Abstract
Objective
To determine the epidemiology and characteristics of transverse myelitis (TM) and neuromyelitis optica spectrum disorders (NMOSD) in Abu Dhabi, United Arab Emirates.
Methods
Retrospective chart review at four large government-run hospitals in Abu Dhabi between 2010 and 2016. Data collected included year of onset, presentation, laboratory results including aquaporin-4 immunoglobulin G (IgG)/myelin oligodendrocyte glycoprotein IgG antibodies and the occurrence of any relapses.
Results
A total of 46 individuals were identified. Of these, 23 (50%) were Emirati citizens. Within the overall group including pediatrics, the crude prevalence rate for monophasic TM was 1.0 per 100 000, and for NMOSD was 0.34 per 100 000. Incidence rates within the overall group for TM and NMOSD were 0.18 per 100 000 and 0.05 per 100 000, respectively. For Emirati citizens aged ≥20 years, the prevalence rate for monophasic TM was 2.46 per 100 000 and 1.76 per 100 000 for NMOSD, and the incidence was 0.57 per 100 000 and 0.17 per 100 000, respectively. The incidence of monophasic TM and NMOSD within the Emirati pediatric population (aged ≤19 years) was 0.18 per 100 000 and 0.06 per 100 000, respectively. The mean age of onset for monophasic TM was 36 years, and for NMOSD was 43 years. Nine patients had a positive aquaporin-4 IgG or anti-myelin oligodendrocyte glycoprotein IgG antibody result. Of the 30 participants with available laboratory cerebrospinal fluid analysis, 36.6% had elevated white blood counts (>5.0 × 106/L), and 43% had elevated protein levels. A total of 19 participants had documentation of oligoclonal bands or IgG index, and just four (21%) had either oligoclonal bands or elevated IgG index.
Conclusion
The present study describes the epidemiology and characteristics of TM and NMOSD among populations in Abu Dhabi. The adult prevalence rate for Emirati citizens was 2.46 per 100 000 for monophasic TM, and 1.76 per 100 000 for NMOSD. The overall incidence was 0.18 per 100 000 and 0.05 per 100 000, respectively.
Keywords: incidence, neuromyelitis optica spectrum disorders, prevalence, transverse myelitis, United Arab Emirates
Introduction
Transverse myelitis (TM) is an acute, inflammatory attack of the spinal cord that typically presents with symptoms developing over hours to days and worsening throughout a time period that can last up to several weeks. These symptoms can include, but are not limited to, ascending paresthesias, pyramidal weakness, bowel or bladder dysfunction, or other autonomic dysfunction.1 Diagnostic criteria from the TM Consortium Working Group include sensory, motor or autonomic dysfunction attributable to the spinal cord, a sensory level, peak symptoms within 4 h to 21 days and evidence of inflammation of the spinal cord on cerebrospinal fluid (CSF) testing or imaging.2
Transverse myelitis is a heterogeneous syndrome, with the main causes of acute TM including relapsing immune-mediated disease, such as multiple sclerosis (MS) or neuromyelitis optica spectrum disorders (NMOSD), and systemic inflammatory conditions, such as systemic lupus erythematosus, toxins or infections.3 If no cause is identified after a thorough work-up, TM can also be categorized as an idiopathic monophasic event, which occurs in approximately 15–30% of cases.2,4,5
There have been few studies characterizing the epidemiology of TM specifically that did not include ischemic myelopathy or documented MS.6 An early study in Israel with 62 Jewish (though ethnically diverse) patients estimated the incidence of acute TM as at 1.34 per 1 000 000, with no evidence of sex, familial or ethnic predisposition.7 Two more recent articles, one from the Kaiser Permanente patient database with a diverse patient population in the USA and one in New Zealand, estimated the incidence at 3.0 of 100 000 per year and 24.6 of 1 000 000, respectively.8,9 However, these studies did not necessarily distinguish among the different etiologies of TM.
Epidemiological data about TM and NMOSD in the Middle East is limited. Abu Dhabi is the largest Emirate in the United Arab Emirates (UAE), and contains 28% of the country’s population.10 Furthermore, it contains a resource rich, comprehensive hospital infrastructure that lends itself well to epidemiological studies. The current study sought to elucidate the prevalence, incidence, and characteristics of TM and NMOSD in this unique Arab population.
