Abstract
Vascular involvement in Late Onset Pompe Disease, glycogen storage disease type II characterized by limb-girdle muscle and diaphragmatic weakness, is well documented. Abnormalities of posterior cerebral circulation have mostly been reported, whereas there are also cases of associated extracerebral arteriopathy. We report the case of a 42-year-old man diagnosed with LOPD a year after renal infarct due to renal artery fibromuscular dysplasia. We propose that the association of LOPD and arteriopathy should always be considered in clinical practice.
Keywords: Muscle disease, Glycogenoses, Metabolic disease (inherited), All clinical neurology
1. Introduction
Glycogen storage disease type II or Pompe disease is an autosomal recessive disorder due to mutations in the GAA gene, encoding the lysosomal enzyme acid alpha-1,4-glucosidase. Intralysosomal storage of glycogen was classically described as the underlying pathogenic mechanism. However, recent data indicate that dysfunctional autophagy may also be responsible for tissue damage [1]. There are two main clinical categories of Pompe disease: infantile onset and late onset Pompe disease (LOPD). Infantile onset disease is characterized by onset of symptoms before the age of 1 year and rapid evolution to heart and respiratory failure and death. Individuals presenting with LOPD have a higher residual enzyme activity and onset of symptoms may occur in childhood, adolescence or in adulthood even up to the 7th decade of life. The main clinical features of LOPD are diaphragmatic and limb-girdle muscle weakness. The diagnosis of LOPD relies on assessment of GAA enzyme activity in blood, skin fibroblasts, or muscle and GAA gene sequencing.
Although skeletal muscles' damage is responsible for most of the clinical manifestations of LOPD, glycogen accumulation has been also found in liver, heart, glial cells, brainstem nuclei, anterior horn cells of spinal cord, smooth muscle, and blood vessels [2,3]. There is growing evidence of both systemic and cerebral vascular involvement in LOPD. Dilation of cerebral arteries, predominant in the posterior circulation and resulting in vertebrobasilar dolichoectasia and basilar artery fusiform aneurysms is mostly reported [4]. Internal carotid dilative arteriopathy, dilation of small cerebral arteries and cerebrovascular stenosis have also been associated with LOPD and complicated in some cases with aneurysm rupture, artery dissection or recurrent stroke [[5], [6], [7], [8]]. Extracerebral vascular involvement may concern cervical arteries, thoracic aorta, whereas there is a single case report of renal and iliac arteriopathy [9].
2. Material and methods
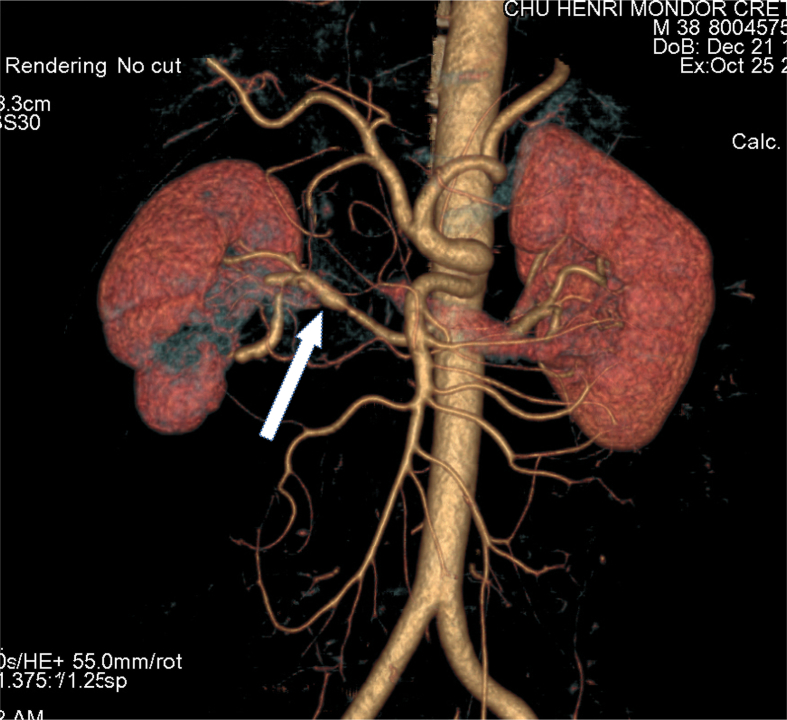
We report the case of a 42 years old man with unremarkable personal and family history who presented at the emergency department at the age of 38 with acute low-back pain. An abdominal Computed Tomography (CT) scan was performed, showing a right renal infarct. The patient had no cardiovascular risk factors. Renal arteriography demonstrated dilation of the distal portion of the right renal artery leading to the diagnosis of focal fibromuscular dysplasia in this young patient presenting no evidence of atherosclerosis. Antiaggregation and anticoagulation therapy was started, according to guidelines. A week later, the patient presented with subacute confusion followed by a generalised tonic clonic seizure with postictal coma. He was admitted to the Intensive Care Unit and hypertensive crisis with hypertensive encephalopathy secondary to acute kidney injury was diagnosed. A cerebral CT scan revealed an hematoma located in the head of the right caudate nucleus, in the territory of the anterior cerebral artery. Interestingly, there were no abnormalities in cerebral CT- and MR-angiography. The patient was thus diagnosed with isolated renal artery fibromuscular dysplasia. A year after this episode, he developed severe dyspnoea with orthopnoea and spirometric analysis showed severe restrictive respiratory insufficiency. Symptoms worsened during follow-up and in less than two years' time he became dependent of nocturnal non-invasive nasal ventilation.
