Abstract
Polychlorinated biphenyls (PCBs) are ubiquitous environmental contaminants that are associated with varied adverse health effects. Lower chlorinated PCBs are prevalent in indoor and outdoor air and can be metabolized to their hydroxylated derivatives (OH-PCBs) followed by sulfation to form PCB sulfates. Sulfation is also a means of signal termination for steroid hormones. The human estrogen sulfotransferase (SULT1E1) and alcohol/hydroxysteroid sulfotransferase (SULT2A1) catalyze the formation of steroid sulfates that are inactive at steroid hormone receptors. We investigated the inhibition of SULT1E1 (IC50s ranging from 7.2 nM to greater than 10 μM) and SULT2A1 (IC50s from 1.3 μM to over 100 μM) by five lower-chlorinated OH-PCBs and their corresponding PCB sulfates relevant to airborne PCB-exposure. Several congeners of lower chlorinated OH-PCBs relevant to airborne PCB exposures were potent inhibitors of SULT1E1 and SULT2A1 and thus have the potential to disrupt regulation of intracellular concentrations of the receptor-active steroid substrates for these enzymes.
Keywords: Sulfotransferase, estradiol, dehydroepiandrosterone, polychlorinated biphenyls, hydroxylated polychlorinated biphenyls, SULT1E1, SULT2A1, endocrine disruption
1. Introduction
Polychlorinated biphenyls (PCBs) remain as persistent environmental toxicants that cause numerous adverse health effects (Ampelman et al., 2015; ATSDR, 2000). PCBs were produced in large quantities in the mid-twentieth century for extensive use in electrical transformers, fluorescent light ballasts, caulk, paint, flame retardants, and many other applications (Erickson and Kaley, 2011). In addition to these legacy sources, there are newly recognized sources of exposures to PCBs, and these include their presence as unintentional byproducts in paints and pigments (Hu and Hornbuckle, 2010; Shanahan et al., 2015).
PCBs undergo enzyme-catalyzed oxidative reactions in biological systems to form hydroxylated PCBs (OH-PCBs), and the formation, physical and chemical properties, and toxicities of these metabolites have been recently reviewed (Dhakal et al., 2017; Grimm et al., 2015b). In many cases it is either the OH-PCBs or their subsequent metabolites that are directly involved in the toxicities observed upon exposure to PCBs. For example, metabolism of OH-PCBs to reactive electrophiles is implicated in genotoxic responses to some PCBs (Robertson and Ludewig, 2011). Metabolism of OH-PCBs can also yield the corresponding sulfuric acid esters (PCB sulfates), and these sulfated metabolites bind with high affinity to human serum proteins such as transthyretin and albumin (Grimm et al., 2013; Rodriguez et al., 2016). Displacement of thyroxine by the binding of OH-PCBs and/or PCB sulfates may disrupt thyroid hormone-dependent processes in susceptible tissues (Brouwer et al., 1998; Grimm et al., 2013). In general, binding to serum proteins by PCB sulfates may also serve as a mechanism for transport of PCB sulfates to tissues, where action of sulfatases may convert the molecule back to OH-PCBs, thus setting up a dynamic cycle between the OH-PCB and the PCB-sulfate. Indeed, studies in rats indicate that intravenous injection of 4-PCB 11 sulfate results in rapid uptake by tissues and conversion to phenolic metabolites (Grimm et al., 2015a).
The mammalian sulfation of OH-PCBs is catalyzed by cytosolic sulfotransferases (SULTs). SULTs catalyze sulfation of xenobiotics such as OH-PCBs as well as endogenous molecules that include steroid hormones, bile acids, catecholamines, neurotransmitters, and others (Coughtrie, 2016; Duffel, 2010; Gamage et al., 2006; Glatt et al., 2001; James and Ambadapadi, 2013). For steroid hormones, SULTs play a key role in signaling processes through the conversion of active steroid hormones to inactive steroid sulfates. Although steroid sulfates are inactive at steroid hormone receptors, they may serve as transport forms for the hormone (Labrie et al., 1995). OH-PCBs interact with SULTs as either substrates or inhibitors, depending upon the specific isoform of the enzyme and the chemical structure of the OH-PCB (Ekuase et al., 2014; Ekuase et al., 2011; Kester et al., 2000; Schuur et al., 1998; Wang and James, 2007; Wang et al., 2005; Wang et al., 2006).
