Abstract
HIV and antiretroviral therapy (ART) together can be far more detrimental to liver cells than either of the two unaided. However, ultrastructural aspects of the synergistic effects of HIV and ART have been understudied. In a patient cohort receiving ART, this study characterizes ultrastructurally sinusoidal degeneration, hepatocytic aberrations, mitochondrial dysfunction, accumulation of bulky lipid droplets (steatosis), and occlusion of sinusoidal lumina. Mitochondrial dysfunction causes the accumulation of acetyl-CoA, which leads to insulin upregulation and resistance, lipid synthesis, and steatosis. Lipid droplets deposited in the sinusoids could be the source of the blood’s lipid profile alterations in HIV patients on ART.
Keywords: Antiretroviral Therapy, Hepatotoxicity, HIV, Liver, Endothelium, Steatosis, Mitochondria, Sinusoid
Introduction
Human immunodeficiency virus (HIV) remains an important health issue with over 35 million currently infected worldwide and more than 40,000 new cases emerging each year in the United States 1,2. While the use of combination antiretroviral therapy (ART) has improved life expectancy of HIV-infected patients and reduced AIDS-related deaths, there has been an increase in morbidity and mortality due to non-opportunistic complications, notably liver and cardiovascular diseases, renal impairment, and hepatocellular carcinoma 3–6. HIV-positive individuals have high rates of liver disease, including viral hepatitis and alcohol-related liver disease, as well as drug-related hepatotoxicity, insulin resistance, non-alcoholic fatty liver disease (NAFLD), and nonalcoholic steatohepatitis (NASH) 3,7,8.
ART is known to disturb liver cells in various ways, some of which include metabolic abnormalities, central fat accumulation, mitochondrial toxicity, insulin resistance, vanishing bile duct syndrome, and NAFLD 9,10. Protease inhibitors (PIs) may cause liver sinusoidal endothelial cells (LSEC), responsible for the transport of nutrients, lipids, and lipoproteins 9,11, to malfunction by reducing nitric oxide production, and/or under-expression of endothelial nitric oxide synthase 12. Injuries to the hepatic sinusoids are initial step for liver cirrhosis and other liver diseases 13. LSEC injury appears during the simple steatosis phase and shortly followed by the activation of Kupffer cells and hepatic stellate cells, and which may all lead to the development of chronic liver diseases such as NAFLD or NASH 11.
HIV infection alone can induce functional changes in the human LSECs 14,15. Lafon et al. examined the in vitro storage and release of endothelial specific factors such as von Willebrand’s factor (vWF), protein S, and endothelin-1 in HIV patients; and found that synthesis of some factors were decreased while crucial functions such as pinocytosis and phagocytosis were preserved 16. Since HIV infection alone may trigger non-lethal functional alterations, the cells can become more aggravated when ART is introduced. Thus, while selected antiretroviral agents are associated with elevated risk of liver disease, the specific role of ART in the pathophysiology of chronic HIV-associated liver injury is yet to be defined.
Our group has previously characterized liver dysfunction in HIV monoinfected adults with chronic elevations in serum aminotransferases on ART, using liver biopsies, biochemical markers, imaging, biometric data, and genetic polymorphisms. We found a high prevalence of NAFLD and NASH in the cohort associated with established fatty liver disease risk factors, including elevated body mass index and the presence of minor alleles in the gene encoding patatin-like phospholipase domain-containing protein 3 (PNPLA3 or adiponutrin) 7.
Here, we describe the ultrastructural findings in livers from a subset of the study participants. Our goal is to elucidate the ultrastructural aberrations in the patients’ livers and discuss them in relation to their biochemical pathways. For example, the spectrum of hepatocytic degeneration (mitochondrial and cytoplasmic) and its relation to sinusoidal dysfunction, elevated aminotransferases, lipid profile alterations, and inflammation have not been shown at the ultrastructural level before.
Materials & Methods
Study Population
This report includes 9 adults enrolled in a study of causes of liver disease in HIV-infected patients receiving ART at the Clinical Research Center of the National Institutes of Health. Selected demographic and clinical parameters on the 9 patients included in this analysis are presented in Table 1. All patients had longstanding HIV infection, with a median time since diagnosis of 19.8 years (range 10.7–25.4 years), and extensive antiretroviral treatment histories, with a median duration of antiretroviral therapy of 13.7 years (range 9.0–15.5 years). Participants had received ART for at least a year, had higher than normal aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels on three or more occasions over a period of at least 6 months, and had no evidence of viral hepatitis or alcohol abuse 7. The protocol was approved by the institutional review board of the National Institute of Allergy and Infectious Diseases and written informed consent was obtained from all participants 7.
Table 1.
