Abstract
Our aim was to elucidate the relationship between the rate of mitochondrial reactive oxygen species (mROS) formation and the reduction level of the mitochondrial coenzyme Q (mQ) pool under various levels of engagement of the mQ-reducing pathway (succinate dehydrogenase, complex II) and mQH2-oxidizing pathways (the cytochrome pathway and alternative oxidase pathway, (AOX)) in mitochondria isolated from the amoeba Acanthamoeba castellanii. The mQ pool was shifted to a more reduced state by inhibition of mQH2-oxidizing pathways (complex III and complex IV of the cytochrome pathway, and AOX) and the oxidative phosphorylation system. The mQ reduction level was lowered by decreasing the electron supply from succinate dehydrogenase and by stimulating the activity of the cytochrome or AOX pathways. The results indicate a direct dependence of mROS formation on the reduction level of the mQ pool for both mQH2-oxidizing pathways. A higher mQ reduction level leads to a higher mROS formation. For the cytochrome pathway, mROS generation depends nonlinearly upon the mQ reduction level, with a stronger dependency observed at values higher than the mQ reduction level of the phosphorylating state (~ 35%). AOX becomes more engaged at higher mQ pool reduction levels (above 40%), when mROS production via the cytochrome pathway increases. We propose that the mQ pool reduction level (endogenous mQ redox state) could be a useful endogenous reporter that allows indirect assessment of overall mROS production in mitochondria.
Abbreviations: AOX, alternative oxidase; FCCP, carbonyl cyanide p-trifluoromethoxyphenylhydrazone; mROS, mitochondrial reactive oxygen species; mQ, mitochondrial coenzyme Q, ubiquinone; mQox, oxidized Q; mQH2, reduced Q (ubiquinol); mQtot, total pool of endogenous Q in the inner mitochondrial membrane; mQH2/mQtot, reduction level of Q; mΔΨ, mitochondrial membrane electric potential; OXPHOS, oxidative phosphorylation; ΔμH+, proton electrochemical gradient
Keywords: Mitochondria, Acanthamoeba castellanii, Reactive oxygen species, Coenzyme Q reduction level
Graphical abstract
Highlights
-
•
mROS generation depends on the reduction level of the endogenous mQ pool.
-
•
A stronger dependency is observed above mQ reduction level of phosphorylating state.
-
•
The mQ reduction level can be an endogenous reporter of overall mROS production.
1. Introduction
Mitochondrial coenzyme Q (mQ, ubiquinone) is an essential electron carrier that plays a central role in the mitochondrial electron transport respiratory chain [1], [2]. Mitochondrial Q shuttles electrons between dehydrogenases and the oxidizing pathway(s) of the mitochondrial respiratory chain and is also involved in the formation of superoxide from semiubiquinone radicals by the respiratory chain, which can lead to mitochondrial oxidative damage [3], [4], [5]. It is widely accepted that mitochondrial reactive oxygen species (mROS) production depends on the level of reduction of mitochondrial electron carriers, especially on mQ. Surprisingly, there are only few data relating mQH2/Q ratio [6] or mQ reduction level [7] with mROS generation.
The amoeba Acanthamoeba castellanii is a nonphotosynthesizing free-living protozoan belonging to the group that diverged from the animal/fungal line after the split from plants [8]. As an opportunistic pathogen that can cause serious diseases in humans, A. castellanii is an evolutionarily and medically important amoebozoan. This species presents features, including mitochondrial physiology, that are common to plants, fungi and animals. In addition to several dehydrogenases, the plant-type respiratory chain of A. castellanii mitochondria contains two ubiquinol (mQH2)-oxidizing pathways, namely, the classical antimycin A- and cyanide-sensitive cytochrome pathway and the alternative benzohydroxamate- and propyl gallate-sensitive ubiquinol oxidase (AOX) pathway [9], [10], [11]. The mQ pool plays a central role in the respiratory chain; respiratory substrate-oxidizing dehydrogenases reduce mQ to mQH2, and the two oxidizing pathways convert mQH2 to mQ. Electron transfer via the AOX pathway does not result in proton pumping and is therefore not coupled to the mitochondrial production of ATP. The study of mitochondrial respiration of succinate (complex II substrate) in A. castellanii allows investigation of the kinetics of two mQH2-oxidizing pathways; one proton electrochemical gradient (ΔμH+)-generating pathway, consisting of the two proton-pumping complexes III and IV (the cytochrome pathway), and one ΔμH+-independent pathway (the AOX pathway). Because mROS production depends on ΔμH+ [4], investigation of A. castellanii mitochondria enables the determination of the relationship between mROS formation and the mQ reduction level at different mitochondrial membrane potential (m∆Ψ) values depending on the engagement of the two mQH2-oxidizing pathways.
The aim of our work was to elucidate the relationship between mROS formation and the reduction level of the mQ pool under a variety of mitochondrial respiration conditions, i.e., at varying degrees of engagement of mQ-reducing the pathway (succinate dehydrogenase, complex II) and mQH2-oxidizing pathways (the cytochrome pathway and AOX) in isolated A. castellanii mitochondria. The mQ reduction level was increased by decreasing electron flow out of the mQ pool via inhibition of the mQH2-oxidizing pathways (complex III, complex IV, or AOX) or inhibition of the oxidative phosphorylation (OXPHOS) system (ATP synthase or ATP/ADP antiporter). The mQ pool was shifted to a more oxidized state by decreasing the electron supply from complex II via inhibition of the mQ-reducing pathway (substrate dehydrogenase) or by stimulation of the activities of the mQH2-oxidizing pathways under uncoupling conditions (the cytochrome pathway) or under GMP activation (AOX). We measured the mQ reduction level under given mitochondrial oxygen consumption and mitochondrial membrane potential (mΔΨ) conditions in terms of H2O2 formation.
2. Materials and methods
2.1. Acanthamoeba castellanii cell culture and isolation of mitochondria
Trophozoites of the A. castellanii strain Neff (ATCC®30010TM) were cultured as described previously [10]. Cells from 72-h cultures were inoculated (time 0) to a final density of approximately 2.5 ± 0.4 × 105 cells × ml−1. After approximately 40 h of exponential growth with a generation time (cell doubling time) of 8 h, the amoeba cultures reached the intermediate growth phase and then the stationary phase, the latter preceding transformation into cysts within a few hours. In this study, trophozoites of A. castellanii were harvested 48 h after inoculation, in the intermediate phase (6.8 ± 0.5 × 106 cells × ml−1). Mitochondria were isolated in an isolation medium containing 0.25 M sucrose, 10 mM Tris/HCl (pH 7.4), 0.5 mM EGTA, and 0.2% bovine serum albumin (BSA) and then purified on a self-generating Percoll gradient (28%) for 45 min at 40,000 g [9]. Purified mitochondria were washed in isolation medium without BSA and EGTA. Protein concentrations of the isolated mitochondria were determined using the biuret method.
