Abstract
The study goal was to determine the intra-individual stability, developmental change, and maternal-reported correlates (socio-demographic, stress experiences, hair characteristics, and care) of hair cortisol in mothers and their infants. To assess cortisol deposition in hair during the periods of 6-to-9 months and 9-to-12 months of age, 3 cm segments of hair samples deemed to represent approximately 3 months of retrospective hair cortisol were sampled longitudinally at 9- and 12-months in 41 mothers and infants. Bivariate correlations and mean level comparisons of log-transformed hair cortisol levels at 9- (T1) and 12-months (T2) in mothers and infants were examined. Hair cortisol values were positively correlated from T1 to T2 for mothers (r = .41, p < .05) and infants (r = .39, p < .05). Hair cortisol values did not significantly differ from T1 to T2 in infants but decreased for mothers (F(1,34) = 9.2, p < .01). Maternal and infant hair cortisol was not associated with each other at either time point. Self-reported measures of stress, and hair characteristics and care were not associated with hair cortisol. This is the first study to obtain hair cortisol from more than one time point within the first year after birth in mothers and infants. The intra-individual stability of hair cortisol suggests that it may be a possible biomarker for detecting change in chronic stress experiences within the first year of life and in the postpartum period.
Keywords: hair cortisol, stress, validity, postpartum, infant
INTRODUCTION
Over the past several decades, much attention has been spent on the impact of adversity on the activation of the hypothalamic-pituitary-adrenal (HPA) axis, a major biological system involved in stress regulation. One of the consequences of HPA axis activation is the release of cortisol, a hormone that serves as a measure of stress.
Major advancements have increased our understanding of capturing the regulation of stress among infants using cortisol. For instance, developmental changes have been documented by decreases in the mean and median basal salivary cortisol levels over the course of the infants’ first year (De Weerth & van Geert, 2002; Gröschl, Rauh, & Dörr, 2003; Grunau et al., 2007; Price, Close, & Fielding, 1983; Tollenaar, Jansen, Beijers, Riksen-Walraven, & de Weerth, 2010). High intra-individual variability in basal levels was observed among 5-to-8 month old infants relative to mothers, over 13 weekly home measures of salivary basal cortisol (De Weerth & van Geert, 2002). Because cortisol secretion follows a circadian rhythm, with a morning peak and lower levels in the evening, capturing intra-individual variability in infancy is challenging given the establishment of this rhythm within the first year of life (De Weerth, Zijl, & Buitelaar, 2003). The longer duration of sleep and other cognitive and socio-emotional changes may dampen infant reactivity overall and help to explain the documented shift in HPA functioning in the second half of the first year of life (Gunnar, Brodersen, Krueger, & Rigatuso, 1996; Lewis & Ramsay, 1995; Tollenaar et al., 2010). HPA activity is also sensitive to other factors such as age, sex, and also breastfeeding and napping, experiences highly relevant to the life of an infant (Kudielka, Hellhammer, & Wüst, 2009; Larson, Gunnar, & Hertsgaard, 1991).
For mothers, the perturbations of the HPA axis from pregnancy and delivery may induce alterations in HPA axis functioning that persist into the postpartum period, with its return to pre-pregnancy functioning occurring weeks to approximately 2 months after birth (D’Anna-Hernandez, Ross, Natvig, & Laudenslager, 2011; Kirschbaum, Tietze, Skoluda, & Dettenborn, 2009; Magiakou et al., 1996; Owens et al., 1987). As the shift toward a biological equilibrium occurs, the HPA axis may undergo additional changes as a result of stressors involved with the transition to motherhood, such as infant caretaking and relatedly, sleep disruption and deprivation. These experiences may contribute to vulnerabilities for chronic stress and stress-related disorders (e.g., depression, anxiety) that can occur in women during the postpartum period (Drevets & Todd, 2005). Furthermore, life adjustments throughout the year, such as increased engagement with others or the return to work for mothers, may also contribute to changes in a mother’s stress experience. Altogether, these events may comprise of chronic stress experiences for mothers and infants in the first year but can be difficult to capture biologically given the unique patterns of HPA activity.
A major question is whether hair cortisol may be a useful measure of chronic stress experiences in mothers and infants during the first year after birth, given the perturbations to the system from biological and psychosocial factors. Hair cortisol is an emerging biomarker that appears to be an effective measure of sustained high levels of HPA activity that may address the multiple limitations of other measures of cortisol. The procedures for obtaining and storing hair cortisol can be less unwieldy than other means (Wosu, Valdimarsdóttir, Shields, Williams, & Williams, 2013). Hair collected at the scalp provides an assessment of long-term cortisol concentrations that may be reliably measured retrospectively up to 3-to-6-months. Although there is some variability in hair growth rates due to race, sex, and age (Stalder & Kirschbaum, 2012), on average, hair grows approximately 1 cm per month (Hayashi, Miyamoto, & Takeda, 1991; Wennig, 2000). This growth rate appears to be true for young children but of course there is variability (Barth, 1987). For example, 1, 3, and 6 cm samples are presumed to reflect chronic stress from the previous 1, 3, and 6 months, respectively, in samples of both adults and children (D’Anna, Ross, Natvig, & Laudenslager, 2011; Gow, Thomson, Rieder, Van Uum, & Koren, 2010; Karlén, Frostell, Theodorsson, Faresjö, & Ludvigsson, 2013; Kirschbaum et al., 2009; Meyer & Novak, 2012; Ouellette et al., 2015; Vaghri et al., 2013; Vanaelst et al., 2013; Veldhorst et al., 2014; Yamada et al., 2007).