Selected characteristics of included HIV monoinfected study participants (n=9).
| Age, years, median (range) | 52 (47–64) |
| Male sex, n (%) | 8 (89%) |
| Time since HIV diagnosis, years, median (range) | 19.8 (10.7–25.4) |
| Antiretroviral therapy duration, years, median (range) | 13.7 (9.0–15.5) |
Liver biopsy diagnosis:
|
|
| 7 (78%) | |
| 5 (56%) | |
| 1 (11%) | |
| 1 (11%) |
Transmission Electron Microscopy (TEM)
Percutaneous liver biopsies were obtained via ultrasound guidance using an 18-gauge needle 7. The tissues were prepared for TEM using the methods described by Ogilvy et al. 17. Briefly, the liver biopsies were doubly-fixed in PBS-buffered glutaraldehyde (2.5%) and PBS-buffered osmium tetroxide (0.5%). They were then embedded in Spurr’s epoxy resin and thin sectioned (90 nm). The sections were collected on 200-mesh copper grids, dried for 24 hours, and double-stained with uranyl acetate and lead citrate. The sections were viewed and photographed using a JEOL JM-1010 electron microscope.
Results
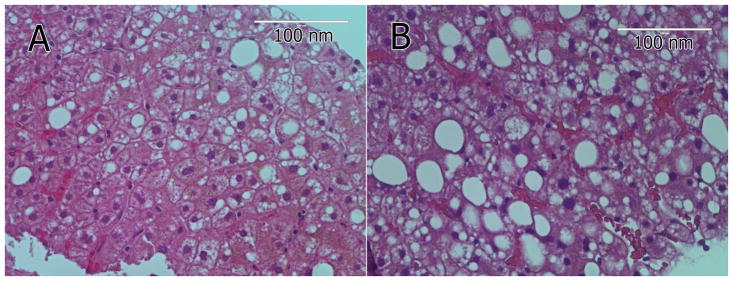
Liver biopsy (light microscopy, Figure 1) identified steatohepatitis in 7 participants (78%), fibrosis in 5. One participant (11%) had minimal steatosis and portal inflammation (non-specific changes) and one participant (11%) had bridging fibrosis without evidence of steatohepatitis. Most of the aberrations described in the TEM images are invisible by light microscopy H&Es with the exception of macrovacuolar steatosis (Figure 1A & B).
Figure 1.
Light microscopy images (H&E) of two liver biopsies from two participating patients in the ultrastructural study (A & B). Most of the aberrations described in the TEM figures are invisible by light microscopy H&Es with the exception of macrovacuolar steatosis.
Changes in hepatocytes were heterogeneous ranging from total cytolysis and necrosis to various degrees of degeneration to almost unaffected. Degeneration was seen as sparse light-density cytoplasm, mitochondria with disintegrated inner membranes, unusually large lipid droplets, many clear vesicles (i.e., cytoplasmic vesication), endothelial cell deterioration, and occluded sinusoids.
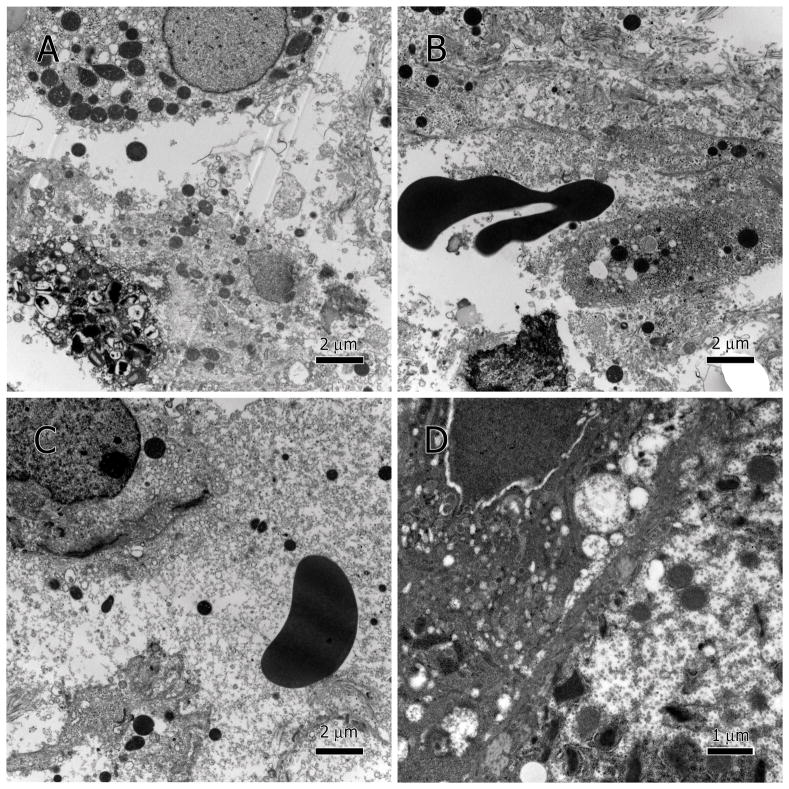
In specimens with extensive hepatocytes damage, cytolysis and necrosis (Figure 2A–C), resulted in the leakage of cellular contents and debris into the sinusoidal lumen (Figure 2C). Red blood cells (RBCs) were found between hepatocytes indicating that necrosis has led to extravasation (Figure 2B). Localized cytoplasmic deterioration was also seen in many hepatocytes (Figure 2D).
