Abstract
Background
To explore potential therapeutic target is one of the areas of great interest in both clinical and basic hepatocellular carcinoma (HCC) studies. Nuclear receptor liver receptor homolog-1 (LRH-1, NR5A2) is proved to play a positive role in several cancers including breast cancer, pancreatic cancer and intestinal cancer in recent years. However, the exact role of LRH-1 in the development and progression of HCC is not fully elucidated.
Methods
The LRH-1 expression level in HCC clinical samples was examined by immunohis-tochemistry (IHC). Stable LRH-1-suppressed HepG2 clones (HepG2LRH-1/-) were generated by transcription activator-like effector nucleases (TALENs) and both in vitro and in vivo experiments were conducted.
Results
We confirmed that LRH-1 showed an increased expression pattern in HCC clinical samples. Our in vitro and in vivo results indicated that suppression of LRH-1 in HepG2 significantly attenuated its proliferation rate and tumorigenic capacity. Gene expression microarray analysis indicated that LRH-1mostly regulated gene expression involved in cell cycle. In addition, our gain-of-function experiments indicated that ectopic expression of LRH-1 dramatically induced the mRNA and protein levels of c-myc and cyclin E1, while attenuating the expression of p21.
Conclusion
Our results suggest that LRH-1 might be a potential therapeutic target for clinical HCC treatment.
Keywords: LRH-1, HCC, c-myc, p21, cyclin E1
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer overall and the second leading cause of cancer-related mortality worldwide.1,2 Surgical removal is currently the preferred option for the treatment of HCC in the majority of cases. However, most patients with HCC are not accessible to curative resection or transplantation due to late detection of this disease.3 Over the past decades, increased understanding of the signaling pathways in HCC development and progression has aroused interest in the identification of molecular biomarkers and potential therapeutic targets.4 Nuclear receptors are a large superfamily of transcriptional factors that function as ligand-dependent or -independent sensor molecules that regulate a broad range of physiological processes such as metabolism, development, and reproduction, but also play various and significant roles in cancer development and progression.5,6 It has been reported that nuclear receptor ERRγ is upregulated in HCC and its inhibition suppresses liver cancer cell proliferation via regulation of p21 and p27.7 Feng et al reported that androgen receptor (AR) regulates cell cycle-related kinase expression to promote hepatocarcinogenesis through the upregulation of β-catenin/TCF signaling.8 HNF4α is the most abundant transcription factor in the liver, a key regulator of hepatic lipid metabolism and a critical determinant of hepatic development. However, in addition to normal physiological function, it has also been reported that HNF4α knockout in adult hepatocytes leads to hepatocyte proliferation and promotes the development of HCC in mice,9 suggesting that HNF4α also plays an important role in HCC.
LRH-1 belongs to the NR5A or Ftz-F1 subfamily, which comprises two identified members (NR5A1/SF-1, NR5A2/LRH-1) in humans. LRH-1 is mostly expressed in tissues derived from endoderm, including intestines, liver, and exocrine pancreas, as well as in the ovaries. In these tissues, LRH-1 plays a predominant role in development,5,10–13 reverse cholesterol transport,14,15 bile-acid homeostasis,16,17 and steroidogenesis.18–20 In addition, LRH-1 has been implicated in tumorigenesis of some cancers, though its exact oncogenic roles are still unclear. Overexpression of LRH-1 is reported in breast cancer, gastric cancer, pancreatic cancer, and ovarian granulosa cell tumors.21–24 Functional studies have demonstrated that LRH-1 promotes breast cancer growth and promotion of cancer cell motility and invasiveness.21,25,26 It has been confirmed that LRH-1 promotes cell proliferation via induction of cyclin D1 and E1 through interaction with β-catenin in intestinal cells,27 and heterozygous LRH-1 knockout in mice markedly prevents intestinal cancer formation.28 Previously, a few studies have been done to explore the functional roles of LRH-1 in HCC. Increased expression of LRH-1 in murine hepatic cell line FL83B can increase its proliferation rate.27 Decreased expression of LRH-1 in liver cancer cell line BEL-7402 prevents the cell proliferation rate and promotes cell apoptosis.29 Recently, a study highlighted the importance of LRH-1 in coordinating glutamine-induced metabolism and signaling to promote hepatocellular carcinogenesis.30 However, the exact functional role of LRH-1 in HCC is not fully understood.
In the present study, we examined the expression level of LRH-1 in clinical HCC specimens and their adjacent non-tumor lesions. Furthermore, we also investigated the functional significance of LRH-1 in the growth regulation of liver cancer cells and determined the potentially crucial genes involved in this process.
Materials and methods
Immunohistochemistry
Immunohistochemistry of LRH-1 and immunoreactivity score (IRS) analysis were performed on human liver cancer tissue microarray slide (Catalog: LV-1505, Alena Bio, Xi’an, China) containing validated cases of HCC (n=50) and adjacent non-tumor tissues (n=50) using an immunohisto-chemistry-validated rabbit polyclonal anti-LRH-1 antibody (Catalog: HPA005455, Sigma-Aldrich Co., St Louis, MO, USA). The LRH-1 immunosignal intensity detected in HCC was assessed by a semi-quantitative IRS analysis, scores were obtained by multiplying the level of staining intensity (negative =0; weak =1; moderate =2; strong =3) and percentage of positive cells (0%=0; 10%=1; 11%–50%=2; 51%–80%=3; 80%=4).
Cell lines and cell culture
Two liver cancer cell lines, HepG2 and HuH7 obtained from ATCC (American Type Culture Collection, Manassas, VA, USA), were used in this study. HepG2 and HuH-7 were cultured in different media according to the original suppliers’ instructions.