Hair cortisol is a relatively new biological measure, with only one published study that we identified which included human infants (younger than 1 year of age) (Yamada et al., 2007), and only a few reporting the association of children and mothers’ cortisol levels (Karlén et al., 2013; Ouellette et al., 2015). Therefore, examining the intra-individual stability of hair cortisol within the first year after birth may help to understand whether it can serve as an integrative measure of cortisol in mothers and infants, given the developmental HPA activity changes that occur during infancy and in women during the postpartum period. Furthermore, the mutual effects of maternal and infant behaviors are known to affect stress regulatory systems. No study to date, however, has examined if cortisol levels in the hair of mothers and infants are related and how those levels might relate to other stress measures.
Present Study
This study is the first to examine hair cortisol longitudinally across two samples within the first year after birth in both mothers and infants. It was designed to determine the intra-individual stability of hair cortisol. We selected 9 months as the first time point for data collection, given that not all infants are born with scalp hair, and that those who do have hair often shed it between 4 and 6 months of age (Barth, 1987; Fletcher, 1998; Sams, 1990). A 3 cm length from the scalp was used for sampling because of prior findings suggesting reliability concerns in lengths beyond this time with greater “wash-out” effects with hair further out from the scalp (Kirschbaum et al., 2009). We hypothesized that the levels of hair cortisol from 9- and 12-month time points of the data collection would be correlated, thus demonstrating stability in the measure within the individual. Due to the documented developmental shifts in HPA activity, we also examined mean differences by time point. In addition, we explored the association between mother and infant hair cortisol levels at each time point given shared environment and genetic factors, and also the association of their hair cortisol levels with maternal report of stress and symptomatology. The data also provided an opportunity to determine the association of mother and infant hair cortisol with socio-demographic data, and hair characteristics and hair care, as these are factors that are commonly evaluated within adult studies of hair cortisol (Wosu et al., 2013).
METHODS
Participants
Recruitment and study approval was obtained from the Institutional Review Board at the University of Massachusetts, Boston (UMB). Mothers were eligible if they were between 20 and 43 years of age and infants were eligible if they were full-term (>37 gestational weeks) and clinically normal at delivery. To minimize the confounding effect of risk variables, mothers and/or infants with Cushing’s disease, asthma, steroid medication, diabetes, and other conditions known to affect cortisol levels were excluded. Mothers and infants were required to have hair length of at least 3 cm on the posterior side of their scalp.
Mothers and their infants were recruited through the newborn unit at the Brigham and Women’s Hospital, Boston. This is a large teaching hospital within an urban setting, with a maternity ward that has a socioeconomically diverse population reflective of the city (approximately 50% of women and infants are White, followed by Hispanic, Black, Asian, and multiracial). Shortly after giving birth, a research assistant requested permission from mothers to be contacted and recruited for our ongoing research studies. Mothers were contacted about the study approximately 7 months later. Data collection for 47 infants was scheduled for when the infants were 39 weeks (9 months) old (T1) and then again at 52 weeks (12 months) old (T2). Mothers and infants who participate in our studies are majority White, and well educated.
Procedures
The data collection took place within the research facilities of the Child Development Unit at University of Massachusetts Boston, and in two cases when the mothers could not come to the laboratory, in the home. The procedure was the same at both T1 and T2. The research assistant explained the procedures, answered questions, and obtained consent from the mother. After hair samples were obtained from mothers and infants the mother then completed a demographic questionnaire and a series of questionnaires on her and her family’s stress experiences over the past 3 months. Mothers were paid $60 per visit.
Measures
Questionnaires were administered to obtain information about the maternal experiences. Given our interest in these experiences from the previous 3 months, instructions of the standardized measures were modified to this timeframe of 3 months.
Affect.
The Positive Affect Negative Affect Scale (PANAS; Watson, Clark, & Tellegen, 1988) is a 20-item self-report measure assessing positive and negative affect, reflecting dispositional dimensions. Items are rated on a five-point scale ranging from 1 = very slightly or not at all to 5 = extremely to indicate feelings about the particular affect. Psychometric properties have been well documented (Thompson, 2007).
Mood Symptomatology.
The Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) and Beck Anxiety Inventory (BAI; Beck & Steer, 1993) are each 21-item questionnaires that assess the severity of depression and anxiety symptoms, respectively, with a score of > 14 on the BDI and a score of > 16 on the BAI corresponding to clinical levels of depression and anxiety. Item-response scales range from 0 to 3, with the higher score indicating more severe symptoms. These instruments have demonstrated solid reliability and validity in clinical and community populations (Beck, Steer, & Carbin, 1988; Fydrich, 1992).
Self-Reported Stress.
The Perceived Stress Scale (PSS; Cohen & Williamson, 1988) is a widely used instrument for assessing perception of chronic stress. Respondents are asked to assess how unpredictable, uncontrollable, and overloaded they are in their lives through 14 items using a five-point scale (1 = never to 5 = very often).
Hair Characteristics and Care.
Mothers reported their hair color (blond, brown, black, and gray), and the frequency of hair washing, and heat use and hair products for styling. Those who washed at least three times per week were considered “frequent” washers while those who washed less than weekly were considered “infrequent” washers. Frequent users of heat and products were those who reported using heat or products more than at least three times per week, whereas everyone else was considered “infrequent” users.