Methods
The present study was an extension of a previously published epidemiological study on demyelinating disorders in Abu Dhabi that found that the characteristics of MS in Abu Dhabi were similar to those of the West.11 A retrospective chart review was carried out on all inpatient and outpatient visits for demyelinating disorders, TM, and NMOSD at four large government hospitals in the Emirate of Abu Dhabi: Tawam Hospital, Sheikh Khalifa Medical Center (SKMC), Mafraq Hospital, and Al Ain Hospital between 2010 and 2016. These hospitals are all managed by the government and use the same electronic medical record system. Records were searched for ICD9 codes from 341.0 (neuromyelitis optica) to 341.9 (demyelinating diseases of the central nervous system unspecified), 323.82 (encephalitis, myelitis, encephalomyelitis), 340 (MS), 323.9 (myelitis, encephalomyelitis) and 377.30 (optic neuritis). Each patient was cross-checked by medical record number, name, cell phone number and birth date to avoid duplication of patient records. Institutional review board approval was obtained from each hospital.
All patient records were reviewed by a neurologist trained in autoimmune and inflammatory neurological diseases (author N.S.). Transverse myelitis was defined using the 2002 diagnostic criteria from the TM Consortium Working Group.2 Data collected included sex, nationality, family history, year of symptom onset, symptom presentation, CSF (including oligoclonal/immunoglobulin G [IgG] positivity) and laboratory data inflammatory markers including ANA, vitamin D levels, serum aquaporin-4 (AQP4) and myelin oligodendrocyte glycoprotein (MOG) IgG autoantibodies (laboratory: Eurofins Biomnis, Biologie Medicale Specialisee), imaging results, treatment, working diagnosis at the end of the work-up, and relapse events. Patients with a clear alternate diagnosis, such as malignancy, were excluded from analysis. If there was any discrepancy with the diagnosis, the case was reviewed by one of the other neurologists (authors M.S., T.A., M.L.). If there was disagreement regarding the diagnosis, the patient was excluded from the study.
Each patient was classified into one of three categories: (i) monophasic TM; (ii) relapsing TM seronegative or untested for AQP4 IgG or MOG IgG antibody; and (iii) NMOSD. The diagnoses were obtained using the 2015 international consensus diagnostic criteria for NMOSD, which allow for diagnosis of seronegative NMOSD or unknown serological status.12 This includes at least two core clinical characteristics (i.e. optic neuritis, acute myelitis or area postrema syndrome), and negative or unavailable testing for AQP4 IgG with exclusion of all other diagnoses.