2.2. General measurement conditions
All measurements were performed in a standard incubation medium (28 °C) containing: 120 mM KCl, 20 mM Tris/HCl (pH 7.4), 3 mM KH2PO4, 8 mM MgCl2, 1 mM EGTA, and 0.2% BSA with continuous stirring. Mitochondria (0.33 mg of protein/ml) were incubated with succinate (5 mM) as an oxidizable substrate of complex II in the presence of rotenone (2 μM) to block electron input from complex I. Respiratory rate, mΔΨ, mQ reduction levels, and mROS formation were measured under (i) resting (nonphosphorylating, State 4) conditions, i.e., in the absence of exogenous ADP, and (ii) phosphorylating (State 3) conditions, i.e., in the presence of 1–2 mM ADP. The mQ-reducing pathway was titrated with increasing concentrations of malonate (an inhibitor of succinate dehydrogenase, complex II). The activity of a major mQH2-oxidizing pathway (the cytochrome pathway) was varied with increasing concentrations of (i) antimycin A or cyanide (inhibitors of complex III and complex IV of the cytochrome pathway, respectively), (ii) oligomycin or carboxyatractyloside (inhibitors of ATP synthase and ATP/ADP antiporter, respectively), or (iii) uncoupler (carbonyl cyanide p-trifluoromethoxyphenylhydrazone, FCCP) in the presence of AOX inhibitor. Parameters of the cytochrome pathway were measured in the presence of benzohydroxamate (1 mM) (respiratory rate, mΔΨ, and mQ reduction level) or propyl gallate (3 µM) (H2O2 formation). The activity of the alternative mQH2-oxidizing pathway (AOX) was varied with increasing concentrations of GMP (an allosteric activator of AOX, [12], [13]) in the presence of 0.65 mM cyanide or 90 nM antimycin A (to exclude the activity of the cytochrome pathway). The relationships between the studied respiratory chain parameters (respiratory rate, mΔΨ, mQ reduction levels, and H2O2 formation) and increasing concentrations of the respiratory chain and OXPHOS system modulators are shown in the Supplementary materials (Figs. S1–S6).
2.3. Mitochondrial oxygen consumption and mΔΨ measurements
Oxygen uptake was measured polarographically with a Clark-type oxygen electrode (Rank Brothers, Cambridge, UK) in 3.0 ml of incubation medium (28 °C) with 1 mg of mitochondrial protein. Only high quality A. castellanii mitochondrial preparations, i.e., those with ADP/O values of ~ 1.40 (with succinate as a respiratory substrate) and respiratory control ratios of ~ 3.5, were used in all the experiments. For OXPHOS control, phosphorylating respiration (State 3) was measured after an ADP pre-pulse (50 μM) using 150 μM ADP as the main pulse. The total amount of oxygen consumed during phosphorylating respiration was used to calculate the ADP/O ratio. The mΔΨ measurements allowed fine control of the duration of phosphorylating respiration. Values of O2 uptake are given in nmol O2 × min−1 × mg protein−1.
The mΔΨ was measured simultaneously with oxygen uptake using a tetraphenylphosphonium (TPP+)-specific electrode as described previously [14], [15], [16]. The TPP+-specific electrode was calibrated with three sequential additions (0.8, 0.8, and 1.6 µM) of TPP+. After each run, 0.5 µM FCCP was added to release TPP+ for baseline correction. For calculation of the ΔΨ value, the matrix volume of the amoeba mitochondria was assumed to be 2.0 μl × mg−1 protein. The calculation assumes that the TPP+ distribution between the mitochondria and medium followed the Nernst equation. The values of ΔΨ are given in mV.
2.4. Assay of H2O2 production by isolated mitochondria
The mitochondrial H2O2 production rate was measured by the Amplex Red-horseradish peroxidase method (Invitrogen) [17]. Horseradish peroxidase (0.1 U × ml−1) catalyzes the H2O2-dependent oxidation of nonfluorescent Amplex Red (5 μM) to fluorescent resorufin red. Fluorescence was kinetically followed for 10 min at 545 nm (excitation), 590 nm (emission), and gain 150 using an Infinite M200 PRO Tecan multimode reader with 24-well plates. Mitochondria (0.17 mg of mitochondrial protein) were incubated in 0.5 ml of the standard incubation medium (see above) with 5 mM succinate as an oxidizable substrate in the presence of rotenone (2 μM). Because, at high concentrations, AOX inhibitors, benzohydroxamate and propyl gallate, are known to inhibit horseradish peroxidase activity and scavenge free radicals [18], [19], [20], [21], H2O2 formation related to the cytochrome pathway activity was measured in the presence of a low concentration of propyl gallate (1.5 µM), which completely inhibits AOX activity. Because cyanide inhibits horseradish peroxidase activity [22], the H2O2 formation rate related to AOX activity was measured in the presence of 90 nM antimycin A to inhibit cytochrome pathway activity. Therefore, titration of H2O2 formation related to cytochrome pathway activity was performed only in the presence of increasing concentrations of antimycin A not cyanide. Reactions were monitored with constant stirring at 28 °C and calibrated with known amounts of H2O2 in the absence or presence of 1.5 µM propyl gallate. H2O2 production rates were determined from slopes calculated from readings obtained from several repeated 10-min measurements. Values of H2O2 production are given in pmol H2O2 × min−1 × mg protein−1.
2.5. Determination of the mQ reduction level
The reduction level of mQ (mQH2/mQtot), i.e., the ratio of reduced mQ (ubiquinol, mQH2) to the total endogenous pool of mQ in the inner mitochondrial membrane under steady-state respiration was determined by extraction followed by HPLC detection [23]. Mitochondria (1–2 mg of mitochondrial protein) in the steady state were chemically quenched with 4 ml of 0.65 M HClO4 in methanol (0 °C), and mQ was subsequently extracted with 3 ml of petroleum ether. Detection of the oxidized and reduced forms of mQ (at 275 nm and 290 nm, respectively) was performed by HPLC with a reverses-phase Lichrosorb 10 RP 18 column (4.6 mm × 250 mm). A completely oxidized extract was obtained during incubation in the absence of a reducing substrate using an evaporation/ventilation step. A completely reduced extract was obtained upon anaerobiosis and in the presence of respiratory substrate (5 mM succinate), 1 mM cyanide and 1 mM benzohydroxamate. As previously determined, the endogenous Q in A. castellanii mitochondria is Q9 [12]. Commercial Q9 (Sigma) was used for peak calibration. The reduction level of mQ is expressed as the percentage of total mQ (mQH2/mQtot).
3. Results
Cytochrome pathway activity was measured in the presence of AOX inhibitor (1 mM benzohydroxamate or 1.5 µM propyl gallate). The activity of the alternative mQH2-oxidizing pathway (AOX) was measured in the presence of 0.65 mM cyanide or 90 nM antimycin A to exclude cytochrome pathway activity.