Hair Sampling
The hair was cut with sterile scissors as close to the scalp as possible. Through pilot work, it was determined that approximately a 1.5-inch strip of hair cut at the level of the scalp, with a length of 3 cm would weigh at least 10 mg, a sufficient weight for the assay and approximately a 3 month retrospective measure of hair cortisol. This was also a sufficient amount when taking into consideration that hair weights vary by individual and in particular, infants who have finer hair than adults (Cone, 1996; Ito et al., 2005; Kalra, Einarson, Karaskov, Van Uum, & Koren, 2007; Kirschbaum et al., 2009; Meyer, Novak, Hamel, & Rosenberg, 2014). The hair was sampled from the vertex posterior of the head, following previous studies indicating lower hair cortisol intra-variation from this region compared to other regions of the head (Steudte et al., 2011).
Hair samples were first obtained from mothers while they were seated. Infant hair sampling took place while the infant was seated on the mother’s lap and when the infant was in a quiet and comfortable state. Directly after the sampling, the sample was cut to a 3 cm length at the distal end and placed directly into a plastic vial. Because cortisol in hair is stable at room temperature (Meyer & Novak, 2012), vials were stored in a locked file cabinet.
Hair Processing and Cortisol Assay
The samples were sent to the laboratory of Dr. Jerrold Meyer at the University of Massachusetts, Amherst for processing and cortisol assay. Procedures followed those described by Meyer et al. (2014). Briefly, samples were washed twice with isopropanol to remove external contaminants, dried, and then ground to a fine powder. Cortisol was extracted into methanol, the methanol was evaporated, and the residue was redissolved in assay buffer. Cortisol content was analyzed using a sensitive and specific enzyme immunoassay (Salimetrics, Carlsbad, CA) with intra- and inter-assay CVs of less than 5% and 10%, respectively. With six samples unusable due to insufficient hair weight at T1, usable data were obtained from 41 mothers and infants at T1. At T2, the hair samples for 31 mothers and infants were obtained. The attrition at T2 was primarily the result of scheduling conflicts (e.g., the follow-up appointments coincided with participant vacations, or weather related concerns), and is typical of challenges in longitudinal studies.
Statistical Analysis
SPSS v22 was used to conduct all the analyses. Data inspection prior to analyses indicated non-normally distributed hair cortisol values. Therefore, the hair cortisol values for mothers and infants were log-transformed for each time point, a practice that is commonly conducted with hair cortisol data (Pereg et al., 2010; Russell, Koren, Rieder, & Van Uum, 2012; Stalder et al., 2012a). Self-reported measures did not statistically differ from T1 to T2, therefore T1 self-reported measures were used as independent variables in analyses. T-tests were conducted to determine hair cortisol level differences based on socio-demographic, parity, and hair characteristics and care variables. Bivariate correlations were conducted to determine associations between all variables.
RESULTS
Descriptives
Table 1 presents participant demographics in addition to their hair care practices. The majority of the participants was White and highly educated. With the exception of one participant, all were college educated.
Table 1.
Descriptives of Demographic and Maternal Hair Care Practices as Reported at T1–9 Months (Percentages Indicated Unless Otherwise Noted)
| Infant | |
| Male | 46.3 |
| Female | 53.6 |
| Mother | |
| Age (Mean years) | 33.4 |
| Age (Range years) | 22.0–43.2 |
| Infant race | |
| Asian | 7.3 |
| Black (Hispanic) | 4.9 |
| Black (Non-Hispanic) | 2.4 |
| White (Hispanic) | 7.3 |
| White (Non-Hispanic) | 56.1 |
| Mixed | 7.3 |
| No response | 14.6 |
| Maternal race | |
| Asian | 7.3 |
| Black (Hispanic) | 4.9 |
| Black (Non-Hispanic) | 2.4 |
| White (Hispanic) | 9.8 |
| White (Non-Hispanic) | 61.0 |
| No response | 14.6 |
| Education | |
| High school | 2.4 |
| College | 39.0 |
| Graduate school | 46.3 |
| No response | 12.2 |
| Marital status | |
| Single | 14.6 |
| Married | 85.3 |
| Parity | |
| Primiparous | 45.7 |
| Multiparous | 54.3 |
| Height (ft.) | |
| Mean | 5.4 |
| Range | 4.9–5.9 |
| Weight (lbs.) | |
| Mean | 149.1 |
| Range | 95.0–250.0 |
| Hair color | |
| Blond | 22.6 |
| Brown | 64.5 |
| Black | 3.2 |
| Gray | 9.8 |
| Hair wash | |
| Infrequent | 29.7 |
| Frequent | 70.3 |
| Hair heat use | |
| Infrequent | 51.4 |
| Frequent | 48.6 |
| Hair product use | |
| Infrequent | 56.8 |
| Frequent | 43.2 |
Table 2 displays means of stress, depression, and anxiety measures. Overall, the mothers in our sample reported low levels of stress.
Table 2.
Means and Range of Stress, Depression, and Anxiety Measures
| 9-Months | 12-Months | |||
|---|---|---|---|---|
| M | Range | M | Range | |
| PANAS | ||||
| Positive | 34.6 | 19–46 | 35.5 | 19–46 |
| Negative | 19.5 | 11–35 | 17.4 | 10–34 |
| Perceived stress | 15.6 | 6–32 | 12.3 | 2–23 |
| Beck anxiety | 7.1 | 0–43 | 6.5 | 0–33 |
| Beck depression | 6.6 | 0–25 | 5.7 | 0–22 |
Table 3 displays means and correlations between key variables, including maternal report and hair cortisol values. Several significant associations were observed among standardized measures of maternal stress, affect, and mood symptomatology. As expected, higher endorsement of positive affect was significantly and negatively associated with higher endorsement of negative affect, depression, and perceived stress, and reported negative affect was positively associated with reported depression, anxiety, and perceived stress. Reported depression and anxiety were positively correlated.
Table 3.