Plasmids’ construction
The plasmids for transcription activator-like effector nucle-ases’ (TALENs) assembly were obtained from Addgene (Cambridge, MA, USA). TALENs targeting LRH-1 were constructed through Golden Gate TALENS Assembly.31 Human LRH-1 cDNA (HsCD00347044, Harvard Cancer Center DNA Resource Core) was FLAG-tagged and subcloned into pBABE-puro as pBABE-FLAG-LRH-1 for retroviral transduction. p21 cDNA was subcloned into pLenti6/v5 as pLenti6/v5-p21 for lentiviral transduction. Nucleotides targeting c-myc were subcloned into pLKO.1 as pLKO.1-sh c-myc for lentiviral transduction.
Reporter assay
HepG2 and HepG2-LRH-1 cells were plated at 5×104 cells/well in 24-well plates and grown in normal media with 10% FBS for 24 hours before transfection. Cells were transfected with 0.2 µg reporter plasmid (pGL3-c-myc/p21/cyclin E1-Luc), and 0.015 µg Renilla luciferase control reporter pRL-CMV using FuGENE 6 transfection reagent. Cells were lysed 48 hours post-transfection for reporter activity determination using the dual luciferase reporter method. All assays were performed in three independent experiments repeated in triplicate and results were presented as mean ± SD.
Generation of LRH-1 overexpressed HepG2 and HuH-7 cells
pBABE-FLAG-LRH-1 and vector pBABE-puro were transfected into PA317 cells for production of retroviruses. For retroviral transduction, HepG2 and HuH-7 cells were co-cultured with the retroviruses-containing medium including 8 µg/mL polybrene for 48 hours, puromycin was added and co-cultured for 10 days.
Cell proliferation assay and colony formation assay
Briefly, HepG2 cells were harvested at 80% confluence and re-suspended in complete medium and inoculated into a 96-well plate at 3×103 cells/well for a duration of 1–5 days. MTT assay was performed as described previously.32 As to colony formation assay, 1×103 HepG2 and HepG2LRH-1/− cells with decreased LRH-1 expression were seeded into a 6-well plate. After incubation for 10 days, cells were fixed with 70% ethanol and subsequently stained by 0.2% crystal violet solution. The images were obtained and the colonies were counted.
In vivo tumor growth assay
In vivo tumorigenicity of HepG2 and HepG2LRH-1/− cells with decreased LRH-1 expression was evaluated by subcutaneous injection of cells (5×106 cells/injection) into the flanks of nude mice and allowing them to grow for 6–7 weeks. Tumor growth and volumes were monitored (mm3 was measured by electronic calipers according to the formula [width × length × height/2]). Finally, mice were sacrificed and the tumors were harvested, weighed, and Ki67-stained by IHC (ab15580). Animals were housed under specific pathogen-free conditions at the Laboratory Animal Service Center (LASEC) and experiments were conducted according to protocols and guidelines of the Animal Experimentation Ethics Committee (AEEC) in The Southern Medical University, which also approved the study.
qRT-PCR
Cells were grown to 80% confluence and collected, total RNA was extracted using Trizol and reverse transcribed to cDNA using M-MLV reverse transcriptase. For qRT-PCR analysis, cDNA samples were PCR-amplified with specific primers in an RT-PCR system (AbI 7500), the expression levels of target genes relative to GAPDH were determined by a SYBR Green-based detection method. The amplification curves were analyzed with ABI7500 SDS Software. The sequences of different specific primers used are listed in Table S1.
Western blotting
Protein expression of LRH-1, p21, cyclin E1, c-myc, and β-actin was detected by conventional Western blotting. Briefly, well-grown HepG2 and HuH-7 cells were collected. Cell lysates were collected and applied for LRH-1, p21, cyclin E1, c-myc, and β-actin detection by using mouse monoclonal LRH-1 antibody (Catalog: ab41901, Abcam, Cambridge, UK), rabbit polyclonal c-myc antibody (Catalog: sc-788, Santa Cruz Biotechnology Inc., Dallas, TX, USA), rabbit polyclonal cyclin E1 antibody (Catalog: sc-198, Santa Cruz Biotechnology Inc.), and rabbit monoclonal p21 antibody (Catalog: 2947, Cell Signaling Technology, Danvers, MA, USA).
Gene expression microarray
HepG2 mRNA microarray was performed with Arraystar Human LncRNA Microarray v4.0 according to the manufacturer’s protocol. All analyses were corrected for multiple hypothesis testing, and effects were determined to be significant when greater than or equal to two-fold with an adjusted P-value ≤0.05. Microarray data have been deposited into the National Center for Biotechnology Information Gene Expression Omnibus (accession no GSE98688).
Statistical analysis
All results shown represent the means ± SEM from triplicate experiments performed in a parallel manner unless otherwise indicated. Statistical analyses were performed using an unpaired, two-tailed Student’s t-test. All comparisons were made relative to untreated controls and significance of difference is indicated as P<0.05, P<0.01.
Ethics statement
All human tissues were obtained from patients after their informed consent according to protocols and guidelines approved by the Institutional Clinical Research Ethics Committees.