3.1. The relationship between mROS formation and the reduction level of the mQ pool under various degrees of engagement of the cytochrome pathway
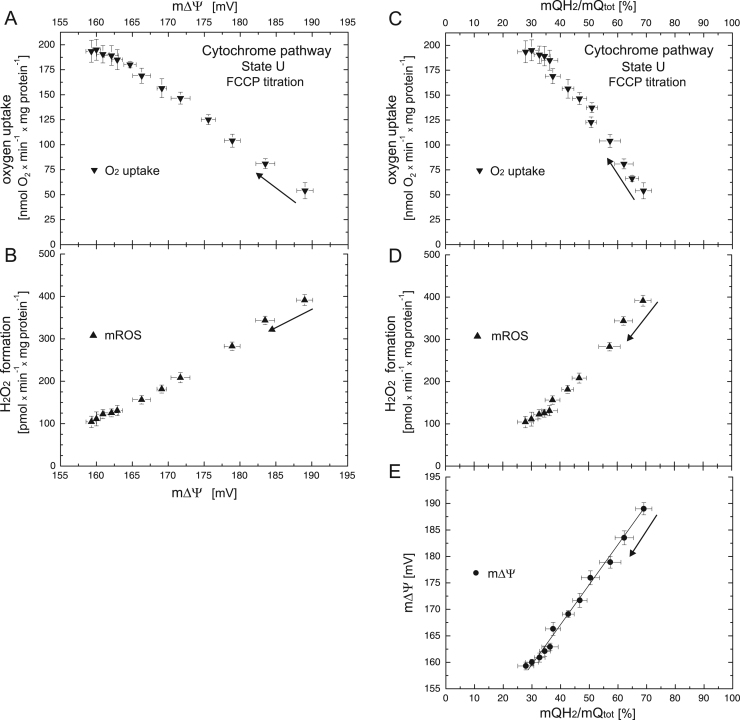
At first, the mQ pool was progressively shifted to a more oxidized state by stimulating the activity of the mQH2-oxidizing pathway (the cytochrome pathway) by uncoupling with increasing concentrations of FCCP (up to 500 nM) (Figs. 1, S1). Fig. 1 and B show changes in respiratory rate (progressively increasing from ~ 55 to ~ 195 nmol O2 × min−1 × mg protein−1) and H2O2 formation rate (progressively decreasing from ~ 100 to ~ 390 pmol H2O2 × min−1 × mg protein−1) when the m∆Ψ of nonphosphorylating mitochondria was gradually decreased (from ~ 189 to ~ 160 mV) by the uncoupler. The uncoupler-induced changes in the respiratory and H2O2 formation rates were accompanied by a gradual decrease in the mQ reduction level from ~ 69 to ~ 28% (Fig. 1C–E). A linear dependence of m∆Ψ on the mQ reduction level for the cytochrome pathway under the uncoupling conditions was observed (Fig. 1E).
Fig. 1.
The relationships between respiratory rate (A) and H2O2 formation (B) versus m∆Ψ and the relationships between respiratory rate (C), H2O2 formation (D), and m∆Ψ (E) versus mQ reduction level when the cytochrome pathway activity of nonphosphorylating mitochondria was varied with increasing concentrations of FCCP (up to 500 nM). Measurements were performed in the presence of 1 mM benzohydroxamate or 1.5 µM propyl gallate (H2O2 formation). Arrows indicate the starting point and direction of titrations.
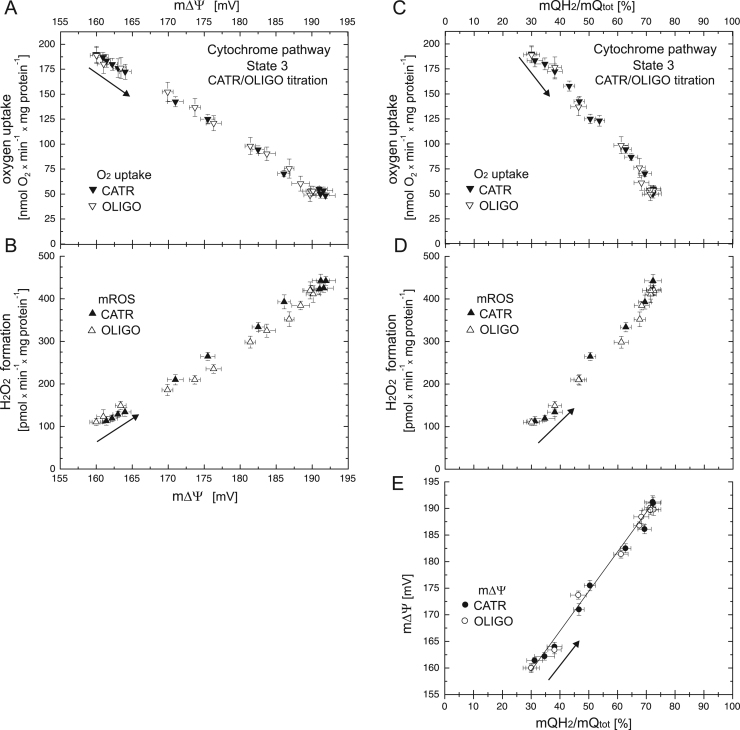
Next, the mQ reduction level of the cytochrome pathway under phosphorylating conditions was gradually increased by inhibition of the OXPHOS system with increasing concentrations of carboxyatractyloside (up to 325 nM) or oligomycin (120 nM) (Fig. S2). Fig. 2 shows the relationships between the respiratory rate and H2O2 formation versus m∆Ψ (Fig. 2A, B) and mQ reduction level (Fig. 2C, D) when the cytochrome pathway activity of phosphorylating mitochondria (in the presence of 1 mM ADP) was gradually varied with the inhibitors of ADP/ATP antiporter and ATP synthase. During carboxyatractyloside/ oligomycin titration, an increase in m∆Ψ from ~ 160 mV (uninhibited phosphorylating state) up to ~ 192 mV (fully inhibited phosphorylating state) was accompanied by an increase in mQ reduction from ~ 30% (uninhibited phosphorylating state) to ~ 72% (nonphosphorylating state in the presence of OXPHOS inhibitors). A linear dependence of m∆Ψ on the mQ reduction level for the cytochrome pathway under phosphorylating conditions (during the carboxyatractyloside/oligomycin-induced State 3 to State 4 transition) is presented in Fig. 1E. Moreover, a progressive decrease in the respiratory rate from ~ 195 to ~ 50 nmol O2 × min−1 × mg protein−1 and a progressive increase in H2O2 formation from 110 to ~ 440 pmol H2O2 × min−1 × mg protein−1 accompanied the carboxyatractyloside/oligomycin-induced State 3 to State 4 transition (Fig. 2A–D).
Fig. 2.
The relationships between respiratory rate (A) and H2O2 formation (B) versus m∆Ψ and the relationships between respiratory rate (C), H2O2 formation (D), and m∆Ψ (E) versus mQ reduction level when the cytochrome pathway activity of phosphorylating mitochondria was varied with increasing concentrations of carboxyatractyloside (CATR, up to 350 nM) or oligomycin (OLIGO, up to 120 nM). Measurements were performed in the presence of 1 mM benzohydroxamate or 1.5 µM propyl gallate (H2O2 formation). Arrows indicate the starting point and direction of titrations.
Rates of H2O2 formation were elevated up to 4 times in the resting State 4 (in the absence or presence of OXPHOS inhibitors) compared to the rates in the phosphorylating State 3 or uncoupled state (Figs. 1, 2), because higher (up to ~ 30 mV) m∆Ψ and higher (up to 40%) mQ reduction levels were observed.