Figure 2.
Hepatocytic cytolysis. Necrotic hepatocytes presented as degenerate cytoplasm and membranes (A–C), extravasation of RBC between hepatocytes (B), as well as leakage of cellular contents into the sinusoidal lumen, note the loose mitochondria in the serum (C). Partial cytoplasmic degeneration (D).
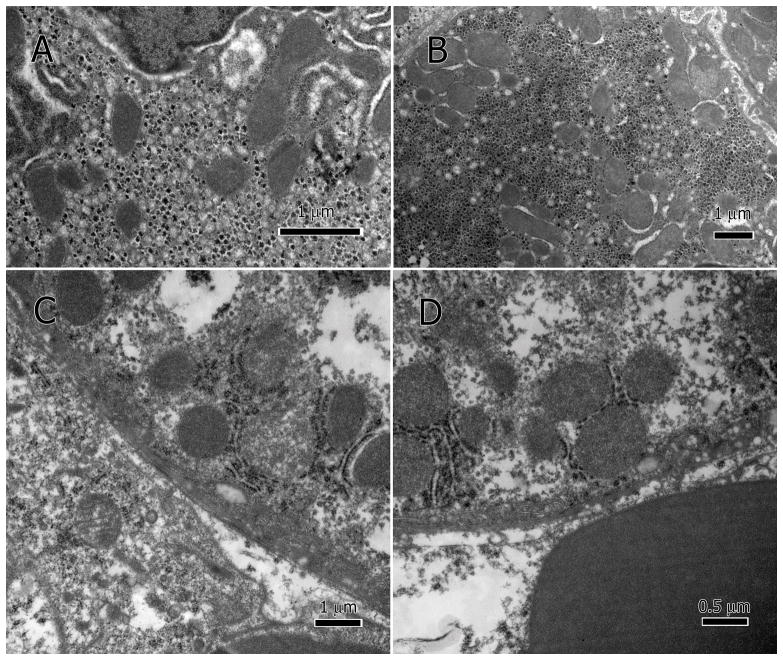
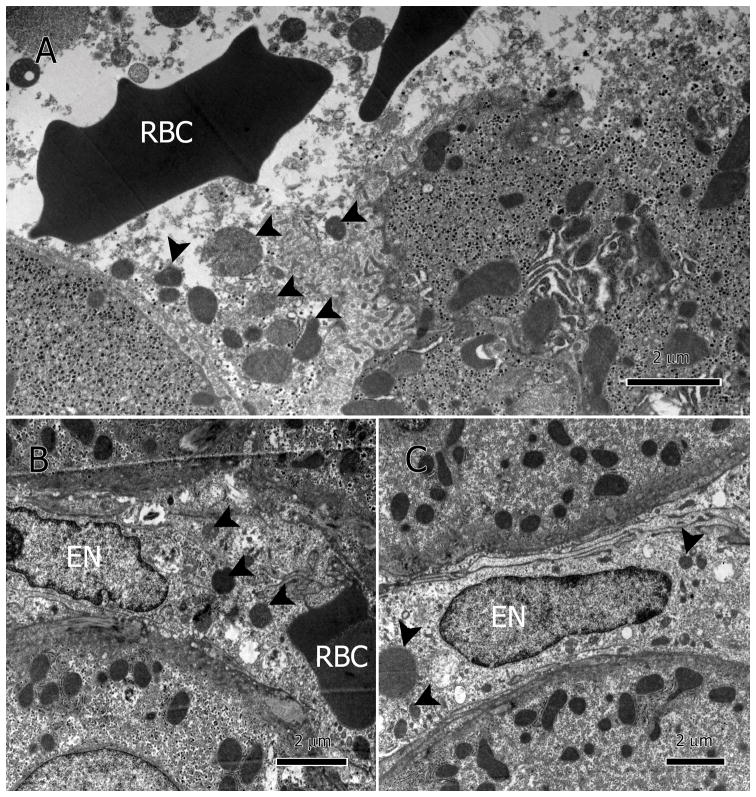
Deterioration of the mitochondria was also heterogeneous. A majority of the mitochondria in the damaged hepatocytes had impaired inner membranes, and an amorphous or granular matrix (Figure 3A&B) while some had reached late stages of destruction (Figure 3C&D). A similar pattern was found in the sinusoidal endothelium where many mitochondria were seen in the sinusoidal lumen with various degrees of deterioration (Figure 4A–C).
