Abstract
The ethosomal carrier system can increase the penetration of azelaic acid into the stratum corneum, but ethosomes have both physical (particle aggregation or fusion) and chemical instability (chemical interaction of active ingredients during storage) that are often encountered in long-term storage. The aim of this study is to acquire proethosome formula of azelaic acid with lyoprotectant which has better stability than ethosomes of azelaic acid. Azelaic acid proethosomes were measured its absorbance using an ultraviolet-visible is spectrophotometer at a wavelength of 204 nm to obtain a percentage of entrapment efficiency (EE%). Proethosomes particle size was obtained from the analysis using particle size analyzer. Proethosomes consisting of phosphatidylcholine, ethanol, and propylene glycol were prepared by a thin-layer hydration process. After that, it was added with lyoprotectants such as trehalose, glucose, and mannitol before it was freeze-dried. Physical stability was studied with physical appearance, EE, and particle size. Chemical stability study determined the level of azelaic acid. Both tests were evaluated every 2 weeks for 8-week storage at 4°C and 27°C. Least entrapment efficiency and particle size changes was proethosomes with trehalose addition from 92.06% and 261.0 nm became 68.92% and 957.7 nm at 27°C, meanwhile at 4°C became 77.47% and 439.4 nm. While the highest percentage of azelaic acid content in proethosomes with trehalose was 62.07% (at 27°C) and without lyoprotectant 69.40%. Based on their characteristic, it can be assumed that, azelaic acid proethosomes with trehalose have the best stability than ethosomes and proethosomes with other lyoprotectants.
Keywords: Azelaic acid, proethosomes, stability
INTRODUCTION
Azelaic acid with poor water solubility makes it difficult to formulate azelaic acid for topical use. Topical use of drug through the skin becomes an obstacle because the human skin has an outer layer of stratum corneum that acts as the main barrier of the human body.[1] The formulation of azelaic acid ethosomes by simple method with adding aqueous phase to ethanol solution with concentration between 20% and 45% v/v of phosphatidylcholine (5%, w/w) and azelaic acid (0.2%, w/w), the ratio of drug release was faster than azelaic acid liposome formulation, and the greater concentration of ethanol, the faster release of azelaic acid.[2]
Ethosomes as a drug carrier system have both physical (particle or fusion particle) and chemical (chemical interaction of active ingredients during storage) exposed to long-term storage. A study by Celia et al. reported an increase in ethosome vesicle size of three formulas with vesicle size after preparation of 310.8, 311.4, and 313.1 nm–315.2, 314.6, and 316.7 nm after storage at 26°C for 10 days.[3] Ethosomes formulation after preparation had an entrapment efficiency (EE) of 57.2 ± 4.1% and decreased after 180 days of storage to 55.4 ± 3.9%.[4] In the storage of ethosomes in the refrigerator, it was reported that ethosomes have better stability than storage at 29°C, seen from the spherical shape and ethosomal integrity after storage for 60 and 275 days. In addition, a better vesicle distribution was also seen in refrigerator storage, indicating the absence of flocculation, and despite the occurrence of creaming on the suspension, the particles are easily dispersed with shaking.[5]
An alternative to overcome this instability is to apply freeze-drying process or lyophilization which is capable of removing water content in the sample through sublimation and desorption under vacuum. The freeze-dried form can provide better stability by preventing particle aggregation, preventing degradation of vesicle-forming polymers, and preventing the release of active substances from vesicles. In addition, the freeze-dried form is able to provide stability in nanovesicles for up to 6 months in extreme storage conditions. During the freeze-drying process, it involves the process of applying various pressures during the cooling and drying stages, so it needs lyoprotectant to protect the ethosomes from the cooling and dehydration process. It has been well known that during the cooling process, there is a phase separation, so it is necessary to add lyoprotectant which can increase the stability during storage. There are several uses of lyoprotectants in the freeze-drying process, among others, sugars such as trehalose, sucrose, dextran, glucose, and mannitol. Trehalose is a lyoprotectant which is more often used because its low hygroscopicity, the absence of hydrogen internal bonds that allow the formation of hydrogen bonds more flexible during freeze drying, very low chemical reactivity, and its higher temperature glass transitions.[6]
SUBJECTS AND METHODS
Material
Azelaic acid (Sigma, United States, CAS No. 123-99-9), Phospholipon 90G (Lipoid, Germany), ethanol 96%, methanol, trehalose, glucose, and mannitol obtained by Merck were used in this study.
