Abstract
Objective:
Continuous thoracic epidural analgesia (TEA) is compared with erector spinae plane (ESP) block for the perioperative pain management in patients undergoing cardiac surgery for the quality of analgesia, incentive spirometry, ventilator duration, and intensive care unit (ICU) duration.
Methodology:
A prospective, randomized comparative clinical study was conducted. A total of 50 patients were enrolled, who were randomized to either Group A: TEA (n = 25) or Group B: ESP block (n = 25). Visual analog scale (VAS) was recorded in both the groups during rest and cough at the various time intervals postextubation. Both the groups were also compared for incentive spirometry, ventilator, and ICU duration. Statistical analysis was performed using the independent Student's t-test. A value of P < 0.05 was considered statistically significant.
Results:
Comparable VAS scores were revealed at 0 h, 3 h, 6 h, and 12 h (P > 0.05) at rest and during cough in both the groups. Group A had a statistically significant VAS score than Group B (P ≤ 0.05) at 24 h, 36 h, and 48 h but mean VAS in either of the Group was ≤4 both at rest and during cough. Incentive spirometry, ventilator, and ICU duration were comparable between the groups.
Conclusion:
ESP block is easy to perform and can serve as a promising alternative to TEA in optimal perioperative pain management in cardiac surgery.
Keywords: Erector spinae plane block, thoracic epidural analgesia, visual analog scale
Introduction
The perioperative pain management plays a vital role in the management of patients undergoing cardiac surgery. Cardiac surgical pain is of moderate-to-severe type accounting to sternotomy, sternal retraction, internal mammary artery harvesting, and chest tube insertions. Ineffective pain management results in hemodynamic perturbations with systemic complications-pulmonary (atelectasis, pneumonia, and stasis of bronchial secretions), cardiovascular (increased oxygen consumption and tachycardia), musculoskeletal (muscle weakness), and increased neurohormonal response.[1] The American Society of Anesthesiologist task force on the management for acute postoperative pain, recommend the use of multimodal techniques for pain management.[2] These include regional analgesia, intravenous (IV), and oral analgesics. Opioids, paracetamol, and nonsteroidal anti-inflammatory drugs have been administered as parenteral analgesics. Opioids can cause nausea, vomiting, pruritus, and respiratory depression when they are used solely for analgesia. Various regional techniques, especially thoracic epidural analgesia (TEA) have been widely described to reduce the postoperative pain in cardiac surgery with improved outcome. Paravertebral blocks (PVBs) have been comparable to TEA in minimally invasive cardiac surgery[3] and thoracotomies. The reported incidence of adverse effects has been minimal with PVB as compared to TEA in thoracotomies.[4] Bilateral PVBs have been described in abdominal vascular surgery and obstetric surgeries.[5] PVBs are associated with complications such as pneumothorax and vascular injuries.
Ultrasound-guided (USG) erector spinae plane (ESP) block is recently introduced technique for regional analgesia in thoracic neuropathic pain, rib fractures, and breast surgeries.[6] ESP block is relatively easier to perform as compared to TEA and PVB in breast surgeries.[6] Hence, this study was performed to compare continuous TEA with ultrasound-guided bilateral erector spinae block for perioperative pain management in cardiac surgical patients. Incentive spirometry, ventilator, and intensive care unit (ICU) duration were also compared between the groups.
Methodology
A total of 50 cardiac surgical patients were recruited in this study after obtaining the Institutional Ethics Committee clearance and written informed consent from the patients. Randomization was performed to two groups of 25 each, Group A (TEA) and Group B (ESP block) using the closed envelope method.
Inclusion criteria
Adult elective cardiac surgical patients underwent median sternotomy.
Exclusion criteria
Emergency surgery, left main coronary artery disease, left ventricular ejection fraction <40%, anomalies of vertebral column, blood or CSF tap during the procedure, failed blocks, patient on anti-coagulants, bleeding diathesis, and patients who expired before extubation.
A day before the surgery, a single anesthesiologist performed either TEA or ESP block. An 18 G IV cannula was inserted and the patient was connected to standard monitors such as noninvasive blood pressure, electrocardiography (ECG), and pulse oximetry (SpO2). In the left lateral decubitus position, either of the block was performed.