Results
LRH-1 is frequently upregulated in HCC clinical specimens
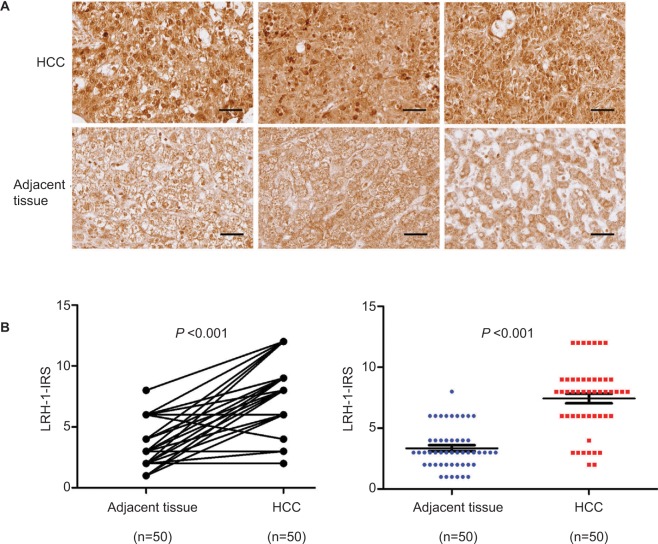
Immunohistochemistry of LRH-1 with tissue array including 50 pairs of HCC and adjacent tissue was first performed in the present study. Our results indicated that a significant increase of malignant cells with intense nuclear immunoreactivity was seen in HCC lesions (Figure 1A). In adjacent liver tissues, the LRH-1 protein was present at relatively low levels in nuclei, while weak immunostaining was also observed in the cytoplasm in some cases. On the contrary, in most HCC tissues, the expression of LRH-1 was dramatically increased, especially in the nuclei. IRS analysis further confirmed that HCC tissues exhibited significantly higher LRH-1 IRS as compared to tumor-adjacent tissue (Figure 1B). These results suggest that increased LRH-1 expression in HCC may play an oncogenic role in the development and progression of carcinogenesis.
Figure 1.
LRH-1 was frequently upregulated in HCC clinical specimens.
Notes: (A) LRH-1 immunohistochemistry. Three representative micrographs of LRH-1-immunostained tissue microarray spots of HCC and adjacent tissues. Magnification, ×400; bars, 30 µm. The cells in tumor-adjacent tissues showed detectable or weak nuclear immunoreactivity. Intense nuclear immunosignals were detected in cancer cells in HCC tissues. (B) LRH-1 IRS analysis performed on HCC and adjacent tissues. Results showed that HCC tissues showed significantly higher LRH-1 expression than tumor-adjacent tissues. P<0.001 vs tumor-adjacent tissues.
Abbreviations: HCC, hepatocellular carcinoma; IRS, immunoreactivity score.
Suppression of LRH-1 in HepG2 prevents its growth and colony formation capacity in vitro
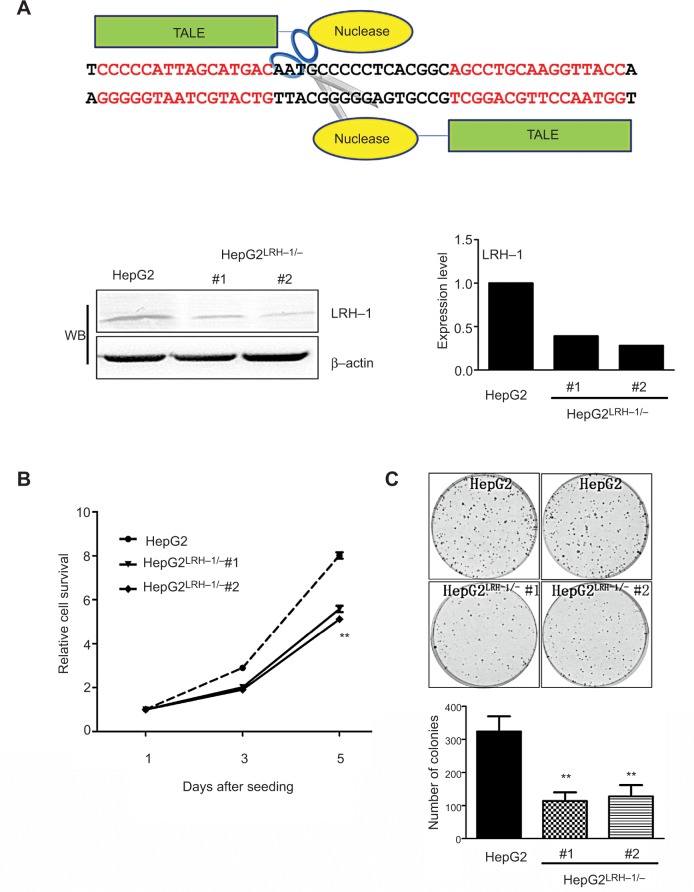
It is hypothesized that LRH-1 might promote the development and progression of HCC based on our previous study. To elucidate the functional significance of LRH-1 in HCC cell growth, we generated stable LRH-1-supressed HepG2 clones (HepG2LRH-1/−) by TALENs. Figure 2A shows a schematic diagram of the TALENs targeting the second exon of LRH-1, and suppression of LRH-1 which was confirmed by Western blotting. The in vitro growth experiment showed that suppression of LRH-1 severely prevented the proliferation rate in HepG2 (Figure 2B). Colony formation assay indicated that HepG2LRH-1/− cells formed less and smaller colonies as compared to parental HepG2 cells (Figure 2C).
Figure 2.
Suppression of LRH-1 in HepG2 prevents its growth and colony formation capacity in vitro.
Notes: (A) Schematic diagram of the TALENs targeting the second exon of LRH-1 and Western blotting (WB) validation of HepG2LRH-1/− cell clones. (B) Proliferation assay by MTT. Two HepG2LRH-1/− cell clones proliferated at slower rates than the parental HepG2 cells. (C) Colony formation assay. Results showed that HepG2LRH-1/− cells formed smaller and less colonies than parental HepG2 cells. ** P<0.01 vs parental HepG2 cells.
Abbreviation: TALENs, transcription activator-like effector nucleases.