Methods
Preparation of azelaic acid proethosomes and subsequent reconstitution
Proethosomes of azelaic acid were obtained by a thin-layer hydration process using a rotary evaporator. Phospholipid 90 G was dissolved in dichloromethane and methanol (2:1). Then, the thin layer was stored in the refrigerator for 24 h before hydration with the water phase. The formed thin layer was hydrated using a phosphate-buffered solution of pH 7.4 containing 1:1 azelaic acid (phospholipid: azelaic acid) and 35% v/v ethanol. The ethosomal suspension was sonicated for 15 min to reduce the particle size, then its been divided into five formulas, each formula was added of 10% w/v trehalose, glucose, and mannitol, while one formula was freeze-dried without lyoprotectant and one formula still in suspended form. Four formulas were freeze-dried gradually. Reconstitution proethosomal powder becomes an ethosomal suspension using phosphate buffer pH 7.4.
Characterization of azelaic acid proethosomes
After obtaining proethosomes of azelaic acid, EE and particle size from five formulas were measured to determine lyoprotectant which gives best stability to azelaic acid proethosomes azelaic acid proethosomes.
Entrapment efficiency test
The standard solution of azelaic acid in phosphate buffer pH 6.8 with various concentrations measured its absorbance using a ultraviolet-visible (UV-V) spectrophotometer at a wavelength of 204 nm and obtained a calibration curve.[7,8] Calculation of indirect EE was obtained by comparison of azelaic acid concentration in sediment with total azelaic acid concentration. Proethosomes were reconstituted in phosphate buffer and then diluted with phosphate buffer pH 6.8–50 ppm. The concentration of azelaic acid in supernatant which was obtained from the centrifugation of sample was analyzed by UV-V spectrophotometer at the maximum wavelength of azelaic acid (204 nm) and determined by calibration curve.[9,10]
Particle size
The particle size from five formulas was analyzed using particle size analyzer. Samples were measured from ethosomes suspension that had been separated from free azelaic acid and diluted with purified water.
RESULTS
Physical stability study
Physical stability study proethosomes and ethosomes of azelaic acid were carried out at refrigerator storage temperature (4°C) and room temperature (27°C) for 8 weeks. Physical appearance, EE, and particle size distribution are evaluated every 2 weeks. Physical appearance showed no change in color and odor on proethosomes and ethosomes. EE of ethosomal immediately after it had been freeze-dried was almost as good as ethosomal suspension; it was about 90%. The highest percentage was obtained from proethosome formula with the addition of mannitol as a lyoprotectant (97.7%). Mean initial EE% of proethosomes with addition of trehalose, glucose, and mannitol and without addition of sugar and ethosome suspension was 92.06%, 89.51%, 97.7%, 91.5%, and 94.48%. After storage for 8 weeks at 4°C, it decreased to 77.47%, 57.56%, 73.2%, 67.56%, and 22.94% [Figure 1], while at 27°C, it was 68.92%, 33.3%, 69.15%, 64.25%, and 15.99%, respectively [Figure 2]. The initial particle size of proethosomes with the addition of trehalose, glucose, mannitol, proethosomes without lyoprotectant, and ethosomes in suspended form were 261.0, 270.2, 247.3, 280.0, and 179.0 nm became 439.4, 148.6, 2230, 162.5, and 1888 nm at 4°C [Figure 3] and were increased to 957.7 nm, 734.6 nm, 1438 nm, 1391 nm, and 808.5 nm at 27°C storage temperature [Figure 4].
Figure 1.
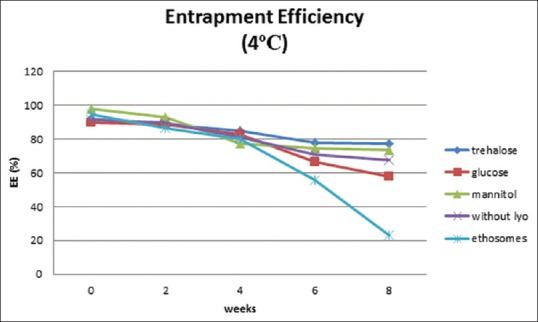
Entrapment efficiency of azelaic acid proethosomes and ethosomes for 8 weeks at 4°C
Figure 2.