In Group A, TEA was performed under the strict aseptic precautions. Local infiltration with 2% of lignocaine under the skin, in C7/T1 intervertebral space was administered. An 18 G Tuohy needle was inserted at C7/T1 intervertebral space to identify epidural space using the hanging-drop technique. 20G catheter was threaded 3–4 cm in caudal direction in the epidural space.
In Group B, USG ESP block was performed under the strict aseptic precautions. A high-frequency 12 MHz linear ultrasound transducer (Philips En Visor CHD, Bothell, Washington, USA 98041) was placed in a longitudinal orientation 3 cm lateral to the T6 spinous process corresponding to the T5 transverse process. Three muscles trapezius (uppermost), rhomboids major (middle), and erector spinae (lowermost) were identified superior to the hyperechoic transverse process. Local infiltration with 2% of lignocaine at the site of needle insertion was administered. Using in-plane approach an 18 G Tuohy needle was inserted in caudal–cephalad direction, until the tip is deep to erector spinae muscle, as evidenced by visible hydrodissection below the muscle plane, and on injection of 5 ml of normal saline. A 20 G epidural catheter was threaded 5 cm in cephalad direction. The same procedure was performed on the opposite side [Figure 1a, b and Video 1].
Figure 1.
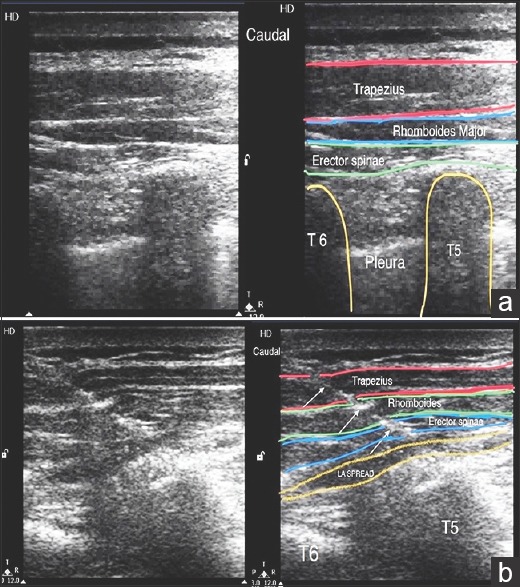
(a) Sonoanatomy at the level of the T6 spinous process while performing erector spinae plane block. (b) Needle path and hydrodissection while performing erector spinae plane block
On the day of surgery, patients were connected to standard monitors such as invasive arterial blood pressure, ECG, and pulse oximetry (SpO2). Before the induction of general anesthesia (GA) in either of the groups, correct placement of the catheters was confirmed by sensation to pinprick after 20–30 min of bolus dose of local anesthetic (LA).
In Group A (TEA), bolus dose of 0.25% plain bupivacaine 15 ml was administered through the catheter after the negative aspiration for blood or cerebrospinal fluid, followed by a continuous infusion of 0.125% plain bupivacaine at the rate of 0.1 ml/kg/h till 48 h postextubation.
In Group B (ESP Block), bolus dose of 0.25% plain bupivacaine 15 ml was injected in each of the catheters after the negative aspiration for blood, followed by a continuous infusion of 0.125% plain bupivacaine at the rate of 0.1 ml/kg/h till 48 h postextubation, through both the catheters.
All patients underwent cardiac surgical procedures under standardized GA protocol with TEA or ESP block. At the end of the surgery, patients were shifted to postoperative cardiac surgical ICU and were extubated after satisfying the extubation criteria.
The pain assessment was performed using 10 cm visual analog scale (VAS) (10 cm-maximum pain and 0-no pain). The postoperative pain assessment using VAS at rest and during cough were performed at 0 h (extubation), 3 h, 6 h, 12 h, 24 h, 36 h, and at 48 h. Peak inspiratory flow spirometry (incentive spirometry) was performed at 3 h, 6 h, 12 h, 24 h, 36 h, and 48 h postextubation to assess the number of balls raised in the spirometer as an indicator of peak inspiratory flow rate (1 ball = 600 ml, 2 balls = 900 ml, and 3balls = 1200 ml). Breakthrough pain was defined as VAS >4 at rest. IV paracetamol 1 g every 6th hourly was administered to both the group of patients. Rescue analgesia was administered, if VAS was >4 at rest or on patient's demand, with IV fentanyl 1 mcg/kg. The second rescue analgesic planned was IV diclofenac 1 mg/kg diluted in 100 ml normal saline and administered slowly, if VAS remained persistently >4 after 30 min of the first rescue analgesic administration. Dynamic pain was defined as the difference in VAS score between rest and cough of >2 points. Pain was classified as mild (VAS 0-4), moderate (VAS 5-7), and severe (VAS 8-10).