Suppression of LRH-1 in HepG2 attenuates its growth in vivo
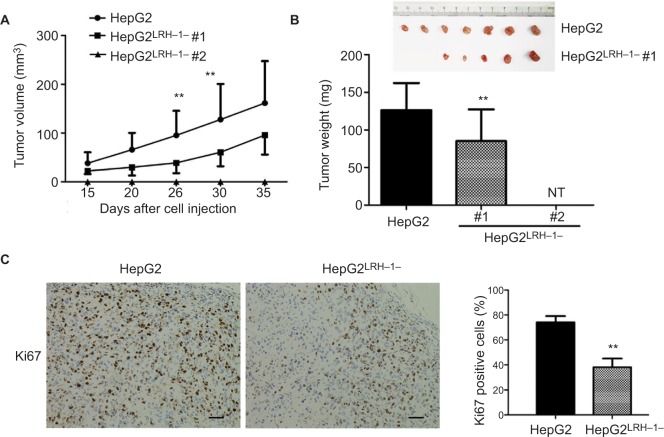
Our previous results suggested that suppressing LRH-1 expression in HepG2 prevented its proliferation rate and colony formation capacity in vitro. To further elucidate the functional role of LRH-1 in HCC, evaluation of in vivo tumorigenicity of LRH-1 was also performed. HepG2 and HepG2LRH-1/− cells were subcutaneously injected into seven nude mice respectively, our results showed that HepG2 cells formed xenograft tumors with larger sizes and at faster rates as compared to HepG2LRH-1/− #1 cell, while HepG2LRH-1/− #2 lost the in vivo tumorigenic capacity in all nude mice (Figure 3A and B). In addition, immunohistochemistry of xenograft tumors with specific Ki67 antibody was also performed, and results suggested that significantly lower and less Ki67 immunoreactivity was seen in xenograft tumors formed from HepG2LRH-1/− than parental HepG2 cells (Figure 3C).
Figure 3.
Suppression of LRH-1 in HepG2 attenuates its growth in vivo.
Notes: (A and B) Growth curve shows the growth patterns of tumors formed by HepG2LRH-1/− cell clones and parental HepG2 cells, HepG2 cells formed xenograft tumors with larger sizes and at faster rates as compared to HepG2LRH-1/− #1 cells, while HepG2LRH-1/− #2 lost the in vivo tumorigenic capacity in all nude mice (NT, no tumor). (C) Ki67 immunohistochemistry. Our results suggested that significantly lower and less Ki67 immunoreactivity was seen in xenograft tumors formed from HepG2LRH-1/− than parental HepG2 cells. Scale bar =100 μm.** P<0.01 vs parental HepG2 cells.
Gene expression microarray analysis for the identification of LRH-1-regulated genes in HepG2 cells
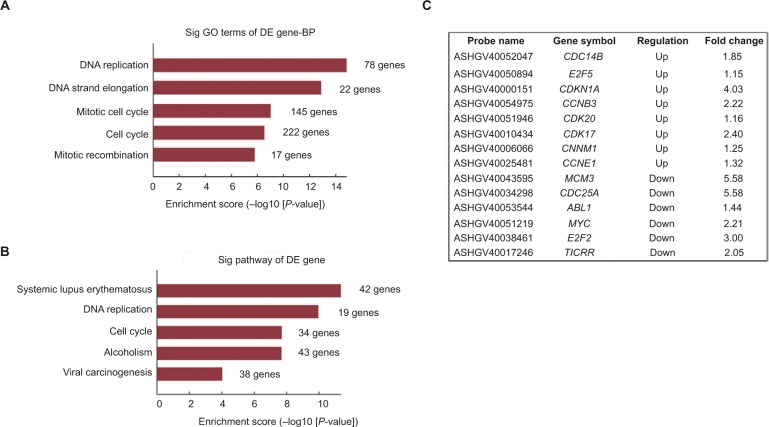
Our previous data proved that suppression of LRH-1 in HepG2 cells compromised its growth capacity both in vitro and in vivo. To identify LRH-1-regulated genes and to determine the possible mechanisms underlying the role of LRH-1 in growth in HepG2 cells, gene expression microarray was performed with HepG2 and HepG2LRH-1/− cells, using the Arraystar Human LncRNA Microarray v4.0. Our results identified that the gene expression levels detected by 2006 probes were significantly (2-folds expression alteration) down-regulated, while a greater number, 2,590 probes, were upregulated following suppression of LRH-1, suggesting that gene repression by LRH-1 is an important feature of its action in HepG2 cells. To explore the signaling pathways that may mediate LRH-1 function in HCC, gene ontology (GO) analyses were subsequently performed. Our results indicated that significant GO biological process groups were related to DNA replication and cell cycle (Figure 4A). These findings were confirmed in analysis of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways, which also incorporated the terms “DNA replication” and “cell cycle” (Figure 4B). Several cell cycle-related genes that altered after suppression of LRH-1 are listed in Figure 4C.
Figure 4.
Gene expression microarray analysis for the identification of LRH-1-regulated genes in HepG2 cells.
Notes: (A) Gene ontology (GO) analysis of genes that are LRH-1-regulated in HepG2 and in HepG2LRH-1/− cells. (B) KEGG pathway enrichment analysis for LRH-1-regulated genes in HepG2 cells are shown. (C) Several cell cycle-related genes that altered after suppression of LRH-1 were listed.
Abbreviations: BP, biological process; DE, differential expression; KEGG, Kyoto Encyclopedia of Genes and Genomes.