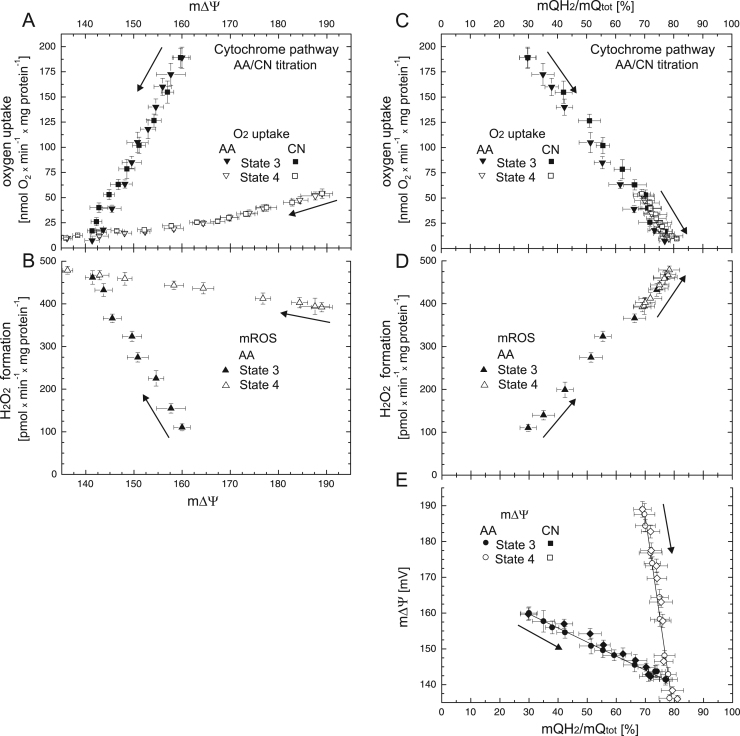
Furthermore, the mQ reduction level of the cytochrome pathway was gradually increased by decreasing the electron flow out of the mQ pool via inhibition of complex III by increasing the concentrations of antimycin A (up to 60 nM) and complex IV by increasing the concentrations of cyanide (up to 0.4 mM) (Fig. S3). Measurements were performed under phosphorylating (State 3) and nonphosphorylating (State 4) conditions. Because cyanide inhibits peroxidase activity, measurements of H2O2 formation were performed only during antimycin A titrations. Fig. 3 shows the relationships between respiratory rate and H2O2 formation versus m∆Ψ (Fig. 3A, B) and versus mQ reduction level (Fig. 3C, D) when the cytochrome pathway activity was gradually inhibited. The relationships obtained during the antimycin A and cyanide titrations were similar. A much steeper linear dependence of m∆Ψ on the mQ reduction level was obtained for the cytochrome pathway under nonphosphorylating conditions than under phosphorylating conditions (Fig. 3E). During antimycin A/cyanide titration under nonphosphorylating conditions, a progressive decrease in m∆Ψ from ~ 189 mV (uninhibited State 4) up to ~ 136 mV (fully inhibited State 4) was accompanied by a relatively moderate increase in mQ reduction level from ~ 69% (uninhibited State 3) to ~ 81% (fully inhibited State 3) (Fig. 1E). This 12% increase in the mQ reduction level was accompanied by an ~ 1.2-fold increase in the H2O2 formation rate (Fig. 3D). Under phosphorylating conditions, a progressive decrease in m∆Ψ from ~ 160 mV (uninhibited State 3) up to ~ 141 mV (fully inhibited State 3) was accompanied by a progressive increase in mQ reduction from ~ 30% (uninhibited State 3) to ~ 77% (fully inhibited State 3) (Fig. 1E). This 47% increase in the mQ reduction level accompanying the inhibition of the cytochrome pathway in State 3 was accompanied by an ~ 4.2-fold increase in the H2O2 formation rate (Fig. 3D).
Fig. 3.
The relationships between respiratory rate (A) and H2O2 formation (B) versus m∆Ψ and the relationship between respiratory rate (C), H2O2 formation (D), and m∆Ψ (E) versus mQ reduction level when the cytochrome pathway activity was varied with increasing concentrations of cyanide (CN, up to 0.4 mM) or antimycin A (AA, up to 60 nM). Measurements were performed under phosphorylating (State 3) and nonphosphorylating (State 4) conditions, in the presence of 1 mM benzohydroxamate or 1.5 µM propyl gallate (H2O2 formation). Arrows indicate the starting point and direction of titrations.
The results indicate a direct dependence of mROS formation on the reduction level of the mQ pool (and m∆Ψ) under various levels of engagement of the cytochrome pathway. The higher mQ reduction level (and the larger m∆Ψ) the bigger mROS production.
3.2. The relationship between mROS formation and the reduction level of the mQ pool under varying activity of complex II when the cytochrome pathway is engaged
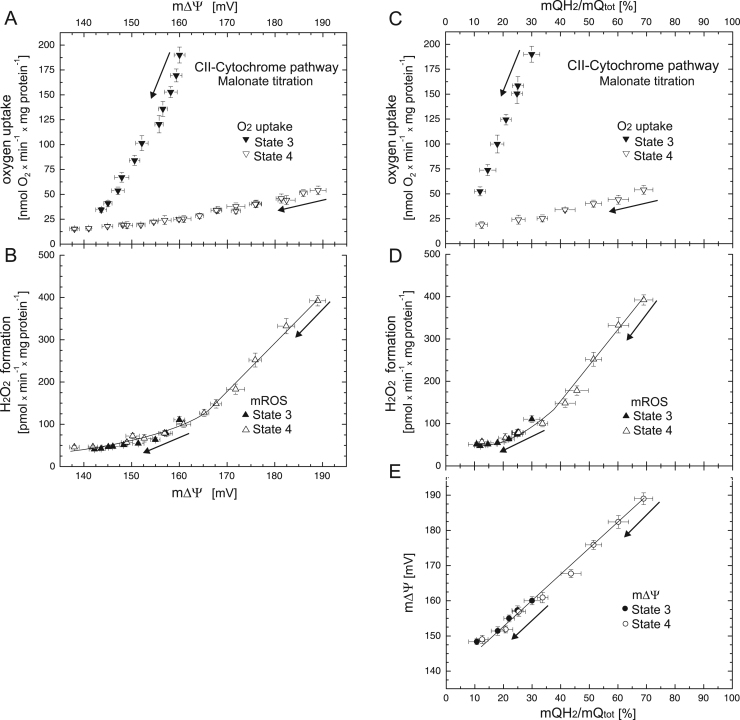
The dependence of the rate of electron transfer through the mQ reducing enzyme (complex II) on the reduction level of the mQ pool (and m∆Ψ) when the cytochrome pathway is active was studied by malonate titration under phosphorylating and nonphosphorylating conditions. Malonate titration enabled lowering of the mQ reduction level below 30% (reached with FCCP titration, Fig. 1C–E), leading to an ~ 10% reduction level for both respiratory states (Fig. 4C–E). At levels below the mQ pool reduction level (and m∆Ψ) of uninhibited State 3, i.e., below 30% of the mQ reduction level (and below ~ 160 mV), the kinetic relationships between H2O2 formation versus m∆Ψ (Fig. 4B) and versus mQ reduction level (Fig. 4D) overlapped for both respiratory states. Under both phosphorylating and nonphosphorylating conditions, H2O2 formation depends nonlinearly upon the mQ pool reduction level (and m∆Ψ). The threshold values for the mQ pool reduction level and m∆Ψ are slightly greater than the values for the uninhibited State 3, i.e., an ~ 35% reduction level of mQ and ~ 165 mV of m∆Ψ. At values greater than these thresholds, a sharply increased dependence of H2O2 formation on the mQ reduction level (and m∆Ψ) was observed (Fig. 4B, D); therefore, a small increase in the mQ reduction level (and m∆Ψ) leads to a high rate of H2O2 production by mitochondria.
Fig. 4.
The relationships between respiratory rate (A) and H2O2 formation (B) versus m∆Ψ and the relationships between respiratory rate (C), H2O2 formation (D), and m∆Ψ (E) versus mQ reduction level when the cytochrome pathway is engaged. Complex II (CII) activity was varied with increasing concentrations of malonate (up to 4 mM). Measurements were performed under phosphorylating (State 3) and nonphosphorylating (State 4) conditions, in the presence of 1 mM benzohydroxamate or 1.5 µM propyl gallate (H2O2 formation). Arrows indicate the starting point and direction of titrations.