Intraoperative fentanyl consumption, ventilator duration, ICU stay, and any complications were recorded.
Data were expressed as a mean ± standard deviation. Categorical data were analyzed using Chi-squared test and Independent t-test was used to analyze the continuous variables. A two-tailed value of P < 0.05 was considered statistically significant. Statistical analysis was performed using MedCalc software version 12.2.1.0 (Ostend, Belgium).
Results
The literature available on ESP was limited to some sporadic case reports and editorials.
Hence, a pilot study was conducted on 50 patients and post hoc analysis was performed using VAS scores obtained from the present study with an alpha error (Type 1) of 0.05 and calculated the beta error (Type II) being 80.4%.
A total of 25 patients in each group completed the study. Both the groups were comparable with respect to the age and gender [Table 1]. Both groups had a comparable VAS scores at 0 h, 3 h, 6 h, and 12 h (P > 0.05) both at rest and during cough. However, Group A had a statistically significant VAS score than Group B (P ≤ 0.05) at 24 h, 36 h, and 48 h, but mean VAS in Group A was ≤4 both at rest and during cough [Tables 2a and b]. Peak inspiratory spirometry was comparable between the two groups (P > 0.05) [Table 3]. None of the patients received rescue analgesia during the VAS assessment; however, there were a total of nine breakthrough pain episodes in Group A and seven breakthrough episodes of pain in Group B, who demanded analgesia [Figure 2]. None of the patients in either of the group required the second rescue analgesia. Total intraoperative fentanyl consumption, ventilator duration, and ICU duration were comparable between the two groups (P > 0.05) [Table 1]. There were no complications reported in either of the groups.
Table 1.
Demography and other parameters between Group A (thoracic epidural analgesia) and Group B (erector spinae plane block)
| Group A (TEA) | Group B (ESP block) | P | |
|---|---|---|---|
| Age (years) | 50.12±15.21 | 45±19.43 | 0.30 |
| Sex | |||
| Male | 13 | 15 | 0.16 |
| Female | 12 | 10 | |
| Intraoperative fentanyl (mcg) | 330±82.92 | 364.4±105.39 | 0.20 |
| Ventilator duration (min) | 295±50.99 | 298.8±55.68 | 0.80 |
| ICU duration (min) | 3843±962.95 | 3270±1209.34 | 0.07 |
TEA: Thoracic epidural analgesia, ESP: Erector spinae plane, ICU: Intensive Care Unit
Table 2a.
Visual analog scale between Group A (thoracic epidural analgesia) and Group B (erector spinae plane block) at rest
| VAS (rest) | 0 h | 3 h | 6 h | 12 h | 24 h | 36 h | 48 h |
|---|---|---|---|---|---|---|---|
| Group A (n=25) | 1.56±1.08 | 1.52±0.65 | 1.64±0.64 | 1.92±0.90 | 2.08±0.64 | 2.24±1.05 | 2±1.32 |
| Group B (n=25) | 1.04±0.98 | 1.4±1.00 | 1.64±1.35 | 1.68±1.35 | 1.44±0.87 | 1.08±0.86 | 0.8±0.64 |
| P | 0.08 | 0.62 | 1.00 | 0.46 | 0.004 | 0.0001 | 0.0002 |
VAS: Visual analog scale
Table 2b.
Visual analog scale between Group A (thoracic epidural analgesia) and Group B (erector spinae plane block) during cough
| VAS (cough) | 0 h | 3 h | 6 h | 12 h | 24 h | 36 h | 48 h |
|---|---|---|---|---|---|---|---|
| Group A (n=25) | 2.16±1.21 | 2.36±0.76 | 2.52±0.87 | 2.76±1.13 | 3.08±0.70 | 2.96±1.21 | 2.72±1.37 |
| Group B (n=25) | 1.88±1.39 | 2.44±0.92 | 2.6±1.29 | 2.4±1.47 | 2.36±1.07 | 1.8±1.08 | 1.36±0.70 |
| P | 0.45 | 0.74 | 0.79 | 0.34 | 0.007 | 0.0008 | 0.0001 |
VAS: Visual analog scale
Table 3.