Correlations Between and Maternal Reported Variables and Maternal and Infant Hair Cortisol (Log Transformed From pg/mg Values)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Maternal positive affect | — | −.41* | −.45** | −.25 | −.59** | −.22 | .16 | .04 | −.27 |
| 2. Maternal negative affect | — | .58** | .71** | .77** | .26 | −.37 | .08 | .07 | |
| 3. Maternal depression | — | .51** | .62** | .17 | −.08 | .18 | .30 | ||
| 4. Maternal anxiety | — | .52** | .11 | −.16 | −.15 | .24 | |||
| 5. Maternal perceived stress | — | .25 | −.27 | −.09 | −.15 | ||||
| 6. T1 Maternal hair cortisol | — | −.08 | .41* | .27 | |||||
| 7. T1 Infant hair cortisol | — | −.07 | .39* | ||||||
| 8. T2 Maternal hair cortisol | — | .06 | |||||||
| 9. T2 Infant hair cortisol | |||||||||
| Means | 34.6 | 19.5 | 6.6 | 7.1 | 15.6 | 3.0 | 4.2 | 2.7 | 4.3 |
| (SD) | (7.4) | (6.5) | (6.1) | (8.3) | (7.5) | (0.8) | (1.4) | (0.6) | (1.3) |
Note: N’s range from 30 to 41 due to occasional missing data;
p < .05,
p < .01,
p < .001.
Maternal and Infant Hair Cortisol
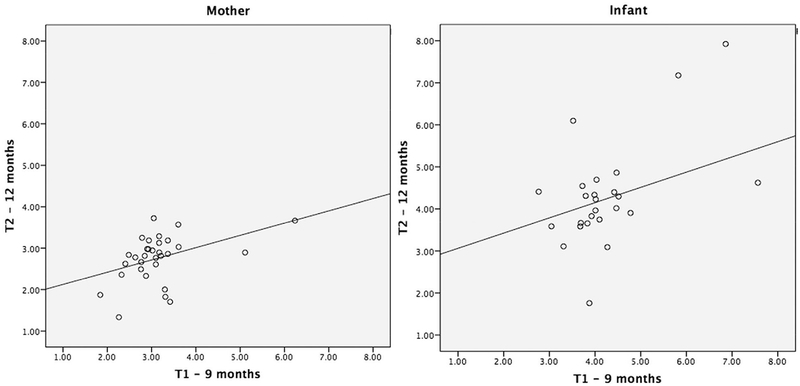
Hair cortisol values were positively correlated at T1 and T2 for both mother and infant. Figure 1 displays scatterplots for the mother and infant. Mother and infant hair cortisol levels were not associated at either time point. Repeated-measures ANOVA were conducted to determine differences from T1 to T2 in mothers and infants. These analyses revealed no mean differences over time in infants but a decrease in hair cortisol levels in mothers (F(1,34) = 9.2, p < .01, T1: M = 3.1, SD = .1; T2: M = 2.7, SD = .1). Paired t-tests comparing mothers and infants hair cortisol level for each time point revealed higher hair cortisol values in infants than in mothers at T1 (t(34) = −1.1, p < .001; Mother: M = 3.1, SD = .8; Infant: M = 4.2, SD = 1.4) and T2 (t(34) = −1.6, p < .001; Mother: M = 2.7, SD = .6; Infant: M = 4.3, SD = 1.3).
FIGURE 1.
Scatterplots of hair cortisol levels across T1 (9-months) to T2 (12-months) in mothers and infants (log transformed values).
Maternal reports of stress, affect, and mood symptomatology were not associated with any of the hair cortisol measures.
Additional repeated-measures ANOVAs showed no mean differences by infant gender, marital status, and hair care practices for either mother or infant at each time point. Because of the small variation and high level of maternal education, we compared mothers who had attended graduate school and those who did not; no differences were observed in this comparison. We could not compare hair cortisol by hair color due to small sample sizes in each group.
Bivariate correlations between maternal age, height, and weight with hair cortisol values showed one significant association, with older mothers having higher levels of hair cortisol at T2 (r = .45, p < .05).
DISCUSSION
Intra-Individual Stability
Our primary goal was to determine the intra-individual stability of hair cortisol in a low-risk sample of mothers and infants during the first year after birth. Determining this stability can help to understand how hair cortisol, as an integrated measure of HPA activity, might serve as a marker for chronic stress during the postpartum period. Our findings showed that for both mothers and infants, the 9-month assessment of hair cortisol (presumably reflecting levels from 6-to-9 months) was positively associated with the 12-month assessment of hair cortisol (presumably reflecting levels from 9-to-12 months). This finding suggests that hair cortisol levels are moderately stable within the second half of the first year after birth for low-risk women and infants. Our findings are consistent with research with adults, which have showed positive associations in hair cortisol levels over time. In one study, two samplings that were 1-year apart and three samplings that were 2-months apart were highly correlated (r’s between .68 and .79) (Stalder et al., 2012b).
Developmental Differences
Another important question was whether the hair cortisol during the first year after birth might reflect any developmental change in cortisol output, given observations of decreases in diurnal basal measures of salivary cortisol within infants and mothers (De Weerth & van Geert, 2002; Gröschl et al., 2003; Grunau et al., 2007; Price et al., 1983). In our study, there were no mean differences in hair cortisol levels collected at the 9- and 12-month time points for infants. Given that the hair cortisol collected represents an integrated accumulation of cortisol levels over time (approximately 6-to-9 months and 9-to-12 months), it may not be sensitive enough to detect the developmental fluctuations of cortisol that might occur on either a daily, weekly, or even monthly basis. Furthermore, given that hair cortisol is a representation of this cortisol output, increase or decreases of cortisol within the timeframe may counteract each other. For instance, cortisol might decrease as a function of physical development, but it may also increase due to other stress demands during this time period. Additional research is needed to determine the extent in which each of the various factors has an effect on hair cortisol change. Shorter term measurements of cortisol may complement the use of hair cortisol.