Our studies focus on the human estrogen- and hydroxysteroid- sulfotransferases SULT1E1 and SULT2A1, respectively. SULT1E1 catalyzes the sulfation of estradiol and other phenolic steroids, and SULT2A1 catalyzes the sulfation of hydroxysteroids such as dehydroepiandrosterone and various alcohol-containing androgens (Coughtrie, 2016; Falany et al., 1995b). SULT1E1 has a high affinity and catalytic efficiency for estradiol as substrate, and can thereby be involved in estrogenic signaling through regulation of intracellular concentrations of the active hormone. SULT2A1 catalyzes the formation of dehydroepiandrosterone sulfate (DHEAS) the most abundant circulating steroid in humans. DHEAS serves as a transport form for dehydroepiandrosterone (DHEA) in the serum. Following uptake of DHEAS by tissues and its subsequent hydrolysis catalyzed by steroid sulfatase, DHEA serves as a precursor for both androgens and estrogens (Labrie et al., 1995). Thus, both SULT1E1 and SULT2A1 can play important roles in steroid signaling through regulation of the active forms of the hormones.
OH-PCBs are known to be endocrine disruptors, wherein they exert multiple varied effects on hormone-dependent physiological processes (Brouwer et al., 1998; Grimm et al., 2015,b; Meerts et al., 2004; Quinete et al., 2014). Inhibition of SULT1E1 has been demonstrated for several OH-PCBs, and inhibition constants at or below physiological concentrations of estradiol suggest that this may be a basis for physiological estrogenic effects observed with some PCBs and OH-PCBs (Kester et al., 2000; Kester et al., 2002). Likewise, inhibition constants for some OH-PCBs as inhibitors of the sulfation of DHEA catalyzed by SULT2A1 are within the range of reported serum concentrations for this steroid hormone (Ekuase et al., 2014; Ekuase et al., 2011).
A less-often studied group of PCBs are those congeners containing fewer than five chlorine atoms. These lower-chlorinated PCBs are highly represented in both indoor and outdoor air samples (Ampleman et al., 2015; Grimm et al., 2015b; Hu et al., 2010), and there is increasing concern about airborne PCB exposures from indoor air of older buildings, in particular within U.S. schools (Herrick et al., 2004; Herrick et al., 2011). We have directed our attention toward inhibition of SULT1E1 and SULT2A1 by OH-PCB metabolites that would likely be derived from human exposure to PCBs in air. Thus, we have examined several para hydroxylated OH-PCBs that would be derived from PCBs 3, 8, 11, and 52 (congeners that are among the ten most commonly observed PCBs in air samples Grimm et al., 2015b). In addition, we have studied 4’-OH-PCB 25, which would be a metabolite of PCB 28, another congener among the ten most commonly encountered PCBs in air. It is noteworthy that 4’-OH-PCB 25 has recently been identified as a metabolite in human plasma with an estimated half-life of 6.5 years (Quinete et al., 2017). Since SULTs may be subject to product inhibition by sulfates (Gulcan and Duffel, 2011; James, 2014; Zhang et al., 1998), we have also explored the potential for the PCB sulfates to inhibit the sulfation of estradiol catalyzed by SULT1E1 and the sulfation of DHEA catalyzed by SULT2A1.