Peak inspiratory flow (spirometry) between Group A (thoracic epidural analgesia) and Group B (erector spinae plane block)
| Spirometry | 3 h | 6 h | 12 h | 24 h | 36 h | 48 h |
|---|---|---|---|---|---|---|
| Group A (n=25) | 750±129.90 | 816±106.77 | 852±94.07 | 858±110.57 | 870±136.93 | 888±96.05 |
| Group B (n=25) | 678±150.75 | 744±175.78 | 780±183.71 | 882±90 | 906±110.23 | 906±110.23 |
| P | 0.07 | 0.08 | 0.08 | 0.40 | 0.31 | 0.54 |
Figure 2.
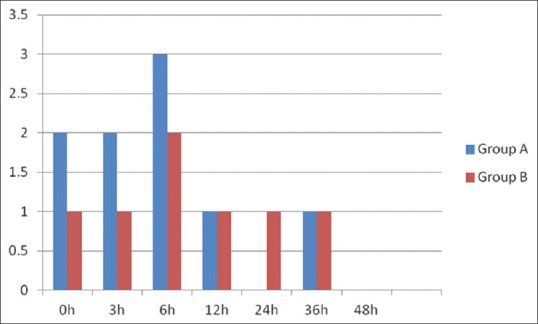
Rescue analgesic requirement between Group A (thoracic epidural analgesia) and Group B (erector spinae plane block). X– axis: time interval in hours postextubation. Y– axis: Number of rescue analgesic episodes
Discussion
In the current era of fast tracking in cardiac surgery, optimal perioperative pain management plays a vital role. In the present study, both groups had VAS ≤4 which signified optimal pain management, facilitating in fast tracking with comparable ventilator duration. Effective pain management also resulted in better pulmonary rehabilitation with an acceptable peak inspiratory flow of around 900 ml in both the groups. Better dynamic pain scores (VAS during cough ≤4) in both the groups had facilitated in expulsion of secretions, preventing postoperative pulmonary complications.
Neuraxial (NA) techniques have been widely researched by various authors in patients undergoing cardiac surgery.[7,8,9] Driving force for the use of NA techniques could be the advent of fast tracking which could facilitate early extubation and shorter ICU stay with decreased hospital duration. Although, there was no significant difference in parameters such as perioperative morbidity and mortality when NA analgesia was performed alone or in combination with GA,[10,11,12,13] time to extubation, and quality of analgesia showed a significant difference.[14,15]
Bracco et al.[16] have reported a minimal postoperative complications such as myocardial dysfunction, pneumonia, acute renal failure, and delirium in cardiac surgical patients who were administered TEA combined with GA compared with GA alone. They also reported a shorter ICU and hospital stay resulting in decreased calculated cost saving per person if the TEA was used. In the present study, authors reported shorter ventilator duration of 295 ± 50.99 min in TEA group and ICU duration of 3843 ± 962.95 min.
In cardiac surgical patients, a major concern in using NA techniques is the safety of the procedure in patients with chronic use of antiplatelet agents, intraoperative systemic anticoagulation, and cardiopulmonary bypass-induced coagulopathy. The incidence of epidural hematoma is unknown in cardiac surgery.[17,18] However, reported estimated risk of epidural hematoma with TEA being 1 in 12,000 and catheter-related epidural hematoma being 1 in 5493.[19]
There has been a resurgence of NA analgesia in cardiac surgery due to the minimally invasive techniques. Recent literature using intrapleural, paravertebral, and intercostal blocks may have a unique clinical advantage over the traditional epidural techniques.
There is limited literature available on the efficacy of ESP block barring only a few case reports in noncardiac surgeries.
Forero et al.[6] have popularized ESP block for patients with chronic thoracic neuropathic pain, who were poorly responsive to oral pharmacotherapy. He revealed an extensive multi-dermatomal sensory block which was investigated in fresh cadavers, for the likely site of action of ESP block, which being dorsal and ventral rami of thoracic spinal nerves. The authors had revealed the extent of the cutaneous sensory block over the anterior-posterior thorax ranging from T1 to T11, spreading cephalocaudal with an injection of 25 ml of LA when administered at the level of T5.