On the other hand, a decrease in hair cortisol levels was observed for mothers across the two time points. While elevated cortisol levels from pregnancy and birth decrease within the first year, this generally occurs within the first several weeks rather than in the second half of the first year (D’Anna-Hernandez et al., 2011; Kirschbaum et al., 2009; Magiakou et al., 1996; Owens et al., 1987). Instead, it is possible that the hair cortisol decline during the 6-to-12 month period reflects lower levels of HPA reactivity over this interval. Although lifestyle or quality of life changes were not detected from our questionnaire measures, our low-risk mothers may be settling into more regular routines with their caretaking and possibly obtaining better quality sleep. Other researchers have found declines in depression scores among middle-class healthy mothers from 2 to 12 months postpartum (Beeghly et al., 2002) and declines in diurnal cortisol patterns among high-SES postpartum mothers from 5 to 20 weeks postpartum (Tu, Lupien, & Walker, 2006).
Aside from the first year after birth, decreases in hair cortisol levels, at least for children, have been observed across a wider period during childhood, as demonstrated recently in hair cortisol levels measured at 1, 3, 5, and 8-year time points (Karlén et al., 2013). In considering wider age differences, specifically the comparison between infants and adults, we found significantly higher levels of infant hair cortisol compared to maternal hair cortisol at both time points. This is also consistent with the few studies showing higher levels of cortisol in children than in adults, obtained through hair (Dettenborn, Tietze, Kirschbaum, & Stalder, 2012; Karlén et al., 2013) and in assessments obtained at midday and evening salivary cortisol assessments (Clearfield, Carter-Rodriguez, Merali, & Shober, 2014; Letourneau, Watson, Duffett-Leger, Hegadoren, & Tryphonopoulos, 2011; Stenius et al., 2008). As concluded by Gunnar and Donzella (2002), with sensitive caregiving, children by the end of age 1 year may show fewer increases in cortisol response, with this hyposensitivity to stressors acting as a buffer to threat. It is also worth noting that levels of corticosteroid binding globulin (CBG), the major plasma cortisol binding protein, are much lower in neonates than in their mothers (Pawluski, Brain, Underhill, Hammond, & Oberlander, 2012). As cortisol accumulation in hair is thought to be derived from the free fraction in the bloodstream (Meyer & Novak, 2012), this difference in CBG may contribute to the higher hair levels in infants compared to adults.
Within just our sample of mothers, we also found that older mothers were more likely to have higher levels of hair cortisol at T2. Age has not been systematically found to be associated with higher levels of cortisol (Wosu et al., 2013); therefore, one possibility, while speculative, is that older mothers are more likely to experience greater stress due to parenting (Ostberg & Hagekull, 2000) and that this may be especially true as their children approach the age of 1 year and are more active. Most mothers in our sample were over the age of 30 years, and therefore older than 27.7 years, the average age in which Massachusetts women give birth for the first time (Mathews & Hamilton, 2009).
Associations Between Maternal and Infant Cortisol
We did not observe any associations between concurrent measures of mother and infant hair cortisol levels at either T1 or T2. Previous work has showed maternal hair cortisol from second and third trimester to be correlated with child hair cortisol at age 1 and 3 (Karlén et al., 2013). The authors of that study suggested that these associations were due to either a maternal calibration of the child’s HPA axis system or heritability of HPA reactivity; however, no concurrent measures of hair cortisol were obtained in that study. One recent study did observe a correlation but with higher-stressed mothers and daughters at age 7 (Ouellette et al., 2015). Such an association may not have been detectable with our low-risk sample and small sample size. Associations of concurrent salivary measures of basal cortisol between mothers and infants have been mixed (Clearfield et al., 2014; De Weerth & van Geert, 2002; Spangler, Schieche, Ilg, Maier, & Ackermann, 1994; Stenius et al., 2008, 2010). Measures of hair cortisol at additional time points, along with other psychobiological measures of stress reactivity, measures of psychosocial environment, and data from other family members may provide more information about the relative environmental and genetic contributions to long-term HPA activity.
Additional Correlates
The hair characteristics and hair care practices measured in our study were not associated with hair cortisol levels in mothers or infants. This result is consistent with several other studies, although most have been conducted with adults and not children (for a review, see Wosu et al., 2013). Notably, self-reported measures of stress or depression and anxiety symptomatology were not associated with hair cortisol. In general, the literature on these associations has been mixed (Wosu et al., 2013). Given our low-risk sample, the reported stress and symptomatology in our sample of mothers may be too low and insufficiently variable to produce any associations with hair cortisol values, especially compared to previous studies that have relied on clinical or highly stressed samples.
LIMITATIONS
As is the case with many other studies on hair cortisol, the associations we found with other variables may be underpowered given the small sample size, and biased towards mothers and children with sufficient amounts of hair and those willing to have their hair sampled. Our findings may not be generalizable to minority participants and those with different color hair as we did not have sufficient number of minority participants. The cortisol values could vary by hair growth rates; we did not have specific growth rates on our participants as with almost all hair cortisol studies. Average hair growth rate in children aged 3–11 years is similar to that of adults (Barth, 1987), but to our knowledge no data have been published on the growth rate in infants during the first postpartum year. Because normative data has not yet been established for hair cortisol concentrations due to differing laboratory processing and assay procedures, there is no existing “standard range” of values to which we could compare our results. Other study limitations include a lack of salivary or plasma cortisol measures to ascertain concordance with the hair values, as well as the possibility of recall bias in retrospective reporting that can occur with the use of questionnaires. Finally, our study examined correlates of hair cortisol values and therefore, we cannot infer causality between the measures of hair cortisol and the other variables.