2. Materials and Methods
2.1. Chemicals and reagents
The synthesis and characterization of each of the following PCB metabolites was conducted by the Synthesis Core of the Iowa Superfund Research Program. Briefly, OH-PCBs were synthesized via the Suzuki coupling of a chlorinated benzene boronic acid with a chlorinated iodo- or bromobenzene, followed by demethylation with BBr3 in dichloromethane as described (Joshi et al. 2011, Lehmler & Robertson 2001, Rodriguez et al. 2016, Zhu et al. 2013). All PCB sulfates were synthesized in two steps by reacting the corresponding OH-PCBs with 2,2,2-trichloroethyl chlorosulfate and, subsequently, removing the 2,2,2-trichloroethyl protective group with zinc powder/ammonium formate (Flor et al. 2015, Grimm et al. 2013, Lehmler et al. 2013, Li et al. 2010, Rodriguez et al. 2016). Adenosine 3’-phosphate 5’-phosphosulfate lithium salt hydrate (PAPS) was purchased from Sigma-Aldrich (St. Louis, MO) and purified as described previously (Sekura, 1981). The purity of the PAPS (99%) was assessed by high performance liquid chromatography using a previously described method (Sheng et al., 2001). [3H]-Dehydroepiadrosterone [1,2,6,7-3H(N)] (49.7 Ci/mmol) and [3H]-estradiol [2,4,6,7- 3H(N)] (81.0 Ci/mmol) were obtained from PerkinElmer (Waltham, MA), and Econo-Safe liquid scintillation cocktail was from Research Products International (Mount Prospect, IL). Absolute ethanol was obtained from Decon Laboratories (King of Prussia, PA). All other chemicals were purchased from ThermoFisher Scientific (Waltham, MA), and were at least ACS grade.
2.2. Expression and Purification of SULT1E1 and SULT2A1
Recombinant human SULT1E1 (Squirewell and Duffel, 2015) and SULT2A1 (Gulcan et al., 2008) were expressed in Escherichia coli BL21 (DE3) cells, purified, and characterized as described previously.
2.3. Inhibition of SULT1E1 by PCB metabolites
Assays for the sulfation of estradiol catalyzed by SULT1E1 were conducted using a previously described method (Squirewell and Duffel, 2015). The 200 μL reactions were carried out in assay mixtures consisting of 0.25 M potassium phosphate (pH 7.4), 8.3 mM 2-mercaptoethanol, 50 μM PAPS, and 7.0 nM [3H] estradiol. [3H] Estradiol and the selected OH-PCBs and PCB sulfates were dissolved in absolute ethanol, and they were added to the reaction mixtures. The concentrations of OH-PCB and PCB sulfate metabolites in these assays ranged from 0.01–10,000 nM. All reactions were performed in triplicate. Following a 3 min equilibration of the reaction mixture at 37oC, 3.0 ng of purified SULT1E1 was added to initiate the reaction, and the solution was incubated for 4 minutes. The reaction was terminated by addition of 800 μL 0.25M Tris-HCl (pH 8.7) and 4.0 mL chloroform. After vortex mixing, the phases were separated by centrifugation at 150 x g for 5 minutes. A 500 μL aliquot of the aqueous layer (containing [3H]-estradiol-3-sulfate) was removed and added to 10 mL of liquid scintillation cocktail for analysis. The amount of estradiol-3-sulfate that was produced in the reaction was then determined using a Tri-CARB 2000TR Liquid Scintillation Analyzer (Packard BioScience Company, Meriden, CT).
2.3. Inhibition of SULT2A1 by PCB metabolites
Assays for the sulfation of DHEA catalyzed by SULT2A1 were carried out as previously described (Squirewell et al., 2014). The 200 μL reactions were conducted in assay mixtures containing 0.25 M potassium phosphate (pH 7.4), 8.3 mM 2-mercaptoethanol, 200 μM PAPS, and 1.0 μM [3H]-DHEA. [3H]-DHEA and the selected OH-PCBs and PCB sulfates were dissolved in absolute ethanol, and they were added to the reaction mixtures. The concentrations of OH-PCB and PCB sulfate metabolites ranged from 10 – 100,000 nM within the assay. All reactions were performed in triplicate. After an initial 2 min temperature equilibration of the assay mixture at 37 oC, the reaction was initiated by addition of 30 ng of purified SULT2A1 and incubated for 4 minutes. The reaction was terminated by the addition of 800 μL 0.50 mM potassium hydroxide and 500 μL chloroform. After vortex mixing, the phases were separated by centrifugation at 150 x g for 5 minutes, and a 500 μL aliquot of the aqueous layer (containing [3H]-DHEA sulfate) was removed added to 10 mL liquid scintillation cocktail for analysis of the amount of product formed in the reaction as described for SULT1E1.
2.4. Data analysis
Analysis of inhibition was based upon the percentage of the control rate of sulfation of the appropriate substrate in the absence of PCB metabolites. Inhibition data were analyzed using SigmaPlot 13.0 software (Systat Software, Inc., San Jose, CA) in order to obtain dose-response curves and determine IC50 values.