2.1. Results
Because of intriguing important diaphragmatic involvement, a thorough inquiry on patient's medical history was performed, which brought to light difficulties during physical exercise since adolescence and progressive muscle weakness concerning mainly pelvic girdle musculature. Mobility problems became significant at the age of 35, when the patient became incapable of running and begun to have considerable difficulties in rising from a chair. Physical examination at the age of 39 years revealed bilateral winged scapula, axial and pelvic girdle weakness. Gait was typical of LODP, waddling with a wider base of support and lumbar hyperlordosis. Muscle biopsy demonstrating a vacuolar myopathy and reduced GAA enzyme activity on dried blood spot led to the diagnosis of Pompe disease. Urinary glucose tetrasaccharide concentrations were, as expected, high. Given the importance of the symptoms, enzyme replacement therapy was initiated at the age of 40 years. The patient reported a benefice of the treatment, with an increasing maximum walking distance.
2.2. Discussion and conclusions
We therefore present the first case of isolated clinically important renal arteriopathy in LOPD. Involvement of cerebral vessels in Pompe disease is well-known. We demonstrate that renal arteries may also be involved, potentially leading to kidney infarct, in the absence of any cerebral arteries abnormalities. Increased awareness of non-cerebral arteries fibromuscular dysplasia is warranted in patients diagnosed with LOPD. Our case also indicates that systemic arteriopathy may be diagnosed early in the course of LOPD, even in the absence of patent muscle weakness or respiratory insufficiency.
Contributor Information
Evangelia Pappa, Email: evapapel@hotmail.com.
Philippe Grimbert, Email: philippe.grimbert@aphp.fr.
Pascal Laforêt, Email: pascal.laforet@aphp.fr.
Guillaume Bassez, Email: guillaume.bassez@aphp.fr.
Appendix
CT angiography showing fibromuscular dysplasia of the right renal artery (arrow).
References
- 1.American Association of Neuromuscular & Electrodiagnostic Medicine Diagnostic criteria for late-onset (childhood and adult) Pompe disease. Muscle Nerve. 2009;40:149–160. doi: 10.1002/mus.21393. [DOI] [PubMed] [Google Scholar]
- 2.Hobson-Webb L.D., Proia A.D., Thurberg B.L., Banugaria S., Prater S.N., Kishnani P.S. Autopsy findings in late-onset Pompe disease: a case report and systematic review of the literature. Mol. Genet. Metab. 2012;106:462–469. doi: 10.1016/j.ymgme.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Kretzschmar H.A., Wagner H., Hübner G., Danek A., Witt T.N., Mehraein P. Aneurysms and vacuolar degeneration of cerebral arteries in late-onset acid maltase deficiency. J Neurol Sci. sept. 1990;98(2–3):169–183. doi: 10.1016/0022-510x(90)90258-o. [DOI] [PubMed] [Google Scholar]
- 4.Laforêt P., Petiot P., Nicolino M. Dilative arteriopathy and basilar artery dolichoectasia complicating late-onset Pompe disease. Neurology. 2008;70:2063–2066. doi: 10.1212/01.wnl.0000313367.09469.13. [DOI] [PubMed] [Google Scholar]
- 5.Montagnese F., Granata F., Musumeci O. Intracranial arterial abnormalities in patients with late onset Pompe disease (LOPD) J. Inherit. Metab. Dis. 2016;39:391–398. doi: 10.1007/s10545-015-9913-x. [DOI] [PubMed] [Google Scholar]
- 6.Hensel O., Hanisch F., Stock K., Stoevesandt D., Deschauer M., Müller T. Morphology and function of cerebral arteries in adults with pompe disease. JIMD Rep. 2015;20:27–33. doi: 10.1007/8904_2014_385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malhotra K., Carrington D.C., Liebeskind D.S. Restrictive Arteriopathy in late-onset Pompe disease: case report and review of the literature. J. Stroke Cerebrovasc. Dis. 2017;26(8):e172–e175. doi: 10.1016/j.jstrokecerebrovasdis.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 8.Anneser J.M.H., Pongratz D.E., Podskarbi T., Shin Y.S., Schoser B.G.H. Mutations in the acid α-glucosidase gene (M. Pompe) in a patient with an unusual phenotype. Neurology. 2005;64(2):368–370. doi: 10.1212/01.WNL.0000149528.95362.20. [DOI] [PubMed] [Google Scholar]
- 9.Quenardelle V., Bataillard M., Bazin D., Lannes B., Wolff V., Echaniz-Laguna A. Pompe disease presenting as an isolated generalized dilative arteriopathy with repeated brain and kidney infarcts. J. Neurol. 2015;262:473–475. doi: 10.1007/s00415-014-7582-6. [DOI] [PubMed] [Google Scholar]