Figure 3.
Damaged hepatocytic mitochondria. The inner membranes of the mitochondria lack definition and the cristae are no longer visible, and matrix amorphous or granular (A–D).
Figure 4.
Mitochondria in sinusoids. Mitochondria leaked into the sinusoidal lumen (A–C, arrowheads). Due to heterogeneous nature of the mitochondrial damage, mitochondria appear at various levels of degeneration. RBC: Red blood cells; EN: endothelial nucleus.
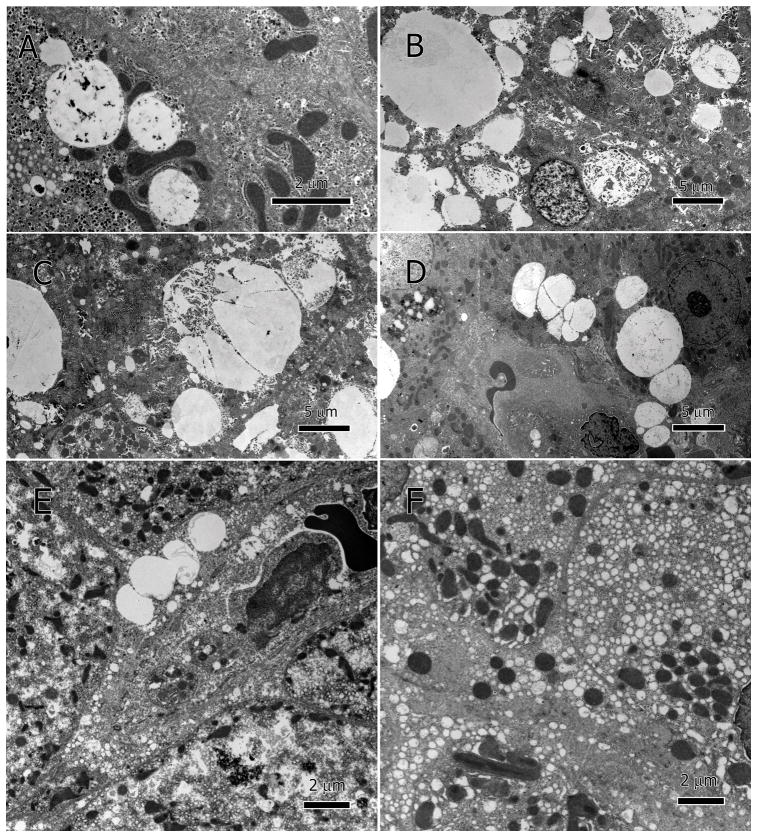
Unusually large lipid-droplets (macrovesicular steatosis) were seen in the cytoplasm of most hepatocytes but not in the LSECs (Figure 5A–E). Lipid droplets coalesced into larger ones (Figure 5C), accumulated on the basal side of sinusoid (adjacent to sinusoidal endothelium), and moved into sinusoidal lumen (Figure 5D&E). Some droplets had dark speckles within and along the droplet margin, which could be remnants of ER membranes (Figure 5C). In some hepatocytes, there was extensive clear cytoplasmic vesicles (<0.5μm in diameter) that did not contain lipid within the vesicles (Figure 5F).
Figure 5.
Hepatocytic lipid inclusions (macrovesicular steatosis) and small vesicles (microvesicular steatosis). Large lipid droplets of various sizes accumulated within hepatocytes; some have dark speckles within and along the droplet margin (A–D). Lipid droplets coalesce into larger ones (C), accumulate on the side of sinusoid and move into sinusoidal lumen (D and E). Microvesicular steatosis, vesication in the hepatocytes, produced numerous vesicles <0.5 μm in diameter (F).