3.3. The relationship between mROS formation and the reduction level of the mQ pool under varying activity of AOX
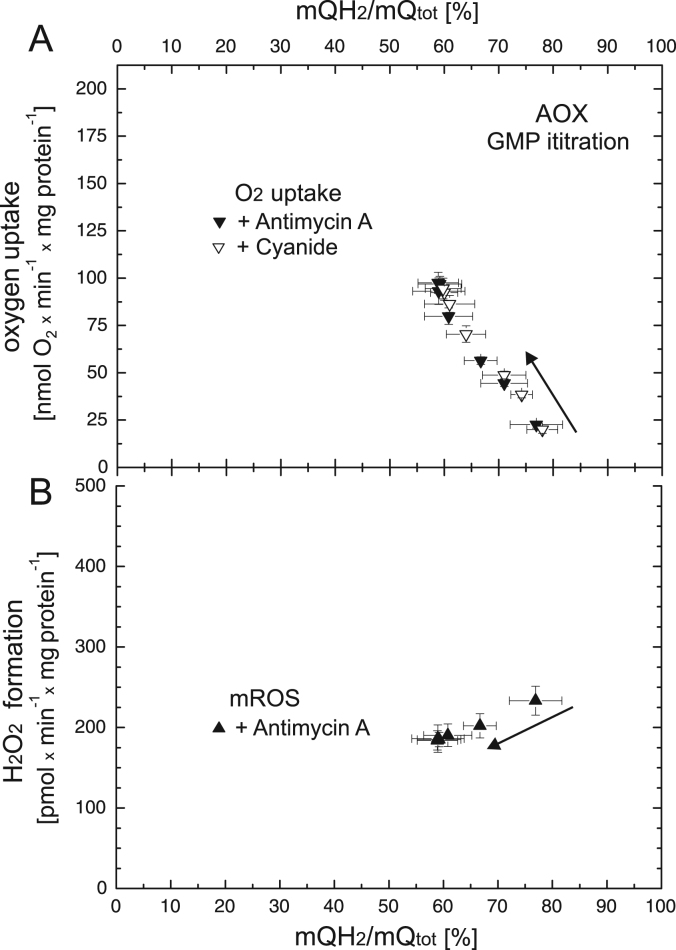
When the cytochrome pathway was inactive (in the presence of high concentrations of cyanide or antimycin A), the activity of the unstimulated AOX pathway led to low levels of oxygen uptake (~ 20 mol O2 × min−1 × mg protein−1) accompanied by very high mQ reduction levels (~ 77%) and H2O2 formation (~ 230 pmol H2O2 × min−1 × mg protein−1) (Fig. 5). The AOX-mediated mROS formation is independent of m∆Ψ (and ΔμH+), since no proton pumping occurs in the respiratory pathway when AOX works with complex II. Increasing the concentration of GMP progressively stimulated AOX activity, leading to up to ~ 4.5 times higher respiratory rates, up to ~ 1.3 times lower H2O2 formation, and a considerable decrease in mQ reduction levels (up to ~ 59%). Fig. 5 shows the approximately linear relationships between respiratory rate (Fig. 5A) and H2O2 formation (5B) versus mQ reduction level (ranging from 59% to 77%) when the AOX activity was gradually stimulated by GMP.
Fig. 5.
The relationships between respiratory rate (A) and H2O2 formation (B) versus mQ reduction level when AOX activity was varied with increasing concentrations of GMP (up to 1 mM). Measurements were performed in the presence of 0.65 mM cyanide or 90 nM antimycin A. Arrows indicate a starting point and direction of titrations.
3.4. The relationship between mROS formation and the reduction level of the mQ pool under varying activity of complex II when AOX is engaged
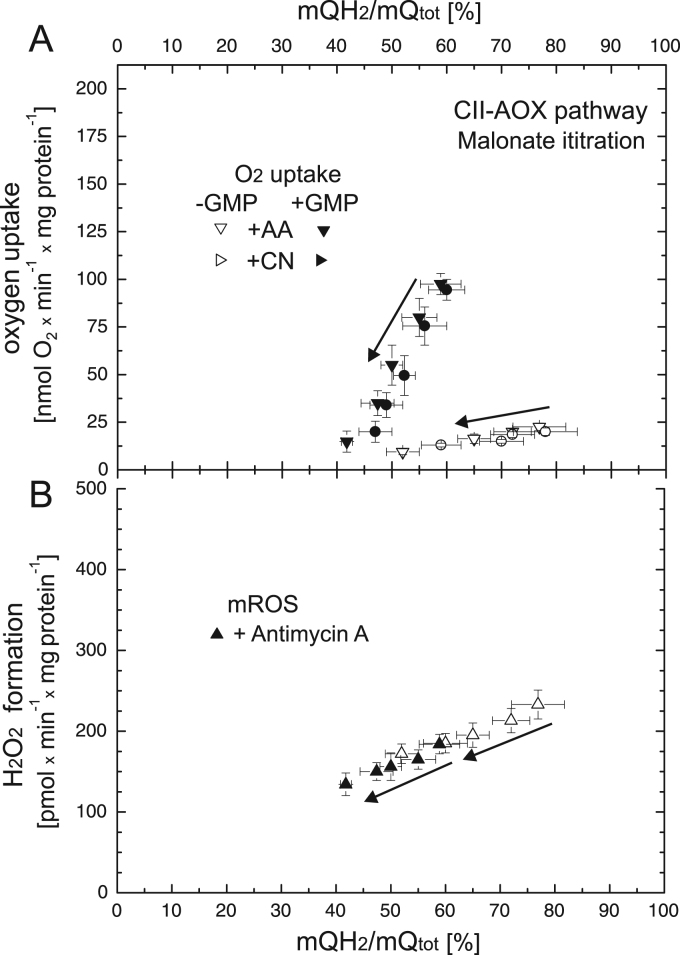
The dependence of the rate of electron transfer through the mQ-reducing enzyme (complex II) on the reduction level of the mQ pool when AOX is active was studied by malonate titration in the absence or presence of GMP (Fig. 6). A progressive decrease in respiratory rate and H2O2 formation was observed with lowering of the mQ pool reduction level from ~ 77 to ~ 52% and from ~ 59 to ~ 42% for unstimulated and GMP-stimulated succinate-sustained AOX, respectively. The kinetic relationships between H2O2 formation versus mQ reduction level (Fig. 6B) overlapped for both conditions and were approximately linear in the range of the mQ reduction level of AOX during succinate oxidation. These results indicate that AOX becomes engaged at only high reduction levels of the mQ pool (greater than 40%).
Fig. 6.
The relationships between respiratory rate (A) and H2O2 formation (B) versus mQ reduction level when AOX is engaged in the absence or presence of 1 mM GMP. Complex II (CII) activity was varied with increasing concentrations of malonate (up to 4 mM). Measurements were performed in the presence of 0.65 mM cyanide or 90 nM antimycin A. Arrows indicate the starting point and direction of titrations.
3.5. Inactive mQ pool
In A. castellanii mitochondria, a completely oxidized mQ pool (0% mQ reduction level) was obtained after incubation of mitochondria in the absence of a reducing substrate. Upon anaerobiosis and in the presence of a respiratory substrate (5 mM succinate), 1 mM cyanide, and 1 mM benzohydroxamate, a completely reduced mQ pool with ~ 85% reduction was obtained. This result indicates that in A. castellanii mitochondria isolated from the intermediate phase of growth, ~ 15% of the mQ pool is unreducible.