Results
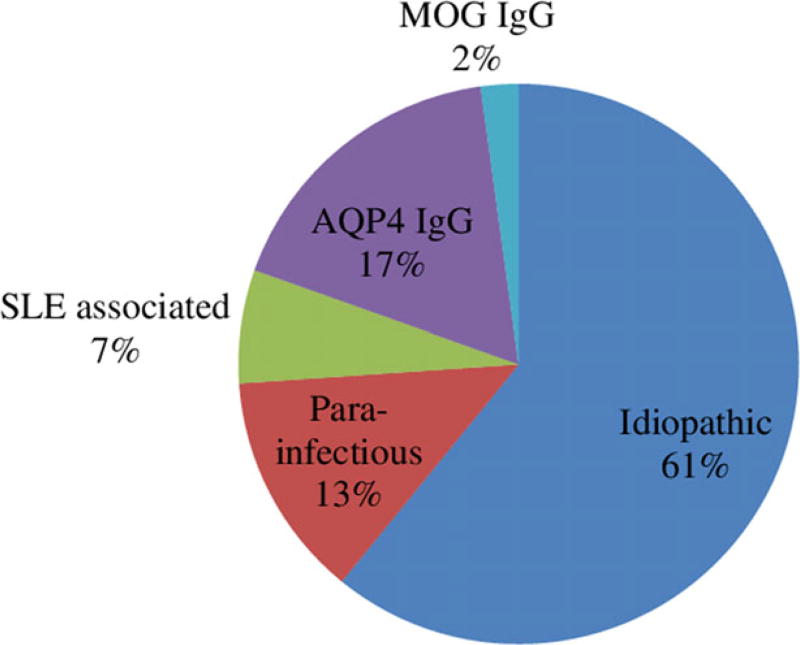
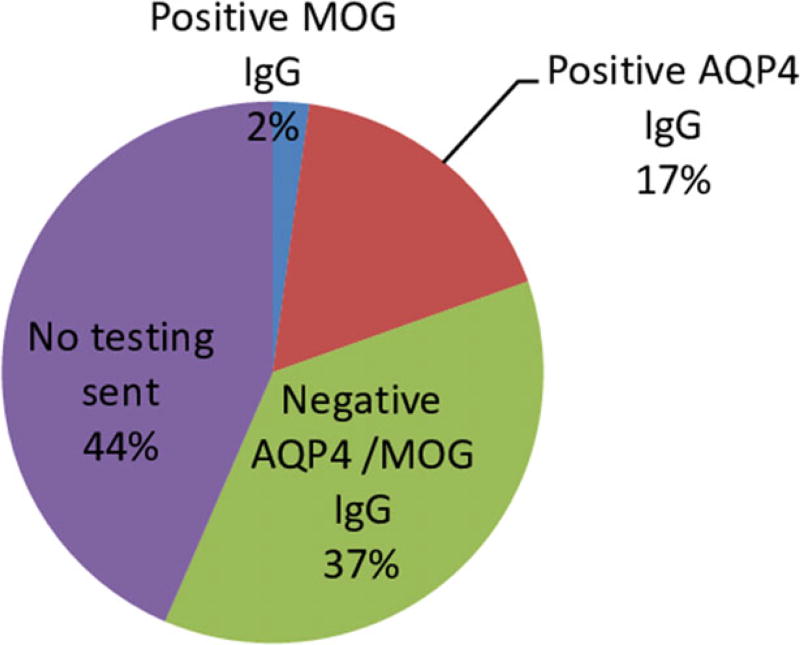
A total of 46 participants were identified with either: (i) monophasic TM (29 patients); (ii) relapsing TM seronegative or untested (seven patients); or (iii) AQP4 IgG/MOG IgG positivity or satisfying NMOSD criteria (10 patients; Fig. 1; Table 1). In 44% of cases, no AQP4 IgG antibodies were sent as part of the medical work-up (Fig. 2). Half (23) of the patients identified were national Emirati citizens. Demographics and characteristics are outlined in Table 2.
Figure 1.
Characteristics of transverse myelitis and neuromyelitis optica spectrum disorders in Abu Dhabi, United Arab Emirates, between 2010 and 2016 (n = 46). AQP4 IgG, aquaporin-4 immunoglobulin G autoantibodies; MOG IgG, myelin oligodendrocyte glycoprotein immunoglobulin G autoantibodies; SLE, systemic lupus erythematosus.
Table 1.
Characteristics of participants fulfilling neuromyelitis optica spectrum disorders criteria in Abu Dhabi, United Arab Emirates
| Sex | Country of origin |
Presenting symptoms | AQP4 IgG | MOG IgG | Satisfies criteria for seronegative NMOSD |
MRI |
|---|---|---|---|---|---|---|
| Female | UAE | Bilateral lower limb weakness | Positive AQP4 IgG | Unknown | Not applicable | Extensive fibrosis and atrophy of cervical/thoracic cord |
| Female | UAE | Vision loss | Positive AQP4 IgG | Unknown | Not applicable | Unknown |
| Female | UAE | ON | Positive AQP4 IgG | Unknown | Not applicable | Optic nerve hyperintensity, otherwise normal spine, brain MRI |
| Female | UAE | Unknown | Positive AQP4 IgG | Unknown | Not applicable | LETM |
| Normal brain MRI | ||||||
| Female | UAE | ON, TM | Positive AQP4 IgG | Unknown | Not applicable | LETM |
| Female | Syria | Lower extremity weakness | Positive AQP4 IgG | Unknown | Not applicable | LETM |
| Male | India | ON | Positive AQP4 IgG | Unknown | Not applicable | Left optic nerve edema/enhancement. Multiple white matter intensities in brain. C spine MRI normal. |
| Male | Pakistan | Lower extremity weakness | Positive AQP4 IgG | Unknown | Not applicable | LETM |
| Female | UAE | ON | Negative AQP4 IgG | Positive MOG IgG | Not applicable | LETM |
| Male | Yemen | Upper and lower extremity weakness, ON, TM | Negative AQP4 IgG | Unknown | Yes | LETM |
Total n = 10. AQP4, aquaporin-4; IgG, immunoglobulin G; LETM, longitudinally extensive transverse myelitis lesion; MOG, myelin oligodendrocyte glycoprotein; MRI, magnetic resonance imaging; NMO, neuromyelitis optica; NMOSD, neuromyelitis optica spectrum disorders; ON, optic neuritis; TM, transverse myelitis.