Hamilton and Manickam,[20] reported a successful ESP block using a continuous catheter for pain relief in patients with multiple unilateral rib fractures. The authors postulated that cephalocaudal spread of the LA is due to its proximity of the costotransverse foramina, where both dorsal and ventral rami of thoracic spinal nerves originated. The spread of LA is also facilitated by thoracolumbar fascia extending from the posterior thorax and abdomen in continuity with the nuchal fascia of the neck superiorly. In the present study, adequate pain relief was obtained, substantiated by VAS scores <4, which persisted for 48 h postextubation using a continuous catheter.
Forero et al.[21] in their case report has revealed the benefit of ESP as rescue analgesic technique in thoracotomy after a failed epidural. TEA and PVB are mostly chosen as the first line regional analgesic techniques in thoracic surgeries for the pain management.[22,23] When there is a contraindication or failure of these blocks, intercostal nerve block remained as an alternative but necessitating multiple injections. ESP block can serve as an alternative either as a single dose or as a continuous catheter based infusion for postthoracotomy pain. In the present study, the authors revealed comparable pain score between TEA and ESP block until 12 h postextubation. The VAS scores remained to be persistently ≤4 until 48 h in either of the group.
ESP block can be performed by inserting the needle 2–3 cm lateral to T6 spinous process, until a bony resistance is obtained which confirms the T5 transverse process. LA is injected by slightly withdrawing the needle, so that it lies between transverse process and ESP muscle. Bonvicini[24] has reported the use of bilateral USG ESP block in the breast cancer reconstructive surgery and suggested it to be an effective alternative to PVB and TEA technique. The sonoanatomy is easily recognizable with no vital structures at the risk of needle injury. The authors have used USG ESP block over the surface landmark technique, which resulted in no failed blocks.
Ho et al.[18] and Bracco and Hemmerling[19] have reported complications with an epidural analgesia in cardiac surgery. In the present study, there were no complications recorded in either of the group. Hence, the authors found ESP block as a promising alternative to TEA for the perioperative analgesia.
Conclusion
ESP block had a comparable pain scores with TEA, and hence proved to be an effective alternative to TEA in an adult cardiac surgery for the perioperative pain management and fast tracking.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Video Available on: www.annals.in
References
- 1.Cogan J. Pain management after cardiac surgery. Semin Cardiothorac Vasc Anesth. 2010;14:201–4. doi: 10.1177/1089253210378401. [DOI] [PubMed] [Google Scholar]
- 2.American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: An updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–73. doi: 10.1097/ALN.0b013e31823c1030. [DOI] [PubMed] [Google Scholar]
- 3.Dhole S, Mehta Y, Saxena H, Juneja R, Trehan N. Comparison of continuous thoracic epidural and paravertebral blocks for postoperative analgesia after minimally invasive direct coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2001;15:288–92. doi: 10.1053/jcan.2001.23271. [DOI] [PubMed] [Google Scholar]
- 4.Mehta Y, Arora D, Sharma KK, Mishra Y, Wasir H, Trehan N, et al. Comparison of continuous thoracic epidural and paravertebral block for postoperative analgesia after robotic-assisted coronary artery bypass surgery. Ann Card Anaesth. 2008;11:91–6. doi: 10.4103/0971-9784.41576. [DOI] [PubMed] [Google Scholar]
- 5.Cantó M, Sánchez MJ, Casas MA, Bataller ML. Bilateral paravertebral blockade for conventional cardiac surgery. Anaesthesia. 2003;58:365–70. doi: 10.1046/j.1365-2044.2003.03082_2.x. [DOI] [PubMed] [Google Scholar]
- 6.Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: A Novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41:621–7. doi: 10.1097/AAP.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 7.Liem TH, Booij LH, Hasenbos MA, Gielen MJ. Coronary artery bypass grafting using two different anesthetic techniques: Part I: Hemodynamic results. J Cardiothorac Vasc Anesth. 1992;6:148–55. doi: 10.1016/1053-0770(92)90189-e. [DOI] [PubMed] [Google Scholar]