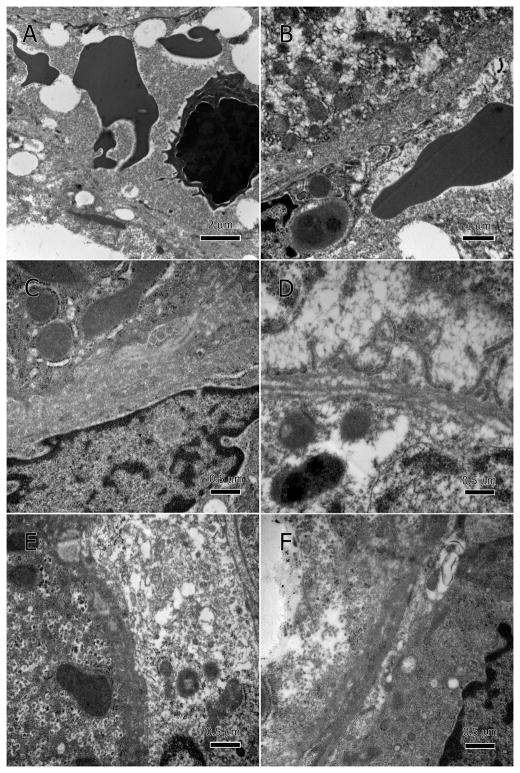
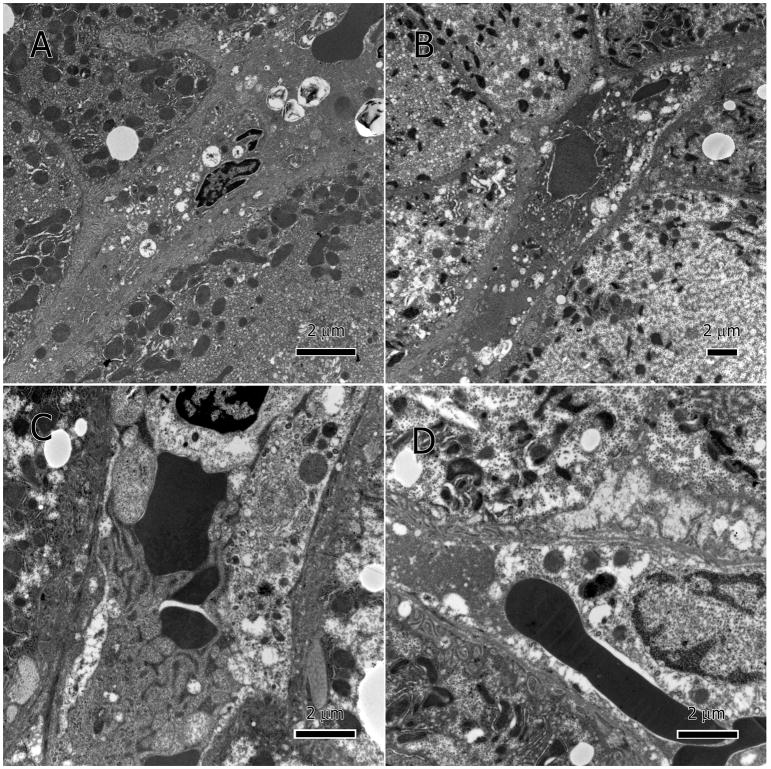
There was also significant damage to the sinusoidal endothelial cells, specifically the membrane and mitochondria (Figure 6A–F). Major deterioration of mitochondria as well as hypertrophic nuclei (Figure 6C) was also present. Leakage of cellular debris and degenerate organelles into the sinusoidal lumina was enabled by the severely deteriorated sinusoidal endothelium (Figure 6D–F). Sinusoidal lumina became occluded with a thick proteinaceous material and had many vesicles and lipid droplets (Figure 7A–D). Additionally, the hepatocytes’ basal surface lost its microvilli and the space of Disse was packed with cellular debris or totally lacking.
Figure 6.
Degenerate sinusoidal endothelium. Endothelial membranes show poorly defined margins, deterioration, and discontinuity; A and B at low magnification, and C–F at higher magnification. The degenerate endothelium has allowed hepatocytic cellular contents to leak into the sinusoidal lumen, especially large lipid droplets (A). Hepatocytes’ basal surface (adjacent to sinusoidal endothelium) lost its microvilli and the space of Disse was restricted or lacking.
Figure 7.
Occluded sinusoids. Sinusoidal lumen filled with cellular debris from necrotic and degenerate hepatocytes (A–D), thick proteinaceous material that may be of hepatocytic cytoplasm (A–D), degenerate endothelium membrane (C), and lipid droplets (A). Some sinusoidal endothelial nuclei are hypertrophic (D).
There were no HIV particles or drug accumulation visible in the examined tissues.
Discussion
Though liver injury is common in HIV-infected patients receiving ART, studies that include transmission electron microscopy (TEM) are limited and largely reflect acute drug-induced liver injury. The ultrastructural aspects of chronic ART-associated liver injury have been understudied and a complete view of their pathology has not been presented in detail before 10,18. ART, despite its life extending benefit, has serious side effects and ramifications 7,19. Our study identified ultrastructural correlates of liver injury in HIV-infected patients who had persistent aminotransferase elevations on ART.
Our findings suggest that ART is associated with heterogeneous effects on the liver cells, manifested in a hepatocytic damage that ranges from slightly affected to totally necrotic. Hepatic cell heterogeneity is suggested as a mechanism to reduce toxicity 20; however, the hepatic effects of ART may reflect two levels of existing heterogeneities: mitochondrial heteroplasmy within a single hepatocyte and mitochondrial heterogeneity between hepatocytes. ART may exert a selective pressure that works on the mitochondrial population and destroys mitochondria that cannot metabolize or neutralize its components, eventually resulting in progressive liver injury.