4. Discussion
The mQ reduction shift that occurs when mitochondria switch from nonphosphorylating conditions (State 4, a more reduced state) to phosphorylating conditions (State 3, a more oxidized state) is of utmost physiological significance. In vivo, mitochondria can shift rapidly between these conditions inducing a change in the mQ reduction level and thereby in mROS formation. Our study combined quantitatively important mitochondrial parameters: mΔΨ, oxygen uptake, mROS formation, and mQ pool reduction level, with a special focus on the relationship between mROS formation and mQ reduction level. In A. castellanii mitochondria, during nonphosphorylating respiration, high mQ reduction levels were accompanied by increased mΔΨ values and mROS formation. In contrast, during phosphorylating respiration, the relative oxidation of the mQ pool was accompanied by decreased mΔΨ values and therefore by decreased mROS formation. In isolated mitochondria, the relationship between mROS formation and the mQ reduction level can be elucidated by varying the mQ reduction level (mQH2/mQtot) by using agents that cause both stimulation and inhibition of respiratory chain electron transport.
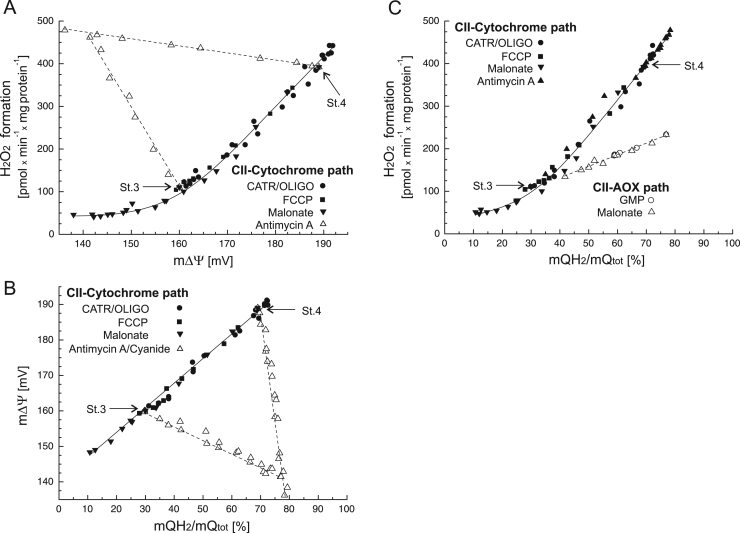
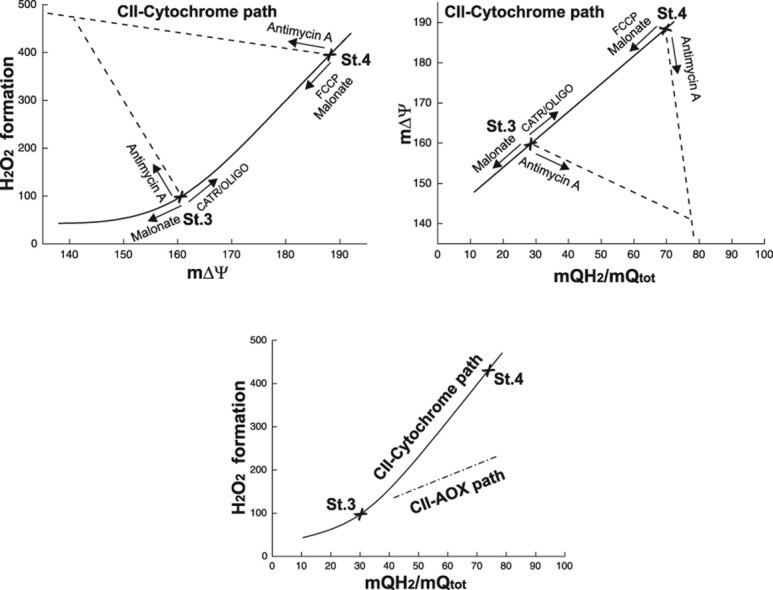
In A. castellanii mitochondria, the respiratory activity of the cytochrome pathway is much higher than that of AOX. The cytochrome pathway becomes engaged at much lower mQ pool reduction levels (from ~ 10%) (Fig. 4C–E) than the AOX pathway (from ~ 40%) (Fig. 6). Comparing the relationships between mROS formation and the reduction level of the mQ pool for both mQH2-oxidizing pathways (Fig. 7C), it is clear that at higher mQ reduction levels, the cytochrome pathway produces much more (even twice as much) mROS compared to AOX. At ~ 40% of mQ reduction level, the mROS formation is similar for both mQH2-oxidizing pathways (~ 130 pmol H2O2 × mg protein−1). The relationship between mROS formation and the mQ pool reduction level is much steeper for the cytochrome pathway than for AOX; for the cytochrome pathway, H2O2 formation depends strongly upon the mQ pool reduction level in the 40–80% range (Fig. 7C). Above the threshold value of the mQ pool reduction level, i.e., slightly above the value for the uninhibited State 3 (~ 35%), which corresponds to ~ 165 mV m∆Ψ (Fig. 7A, B), a small increase in the mQ reduction level gives rise to a high rate of H2O2 production by mitochondria, with oxidation occurring via the cytochrome pathway. It has been shown previously that in nonphosphorylating rat heart mitochondria, mROS generation strongly but nonlinearly depends upon mΔΨ, increasing at mΔΨ greater than that of State 3 [24]. Our results confirm this observation, however indicating the contribution of the mQ reduction level to mROS formation.
Fig. 7.
Summative graphs. The relationships between H2O2 formation versus m∆Ψ (A) and m∆Ψ versus mQ reduction level (B) under various levels of engagement of the complex II (CII)-fueled cytochrome pathway. (A,B) Titrations with carboxyatractyloside (CATR), oligomycin (OLIGO), FCCP, malonate, and antimycin A/cyanide are all shown here. Measurements were performed under phosphorylating (St.3) and nonphosphorylating (St. 4) conditions, in the presence of 1 mM benzohydroxamate or 1.5 µM propyl gallate (H2O2 formation) to exclude AOX activity. (C) The relationships between H2O2 formation versus m∆Ψ under various levels of engagement of the complex II (CII)-fueled cytochrome pathway (full symbols, solid curve) and the complex II (II)-fueled AOX (empty symbols, dashed line). For the cytochrome pathway, titrations (as in A and B) were performed in the presence of 1.5 µM propyl gallate to exclude AOX activity. For AOX, titrations with GMP and malonate (in the presence or absence of GMP) were performed in the presence of antimycin A to exclude the cytochrome pathway.
To date, at least 11 sites that produce superoxide anion and/or H2O2 have been identified in mammalian mitochondria [25]. Under our experimental conditions, during succinate oxidation, the flavin site of mitochondrial complex II (site IIF of complex II) produced mROS when AOX was active, while site IIF of complex II and the Qo site of mitochondrial complex III (site IIIQo) participated in mROS production when the cytochrome pathway was active. The highest H2O2 formation was observed in the presence of antimycin A (Fig. 7A, C), a Qi site inhibitor of complex III. It has been shown that contributions of specific sites of the mitochondrial respiratory chain to the production of ROS in mitochondria depend very strongly on the substrates being oxidized [26]. Our results indicate that the production of ROS in mitochondria depends not only on the engagement of mROS formation sites but also on the engagement of QH2-oxidizing pathways.
There are only few studies relating mQ reduction level with mROS generation, which is likely due to difficulties in the measurement of the mQ reduction level. In different cell lines, it has been shown how the mitochondrial electron transport chain is optimized to better oxidize different fuels using the reducing status of mQ (mQH2/Q ratio) as a metabolic sensor and mROS generated by complex I by reverse electron transport as an executor [6]. In rat heart mitochondria, mROS production by reverse electron transport at complex I is steeply dependent on m∆Ψ and on mQ reduction level, indicating the sensitivity of superoxide anion production by this respiratory chain site to the two physiological variables [7]. Marphy's group has shown that in the presence of antimycin A, the production of superoxide at the Qo site is specifically linked to local accumulation of reduced cytochrome b566 when the Qi site is inhibited by antimycin A, and this effect is independent of m∆Ψ [7]. Our results indicate that mROS production is a direct function of the mQ pool reduction level and is independent of m∆Ψ when the Qi site is inhibited by antimycin A and when reverse electron transport is excluded by rotenone during succinate oxidation.