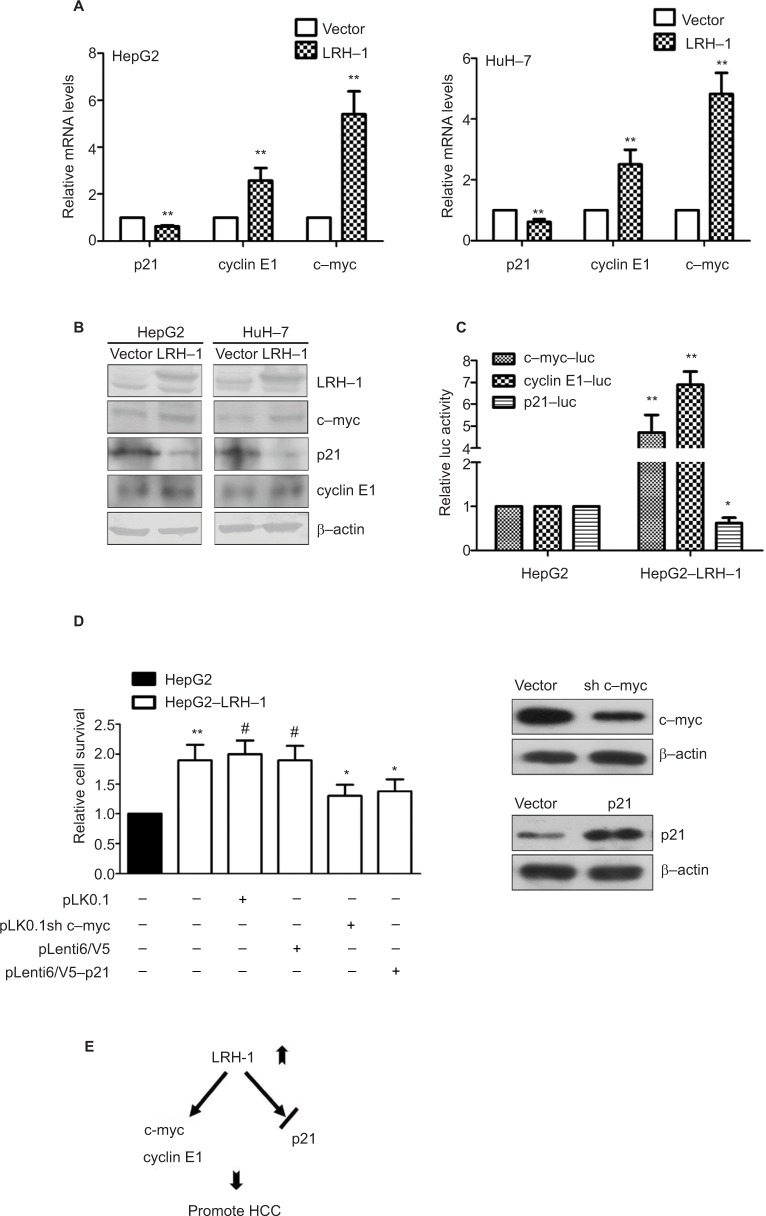
LRH-1 promotes the development and progression of HCC through induction of c-myc and cyclin E1, and suppression of p21
To further verify the results obtained from the gene expression microarray, we generated stable LRH-1-transdued infectants in two HCC cell lines, HepG2 and HuH-7, to elucidate the molecules mediated by LRH-1 in HCC. Ectopic expression of LRH-1 in HepG2 and HuH-7 cells was confirmed by Western blotting (Figure 5B). Our data indicated that ectopic expression of LRH-1 dramatically induced the mRNA and protein levels of c-myc and cyclin E1, and attenuated, in the meanwhile, the expression of p21 (Figure 5A and B). In addition, the protein expression levels of c-myc and cyclin E1 were decreased, while p21 expression was increased in HepG2LRH-1/− cells (Figure S1). We next investigated whether LRH-1 regulates these genes’ expression through its transcriptional activity, and the reporter assay showed that luciferase activity of c-myc and cyclin E1 promoter-luciferase reporter was significantly enhanced, while that of p21 was dramatically suppressed, in HepG2-LRH-1 cells (Figure 5C). To further elucidate the functional role of these factors, we suppressed the c-myc and restored p21 expression in HepG2-LRH-1 cells by lentivirus-mediated knockdown and overexpression, and the results indicated that the increased growth ability of HepG2-LRH-1 cells could be, at least partially, reversed by suppressing the expression of c-myc or restoring the expression of p21 (Figure 5D). Taken together, our results suggested that LRH-1 may promote the development and progression of HCC through induction of c-myc and cyclin E1, and suppression of p21 (Figure 5E).
Figure 5.
LRH-1 promotes the development and progression of HCC through induction of c-myc and cyclin E1, and suppression of p21.
Notes: (A) qRT-PCR analysis of c-myc, cyclin E1, and p21 genes in HepG2 and HuH-7 cells with ectopic LRH-1 expression. Our data indicated that ectopic expression of LRH-1 dramatically induced the mRNA and protein levels of c-myc and cyclin E1, and attenuated, in the meanwhile, the expression of p21. (B) Western blotting validation of c-myc, cyclin E1, and p21 in HepG2 and HuH-7 with ectopic LRH-1 expression. (C) Reporter assay showed that luciferase activity of c-myc and cyclin E1 promoter-luc reporter was significantly enhanced, while that of p21 was dramatically suppressed, in HepG2-LRH-1 cells. (D) MTT results indicated that the increased growth ability of HepG2-LRH-1 cells could be, at least partially, reversed by suppressing the expression of c-myc or restoring the expression of p21. (E) Schematic diagram showing the hypothesized pathways of LRH-1-mediated cell growth in HCC via its transcriptional repression of p21 and transactivation of c-myc and cyclin E1. * P<0.05; ** P<0.01; #not significant.
Abbreviations: HCC, hepatocellular carcinoma; luc, luciferase.
Discussion
LRH-1 has been reported to be implicated in tumorigenesis of several cancers including breast cancer, gastric cancer, pancreatic cancer, intestinal cancer, and ovarian granulosa cell tumors recently.21–24,27,28 In HCC, few studies have been undertaken to explore the potential function of LRH-1. It is reported that ectopic expression of LRH-1 in FL83B promotes its proliferation capacity, while suppression of LRH-1 in BEL-7402 attenuates its proliferation rate and promotes cell apoptosis.27,29 In addition, it has been shown that LRH-1 regulates the glutamine-induced metabolism and signaling to promote hepatocellular carcinogenesis.30 However, whether LRH-1 expression is increased or not in clinical HCC tissue, and the involved mechanism concerning the participation of LRH-1 in HCC, remain largely unknown.