Our TEM images show that in the majority of damaged hepatocytes and endothelial cells, the inner mitochondrial membranes and cristae are no longer identifiable due to deterioration. Mitochondrial injury and resulting dysfunction may initiate a cascade of events starting with lipid accumulation, membrane deterioration, and the disruption of other energy-dependent processes that eventually lead to necrosis as we have observed in the examined specimens 21. The impairment of mitochondrial fatty acid β-oxidation, TCA cycle, and oxidative phosphorylation lead to intracellular lipid accumulation and greatly contribute to the resulting hepatotoxicity. However, the etiology of these abnormalities cannot be established by our cross-sectional study; HIV, ART, other medications, historic use of alcohol and/or illicit drugs, fatty liver disease, and aging are all potential contributors.
HIV infection, per se, contributes to mitochondrial damage. Our group has previously demonstrated that HIV infection has direct effects on mitochondria, associated in part with immune cell activation and systemic inflammation. Mitochondrial genes were down-regulated in peripheral blood mononuclear cells and adipose tissue from untreated HIV-infected adults compared to HIV-negative and HIV-infected patients receiving ART 22.
ART can cause mitochondrial toxicity independent of HIV-infection, as demonstrated in vitro and animal models 23–25. Nucleotide reverse transcriptase inhibitors (NRTIs), most notably stavudine, are known to affect mitochondrial DNA replication and cause their DNA depletion, and leading, through this or other mechanisms, to mitochondrial dysfunction with anaerobic respiration, lactic acidosis, and hepatosteatosis 26. NRTIs are also associated with peripheral lipoatrophy and insulin resistance, and may contribute to liver injury, including fatty liver disease, through alterations in fat and sugar metabolism 27. Other antiretroviral agents, including the non-nucleoside reverse transcriptase inhibitor (NNRTI) efavirenz and protease inhibitors, are also associated with liver injury, including mitochondrial toxicity 28.
Cytoplasmic accumulation of lipid droplets usually signifies dysfunctional mitochondrial TCA cycle (or hypoxia); and therefore indicates an unhealthy struggling cell and the beginning of steatosis. Furthermore, lipid droplets in a fatty liver can also function as a sink for the sequestration fat-soluble toxic drugs 29. Some ART components are deposited into lipid, and the drug-laden droplets are secreted in the sinusoidal lumina and disseminated through the blood to be dumped into the intestine, or appear as a skin rash 23. In the HIV-infected patients receiving ART, the lipid droplets’ quantity and size may be influenced by the scope of mitochondrial dysfunction within the cell and by the cells’ ongoing attempt to sequester the ART drugs in lipid droplets (or sometimes in aqueous vesicles depending on the chemical properties of the drug).
When the hepatocyte is unable to sequester the drug within lipid droplets, vesication occurs and the cytoplasm appears filled with tiny vesicles as seen in Figure 5F. Lipid droplets appear when the mitochondrial TCA are too impaired and acetyl-CoA, the main component of fatty acid synthesis, accumulates outside the mitochondria in the cytoplasm, and thus promotes fatty acid synthesis 30,31. This combination of mitochondrial dysfunction and lipid droplet accumulation progresses into a fatty liver and is the precursor to other liver diseases as well. Additionally, mitochondrial dysfunction could be responsible for insulin resistance since it causes the accumulation of a high level of acetyl-CoA, which gets converted to malonyl acetyl-CoA by its carboxylase enzyme, and is used for fatty acid synthesis. The conversion to malonyl acetyl-CoA requires high level of plasma insulin 32. The emerging sequence here is that mitochondrial dysfunction causes the accumulation of acetyl-CoA, which leads to insulin upregulation and resistance, fat synthesis, and steatosis.
In response to ART, the hepatocytes that produce cytoplasmic vesicles rather than lipid droplets could undergo cytolysis and necrosis when vesication continues for a long period of time. This may be a mechanism by which ART increases the risk of microvesicular steatosis and liver disease in HIV-infected patients.
Another key physiological change seen in these biopsies is endothelial cell damage in the sinusoid and other hepatic vessels. Ultrastructurally, this damage manifests in degeneration of the endothelial membrane, mitochondria, and hypertrophic nuclei. These changes may represent a toxic injury from ART. Protease inhibitors may target the endothelial membrane as well 9. The hypothesized mechanism is that it reduces the production of nitric oxide within the endothelium 12.
The destructive effect of ART on the cells may occur at a rate faster than what the lipid droplets can sequester and Kupffer cells can engulf. A buildup of necrotic cells and leakage of cellular debris into the lumina of sinusoids and vessels cause their occlusion. The vascular supply of hepatocytes becomes insufficient due to dysfunctional sinusoids and vessels and leads to abnormal transportation throughout the liver. Additionally, transport of drug-laden lipid droplets and clearance of cellular debris through the sinusoids is hindered by the abnormally dense serum; this only contributes to the buildup of lipid droplets within hepatocytes.