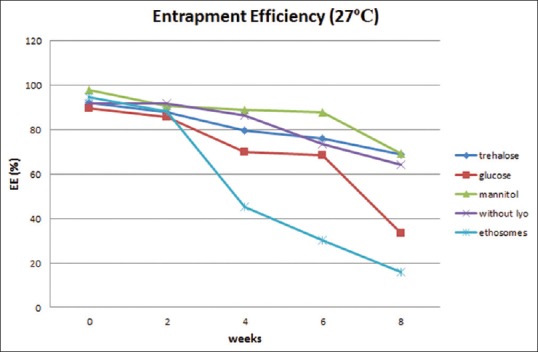
Entrapment efficiency of azelaic acid proethosomes and ethosomes for 8 weeks at 27°C
Figure 3.
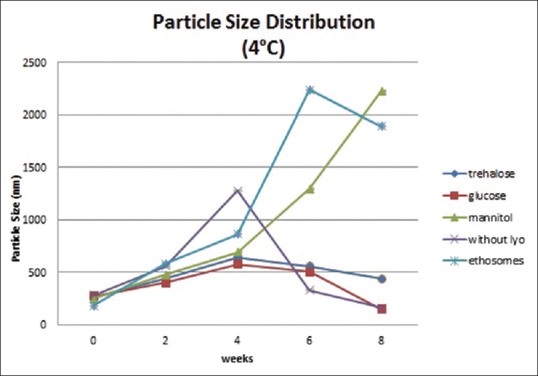
Particle size of azelaic acid proethosomes and ethosomes for 8 weeks at 4°C
Figure 4.
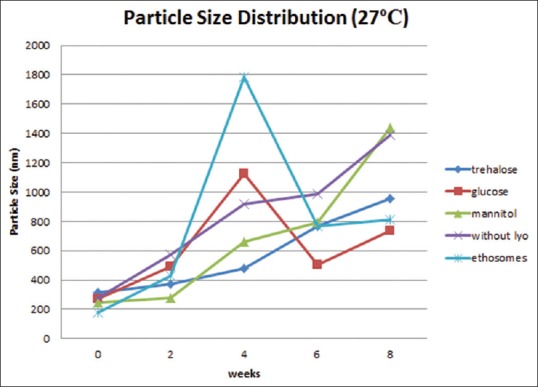
Particle size of azelaic acid proethosomes and ethosomes for 8 weeks at 27°C
Chemical stability test
After 8 weeks, the percentage of azelaic acid content in proethosome with trehalose, glucose , mannitol, proethosomes without lyoprotectant, and ethosomes in suspended form became 59.14%, 47.70%, 48.87%, 69.4%, and 47.24% at 4°C. While at 27°C were 62.07%, 35.4%, 52.53%, 36.43%, 23.35% respectively.
DISCUSSION
Lyophilization enhances the shelf-life of the ethosome and protects proethosomes in the form of lyophilized powder when used in the formula. Lyophilization process can disturb the ethosomes integrity and decrease EE of lyophilization product[11] as EE of proethosome formula without lyoprotectant that was decreased to 91.5%. Freeze-drying leads to malfunction of bilayer phospholipid membranes, and it is well known that sugar group lyoprotectants are able to prevent the aggregation of nanoparticles during drying and storage.[11] EE of ethosomal suspension formula decreased weekly, and after 8 weeks, the ethosomal suspension efficiency rate decreased to 23.24%, and it was the lowest compared with other formulas obtained from the lyophilization process. The biggest changes of EE and particle size occurred on azelaic acid ethosomes from 94.48% to 15.99% and from 179.3 nm to 1888 nm at temperature 27°C, became 22.94% and 808.5 nm at 4°C during 8 weeks storage. An increase in particle size may be due to leakage of vesicles and azelaic acid aggregates.
Least EE and particle size changes occurred in proethosome formula with trehalose addition of 92.06% and 957.7 nm at 27°C and 68.92%, 77.47%, and 439.4 nm at 4°C. Several types of sugar have the ability to protect liposomes from fusion and leakage of vesicles during lyophilization process.[12] From this study it can be assumed if trehalose give the best lyoprotectives effect, which can protect the integrity of the vesicles and prevent any leakage of the active substance among other disaccharides.[13] Based on Jaeghere et al., a complete redispersion of poly (Lactic acid-co-ethylene oxide)nanoparticles is obtained in the addition of trehalose into a nanoparticle suspension with trehalose:nanoparticles ratio of 1:1.[14] In addition, there was virtually no leakage of active substance from vesicles when 9% trehalose was added in the freeze-drying process.[13] The second highest EE was proethosomes with mannitol. Mannitol commonly used other than as a stabilizer of cooling and drying pressures, as well as bulking agents in the formulation especially when the sample to be in freeze dry is small, and as tonicity adjusters that are able to keep the isotonic solution and control the pressure osmosis.[6] The use of glucose in nanoparticles can also provide a good reconstitution effect in the absence of macroscopic aggregation.[6]
Chemical stability tests showed the lowest percentage of azelaic acid content after 8 weeks for suspended form, 23.35% (27°C) and 47.24% (4°C). While the highest percentage of azelaic acid content in proethosomes with trehalose addition was 62.067% at 27°C and proethosome without addition of lyoprotectant 69.4% followed by proethosome with trehalose addition 59.14% at 4°C.