In this study, we first confirmed that LRH-1 showed an increased expression pattern in 50 HCC clinical samples as compared to corresponding tumor-adjacent tissues, supporting that LRH-1 may be associated with the development and progression of HCC. This result is consistent with the statistical data obtained from oncomine database (Figure S2). Indeed, overexpression of LRH-1 has previously been reported in breast cancer, gastric cancer, pancreatic cancer, and ovarian granulosa cell tumors.21–24 However, how LRH-1 expression level is increased in these cancers, as well as in HCC, is still unclear. A few studies have shown that LRH-1 can be induced by some upstream molecules involved in several signaling pathways including p38/MAPK pathway and ERK1/2 pathway.33,34 In addition, LRH-1 has been proven to be a direct target gene of β-catenin, PDX-1, SF-1, and ER.13,35–37 Interestingly, in the present study, we generated several HepG2LRH-1/− clones by TALENs which cut the specific DNA site in the second exon of LRH-1, and up to five different kinds of repair situations were detected in one HepG2LRH-1/− clone derived from a single cell, which indicates that the LRH-1 gene locus in HepG2 genome may be multiple (data not shown), suggesting that gene amplification might be one potential reason for the elevated expression of LRH-1 in HCC. Further efforts remain to be taken to determine the mechanism concerning the elevated LRH-1 expression in HCC and other cancers.
In our in vitro experiment, we proved that suppression of LRH-1 in HepG2 prevents its growth and colony-formation capacity. These results are consistent with a previous report which indicated that suppression of LRH-1 in liver cancer cell line BEL-7402 attenuates the cell proliferation rate and promotes cell apoptosis.29 It has also been suggested that LRH-1 promotes the cell proliferation in breast and intestinal cancers.21,25–27 In addition, our in vivo results show that suppression of LRH-1 in HepG2 dramatically compromised its tumorigenic capacity in nude mice. Recently, it has been elucidated that hepatic loss of LRH-1 prevents DEN-induced liver carcinogenesis in liver-specific LRH-1-deficient (LRH-1hep−/−) mice.30
To further demonstrate LRH-1-regulated genes and to determine the possible signaling pathway underlying the role of LRH-1 in liver cancer cells, gene expression pattern was analyzed between HepG2 and HepG2LRH-1/− cells. GO and KEGG pathway analyses suggest that significant biological process groups are related to DNA replication and cell cycle. This result is consistent with a previous study which suggests that “cell cycle” is the top significant GO biological process for LRH-1-regulated genes identified in colon cancer cell line HCT116 by gene expression microarray.38 To further verify that LRH-1 regulates cell cycle genes in liver cancer cells, we overexpressed LRH-1 in HepG2 and HuH-7 cell lines by retrovirus-mediated transduction. Our RT-PCR and Western blotting results showed that oncogenic c-myc and G1/S-specific cyclin E1 were obviously induced after LRH-1 overexpression in both HepG2 and HuH-7 cells, meanwhile, the mRNA and protein expression levels of cyclin-dependent kinase inhibitor p21 were significantly reduced. Consistently, cyclin E1 has previously been determined to be an LRH-1 direct target gene,27 and knockdown of LRH-1 in pancreatic cancer cells lead to suppression of c-myc.23 In colon cancer cells, it has also been reported that LRH-1 is recruited to the p21 promoter to repress its expression in a p53-dependent manner.38
Conclusion
To sum up, our study provides evidence that LRH-1 expression level is upregulated in clinical HCC tissues; suppression of LRH-1 in liver cancer cells significantly attenuates its proliferation rate and tumorigenic capacity both in vitro and in vivo; LRH-1 mainly regulates the expression of genes involved in cell cycles including c-myc, cyclin E1, and p21. Given that LRH-1 inverse agonist has been successfully developed,39 LRH-1 might be a promising therapeutic target for HCC treatment.
Supplementary materials
Western blotting validation of c-myc, cyclin E1, and p21 in HepG2 and HepG2LRH-1/− cells.
Note: The expression level of c-myc and cyclin E1 was decreased, while that of p21 was increased, in HepG2LRH-1/− cells.
LRH-1 was frequently upregulated in hepatocellular carcinoma clinical specimens.
Note: LRH-1 showed increased expression pattern according to several independent cases derived from oncomine database (www.oncomine.org).
Table S1.
Nucleotide sequences information
| Name | Genomic sites for TALE targeting LRH-1 |
|---|---|
| LRH-1 TALE F | CCCCCATTAGCATGAC |
| LRH-1 TALE R | AGCCTGCAAGGTTACC |
| Primer sequences used for qRT-PCR | |
| c-MYC _F | GGACTATCCTGCTGCCAAGA |
| c-MYC _R | CGCCTCTTGACATTCTCCTC |
| p21_F | TGCCCAAGCTCTACCTTCC |
| p21_R | CAGGTCCACATGGTCTTCCT |
| cyclin E1_F | CGGTATATGGCGACACAAGA |
| cyclin E1_R | ACATACGCAAACTGGTGCAA |
| Nucleotides for c-myc knockdown | |
| sh c-myc S | ccggGATGAGGAAGAAATCGATGctcgagcatcgatttcttcctcatctttttg |
| sh c-myc AS | aattcaaaaaGATGAGGAAGAAATCGATGctcgagcatcgatttcttcctcatc |
Acknowledgments