TEM does not resolve whether this damage occurs first within the endothelial cells or the hepatocytes; however, it has provided a panoramic view of events that take place within the patients’ livers. When TEM results are coupled with biochemical pathways, they point out that the majority of aberrant events are related to mitochondrial dysfunction and its consequences.
The effects of hepatotoxicity and risk of liver disease may be attenuated in the setting of newer antiretroviral therapies and may be lowered if additional contributors to liver disease can be avoided, for example, weight gain. Longitudinal liver biopsies would be the most helpful in monitoring the abnormal deviations in the pathways involved in the pathological process. However, a less invasive monitoring method would be mass spectrometry of metabolomics, or proteomics (total proteomic profile) of the serum, which would detect the deviation due to drug effects; this could lead to an adjustment in dosage that may reduce the deleterious consequences of ART and related drugs.
Footnotes
Financial Disclosures
The authors declare they have no conflicting financial interests.
References
- 1.Sherman KE, Rockstroh J, Thomas D. Human immunodeficiency virus and liver disease: An update. Hepatology. 2015;62(6):1871–1882. doi: 10.1002/hep.28150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prevention CfDCa. Diagnoses of HIV Infection in the United States and Dependent Areas, 2014. Centers for Disease Control and Prevention; 2015. p. 26. [Google Scholar]
- 3.Joshi D, O’Grady J, Dieterich D, Gazzard B, Agarwal K. Increasing burden of liver disease in patients with HIV infection. Lancet. 2011;377(9772):1198–1209. doi: 10.1016/S0140-6736(10)62001-6. [DOI] [PubMed] [Google Scholar]
- 4.Funderburg NT, Mehta NN. Lipid Abnormalities and Inflammation in HIV Inflection. Curr HIV/AIDS Rep. 2016;13(4):218–225. doi: 10.1007/s11904-016-0321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryom L, Lundgren JD, Ross M, et al. Renal Impairment and Cardiovascular Disease in HIV-positive Individuals; The D:A:D Study. J Infect Dis. 2016 doi: 10.1093/infdis/jiw342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryom L, Lundgren JD, De Wit S, et al. Use of antiretroviral therapy and risk of end-stage liver disease and hepatocellular carcinoma in HIV-positive persons. Aids. 2016;30(11):1731–1743. doi: 10.1097/QAD.0000000000001018. [DOI] [PubMed] [Google Scholar]
- 7.Morse CG, McLaughlin M, Matthews L, et al. Nonalcoholic Steatohepatitis and Hepatic Fibrosis in HIV-1-Monoinfected Adults With Elevated Aminotransferase Levels on Antiretroviral Therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60(10):1569–1578. doi: 10.1093/cid/civ101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller KD, Cameron M, Wood LV, Dalakas MC, Kovacs JA. Lactic acidosis and hepatic steatosis associated with use of stavudine: report of four cases. Ann Intern Med. 2000;133(3):192–196. doi: 10.7326/0003-4819-133-3-200008010-00010. [DOI] [PubMed] [Google Scholar]
- 9.Hurlimann D, Weber R, Enseleit F, Luscher TF. HIV infection, antiretroviral therapy, and endothelium. Herz. 2005;30(6):472–480. doi: 10.1007/s00059-005-2740-3. [DOI] [PubMed] [Google Scholar]
- 10.Oppenheimer AP, Koh C, McLaughlin M, et al. Vanishing bile duct syndrome in human immunodeficiency virus infected adults: a report of two cases. World J Gastroenterol. 2013;19(1):115–121. doi: 10.3748/wjg.v19.i1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyao M, Kotani H, Ishida T, et al. Pivotal role of liver sinusoidal endothelial cells in NAFLD/NASH progression. Laboratory investigation; a journal of technical methods and pathology. 2015;95(10):1130–1144. doi: 10.1038/labinvest.2015.95. [DOI] [PubMed] [Google Scholar]
- 12.Dube MP, Lipshultz SE, Fichtenbaum CJ, et al. Effects of HIV infection and antiretroviral therapy on the heart and vasculature. Circulation. 2008;118(2):e36–40. doi: 10.1161/CIRCULATIONAHA.107.189625. [DOI] [PubMed] [Google Scholar]
- 13.Greuter T, Shah VH. Hepatic sinusoids in liver injury, inflammation, and fibrosis: new pathophysiological insights. Journal of gastroenterology. 2016;51(6):511–519. doi: 10.1007/s00535-016-1190-4. [DOI] [PubMed] [Google Scholar]