3. Results and Discussion
We have hypothesized that the inhibition of SULT1E1 and SULT2A1 by metabolites of lower-chlorinated PCBs may occur at concentrations that are relevant for potential disruption of steroid hormone activity or transport. Thus, we focused on five OH-PCBs and their corresponding PCB sulfates that would be derived from PCBs that are among the most commonly encountered in indoor and outdoor air samples.
3.1. Inhibition of SULT1E1 by OH-PCB and PCB Sulfate Metabolites.
OH-PCBs and PCB-sulfates were analyzed to determine their inhibitory effects on the sulfation of estradiol catalyzed by SULT1E1. We used a concentration of 7.0 nM of estradiol to measure the inhibition of SULT1E1, since this approximates the substrate concentration required for half of the maximal velocity for the sulfation of estradiol catalyzed by SULT1E1 at pH 7.4 and 37oC (Squirewell and Duffel, 2015). For determination of the effects of OH-PCBs on estradiol sulfation, the concentrations of OH-PCBs and PCB sulfates used in the reactions were 0.01 nM to 10 μM (Figure 2a). As seen in Table 1, the IC50 values observed for the OH-PCBs ranged between 7.2 nM and 1300 nM. 4-OH-PCB 11 and 4’-OH-PCB 25 had the lowest IC50 values (i.e., the most potent inhibitors), while 4’-OH-PCB 3 was the least potent inhibitor. In contrast to the OH-PCBs, 4’-PCB 25 sulfate was the only PCB sulfate examined that displayed inhibition of SULT1E1, although it was less potent than most of the OH-PCBs examined (Table 1 and Figure 2b).
Fig. 2.
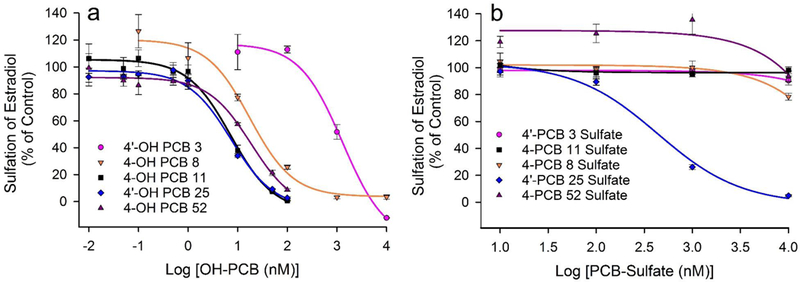
Inhibition of SULT1E1-catalyzed sulfation of 7.0 nM estradiol by PCB metabolites. (a) Inhibition of SULT1E1 by 4’-OH PCB 3, 4-OH-PCB 8, 4-OH-PCB 11, 4’-OH-PCB 25 and 4-OH-PCB 52. Control rates for sulfation of estradiol were 37.1, 27.9, 56.0, 71.4, and 62.8 (nmol/min/mg), respectively. (b) Inhibition of SULT1E1 by 4’-PCB 3-sulfate, 4-PCB 8-sulfate, 4-PCB 11-sulfate, 4’-PCB 25-sulfate and 4-PCB 52-sulfate. Control rates for sulfation of estradiol were 58.1, 57.3, 67.2, 75.8, and 20.2 (nmol/min/mg), respectively.
Table 1.
PCB Metabolites as Inhibitors of SULT1E1
| PCB Metabolite a | IC50 (nM) |
|---|---|
| 4’-OH-PCB 3 | 1300 ± 490 |
| 4’-PCB 3 Sulfate | > 10,000 b |
| 4’-OH-PCB 8 | 18 ± 6 |
| 4’-PCB 8 Sulfate | > 10,000 b |
| 4-OH-PCB 11 | 7.2 ± 1.4 |
| 4-PCB 11 Sulfate | > 10,000 b |
| 4’-OH-PCB 25 | 7.3 ± 1.7 |
| 4’-PCB 25 Sulfate | 440 ± 100 |
| 4-OH-PCB 52 | 19 ± 7 |
| 4-PCB 52 Sulfate | > 10,000 b |
The concentrations of PCB metabolites for the determination of IC50 values were 0.01 nM to 10 μM at an estradiol concentration of 7nM.