- 8.Liem TH, Hasenbos MA, Booij LH, Gielen MJ. Coronary artery bypass grafting using two different anesthetic techniques: Part 2: Postoperative outcome. J Cardiothorac Vasc Anesth. 1992;6:156–61. doi: 10.1016/1053-0770(92)90190-i. [DOI] [PubMed] [Google Scholar]
- 9.Liem TH, Booij LH, Gielen MJ, Hasenbos MA, van Egmond J. Coronary artery bypass grafting using two different anesthetic techniques: Part 3: Adrenergic responses. J Cardiothorac Vasc Anesth. 1992;6:162–7. doi: 10.1016/1053-0770(92)90191-9. [DOI] [PubMed] [Google Scholar]
- 10.Slogoff S, Keats AS. Randomized trial of primary anesthetic agents on outcome of coronary artery bypass operations. Anesthesiology. 1989;70:179–88. doi: 10.1097/00000542-198902000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Tuman KJ, McCarthy RJ, Spiess BD, DaValle M, Dabir R, Ivankovich AD, et al. Does choice of anesthetic agent significantly affect outcome after coronary artery surgery? Anesthesiology. 1989;70:189–98. doi: 10.1097/00000542-198902000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Royse C, Royse A, Soeding P, Blake D, Pang J. Prospective randomized trial of high thoracic epidural analgesia for coronary artery bypass surgery. Ann Thorac Surg. 2003;75:93–100. doi: 10.1016/s0003-4975(02)04074-2. [DOI] [PubMed] [Google Scholar]
- 13.Salvi L, Parolari A, Veglia F, Brambillasca C, Gregu S, Sisillo E, et al. High thoracic epidural anesthesia in coronary artery bypass surgery: A propensity-matched study. J Cardiothorac Vasc Anesth. 2007;21:810–5. doi: 10.1053/j.jvca.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Onan B, Onan IS, Kilickan L, Sanisoglu I. Effects of epidural anesthesia on acute and chronic pain after coronary artery bypass grafting. J Card Surg. 2013;28:248–53. doi: 10.1111/jocs.12086. [DOI] [PubMed] [Google Scholar]
- 15.Scott NB, Turfrey DJ, Ray DA, Nzewi O, Sutcliffe NP, Lal AB, et al. Aprospective randomized study of the potential benefits of thoracic epidural anesthesia and analgesia in patients undergoing coronary artery bypass grafting. Anesth Analg. 2001;93:528–35. doi: 10.1097/00000539-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Bracco D, Noiseux N, Dubois MJ, Prieto I, Basile F, Olivier JF, et al. Epidural anesthesia improves outcome and resource use in cardiac surgery: A single-center study of a 1293-patient cohort. Heart Surg Forum. 2007;10:E449–58. doi: 10.1532/HSF98.20071126. [DOI] [PubMed] [Google Scholar]
- 17.Rosen DA, Hawkinberry DW, 2nd, Rosen KR, Gustafson RA, Hogg JP, Broadman LM, et al. An epidural hematoma in an adolescent patient after cardiac surgery. Anesth Analg. 2004;98:966–9. doi: 10.1213/01.ANE.0000103267.37895.5B. [DOI] [PubMed] [Google Scholar]
- 18.Ho AM, Chung DC, Joynt GM. Neuraxial blockade and hematoma in cardiac surgery: Estimating the risk of a rare adverse event that has not (yet) occurred. Chest. 2000;117:551–5. doi: 10.1378/chest.117.2.551. [DOI] [PubMed] [Google Scholar]
- 19.Bracco D, Hemmerling T. Epidural analgesia in cardiac surgery: An updated risk assessment. Heart Surg Forum. 2007;10:E334–7. doi: 10.1532/HSF98.20071077. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton DL, Manickam B. Erector spinae plane block for pain relief in rib fractures. Br J Anaesth. 2017;118:474–5. doi: 10.1093/bja/aex013. [DOI] [PubMed] [Google Scholar]
- 21.Forero M, Rajarathinam M, Adhikary S, Chin KJ. Continuous erector spinae plane block for rescue analgesia in thoracotomy after epidural failure: A Case report. A A Case Rep. 2017;8:254–6. doi: 10.1213/XAA.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 22.Romero A, Garcia JE, Joshi GP. The state of the art in preventing postthoracotomy pain. Semin Thorac Cardiovasc Surg. 2013;25:116–24. doi: 10.1053/j.semtcvs.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Yeung JH, Gates S, Naidu BV, Wilson MJ, Gao Smith F. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev. 2016;2:CD009121. doi: 10.1002/14651858.CD009121.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonvicini D, Tagliapietra L, Giacomazzi A, Pizzirani E. Bilateral ultrasound-guided erector spinae plane blocks in breast cancer and reconstruction surgery. J Clin Anesth. 2018;44:3–4. doi: 10.1016/j.jclinane.2017.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