SUMMARY
The present study is the first to examine hair cortisol samples across more than one time point within the first year after birth for a group of mother–infant dyads. Our findings with this low-risk sample indicate intra-individual stability in hair cortisol that represents the period of 6-to-12 months after birth in both mothers and infants. No mean level changes in the infants were detected; however, maternal hair cortisol declined significantly from the first to the second time period. There remains the need to specify the factors that contribute to the levels of cortisol accumulation in the hair of infants and mothers during the postpartum period. Overall, the intra-individual stability of hair cortisol suggests its potential usefulness as a biomarker for detecting changes in chronic stress experiences within the first year after birth. This is a promising direction for future studies aimed at understanding chronic stress exposure and the effectiveness of intervention for mothers and infants.
Acknowledgments
This project was supported by NIH grant 1R21HD072448–01 and a Robert Wood Johnson Faculty Seed Grant through the Harvard Center for Population Studies, awarded to Cindy H. Liu (PI) and Ed Tronick (PI). Funding for the hair cortisol analyses was provided by the Hormone Assay Laboratory, Department of Psychological and Brain Sciences, University of Massachusetts—Amherst, which is supported in part by NIH grant OD011180 to Melinda Novak (PI) and Jerrold Meyer (co-investigator).
Contract grant sponsor: NIH
Contract grant number: 1R21HD072448–01
Contract grant sponsor: Robert Wood Johnson Faculty Seed Grant
Contract grant sponsor: NIH
Contract grant number: OD011180
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
REFERENCES
- Barth JH (1987). Normal hair growth in children. Pediatric Dermatology, 4(3), 4173–4184. [DOI] [PubMed] [Google Scholar]
- Beck AT, & Steer RA (1993). Beck anxiety inventory manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Beck AT, Steer RA, & Carbin MG (1988). Psychometric properties of the Beck depression iventory: Twenty-five years of evaluation. Clinical Psychology Review, 8(1), 77–100. doi: 10.1016/0272-7358(88)90050-5 [DOI] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, & Erbaugh J (1961). An inventory for measuring depression. Archives of General Psychiatry, 4, 561–571. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/13688369 [DOI] [PubMed] [Google Scholar]
- Beeghly M, Weinberg MK, Olson KL, Kernan H, Riley J, & Tronick EZ (2002). Stability and change in level of maternal depressive symptomatology during the first postpartum year. Journal of Affective Disorders, 71(1–3), 169–180. doi: 10.1016/S0165-0327(01)00409-8 [DOI] [PubMed] [Google Scholar]
- Clearfield MW, Carter-Rodriguez A, Merali A-R, & Shober R (2014). The effects of SES on infant and maternal diurnal salivary cortisol output. Infant Behavior & Development, 37(3), 298–304. doi: 10.1016/j.infbeh.2014.04.008 [DOI] [PubMed] [Google Scholar]
- Cohen S, & Williamson G, (1988). Perceived stress in a probability sample of the US In Spacapan S & Oskamp S (Eds.), The social psychology of health. Claremont symposium on applied social psychology (pp. 31–67). Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Cone EJ (1996). Mechanisms of drug incorporation into hair. Therapeutic Drug Monitoring, 18(4), 438–443. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8857565 [DOI] [PubMed] [Google Scholar]
- D’Anna KL, Ross RG, Natvig CL, & Laudenslager ML (2011). Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: Comparison to salivary cortisol. Physiology & Behavior, 2–7. doi: 10.1016/j.physbeh.2011.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Anna-Hernandez KL, Ross RG, Natvig CL, & Laudenslager ML (2011). Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: Comparison to salivary cortisol. Physiology & Behavior, 104(2), 348–353. doi: 10.1016/j.physbeh.2011.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weerth C, & van Geert P (2002). A longitudinal study of basal cortisol in infants: Intra-individual variability, circadian rhythm and developmental trends. Infant Behavior and Development, 25(4), 375–398. doi: 10.1016/S0163-6383(02)00141-8 [DOI] [Google Scholar]
- De Weerth C, Zijl RH, & Buitelaar JK (2003). Development of cortisol circadian rhythm in infancy. Early Human Development, 73(1–2), 39–52. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12932892 [DOI] [PubMed] [Google Scholar]
- Dettenborn L, Tietze A, Kirschbaum C, & Stalder T (2012). The assessment of cortisol in human hair: Associations with sociodemographic variables and potential confounders. Stress (Amsterdam, Netherlands), 15(6), 578–588. doi: 10.3109/10253890.2012.654479 [DOI] [PubMed] [Google Scholar]
- Drevets WC, & Todd RD, (2005). Depression, mania, and related disorders In Rubin E & Zorumski C (Eds.), Adult psychiatry (2nd ed., pp. 91–129). Malden, MA: Blackwell Publishing. [Google Scholar]
- Fletcher MA, (1998). Head and neck region In Fletcher MA (Ed.), Physical diagnosis in neonatology (pp. 173–235). Philadelphia, PA: Lippincott-Raven. [Google Scholar]
- Fydrich T (1992). Reliability and validity of the Beck anxiety inventory. Journal of Anxiety Disorders, 6(1), 55–61. [Google Scholar]