No inhibition was detected at a 10 μM concentration of the PCB metabolites.
The choice of a concentration of estradiol that is approximately equal to the concentration required for half-maximal velocity of sulfation catalyzed by SULT1E1 was important, since the rate of estradiol-sulfate formation under these conditions obeys first order kinetics with respect to the substrate, estradiol. This is important for physiological control of the rate of reaction where concentrations of estradiol are at or below this value. At this concentration of substrate, IC50 values for inhibitors of the reaction that are similar to the concentration of estradiol are particularly relevant. This indicates that concentrations of the inhibitor that are equal to the intracellular concentration of hormone would inhibit the sulfation (inactivation) of the estradiol by 50%. Thus, the IC50 values for 4-OH-PCB 11 and 4’-OH PCB 25 suggest that these PCB metabolites at similar concentrations to that of intracellular estradiol would lead to a 50% decrease in the inactivation of estradiol through sulfation. On the other hand, the nearly 200-fold higher IC50 value observed for 4’-OH-PCB 3 make it less likely to have a physiologically relevant effect on estradiol sulfation. Significant inhibition of SULT1E1-catalyzed sulfation of estradiol by either 4’-OH-PCB 8 or 4-OH-PCB 52 might occur, but it would require an approximately 2–3 fold greater intracellular concentration of the OH-PCB relative to that of estradiol. With PCB sulfates, however, only 4’-PCB 25 sulfate showed any inhibition of SULT1E1, and that was with an IC50 value over 50-fold greater than the estradiol.
A full determination of relevance to physiological estradiol signaling is dependent upon the intracellular concentrations of estradiol and the OH-PCB. For estradiol, measurement of intracellular concentrations has been particularly difficult due to low concentrations, although it is assumed that in many cases it is below the serum concentration (Rosner et al., 2013). Serum concentrations of estradiol are known to vary with age, sex, and various physiological and pathophysiological states with values that range from less than 20 pM to over 5.0 nM (Rosner et al., 2013). Likewise, it is currently difficult to determine intracellular and serum concentrations of OH-PCBs, however, estimates of concentrations of total OH-PCBs in human serum vary greatly from 70 pM to 40 nM depending upon the populations examined and the types of exposures to PCBs involved (Guvenius et al., 2003; Koh et al., 2016; Sandau et al., 2000). Under the kinetic assumptions presented above, one would expect that pM to nM concentrations of 4-OH-PCB 11 and 4’-OH-PCB 25 might affect SULT1E1-catalyzed sulfation of estradiol. Moreover, the lower concentrations of serum estradiol in pre-pubertal children (Elmlinger et al., 2002) might also make them more susceptible to inhibition of SULT1E1 by those OH-PCBs with high affinity for the enzyme.
A change in the intracellular concentrations of estradiol through inhibition of its sulfation might have physiological effects on processes that have been linked to SULT1E1. For example, gene-deletion studies in mice have shown that SULT1E1 is an important modulator of estrogen in the placenta, and a role in thrombotic fetal loss has been suggested (Tong et al., 2005). Furthermore, knockout of this gene in mice impairs ovulation and is linked to alterations in estrogen signaling and cyclooxygenase-2 expression (Gershon et al., 2007). Other studies indicate that SULT1E1 has a role in regulating adipocyte differentiation through altering cellular concentrations of active estradiol (Ihunnah et al., 2014; Wada et al., 2011). Moreover, regulatory roles for SULT1E1 have also been identified in adipose tissue and glucose homeostasis (Khor et al., 2010), in endothelial cell function (Xu et al., 2013), and in cystic fibrosis (Falany et al., 2009). Inhibition of the catalytic function of SULT1E1 represents one potential mechanism for regulation of intracellular estradiol concentration in these metabolic processes.