Complex I is believed to be a major site of mROS production in the mitochondrial electron transport chain, either from FAD- or NAD-driven electron flux [3]. It is also generally assumed that the generation of mROS when succinate is used as the substrate depends mostly on reverse electron transfer through complex I. Our study shows that during succinate oxidation, the production of mROS may be very high even when complex I is inhibited by rotenone. Further studies are needed to elucidate whether reverse electron transfer through complex I is the major source of mROS in A. castellanii mitochondria.
The present study indicates that in A. castellanii mitochondria, AOX becomes more engaged at higher mQ pool reduction levels, when mROS production via the cytochrome pathway increases (Fig. 7C). Activation of cyanide- and antimycin A-resistant AOX-mediated respiration by GMP significantly decreased mQ reduction levels and H2O2 formation (Figs. 5, 4), confirming the antioxidant role of the oxidase. It has been previously shown that in A. castellanii mitochondria, AOX may play a role not only in the energetic status of the cell by decreasing the yield of OXPHOS [12] but also in preventing the generation of mROS [27], which are maintained at a constant level throughout the growth cycle of amoeba batch culture [28]. In the present study, we found an ~15% unreducible mQ pool in mitochondria isolated from A. castellanii cells from an intermediate phase of growth. A question arises as to whether the size of the unreducible mQ pool in the inner mitochondrial membrane influences mQ reduction level-dependent mROS formation. Further studies are needed to answer this question. Our previous study indicates that, compared to A. castellanii cells from the intermediate phase of growth, decreased reducible mQ levels in intensively dividing cells (i.e., at the exponential phase of growth) accompanied by increased mQ reduction levels could lead to increased mROS formation and thereby to increased AOX activity (which depends on the mQ reduction level) [11], [28].
Mitochondrial ROS production has been described to be a direct function of m∆Ψ [4], [29]. Fig. 7A is a summative description of the relationships between H2O2 formation versus m∆Ψ under various levels of engagement of the complex II-supplied cytochrome pathway during titrations with carboxyatractyloside/oligomycin, FCCP, malonate, and antimycin A under nonphosphorylating and phosphorylating conditions. The relationship between H2O2 formation and m∆Ψ obtained for antimycin A titration, in which a decrease in m∆Ψ was accompanied by an increase in mQ reduction level, does not fit the relationship obtained for other conditions, in which lower m∆Ψ values were accompanied by lower mQ reduction levels (Fig. 7A); this difference is also evident when m∆Ψ is plotted against the mQ reduction level (Fig. 7B). Given these observations and the fact that AOX-mediated mROS formation is m∆Ψ independent, we postulate that mROS production is a direct function of the mQ pool reduction level rather than of m∆Ψ. The dependence of H2O2 production on the mQ reduction level is clearly evidenced in this study for both QH2-oxidizing pathways (Fig. 7C). Therefore, the mQ pool reduction level (endogenous mQ redox state) could be a useful endogenous reporter that allows indirect assessment of overall mROS production in mitochondria. Most of the mitochondrial sites of superoxide anion/H2O2 formation are related to the reduction level of the mQ pool that connects the dehydrogenase and oxidase sides of the electron transport chain. For example, the rates of mitochondrial superoxide anion/H2O2 production from site IF of complex I and site Qo of complex III can be assessed indirectly by measuring endogenous reporters such as the mitochondrial NAD(P)H redox state and the cytochrome b566, respectively [25], [26]. Measurement of the endogenous mQ pool reduction level could provide insight into overall mROS production related to mitochondrial respiratory chain complexes. The present study analyses the dependence of H2O2 production on the mQ pool reduction level in mitochondria, by using A. castellanii mitochondria as an example, under conditions in which concentrations of agents that cause both stimulation and inhibition of respiratory chain electron transport (substrate, inhibitors and activators) were varied. In future studies, the measurements of the mQ pool reduction level under physiological conditions in the absence of inhibitors of electron transport may be helpful for the assessment of overall intrinsic production of mROS in mitochondria and the physiological role of these species as signaling molecules and in pathologies.
5. Conclusions
We elucidated for the first time the relationship between the rate of mROS formation and the reduction level of the endogenous mQ pool varied by agents that cause both stimulation and inhibition of the mitochondrial respiratory electron transport chain. Our results indicate that mROS production is a direct function of the mQ pool reduction level rather than of m∆Ψ. The production of ROS in mitochondria depends not only on the engagement of mROS formation sites but also on the engagement of QH2-oxidizing pathways. The reduction level of the mQ pool could be a useful endogenous reporter that allows indirect assessment of overall mROS production in mitochondria.
Funding
This work was supported by the National Science Centre, Poland (OPUS 2016/21/B/NZ3/00333).
Conflict of interest/declarations of interest
The authors declare no conflict of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.07.018.
Appendix A. Supplementary material
Supplementary material
References
- 1.James A.M., Smith R.A., Murphy M.P. Antioxidant and prooxidant properties of mitochondrial coenzyme Q. Arch. Biochem. Biophys. 2004;423(1):47–56. doi: 10.1016/j.abb.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 2.Genova M.L., Lenaz G. New developments on the functions of coenzyme Q in mitochondria. Biofactors. 2011;37(5):330–354. doi: 10.1002/biof.168. [DOI] [PubMed] [Google Scholar]
- 3.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kowaltowski A.J., de Souza-Pinto N.C., Castilho R.F., Vercesi A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009;47(4):333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Quinlan C.L., Perevoshchikova I.V., Hey-Mogensen M., Orr A.L., Brand M.D. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013;1:304–312. doi: 10.1016/j.redox.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guaras A., Perales-Clemente E., Calvo E., Acin-Perez R., Loureiro-Lopez M., Pujol C., Martinez-Carrascoso I., Nunez E., Garcia-Marques F., Rodriguez-Hernandez M.A., Cortes A., Diaz F., Perez-Martos A., Moraes C.T., Fernandez-Silva P., Trifunovic A., Navas P., Vazquez J., Enriquez J.A. The CoQH2/CoQ ratio serves as a sensor of respiratory chain efficiency. Cell Rep. 2016;15(1):197–209. doi: 10.1016/j.celrep.