- Gow R, Thomson S, Rieder M, Van Uum S, & Koren G (2010). An assessment of cortisol analysis in hair and its clinical applications. Forensic Science International, 196(1–3), 32–37. doi: 10.1016/j.forsciint.2009.12.040 [DOI] [PubMed] [Google Scholar]
- Gröschl M, Rauh M, & Dörr H-G (2003). Circadian rhythm of salivary cortisol, 17alpha-hydroxyprogesterone, and progesterone in healthy children. Clinical Chemistry, 49(10), 1688–1691. doi: http://www.ncbi.nlm.nih.gov/pubmed/14500602 [DOI] [PubMed] [Google Scholar]
- Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, & Thiessen P (2007). Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. The Journal of Pediatrics, 150(2), 151–156. doi: 10.1016/j.jpeds.2006.10.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Krueger K, & Rigatuso J (1996). Dampening of adrenocortical responses during infancy: normative changes and individual differences. Child Development, 67(3), 877–889. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8706532 [PubMed] [Google Scholar]
- Gunnar MR, & Donzella B (2002). Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology, 27(1–2), 199–220. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11750779. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Miyamoto I, & Takeda K (1991). Measurement of human hair growth by optical microscopy and image analysis. The British Journal of Dermatology, 125(2), 123–129. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1911294 [DOI] [PubMed] [Google Scholar]
- Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, … Paus R (2005). Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB Journal, 19(10), 1332–1334. doi: 10.1096/fj.04-1968fje [DOI] [PubMed] [Google Scholar]
- Kalra S, Einarson A, Karaskov T, Van Uum S, & Koren G (2007). The relationship between stress and hair cortisol in healthy pregnant women. Clinical and Investigative Medicine, Médecine Clinique et Experimentale 30(2), E103–E107. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17716540 [DOI] [PubMed] [Google Scholar]
- Karlén J, Frostell A, Theodorsson E, Faresjö T, & Ludvigsson J (2013). Maternal influence on child HPA axis: A prospective study of cortisol levels in hair. Pediatrics, 132(5), e1333–e1340. doi: 10.1542/peds.2013-1178 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Tietze A, Skoluda N, & Dettenborn L (2009). Hair as a retrospective calendar of cortisol production-increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology, 34(1), 32–37. doi: 10.1016/j.psyneuen.2008.08.024 [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, & Wüst S (2009). Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology, 34(1), 2–18. doi: 10.1016/j.psyneuen.2008.10.004 [DOI] [PubMed] [Google Scholar]
- Larson MC, Gunnar MR, & Hertsgaard L (1991). The effects of morning naps, car trips, and maternal separation on adrenocortical activity in human infants. Child Development, 62(2), 362–372. doi: 10.1111/j.1467-8624.1991.tb01537.x [DOI] [PubMed] [Google Scholar]
- Letourneau N, Watson B, Duffett-Leger L, Hegadoren K, & Tryphonopoulos P (2011). Cortisol patterns of depressed mothers and their infants are related to maternal-infant interactive behaviours. Journal of Reproductive and Infant Psychology, 29(5), 439–459. doi: 10.1080/02646838.2011.649474 [DOI] [Google Scholar]
- Lewis M, & Ramsay DS (1995). Developmental change in infants’ responses to stress. Child Development, 66(3), 657–670. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7789193 [DOI] [PubMed] [Google Scholar]
- Magiakou MA, Mastorakos G, Rabin D, Dubbert B, Gold PW, & Chrousos GP (1996). Hypothalamic corticotropin-releasing hormone suppression during the postpartum period: Implications for the increase in psychiatric manifestations at this time. The Journal of Clinical Endocrinology and Metabolism, 81(5), 1912–1917. doi: 10.1210/jcem.81.5.8626857 [DOI] [PubMed] [Google Scholar]
- Mathews TJ, & Hamilton BE (2009). Delayed childbearing: More women are having their first child later in life U.S. Department of Health and Human Service, Centers for Disease Control NCHS data brief, no 21. Hyattsville, MD. [PubMed] [Google Scholar]
- Meyer J, Novak M, Hamel A, & Rosenberg K (2014). Extraction and analysis of cortisol from human and monkey hair. Journal of Visual Experiments, 83, e50882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, & Novak MA (2012). Minireview: Hair cortisol: A novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology, 153(9), 4120–4127. doi: 10.1210/en.2012-1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostberg M, & Hagekull B (2000). A structural modeling approach to the understanding of parenting stress. Journal of Clinical Child Psychology, 29(4), 615–625. doi: 10.1207/S15374424JCCP2904_13 [DOI] [PubMed] [Google Scholar]
- Ouellette SJ, Russell E, Kryski KR, Sheikh HI, Singh SM, Koren G, & Hayden EP (2015). Hair cortisol concentrations in higher- and lower-stress mother-daughter dyads: A pilot study of associations and moderators. Developmental Psychobiology, 57(5), 519–534. doi: 10.1002/dev.21302 [DOI] [PubMed] [Google Scholar]
- Owens PC, Smith R, Brinsmead MW, Hall C, Rowley M, Hurt D, … Lewin T (1987). Postnatal disappearance of the pregnancy-associated reduced sensitivity of plasma cortisol to feedback inhibition. Life Sciences, 41(14), 1745–1750. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3657381 [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Brain UM, Underhill CM, Hammond GL, & Oberlander TF (2012). Prenatal SSRI exposure alters neonatal corticosteroid binding globulin, infant cortisol levels, and emerging HPA function. Psychoneuroendocrinology, 37(7), 1019–1028. doi: 10.1016/j.psyneuen.2011.11.011 [DOI] [PubMed] [Google Scholar]