Figure 2.
Neuromyelitis optica antibody testing in transverse myelitis/optic neuritis cases in Abu Dhabi, United Arab Emirates, between 2010 and 2016 (n = 46). AQP4 IgG, aquaporin-4 immunoglobulin G autoantibodies; MOG IgG, myelin oligodendrocyte glycoprotein immunoglobulin G autoantibodies.
Table 2.
Demographics and characteristics of transverse myelitis and neuromyelitis optica spectrum disorders patients in Abu Dhabi, United Arab Emirates, from 2010 to 2016
| Characteristic, n (%) | Monophasic TM (n = 29) |
NMOSD (n = 10) | Relapsing TM seronegative or untested (n = 7) |
|---|---|---|---|
| Age at presentation (years) | |||
| 0–19 | 5 (17) | 1 (10) | 1 (14) |
| 20–24 | 4 (14) | 0 | 1 (14) |
| 25–34 | 6 (21) | 2 (20) | 1 (14) |
| 35–44 | 6 (21) | 3 (30) | 2 (28) |
| 45–54 | 3 (10) | 1 (10) | 2 (28) |
| 55–64 | 1 (3) | 2 (20) | 0 |
| 65–89 | 3 (10) | 1 (10) | 0 |
| Unknown | 1 (3) | 0 | 0 |
| Age at presentation – median (range), mean (SD) | 34.0 (12.0–89.0) | 43.0 (8.0–74.0) | 38.0 (15.0–49.0) |
| 36.1 (18.4) | 43 (18.7) | 34.4 (12) | |
| Pediatric incidence, ≤19 years (Emirati nationals only) | 1.76/1 000 000 | 0.59/1 000 000 | 0 |
| (3 patients total) | (1 patient only – AQP4 IgG positive) | ||
| Sex | |||
| Female | 12 (41) | 7 (70) | 6 (86) |
| Male | 17 (59) | 3 (30) | 1 (14) |
| Nationality | |||
| UAE | 12 (41) | 6 (60) | 5 (71) |
| Non-UAE | 17 (59) | 4 (40) | 2 (29) |
Total n = 46. AQP4, aquaporin-4; IgG, immunoglobulin G; NMOSD, neuromyelitis optica spectrum disorders; TM, transverse myelitis; UAE, United Arab Emirates.
Prevalence
The total mid-year population of Abu Dhabi in 2016 was 2 908 173, of which 551 535 were Emirati citizens, giving a crude total population prevalence rate for monophasic TM of 1.00 per 100 000 and NMOSD of 0.34 per 100 000 (Table 3). However, because approximately 50% of Emirati citizens in Abu Dhabi are aged <19 years, and the vast majority of participants in the present study were adults, the adjusted prevalence rate for Emirati adults aged ≥20 was 2.46 per 100 000 for monophasic TM, and 1.76 per 100 000 for NMOSD. Of the 10 total patients with a positive AQP4 IgG/MOG IgG result or who fulfilled criteria for NMOSD, six (60%) were Emirati citizens.
Table 3.
Prevalence and incidence of monophasic transverse myelitis and neuromyelitis optica spectrum disorders among the general population and Emirati citizen population in Abu Dhabi, United Arab Emirates (per 100 000)
| Monophasic TM | NMOSD | |
|---|---|---|
| Total population prevalence | 1.00 | 0.34 |
| Adult prevalence in total population (aged ≥20 years) | 1.00 | 0.39 |
| Adult prevalence in Emirati population (aged ≥20 years) | 2.46 | 1.76 |
| Adult incidence in Emirati population (aged ≥20 years) | 0.57 | 0.17 |
NMOSD, neuromyelitis optica spectrum disorders; TM, transverse myelitis.
Incidence
The incidence of monophasic and relapsing TM (excluding NMOSD) between 2010 and 2016 within the overall group was 0.18 per 100 000, and for NMOSD specifically was 0.05 per 100 000 (Table S1). Within the Emirati nationals pediatric (aged ≤19 years) population, the incidence of monophasic TM was 1.76 per 1 000 000, and NMOSD was 0.59 per 1 000 000. Only one pediatric patient fit the NMOSD criteria, and this patient tested positive for MOG IgG (Tables 1,2; Table S1).