- 14.Steffan AM, Lafon ME, Gendrault JL, et al. Primary cultures of endothelial cells from the human liver sinusoid are permissive for human immunodeficiency virus type 1. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(5):1582–1586. doi: 10.1073/pnas.89.5.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francisci D, Giannini S, Baldelli F, et al. HIV type 1 infection, and not short-term HAART, induces endothelial dysfunction. Aids. 2009;23(5):589–596. doi: 10.1097/QAD.0b013e328325a87c. [DOI] [PubMed] [Google Scholar]
- 16.Lafon ME, Steffan AM, Royer C, et al. HIV-1 infection induces functional alterations in human liver endothelial cells in primary culture. Aids. 1994;8(6):747–752. doi: 10.1097/00002030-199406000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Ogilvy AJ, Shen D, Wang Y, Chan CC, Abu-Asab MS. Implications of DNA leakage in eyes of mutant mice. Ultrastructural pathology. 2014;38(5):335–343. doi: 10.3109/01913123.2014.927406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duong Van Huyen JP, Landau A, Piketty C, et al. Toxic effects of nucleoside reverse transcriptase inhibitors on the liver. Value of electron microscopy analysis for the diagnosis of mitochondrial cytopathy. Am J Clin Pathol. 2003;119(4):546–555. doi: 10.1309/8B8B-J6AP-5KGV-7C1H. [DOI] [PubMed] [Google Scholar]
- 19.Foufelle F, Fromenty B. Role of endoplasmic reticulum stress in drug-induced toxicity. Pharmacol Res Perspect. 2016;4(1):e00211. doi: 10.1002/prp2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herms A, Bosch M, Ariotti N, et al. Cell-to-cell heterogeneity in lipid droplets suggests a mechanism to reduce lipotoxicity. Curr Biol. 2013;23(15):1489–1496. doi: 10.1016/j.cub.2013.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicological sciences : an official journal of the Society of Toxicology. 2002;65(2):166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 22.Morse CG, Voss JG, Rakocevic G, et al. HIV infection and antiretroviral therapy have divergent effects on mitochondria in adipose tissue. J Infect Dis. 2012;205(12):1778–1787. doi: 10.1093/infdis/jis101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shenton JM, Teranishi M, Abu-Asab MS, Yager JA, Uetrecht JP. Characterization of a potential animal model of an idiosyncratic drug reaction: nevirapine-induced skin rash in the rat. Chemical research in toxicology. 2003;16(9):1078–1089. doi: 10.1021/tx034064+. [DOI] [PubMed] [Google Scholar]
- 24.Nagiah S, Phulukdaree A, Chuturgoon A. Mitochondrial and Oxidative Stress Response in HepG2 Cells Following Acute and Prolonged Exposure to Antiretroviral Drugs. J Cell Biochem. 2015;116(9):1939–1946. doi: 10.1002/jcb.25149. [DOI] [PubMed] [Google Scholar]
- 25.Bishop JB, Tani Y, Witt K, et al. Mitochondrial damage revealed by morphometric and semiquantitative analysis of mouse pup cardiomyocytes following in utero and postnatal exposure to zidovudine and lamivudine. Toxicological sciences : an official journal of the Society of Toxicology. 2004;81(2):512–517. doi: 10.1093/toxsci/kfh208. [DOI] [PubMed] [Google Scholar]
- 26.Kohler JJ, Lewis W. A brief overview of mechanisms of mitochondrial toxicity from NRTIs. Environ Mol Mutagen. 2007;48(3–4):166–172. doi: 10.1002/em.20223. [DOI] [PubMed] [Google Scholar]
- 27.Stanley TL, Grinspoon SK. Body composition and metabolic changes in HIV-infected patients. J Infect Dis. 2012;205(Suppl 3):S383–390. doi: 10.1093/infdis/jis205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soriano V, Puoti M, Garcia-Gasco P, et al. Antiretroviral drugs and liver injury. Aids. 2008;22(1):1–13. doi: 10.1097/QAD.0b013e3282f0e2fd. [DOI] [PubMed] [Google Scholar]
- 29.Chudasama AS, Patel VV, Nivsarkar M, Vasu KK, Shishoo CJ. In vivo Evaluation of Self Emulsifying Drug Delivery System for Oral Delivery of Nevirapine. Indian J Pharm Sci. 2014;76(3):218–224. [PMC free article] [PubMed] [Google Scholar]
- 30.Sookoian S, Castano GO, Scian R, et al. Serum aminotransferases in nonalcoholic fatty liver disease are a signature of liver metabolic perturbations at the amino acid and Krebs cycle level. Am J Clin Nutr. 2016;103(2):422–434. doi: 10.3945/ajcn.115.118695. [DOI] [PubMed] [Google Scholar]
- 31.Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002;277(34):30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 32.Berg JM, Tymoczko JL, Gatto GJ, Stryer L. Biochemistry. 8 [Google Scholar]