- Pereg D, Gow R, Mosseri M, Lishner M, Rieder M, Van Uum S, & Koren G (2010). Hair cortisol and the risk for acute myocardial infarction in adult men. Stress, 14(1), 73–81. doi: 10.3109/10253890.2010.511352 [DOI] [PubMed] [Google Scholar]
- Price DA, Close GC, & Fielding BA (1983). Age of appearance of circadian rhythm in salivary cortisol values in infancy. Archives of Disease in Childhood, 58(6), 454–456. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1628010&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E, Koren G, Rieder M, & Van Uum S (2012). Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology, 37(5), 589–601. doi: 10.1016/j.psyneuen.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Sams WM, (1990). Structure and function of the skin In Sams WM & Lynch PJ (Eds.), Principles and practice of dermatology (pp. 3–14). New York, NY: Churchill-Livingstone. [Google Scholar]
- Spangler G, Schieche M, Ilg U, Maier U, & Ackermann C (1994). Maternal sensitivity as an external organizer for biobehavioral regulation in infancy. Developmental Psychobiology, 27(7), 425–437. doi: 10.1002/dev.420270702 [DOI] [PubMed] [Google Scholar]
- Stalder T, & Kirschbaum C (2012). Analysis of cortisol in hair-state of the art and future directions. Brain, Behavior, and Immunity, 26(7), 1019–1029. doi: 10.1016/j.bbi.2012.02.002 [DOI] [PubMed] [Google Scholar]
- Stalder T, Steudte S, Alexander N, Miller R, Gao W, Dettenborn L, & Kirschbaum C (2012a). Cortisol in hair, body mass index and stress-related measures. Biological Psychology, 90(3), 218–223. doi: 10.1016/j.biopsycho.2012.03.010 [DOI] [PubMed] [Google Scholar]
- Stalder T, Steudte S, Miller R, Skoluda N, Dettenborn L, & Kirschbaum C (2012b). Intraindividual stability of hair cortisol concentrations. Psychoneuroendocrinology, 37(5), 602–610. doi: 10.1016/j.psyneuen.2011.08.007 [DOI] [PubMed] [Google Scholar]
- Stenius F, Swartz J, Lindblad F, Pershagen G, Scheynius A, Alm J, & Theorell T (2010). Low salivary cortisol levels in infants of families with an anthroposophic lifestyle. Psychoneuroendocrinology, 35(10), 1431–1437. doi: 10.1016/j.psyneuen.2010.05.010 [DOI] [PubMed] [Google Scholar]
- Stenius F, Theorell T, Lilja G, Scheynius A, Alm J, & Lindblad F (2008). Comparisons between salivary cortisol levels in six-months-olds and their parents. Psychoneuroendocrinology, 33(3), 352–359. doi: 10.1016/j.psyneuen.2007.12.001 [DOI] [PubMed] [Google Scholar]
- Steudte S, Kolassa I-T, Stalder T, Pfeiffer A, Kirschbaum C, & Elbert T (2011). Increased cortisol concentrations in hair of severely traumatized Ugandan individuals with PTSD. Psychoneuroendocrinology, 36(8), 1193–2000. doi: 10.1016/j.psyneuen.2011.02.012 [DOI] [PubMed] [Google Scholar]
- Thompson ER (2007). Development and validation of an internationally reliable short-form of the positive and negative affect schedule (PANAS). Journal of Cross-Cultural Psychology, 38(2), 227–242. doi: 10.1177/0022022106297301 [DOI] [Google Scholar]
- Tollenaar MS, Jansen J, Beijers R, Riksen-Walraven JM, & de Weerth C (2010). Cortisol in the first year of life: normative values and intra-individual variability. Early Human Development, 86(1), 13–16. doi: 10.1016/j.earlhumdev.2009.12.003 [DOI] [PubMed] [Google Scholar]
- Tu MT, Lupien SJ, & Walker C-D (2006). Diurnal salivary cortisol levels in postpartum mothers as a function of infant feeding choice and parity. Psychoneuroendocrinology, 31(7), 812–824. doi: 10.1016/j.psyneuen.2006.03.006 [DOI] [PubMed] [Google Scholar]
- Vaghri Z, Guhn M, Weinberg J, Grunau RE, Yu W, & Hertzman C (2013). Hair cortisol reflects socio-economic factors and hair zinc in preschoolers. Psychoneuroendocrinology, 38(3), 331–340. doi: 10.1016/j.psyneuen.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanaelst B, Rivet N, Huybrechts I, Ludes B, De Henauw S, & Raul JS (2013). Measurement of cortisol and cortisone in children’s hair using ultra performance liquid chromatography and tandem mass spectrometry. Analytical Methods, 5(8), 2074. doi: 10.1039/c3ay26570f [DOI] [Google Scholar]
- Veldhorst MAB, Noppe G, Jongejan MHTM, Kok CBM, Mekic S, Koper JW, … van den Akker ELT (2014). Increased scalp hair cortisol concentrations in obese children. The Journal of Clinical Endocrinology and Metabolism, 99(1), 285–290. doi: 10.1210/jc.2013-2924 [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3397865 [DOI] [PubMed] [Google Scholar]
- Wennig R (2000). Potential problems with the interpretation of hair analysis results. Forensic Science International, 107(1–3), 5–12. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10689559 [DOI] [PubMed] [Google Scholar]
- Wosu AC, Valdimarsdóttir U, Shields AE, Williams DR, & Williams MA (2013). Correlates of cortisol in human hair: Implications for epidemiologic studies on health effects of chronic stress. Annals of Epidemiology, 23(12), 797–811. doi: 10.1016/j.annepidem.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Stevens B, de Silva N, Gibbins S, Beyene J, Taddio A, … Koren G (2007). Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatology, 92(1), 42–49. doi: 10.1159/000100085 [DOI] [PubMed] [Google Scholar]