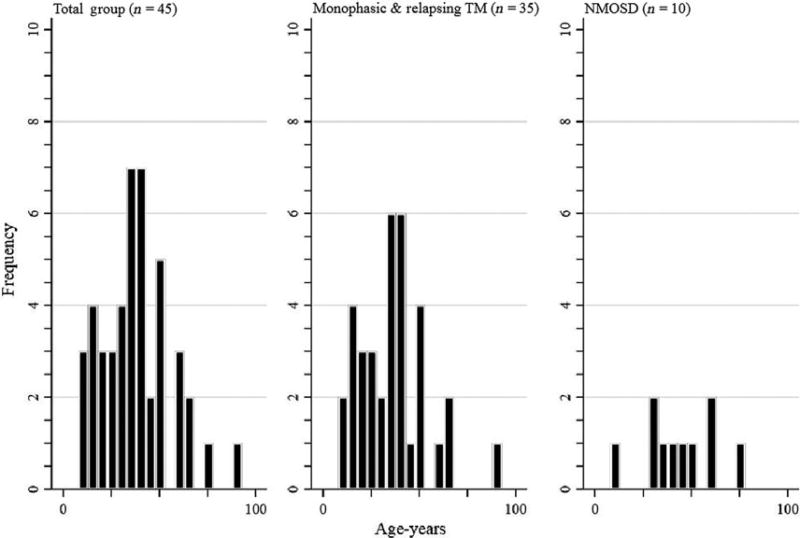
Within the total group, the mean age was 42 ± 17.6 years, with a mean age of onset of 37 ± 18 years (Table 2). Within the Emirati group only, the mean age was 42 ± 20 years, and mean age of onset was 36 ± 20 years (Fig. 3).
Figure 3.
Age of onset distribution of transverse myelitis (TM) and neuromyelitis optica spectrum disorders (NMOSD) in Abu Dhabi, United Arab Emirates.
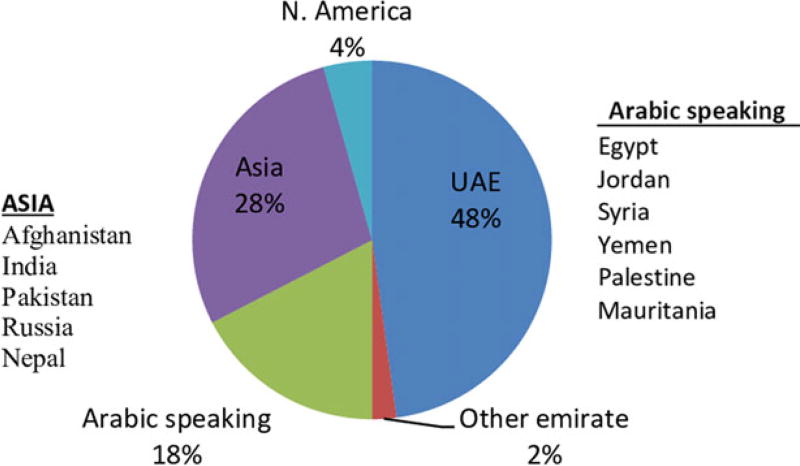
A total of 25 patients (54%) were female and 21 (46%) were male, giving a female-to-male ratio of 1.2:1. Within the local Emirati population 19 of 23 (82%) were female and just four of 23 (17%) were male, giving a female-to-male ratio of 4.75:1. Of the 23 Emirati patients whose family history was known, only one had a family history of autoimmune disease (systemic lupus erythematosus in a brother). While 50% (23/46) of participants were UAE citizens, 17% (8/46) came from another Arabic-speaking country (Fig. 4). Asia was the region of origin for 13 (28%), and two (4%) were from North America.
Figure 4.
Country of origin for people admitted with transverse myelitis or neuromyelitis optica spectrum disorders (n = 46) in Abu Dhabi, United Arab Emirates (UAE) between 2010 and 2016.
Of the 30 participants with available laboratory CSF analysis, 11 of 30 (36.6%) had elevated white blood counts (>5.0 × 106/L) and 13 of 30 (43%) had elevated protein levels. A total of 19 participants had documentation of oligoclonal bands (OCB) or IgG index being carried out and, of these, just four of 19 (21%) had either OCB or elevated IgG index. Of these four patients, two were classified as monophasic TM and two as relapsing TM untested or seronegative. We were unable to determine the timing of the CSF analysis with the clinical presentations due to the limitations of the chart review.