This work was funded by research grant from the National Natural Science Foundation of China (no 81502061 and 81402370); The Science and Technology Project of Shen-zhen (no KQJSCX20160222170616). Grant supports: L Xiao, C Zou.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 4.Stotz M, Gerger A, Haybaeck J, Kiesslich T, Bullock MD, Pichler M. Molecular Targeted Therapies in Hepatocellular Carcinoma: Past, Present and Future. Anticancer Res. 2015;35(11):5737–5744. [PubMed] [Google Scholar]
- 5.Francis GA, Fayard E, Picard F, Auwerx J. Nuclear receptors and the control of metabolism. Annu Rev Physiol. 2003;65:261–311. doi: 10.1146/annurev.physiol.65.092101.142528. [DOI] [PubMed] [Google Scholar]
- 6.Tang XH, Gudas LJ. Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol. 2011;6:345–364. doi: 10.1146/annurev-pathol-011110-130303. [DOI] [PubMed] [Google Scholar]
- 7.Kim JH, Choi YK, Byun JK, et al. Estrogen-related receptor γ is upregulated in liver cancer and its inhibition suppresses liver cancer cell proliferation via induction of p21 and p27. Exp Mol Med. 2016;48:e213. doi: 10.1038/emm.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng H, Cheng AS, Tsang DP, et al. Cell cycle-related kinase is a direct androgen receptor-regulated gene that drives β-catenin/T cell factor-dependent hepatocarcinogenesis. J Clin Invest. 2011;121(8):3159–3175. doi: 10.1172/JCI45967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walesky C, Edwards G, Borude P, et al. Hepatocyte nuclear factor 4 alpha deletion promotes diethylnitrosamine-induced hepatocellular carcinoma in rodents. Hepatology. 2013;57(6):2480–2490. doi: 10.1002/hep.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pare JF, Roy S, Galarneau L, Belanger L. The mouse fetoprotein transcription factor (FTF) gene promoter is regulated by three GATA elements with tandem E box and Nkx motifs, and FTF in turn activates the Hnf3beta, Hnf4alpha, and Hnf1alpha gene promoters. J Biol Chem. 2001;276(16):13136–13144. doi: 10.1074/jbc.M010737200. [DOI] [PubMed] [Google Scholar]
- 11.Falender AE, Lanz R, Malenfant D, Belanger L, Richards JS. Differential expression of steroidogenic factor-1 and FTF/LRH-1 in the rodent ovary. Endocrinology. 2003;144(8):3598–3610. doi: 10.1210/en.2002-0137. [DOI] [PubMed] [Google Scholar]
- 12.Rausa FM, Galarneau L, Bélanger L, Costa RH. The nuclear receptor fetoprotein transcription factor is coexpressed with its target gene HNF-3beta in the developing murine liver, intestine and pancreas. Mech Dev. 1999;89(1–2):185–188. doi: 10.1016/s0925-4773(99)00209-9. [DOI] [PubMed] [Google Scholar]
- 13.Annicotte JS, Fayard E, Swift GH, et al. Pancreatic-duodenal homeobox 1 regulates expression of liver receptor homolog 1 during pancreas development. Mol Cell Biol. 2003;23(19):6713–6724. doi: 10.1128/MCB.23.19.6713-6724.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo Y, Liang CP, Tall AR. The orphan nuclear receptor LRH-1 potentiates the sterol-mediated induction of the human CETP gene by liver X receptor. J Biol Chem. 2001;276(27):24767–24773. doi: 10.1074/jbc.M100912200. [DOI] [PubMed] [Google Scholar]
- 15.Schoonjans K, Annicotte JS, Huby T, et al. Liver receptor homolog 1 controls the expression of the scavenger receptor class B type I. EMBO Rep. 2002;3(12):1181–1187. doi: 10.1093/embo-reports/kvf238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Zhang M, Eggertsen G, Chiang JY. On the mechanism of bile acid inhibition of rat sterol 12alpha-hydroxylase gene (CYP8B1) transcription: roles of alpha-fetoprotein transcription factor and hepatocyte nuclear factor 4alpha. Biochim Biophys Acta. 2002;1583(1):63–73. doi: 10.1016/s1388-1981(02)00186-5. [DOI] [PubMed] [Google Scholar]
- 17.Inokuchi A, Hinoshita E, Iwamoto Y, Kohno K, Kuwano M, Uchiumi T. Enhanced expression of the human multidrug resistance protein 3 by bile salt in human enterocytes. A transcriptional control of a plausible bile acid transporter. J Biol Chem. 2001;276(50):46822–46829. doi: 10.1074/jbc.M104612200. [DOI] [PubMed] [Google Scholar]
- 18.Peng N, Kim JW, Rainey WE, Carr BR, Attia GR. The role of the orphan nuclear receptor, liver receptor homologue-1, in the regulation of human corpus luteum 3beta-hydroxysteroid dehydrogenase type II. J Clin Endocrinol Metab. 2003;88(12):6020–6028. doi: 10.1210/jc.2003-030880. [DOI] [PubMed] [Google Scholar]
- 19.Yazawa T, Inanoka Y, Mizutani T, Kuribayashi M, Umezawa A, Miyamoto K. Liver receptor homolog-1 regulates the transcription of steroidogenic enzymes and induces the differentiation of mesenchymal stem cells into steroidogenic cells. Endocrinology. 2009;150(8):3885–3893. doi: 10.1210/en.2008-1310. [DOI] [PubMed] [Google Scholar]
- 20.Sierens J, Jakody I, Poobalan Y, et al. Localization and regulation of aromatase liver receptor homologue-1 in the developing rat testis. Mol Cell Endocrinol. 2010;323(2):307–313. doi: 10.1016/j.mce.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Suzuki T, Kovacic A, et al. Interactions between prostaglandin E(2), liver receptor homologue-1, and aromatase in breast cancer. Cancer Res. 2005;65(2):657–663. [PubMed] [Google Scholar]
- 22.Wang SL, Zheng DZ, Lan FH, et al. Increased expression of hLRH-1 in human gastric cancer and its implication in tumorigenesis. Mol Cell Biochem. 2008;308(1–2):93–100. doi: 10.1007/s11010-007-9616-1. [DOI] [PubMed] [Google Scholar]