CONCLUSION
Based on the physics and chemical stability tests of five formulas for 8 weeks, the characteristic of ethosomes and percentage of azelaic acid content were changed every week, especially for suspended form that has the greatest change compared to other proethosomes formula. Generally, azelaic acid proethosomes with trehalose as lyoprotectant have the highest EE at both temperatures, and it has a relative stable of particle size changes every week. From this study it can be assumed that azelaic acid proethosomes with trehalose have the best stability than ethosomes and proethosomes with other lyoprotectants.
Financial support and sponsorship
This study was financially supported by DRPM UI.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The author would like to thank Malvern, Jakarta for their research opportunities and thank to Pharmaceutical Research Laboratory's staff, Pharmacy Faculty, University of Indonesia who have assisted during this research.
REFERENCES
- 1.Liu J, Hu G. Advances in studies of phospholipids as carriers in skin topical application. J Nanjing Med Univ. 2007;21:349–53. [Google Scholar]
- 2.Esposito E, Menegatti E, Cortesi R. Ethosomes and liposomes as topical vehicles for azelaic acid: A preformulation study. J Cosmet Sci. 2004;55:253–64. [PubMed] [Google Scholar]
- 3.Celia C, Trapasso E, Cosco D, Paolino D, Fresta M. Turbiscan lab expert analysis of the stability of ethosomes and ultradeformable liposomes containing a bilayer fluidizing agent. Colloids Surf B Biointerfaces. 2009;72:155–60. doi: 10.1016/j.colsurfb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Jain S, Tiwary AK, Sapra B, Jain NK. Formulation and evaluation of ethosomes for transdermal delivery of lamivudine. AAPS PharmSciTech. 2007;8:E111. doi: 10.1208/pt0804111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mbah C, Builders P, Nzekwe I, Kunle O, Adikwu M, Attama A. Formulation and in vitro evaluation of pH-responsive ethosomes for vaginal delivery of metronidazole. J Drug Deliv Sci Technol. 2014;24:565–71. [Google Scholar]
- 6.Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze drying of nanoparticles: Formulation, process, storage considerations. Adv Drug Deliv Rev. 2006;58:1688–713. doi: 10.1016/j.addr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Kishore M, Jayaprakash M, Reddy TV. Spectrophotometric determination of azelaic acid in pharmaceutical formulations. J Pharm Res. 2010;3:3090–2. [Google Scholar]
- 8.Kadam TV, Darekar AB, Gondkar SB, Saudagar RB. Development and validation of spectrophotometric method for determination of azelaic acid. Asian J Res Pharm Sci. 2015;5:83–5. [Google Scholar]
- 9.Dave V, Pareek ASP. Ethosome: A novel approach of transdermal drug delivery system. Int J Adv Res Pharm Bio Sci. 2012;1:439–52. [Google Scholar]
- 10.Thohiroh A. Lipstick Formulation Using Xanton Transfersomes with Thin Layer Lipid Hydration Methode. Pharmacy Faculty, University of Indonesia. 2015 [Google Scholar]
- 11.Ghanbarzadeh S, Valizadeh H, Zakeri-Milani P. The effects of lyophilization on the physico-chemical stability of sirolimus liposomes. Adv Pharm Bull. 2013;3:25–9. doi: 10.5681/apb.2013.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumara BC, Parthiban S, Senthil kumar GP, Tamiz Mani T. Proliposome: A novel approach to carrier drug delivery system. Int J Biopharm. 2015;6:98–106. [Google Scholar]
- 13.Chen C, Han D, Cai C, Tang X. An overview of liposome lyophilization and its future potential. J Control Release. 2010;142:299–311. doi: 10.1016/j.jconrel.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Jaeghere FD, Allemann E, Leroux JC, Stevels W, Feijen J, Doelker E, et al. Formulation and lyoprotection of poly (Lactic acid-co-ethylene oxide) nanoparticles: influence on physical stability and in vitro cell uptake. Pharm Res. 1999;16:859–66. doi: 10.1023/a:1018826103261. [DOI] [PubMed] [Google Scholar]