Discussion
Because TM and NMOSD are rare disorders with heterogeneous pathophysiology, there remains a paucity of clear epidemiological data on these diseases. Furthermore, some of these studies did not separate cases of documented multiple sclerosis, confounding accurate epidemiological measurements.
A recent epidemiology paper described a prevalence of NMOSD in Olmstead County, Minnesota, of 3.9 per 100 000 compared with 10 per 100 000 in Martinique, an island in the Caribbean.13 Another study from Australia and New Zealand identified a prevalence of 0.70 per 100 000 and an incidence of 0.37 per 1 000 000.14 The overall crude prevalence of NMO in Isfahan, Iran, was found to be 1.9 per 100 000.15 Our prevalence results within the local adult Emirati citizen population were found to be within that range at 1.76 per 100 000, with a lower total population prevalence of 0.34 per 100 000.
A frequently cited prior study between 1955 and 1975 in Israel found an incidence of TM of 1.34 per 1 000 000,7,16 whereas another study carried out between 1998 and 2004 in California estimated an incidence of TM of 3.1 per 100 000.8 A more recent single-center study examined 60 acute TM patients between 2001 and 2005 in New Zealand, finding an incidence rate for idiopathic acute TM of 6.3 per 1 000 000.9 None of these studies calculated prevalence rates within their population.
Within the pediatric population, incidence rates of TM in Canadian children aged <18 years was estimated to be 0.2 per 100 000.17 The present study found a similar incidence rate of 0.24 per 100 000 of TM in the Emirati pediatric population (age ≤19 years; Table S1).
The present study showed a mean age of onset for monophasic TM of 36.0 years and 43 years for the NMOSD population (Table 2; Fig. 3). This is similar to an age of onset of 41.4 years found in a large multicenter study on NMOSD in the USA,18 but higher than that found in a cohort of NMO patients in Iran whose age of onset mean was 30 years.15
Although several older studies19,20 and one 2007 study of 20 TM patients at Aga Khan University Hospital in Pakistan show no large sex difference in the incidence of TM,21 in other more recent studies a female predominance has been shown.4,9,22,23 For example, in the Kaiser Permanente study, females represented 71% of the cases with TM.8 However, these studies do not control for patients who were ultimately diagnosed with MS, which has a strong female predominance.24 Of four class III retrospective cohort surveys examined in a recent evidence-based guideline,25 two reported that more women than men are diagnosed with inflammatory myelopathies in association with MS,4,26 but none showed a sex association in idiopathic TM.4,16,26,27 In this total study population, 54% were female, but within the local Emirati population, 82% of participants were female.
In prior studies, idiopathic TM has accounted for between 15% and 30% of cases,2,4,5 although in one series of 354 patients, 64% were described as idiopathic.28 In one large retrospective study that applied TM Consortium Working Group criteria to a cohort of 288 patients, 15.6% were idiopathic, 10.8% developed MS, 19% had spinal cord infarcts, 17.3% were infectious or parainfectious, 17% had NMO and 20.5% had systemic autoimmune disease.22 In our cohort, 28 of 46 (61%) cases were idiopathic, six of 46 (13%) were parainfectious, three of 46 (7%) were systemic lupus erythematosus associated and nine of 46 (19%) were AQP4 IgG/MOG IgG-positive (Fig. 1). It is possible that these discrepancies in the number of idiopathic TM cases might reflect variation in disease criteria definition, possible undiagnosed underlying causes (e.g. frequency of infectious diseases across the world) and available diagnostic technology. They could also represent geographic variability in the proportion of idiopathic TM, though small sample size makes this difficult to ascertain.
The present study had several limitations. The first is the small sample size. However, TM and NMOSD are rare diseases and, as previously discussed, we feel confident that within the Emirati population of Abu Dhabi, we captured almost all of the reported cases of TM between 2010 and 2016. However, it is possible that some patients were evaluated solely at outside hospitals either in the UAE or abroad, or did not present to the hospital when they fell ill. Additionally, the method of retrospective chart review limits data gathering ability, with the level of note detail and tests and imaging ordered varying greatly between providers. Considering that 44% of patients in the database were not tested for AQP4 IgG or MOG IgG antibodies (Fig. 1), we feel that the estimated prevalence of NMOSD is likely an underestimation of the true prevalence in this population. In addition, many of the monophasic TM and seronegative TM patients did not have a complete work-up for possible seronegative NMOSD (such as brain magnetic resonance imaging for brainstem, area postrema syndromes or MOG IgG antibodies), and therefore it is possible that these patients would also fall into the NMOSD category despite being anti-NMO seronegative. Finally, because of the transient nature of the expatriate community in the UAE, many patients return home for care after their diagnosis. Thus, for some patients, we had limited follow-up data. Also, some Emirati nationals also receive their care internationally – often in the USA or Germany, therefore some of their medical records might not be complete.