3.2. Inhibition of SULT2A1 by OH-PCB and PCB-Sulfate Metabolites
DHEA was chosen as the substrate for studies on the inhibition of SULT2A1 due to the fact that it is among those hydroxysteroids that display the highest catalytic efficiency with the enzyme. Moreover, it is physiologically significant as a metabolic precursor to both androgens and estrogens. We employed a substrate concentration of 1.0 μM DHEA for the inhibition studies on OH-PCBs and PCB sulfates, since this is the approximate concentration required for half-maximal velocity observed for the SULT2A1 catalyzed formation of DHEA sulfate (Squirewell et al., 2014). The concentrations of OH-PCBs and PCB sulfates utilized for determination of the potential inhibition of SULT2A1 at this concentration of DHEA were varied from 0.01 μM to 200 μM. As seen in Table 2 and Figure 3, IC50 values for all OH-PCBs examined were between 1.3 μM and 23 μM, with the most potent inhibitors of SULT2A1 being 4’-OH-PCB 8 and 4-OH-PCB 52. In contrast, three of the PCB sulfates had IC50 values greater than 100 μM, while 4’-PCB 8 sulfate and 4-PCB 52 sulfate had IC50 values of 45.8 μM and 29.5 μM, respectively.
Table 2.
PCB Metabolites as Inhibitors of SULT2A1
| PCB Metabolite a | IC50 (μM) |
|---|---|
| 4’-OH-PCB 3 | 12 ± 5 |
| 4’-PCB 3 Sulfate | > 100 b |
| 4’-OH-PCB 8 | 1.5 ± 0.3 |
| 4’-PCB 8 Sulfate | 46 ± 11 |
| 4-OH-PCB 11 | 23 ± 4 |
| 4-PCB 11 Sulfate | > 100 b |
| 4’-OH-PCB 25 | 4.9 ± 0.9 |
| 4’-PCB 25 Sulfate | > 100 b |
| 4-OH-PCB 52 | 1.3 ± 0.4 |
| 4-PCB 52 Sulfate | 30 ± 11 |
The concentrations of PCB metabolites for the determination of IC50 values were 0.01 μM to 200 μM for inhibition of SULT2A1 at a concentration of 1.0 μM DHEA.
The IC50 value for the PCB metabolite was greater than 100 μM.
Fig. 3.
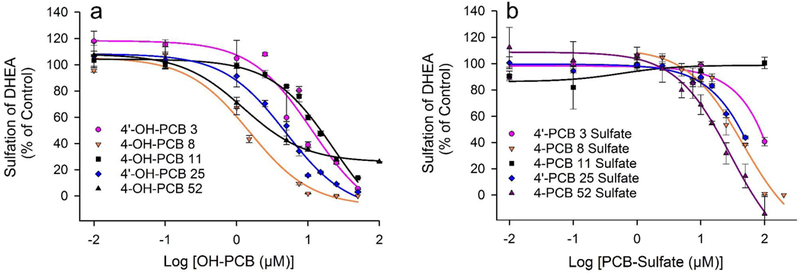
Inhibition of SULT2A1-catalyzed sulfation of 1.0 μM DHEA by PCB metabolites. (a) Inhibition of SULT2A1 by 4’-OH PCB 3, 4-OH-PCB 8, 4-OH-PCB 11, 4’-OH-PCB 25 and 4-OH-PCB 52. Control rates for sulfation of DHEA were 63.8, 69.6, 69.1, 74.6, and 84.4 (nmol/min/mg), respectively. (b) Inhibition of SULT2A1 by 4’-PCB 3 sulfate, 4-PCB 8 sulfate, 4-PCB 11 sulfate, 4’-PCB 25 sulfate and 4-PCB 52 sulfate. Control rates for sulfation of DHEA were 63.1, 44.6, 55.2, 89.4, and 40.8 (nmol/min/mg), respectively.
As described above in our experimental design for inhibition of SULT1E1, it was important to select a concentration of DHEA for the analysis of the SULT2A1-catalyzed reaction where the reaction displays first order kinetics with respect to substrate (i.e., near the apparent Km for DHEA). Therefore, even at concentrations of DHEA well below that used in these assays, inhibitors with IC50 values similar to the apparent Km for DHEA would be expected to inhibit the reaction rate by 50% when the inhibitor is present at the same concentration as the DHEA. Thus, even at much lower concentrations than 1.0 μM, OH-PCBs such as 4’-OH-PCB 8 and 4’-OH-PCB 52 would inhibit the sulfation of an equal concentration of DHEA by approximately 50%. While OH-PCBs or PCB sulfates with higher IC50 values could also inhibit sulfation of low concentrations of DHEA catalyzed by SULT2A1, the relative ratio of inhibitor to DHEA required to do so would have to be proportionately higher.
