have a small number of these ventilators available for high-risk cases. Patients with indicators of severe CM could be triaged to these higher levels of care, potentially even to be placed on mechanical ventilation for 24–48 hr to help reduce intracranial pressure by hyperventilation. While many interventions have not been helpful when applied to less-well-selected groups of severe malaria patients, these extremely high-risk patients may benefit from receiving interventions to reduce brain swelling—such as hypertonic saline, osmotic agents like mannitol, or corticosteroids to reduce inflammation. Potentially, there may also be a role for therapeutics that alter the coagulation state of these patients. The current study raises hope that therapeutics like small molecules or antibodies that would disrupt PfEMP1-binding to EPCR might reduce the fatality rate in CM. This study encourages the discovery and testing of a vaccine that induces antibodies directed against EPCR-binding domains of PfEMP1. Such a vaccine would not prevent clinical malaria but may reduce the morbidity and mortality of CM. In conclusion, the study by Kessler et al. (2017) provides convincing insights into the pathogenesis of CM and may inspire future novel therapeutics.
Figure 1. Pathogenesis of Cerebral Malaria and Possible Interventions to Reduce Mortality.
The pathogenesis of cerebral malaria involves both parasite and host factors. Parasite sequestration plays a central role in this pathogenesis. Possible interventions are indicated in green.
ACKNOWLEDGMENTS
J.D.D. is supported by grants from the NIH (R01AI102907 and DP2AI112219). I apologize that many important studies could not be cited, given the space constraints. I thank Rachel Rudlaff and Manoj Duraisingh for critical reading and comments.
REFERENCES
- Bernabeu M, and Smith JD (2017). Trends Parasitol. 33, 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondorp AM, Desakorn V, Pongtavornpinyo W, Sahassananda D, Silamut K, Chotivanich K, Newton PN, Pitisuttithum P, Smithyman AM, White NJ, and Day NPJ (2005). PLoS Med. 2, e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondorp AM, Lee SJ, Faiz MA, Mishra S, Price R, Tjitra E, Than M, Htut Y, Mohanty S, Yunus EB, et al. (2008). Clin. Infect. Dis 47, 151–157. [DOI] [PubMed] [Google Scholar]
- Dzikowski R, Frank M, and Deitsch K (2006). PLoS Pathog. 2, e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Dankwa S, Bernabeu M, Harawa V, Danziger SA, Duffy F, Kampondeni SD, Potchen MJ, Dambrauskas N, Vigdorovich V, et al. (2017). Cell Host Microbe 22, this issue, 601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf A, Hernandez-Rivas R, Buffet P, Bottius E, Benatar C, Pouvelle B, Gysin J, and Lanzer M (1998). EMBO J. 17, 5418–5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha I, Ekapirat N, Dondorp AM, and Woodrow CJ (2015). Malar. J 14, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TE, and Molyneux ME (2015). Ann. N Y Acad. Sci 1342, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, Lewallen S, Liomba NG, Molyneux ME, and Mueller JG (2004). Nat. Med 10, 143–145. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2016). World Malaria Report 2016 (World Health Organization; ). [Google Scholar]