In conclusion, the present study examines the prevalence and incidence of monophasic TM and NMOSD in adult and pediatric populations in the UAE, and describes the TM/NMOSD epidemiology in Abu Dhabi, UAE. Because healthcare in Abu Dhabi is free and Emiratis are required to be seen once at a government hospital, we believe this study to be an accurate representation of disease prevalence in the Abu Dhabi Emirate within the local citizen population. However, further study on the epidemiology of these disorders with age- and sex-adjusted incidence is required both in the Middle East and elsewhere.
Supplementary Material
Table S1. Incidence rates for monophasic transverse myelitis (TM), relapsing TM and neuromyelitis optica spectrum disorders (NMOSD) between 2010 and 2016 in the general and local Emirati populations, and pediatric populations in Abu Dhabi, United Arab Emirates. Cases that presented before 2010 are not included in calculations (n = 35).
Footnotes
Disclosure of ethical statement
This study was approved by the institutional review boards of each of the four hospitals at which the study was carried out.
Conflict of interest
None declared.
Additional supporting information may be found online in the Supporting Information section at the end of the article:
References
- 1.West TW. Transverse myelitis–a review of the presentation, diagnosis, and initial management. Discov Med. 2013;16:167–77. [PubMed] [Google Scholar]
- 2.Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59:499–505. doi: 10.1212/wnl.59.4.499. [DOI] [PubMed] [Google Scholar]
- 3.Jacob A, Weinshenker BG. An approach to the diagnosis of acute transverse myelitis. Semin Neurol. 2008;28:105–20. doi: 10.1055/s-2007-1019132. [DOI] [PubMed] [Google Scholar]
- 4.de Seze J, Stojkovic T, Breteau G, et al. Acute myelopathies: clinical, laboratory and outcome profiles in 79 cases. Brain. 2001;124(Pt 8):1509–21. doi: 10.1093/brain/124.8.1509. [DOI] [PubMed] [Google Scholar]
- 5.Beh SC, Greenberg BM, Frohman T, Frohman EM. Transverse myelitis. Neurol Clin. 2013;31:79–138. doi: 10.1016/j.ncl.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhat A, Naguwa S, Cheema G, Gershwin ME. The epidemiology of transverse myelitis. Autoimmun Rev. 2010;9:A395–9. doi: 10.1016/j.autrev.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Berman M, Feldman S, Alter M, Zilber N, Kahana E. Acute transverse myelitis: incidence and etiologic considerations. Neurology. 1981;31:966–71. doi: 10.1212/wnl.31.8.966. [DOI] [PubMed] [Google Scholar]
- 8.Klein NP, Ray P, Carpenter D, et al. Rates of autoimmune diseases in Kaiser Permanente for use in vaccine adverse event safety studies. Vaccine. 2010;28:1062–8. doi: 10.1016/j.vaccine.2009.10.115. [DOI] [PubMed] [Google Scholar]
- 9.Young J, Quinn S, Hurrell M, Taylor B. Clinically isolated acute transverse myelitis: prognostic features and incidence. Mult Scler. 2009;15:1295–302. doi: 10.1177/1352458509345906. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO) [Accessed 12/13, 2015];United Arab Emirates. Available at: http://www.who.int/countries/are/en/
- 11.Schiess N, Huether K, Fatafta T, et al. How global MS prevalence is changing: a retrospective chart review in the United Arab Emirates. Mult Scler Relat Disord. 2016;9:73–9. doi: 10.1016/j.msard.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–89. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flanagan EP, Cabre P, Weinshenker BG, et al. Epidemiology of aquaporin-4 autoimmunity and neuromyelitis optica spectrum. Ann Neurol. 2016;79:775–83. doi: 10.1002/ana.24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bukhari W, Prain KM, Waters P, et al. Incidence and prevalence of NMOSD in Australia and New Zealand. J Neurol Neurosurg Psychiatry. 2017;88:632–8. doi: 10.1136/jnnp-2016-314839. [DOI] [PubMed] [Google Scholar]