DHEA is a major substrate for SULT2A1, and it serves as a precursor for synthesis of both androgens and estrogens (Falany et al., 1995a; Labrie et al., 2003). Thus, alteration of the concentration of DHEA by sulfation and by hydrolysis of the resulting sulfate ester would be one component in local intracellular regulation of these biosynthetic pathways. Additionally, SULT2A1 also catalyzes sulfation of bile acids, and gene knockout studies in the mouse have indicated an important physiological role for hepatic SULT2A1 and multidrug resistance protein 4 (Mrp4) in the elimination of bile acids (Assem et al., 2004). Thus, OH-PCB-mediated interference with sulfation of DHEA could play critical roles in the overall effects of these toxicants.
The physiological significance of any inhibition of SULT2A1-catalyzed sulfation of DHEA is, therefore, dependent upon the intracellular concentrations of both the DHEA and the PCB metabolite. As noted above for the analogous situation with estradiol metabolism, the intracellular concentrations of either DHEA or a PCB metabolite are not usually known. Consequently, serum concentrations are often used as starting points for estimating a potential range of tissue concentrations. Such an assumption, however, must always be considered with the caveat that an actual intracellular concentration is likely to be either higher or lower than the serum concentration. In the case of DHEA, human serum concentrations vary with the age and sex of an individual, with values ranging from 5 nM to 24 nM (Labrie et al., 1997). DHEA sulfate, however, is present in human serum at concentrations from 1.5 μM to 11.5 μM (Labrie et al., 1997). As noted earlier, reports of serum concentrations of OH-PCBs exhibit an even larger range of concentrations (70 pM to 40 nM) depending upon various factors (Guvenius et al., 2003; Koh et al., 2016; Liu et al., 2006; Sandau et al., 2000). Our present data on the dependence of inhibition on the structures of lower-chlorinated OH-PCB congeners, however, suggest that total OH-PCB concentration may not be as important as the concentrations of several key individual OH-PCB congeners that may be particularly good inhibitors. Such evaluations will await additional analyses of specific congeners both with respect to tissue concentrations and interactions with SULT2A1.
4. Conclusions
Our studies on two major human sulfotransferases involved in metabolic sulfation of steroid hormones indicated that 4-OH-PCB 11 and 4’-OH-PCB 25 were the most potent inhibitors of SULT1E1, while 4’-OH-PCB 8 and 4-OH-PCB 52 were the most potent inhibitors of SULT2A1. For these inhibitors of the respective SULTs, IC50 values were approximately the same as the concentration of steroid substrate required for half-maximal rates of sulfation. This indicated that concentrations of these OH-PCBs similar to physiological concentrations of the steroid hormone substrates might reduce the rate of hormonal inactivation of the hormone through sulfation catalyzed by these enzymes.
While the PCB sulfates examined here were generally not potent inhibitors, some limited inhibition of SULT1E1 and SULT2A1 was observed. It is likely, however, that a major importance of the PCB sulfates may be either as transport forms for delivery to tissues and/or as precursors to OH-PCBs through the action of intracellular sulfatases. This type of transport would be analogous to the function of DHEA-sulfate, the highest concentration circulating steroid in humans. It binds to serum proteins and is transported to tissues where localized synthesis of estrogens and androgens occur (Labrie et al., 2003). For example, DHEA-sulfate binds to human serum albumin with high affinity and has a half-life of 7–10 hours, while DHEA binds with much lower affinity and has a half-life of 15–30 minutes (White and Porterfield, 2013). By analogy, OH-PCBs and PCB sulfates also bind with high affinity to serum proteins such as human serum albumin (Rodriguez et al., 2016) and transthyretin (Brouwer et al., 1998; Grimm et al., 2013).
Finally, these studies focus on specific congeners of OH-PCBs that would be derived from lower-chlorinated PCBs that are commonly present in air. This suggests that for some of these specific OH-PCBs there is the potential to alter concentrations of active steroid hormones through interference with their inactivation through sulfation. Important future studies will include the identification of other inhibitory OH-PCB congeners that would be metabolically derived from airborne exposures to PCBs, the concentration of those OH-PCBs in tissues, and the relative concentrations of key steroid hormones that would serve as substrates for these SULTs within tissues.