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Robb E.L., Hall A.R., Prime T.A., Eaton S., Szibor M., Viscomi C., James A.M., Murphy M.P. Control of mitochondrial superoxide production by reverse electron transport at complex I. J. Biol. Chem. 2018;293(25):9869–9879. doi: 10.1074/jbc.RA118.003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichinger L., Pachebat J.A., Glockner G., Rajandream M.A., Sucgang R., Berriman M., Song J., Olsen R., Szafranski K., Xu Q., Tunggal B., Kummerfeld S., Madera M., Konfortov B.A., Rivero F., Bankier A.T., Lehmann R., Hamlin N., Davies R., Gaudet P., Fey P., Pilcher K., Chen G., Saunders D., Sodergren E., Davis P., Kerhornou A., Nie X., Hall N., Anjard C., Hemphill L., Bason N., Farbrother P., Desany B., Just E., Morio T., Rost R., Churcher C., Cooper J., Haydock S., van Driessche N., Cronin A., Goodhead I., Muzny D., Mourier T., Pain A., Lu M., Harper D., Lindsay R., Hauser H., James K., Quiles M., Madan Babu M., Saito T., Buchrieser C., Wardroper A., Felder M., Thangavelu M., Johnson D., Knights A., Loulseged H., Mungall K., Oliver K., Price C., Quail M.A., Urushihara H., Hernandez J., Rabbinowitsch E., Steffen D., Sanders M., Ma J., Kohara Y., Sharp S., Simmonds M., Spiegler S., Tivey A., Sugano S., White B., Walker D., Woodward J., Winckler T., Tanaka Y., Shaulsky G., Schleicher M., Weinstock G., Rosenthal A., Cox E.C., Chisholm R.L., Gibbs R., Loomis W.F., Platzer M., Kay R.R., Williams J., Dear P.H., Noegel A.A., Barrell B., Kuspa A. The genome of the social amoeba Dictyostelium discoideum. Nature. 2005;435(7038):43–57. doi: 10.1038/nature03481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarmuszkiewicz W., Wagner A.M., Wagner M.J., Hryniewiecka L. Immunological identification of the alternative oxidase of Acanthamoeba castellanii mitochondria. FEBS Lett. 1997;411(1):110–114. doi: 10.1016/s0014-5793(97)00676-5. [DOI] [PubMed] [Google Scholar]
- 10.Antos-Krzeminska N., Jarmuszkiewicz W. External NAD(P)H dehydrogenases in Acanthamoeba castellanii mitochondria. Protist. 2014;165(5):580–593. doi: 10.1016/j.protis.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Jarmuszkiewicz W., Czarna M., Sluse F.E. Substrate kinetics of the Acanthamoeba castellanii alternative oxidase and the effects of GMP. Biochim. Biophys. Acta. 2005;1708(1):71–78. doi: 10.1016/j.bbabio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Jarmuszkiewicz W., Sluse-Goffart C.M., Hryniewiecka L., Michejda J., Sluse F.E. Electron partitioning between the two branching quinol-oxidizing pathways in Acanthamoeba castellanii mitochondria during steady-state state 3 respiration. J. Biol. Chem. 1998;273(17):10174–10180. doi: 10.1074/jbc.273.17.10174. [DOI] [PubMed] [Google Scholar]
- 13.Woyda-Ploszczyca A.M., Sluse F.E., Jarmuszkiewicz W. Regulation of Acanthamoeba castellanii alternative oxidase activity by mutual exclusion of purine nucleotides; ATP's inhibitory effect. Biochim. Biophys. Acta. 2009;1787(4):264–271. doi: 10.1016/j.bbabio.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Jarmuszkiewicz W., Swida A., Czarna M., Antos N., Sluse-Goffart C.M., Sluse F.E. In phosphorylating Acanthamoeba castellanii mitochondria the sensitivity of uncoupling protein activity to GTP depends on the redox state of quinone. J. Bioenerg. Biomembr. 2005;37(2):97–107. doi: 10.1007/s10863-005-4133-y. [DOI] [PubMed] [Google Scholar]
- 15.Swida A., Woyda-Ploszczyca A., Jarmuszkiewicz W. Redox state of quinone affects sensitivity of Acanthamoeba castellanii mitochondrial uncoupling protein to purine nucleotides. Biochem. J. 2008;413(2):359–367. doi: 10.1042/BJ20080333. [DOI] [PubMed] [Google Scholar]
- 16.Woyda-Ploszczyca A., Jarmuszkiewicz W. Ubiquinol (QH(2)) functions as a negative regulator of purine nucleotide inhibition of Acanthamoeba castellanii mitochondrial uncoupling protein. Biochim. Biophys. Acta. 2011;1807(1):42–52. doi: 10.1016/j.bbabio.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Jarmuszkiewicz W., Woyda-Ploszczyca A., Koziel A., Majerczak J., Zoladz J.A. Temperature controls oxidative phosphorylation and reactive oxygen species production through uncoupling in rat skeletal muscle mitochondria. Free Radic. Biol. Med. 2015;83:12–20. doi: 10.1016/j.freeradbiomed.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Ye X., Yu A., Champion P.M. Dynamics of Nitric Oxide Rebinding and Escape in Horseradish Peroxidase. J. Am. Chem. Soc. 2006;128(5):1444–1445. doi: 10.1021/ja057172m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adjimani J.P., Asare P. Antioxidant and free radical scavenging activity of iron chelators. Toxicol. Rep. 2015:721–728. doi: 10.1016/j.toxrep.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naumchik I.V., Karasyova E.I., Metelitza D.I., Edimecheva I.P., Sorokin V.L., Shadyro O.I. Inhibition of peroxidase-catalyzed oxidation of 3,3,5,5 -tetramethylbenzidine by aminophenols. Biochemistry. 2005;70(3):322–329. doi: 10.1007/s10541-005-0118-z. [DOI] [PubMed] [Google Scholar]
- 21.Yang J.T., Lee I.N., Lu F.J., Chung C.Y., Lee M.H., Cheng Y.C., Chen K.T., Chen C.H. Propyl gallate exerts an antimigration effect on temozolomide-treated malignant glioma cells through inhibition of ROS and the NF-kappaB pathway. J. Immunol. Res. 2017;2017:9489383. doi: 10.1155/2017/9489383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Attar A., Cubillana-Aguilera L., Naranjo-Rodriguez I., de Cisneros J.L., Palacios-Santander J.M., Amine A. Amperometric inhibition biosensors based on horseradish peroxidase and gold sononanoparticles immobilized onto different electrodes for cyanide measurements. Bioelectrochemistry. 2015;101:84–91. doi: 10.1016/j.bioelechem.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Van den Bergen C.W., Wagner A.M., Krab K., Moore A.L. The relationship between electron flux and the redox poise of the quinone pool in plant mitochondria. Interplay between quinol-oxidizing and quinone-reducing pathways. Eur. J. Biochem. 1994;226(3):1071–1078. doi: 10.1111/j.1432-1033.1994.01071.x. [DOI] [PubMed] [Google Scholar]
- 24.Korshunov S.S., Skulachev V.P., Starkov A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416(1):15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 25.Wong H.S., Dighe P.A., Mezera V., Monternier P.A., Brand M.D. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J. Biol. Chem. 2017;292(41):16804–16809. doi: 10.1074/jbc.R117.789271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinlan C.L., Perevoshchikova I.V., Hey-Mogensen M., Orr A.L., Brand M.D. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013;1(1):304–312. doi: 10.1016/j.redox.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Czarna M., Jarmuszkiewicz W. Activation of alternative oxidase and uncoupling protein lowers hydrogen peroxide formation in amoeba Acanthamoeba castellanii mitochondria. FEBS Lett. 2005;579(14):3136–3140. doi: 10.1016/j.febslet.2005.04.081. [DOI] [PubMed] [Google Scholar]
- 28.Czarna M., Sluse F.E., Jarmuszkiewicz W. Mitochondrial function plasticity in Acanthamoeba castellanii during growth in batch culture. J. Bioenerg. Biomembr. 2007;39(2):149–157. doi: 10.1007/s10863-007-9073-2. [DOI] [PubMed] [Google Scholar]
- 29.Figueira T.R., Barros M.H., Camargo A.A., Castilho R.F., Ferreira J.C., Kowaltowski A.J., Sluse F.E., Souza-Pinto N.C., Vercesi A.E. Mitochondria as a source of reactive oxygen and nitrogen species: from molecular mechanisms to human health. Antioxid. Redox Signal. 2013;18(16):2029–2074. doi: 10.1089/ars.2012.4729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material