- 15.Etemadifar M, Dashti M, Vosoughi R, Abtahi SH, Ramagopalan SV, Nasr Z. An epidemiological study of neuromyelitis optica in Isfahan. Mult Scler. 2014;20:1920–2. doi: 10.1177/1352458514537699. [DOI] [PubMed] [Google Scholar]
- 16.Jeffery DR, Mandler RN, Davis LE. Transverse myelitis. Retrospective analysis of 33 cases, with differentiation of cases associated with multiple sclerosis and parainfectious events. Arch Neurol. 1993;50:532–5. doi: 10.1001/archneur.1993.00540050074019. [DOI] [PubMed] [Google Scholar]
- 17.Banwell B, Kennedy J, Sadovnick D, et al. Incidence of acquired demyelination of the CNS in Canadian children. Neurology. 2009;72:232–9. doi: 10.1212/01.wnl.0000339482.84392.bd. [DOI] [PubMed] [Google Scholar]
- 18.Mealy MA, Wingerchuk DM, Greenberg BM, Levy M. Epidemiology of neuromyelitis optica in the United States: a multicenter analysis. Arch Neurol. 2012;69:1176–80. doi: 10.1001/archneurol.2012.314. [DOI] [PubMed] [Google Scholar]
- 19.Lipton HL, Teasdall RD. Acute transverse myelopathy in adults. A follow-up study. Arch Neurol. 1973;28:252–7. doi: 10.1001/archneur.1973.00490220060009. [DOI] [PubMed] [Google Scholar]
- 20.Christensen PB, Wermuth L, Hinge HH, Bomers K. Clinical course and long-term prognosis of acute transverse myelopathy. Acta Neurol Scand. 1990;81:431–5. doi: 10.1111/j.1600-0404.1990.tb00990.x. [DOI] [PubMed] [Google Scholar]
- 21.Kahloon AA, Arif H, Baig SM, Khawaja MR. Characteristics of acute transverse myelitis at Aga Khan University Hospital, Karachi. J Pak Med Assoc. 2007;57:215–8. [PubMed] [Google Scholar]
- 22.de Seze J, Lanctin C, Lebrun C, et al. Idiopathic acute transverse myelitis: application of the recent diagnostic criteria. Neurology. 2005;65:1950–3. doi: 10.1212/01.wnl.0000188896.48308.26. [DOI] [PubMed] [Google Scholar]
- 23.Perumal J, Zabad R, Caon C, et al. Acute transverse myelitis with normal brain MRI : long-term risk of MS. J Neurol. 2008;255:89–93. doi: 10.1007/s00415-007-0686-5. [DOI] [PubMed] [Google Scholar]
- 24.Harbo HF, Gold R, Tintore M. Sex and gender issues in multiple sclerosis. Ther Adv Neurol Disord. 2013;6:237–48. doi: 10.1177/1756285613488434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott TF, Frohman EM, De Seze J, Gronseth GS, Weinshenker BG Therapeutics and Technology Assessment Subcommittee of American Academy of Neurology. Evidence-based guideline: clinical evaluation and treatment of transverse myelitis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011;77:2128–34. doi: 10.1212/WNL.0b013e31823dc535. [DOI] [PubMed] [Google Scholar]
- 26.Scott TF, Bhagavatula K, Snyder PJ, Chieffe C. Transverse myelitis. Comparison with spinal cord presentations of multiple sclerosis. Neurology. 1998;50:429–33. doi: 10.1212/wnl.50.2.429. [DOI] [PubMed] [Google Scholar]
- 27.Bakshi R, Kinkel PR, Mechtler LL, et al. Magnetic resonance imaging findings in 22 cases of myelitis: comparison between patients with and without multiple sclerosis. Eur J Neurol. 1998;5:35–48. doi: 10.1046/j.1468-1331.1998.510035.x. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan C, Kaplin AI, Pardo CA, Kerr DA, Keswani SC. Demyelinating disorders: update on transverse myelitis. Curr Neurol Neurosci Rep. 2006;6:236–43. doi: 10.1007/s11910-006-0011-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Incidence rates for monophasic transverse myelitis (TM), relapsing TM and neuromyelitis optica spectrum disorders (NMOSD) between 2010 and 2016 in the general and local Emirati populations, and pediatric populations in Abu Dhabi, United Arab Emirates. Cases that presented before 2010 are not included in calculations (n = 35).