- 23.Benod C, Vinogradova MV, Jouravel N, Kim GE, Fletterick RJ, Sablin EP. Nuclear receptor liver receptor homologue 1 (LRH-1) regulates pancreatic cancer cell growth and proliferation. Proc Natl Acad Sci U S A. 2011;108(41):16927–16931. doi: 10.1073/pnas.1112047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chand AL, Pathirage N, Lazarus K, et al. Liver receptor homologue-1 expression in ovarian epithelial and granulosa cell tumours. Steroids. 2013;78(7):700–706. doi: 10.1016/j.steroids.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Chand AL, Herridge KA, Thompson EW, Clyne CD. The orphan nuclear receptor LRH-1 promotes breast cancer motility and invasion. Endocr Relat Cancer. 2010;17(4):965–975. doi: 10.1677/ERC-10-0179. [DOI] [PubMed] [Google Scholar]
- 26.Bianco S, Jangal M, Garneau D, Gévry N. LRH-1 controls proliferation in breast tumor cells by regulating CDKN1A gene expression. Oncogene. 2015;34(34):4509–4518. doi: 10.1038/onc.2014.382. [DOI] [PubMed] [Google Scholar]
- 27.Botrugno OA, Fayard E, Annicotte JS, et al. Synergy between LRH-1 and beta-catenin induces G1 cyclin-mediated cell proliferation. Mol Cell. 2004;15(4):499–509. doi: 10.1016/j.molcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Schoonjans K, Dubuquoy L, Mebis J, et al. Liver receptor homolog 1 contributes to intestinal tumor formation through effects on cell cycle and inflammation. Proc Natl Acad Sci U S A. 2005;102(6):2058–2062. doi: 10.1073/pnas.0409756102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S, Lan F, Huang L, et al. Suppression of hLRH-1 mediated by a DNA vector-based RNA interference results in cell cycle arrest and induction of apoptosis in hepatocellular carcinoma cell BEL-7402. Biochem Biophys Res Commun. 2005;333(3):917–924. doi: 10.1016/j.bbrc.2005.05.186. [DOI] [PubMed] [Google Scholar]
- 30.Xu P, Oosterveer MH, Stein S, et al. LRH-1-dependent programming of mitochondrial glutamine processing drives liver cancer. Genes Dev. 2016;30(11):1255–1260. doi: 10.1101/gad.277483.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cermak T, Doyle EL, Christian M, et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011;39(12):e82. doi: 10.1093/nar/gkr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu S, Wang MW, Yao X, Chan FL. Establishment of a novel immortalized human prostatic epithelial cell line stably expressing androgen receptor and its application for the functional screening of androgen receptor modulators. Biochem Biophys Res Commun. 2009;382(4):756–761. doi: 10.1016/j.bbrc.2009.03.110. [DOI] [PubMed] [Google Scholar]
- 33.Yu FQ, Han CS, Yang W, Jin X, Hu ZY, Liu YX. Activation of the p38 MAPK pathway by follicle-stimulating hormone regulates steroidogenesis in granulosa cells differentially. J Endocrinol. 2005;186(1):85–96. doi: 10.1677/joe.1.05955. [DOI] [PubMed] [Google Scholar]
- 34.Guo J, Tao SX, Chen M, et al. Heat treatment induces liver receptor homolog-1 expression in monkey and rat sertoli cells. Endocrinology. 2007;148(3):1255–1265. doi: 10.1210/en.2006-1004. [DOI] [PubMed] [Google Scholar]
- 35.Wagner RT, Xu X, Yi F, Merrill BJ, Cooney AJ. Canonical Wnt/β-catenin regulation of liver receptor homolog-1 mediates pluripotency gene expression. Stem Cells. 2010;28(10):1794–1804. doi: 10.1002/stem.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawabe S, Yazawa T, Kanno M, et al. A novel isoform of liver receptor homolog-1 is regulated by steroidogenic factor-1 and the specificity protein family in ovarian granulosa cells. Endocrinology. 2013;154(4):1648–1660. doi: 10.1210/en.2012-2008. [DOI] [PubMed] [Google Scholar]
- 37.Annicotte JS, Chavey C, Servant N, et al. The nuclear receptor liver receptor homolog-1 is an estrogen receptor target gene. Oncogene. 2005;24(55):8167–8175. doi: 10.1038/sj.onc.1208950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kramer HB, Lai CF, Patel H, et al. LRH-1 drives colon cancer cell growth by repressing the expression of the CDKN1A gene in a p53-dependent manner. Nucleic Acids Res. 2016;44(2):582–594. doi: 10.1093/nar/gkv948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Busby S, Nuhant P, Cameron M, et al. Probe Reports from the NIH Molecular Libraries Program. Bethesda, MD: National Center for Biotechnology Information (US); 2010. Discovery of Inverse Agonists for the Liver Receptor Homologue-1 (LRH1; NR5A2) [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Western blotting validation of c-myc, cyclin E1, and p21 in HepG2 and HepG2LRH-1/− cells.
Note: The expression level of c-myc and cyclin E1 was decreased, while that of p21 was increased, in HepG2LRH-1/− cells.
LRH-1 was frequently upregulated in hepatocellular carcinoma clinical specimens.
Note: LRH-1 showed increased expression pattern according to several independent cases derived from oncomine database (www.oncomine.org).
Table S1.
Nucleotide sequences information
| Name | Genomic sites for TALE targeting LRH-1 |
|---|---|
| LRH-1 TALE F | CCCCCATTAGCATGAC |
| LRH-1 TALE R | AGCCTGCAAGGTTACC |
| Primer sequences used for qRT-PCR | |
| c-MYC _F | GGACTATCCTGCTGCCAAGA |
| c-MYC _R | CGCCTCTTGACATTCTCCTC |
| p21_F | TGCCCAAGCTCTACCTTCC |
| p21_R | CAGGTCCACATGGTCTTCCT |
| cyclin E1_F | CGGTATATGGCGACACAAGA |
| cyclin E1_R | ACATACGCAAACTGGTGCAA |
| Nucleotides for c-myc knockdown | |
| sh c-myc S | ccggGATGAGGAAGAAATCGATGctcgagcatcgatttcttcctcatctttttg |
| sh c-myc AS | aattcaaaaaGATGAGGAAGAAATCGATGctcgagcatcgatttcttcctcatc |