Fig. 1.
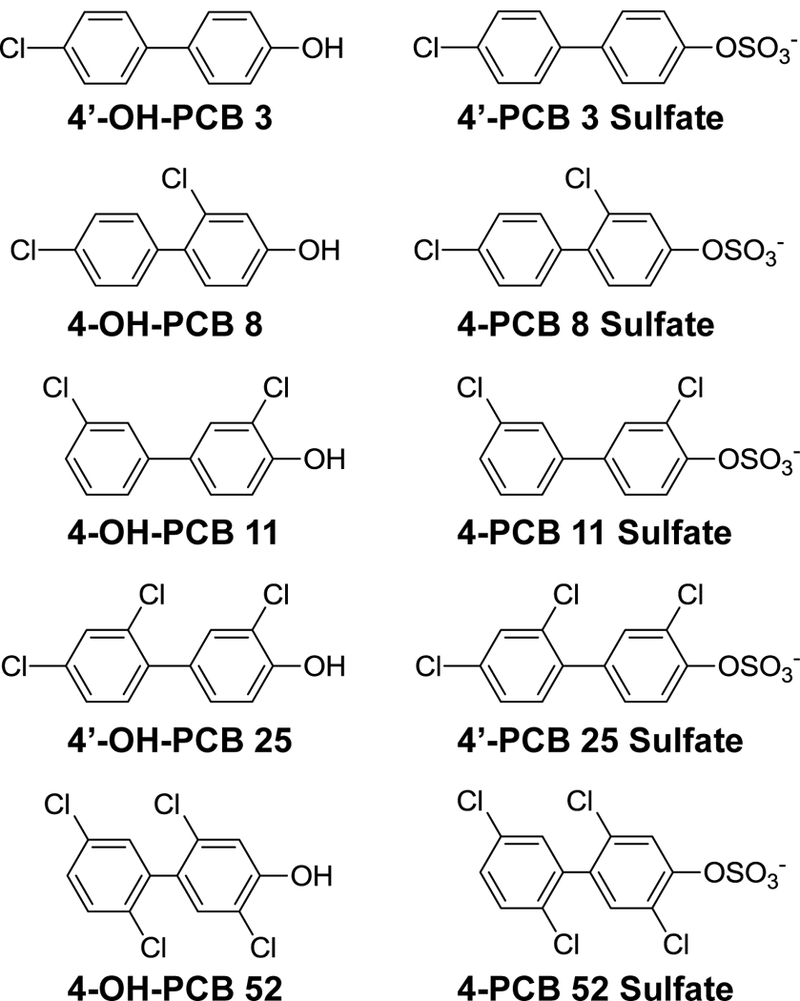
Structures of PCB Metabolites
Highlights.
Hydroxylated metabolites of airborne PCBs inhibited steroid sulfotransferases.
SULT1E1 and SULT2A1 differed in their specificities for OH-PCBs as inhibitors.
The corresponding PCB sulfates were either not inhibitory or were weak inhibitors.
Metabolites of these PCBs have the potential to affect steroid hormone signaling.
Acknowledgements
The authors thank Dr. Xueshu Li of the Synthesis Core of the Iowa Superfund Research Program for providing the PCB metabolites used in these experiments.
Funding Sources
This work was supported by NIH grant P42 ES013661 from the National Institute of Environmental Health Sciences and the University of Iowa Environmental Health Sciences Research Center (NIH P30 ES005605). Fellowship support (to V.S.P.) from the American Physiological Society (William Townsend Porter Physiology Development Fellowship) and the Iowa Biosciences Advantage Program is also gratefully acknowledged. The Iowa Biosciences Advantage program is supported by the National Institute of General Medical Sciences of the NIH (R25 GM058939), the University of Iowa Office of the Vice President for Research, and the UI Chief Diversity Office.
Abbreviations
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone sulfate
- OH-PCB
hydroxylated polychlorinated biphenyl
- 4’-OH PCB 3
4-chloro-4’-hydroxybiphenyl
- 4-OH-PCB 8
2,4’-dichloro-4-hydroxybiphenyl
- 4-OH-PCB 11
3,3’-dichloro-4-hydroxybiphenyl
- 4’-OH PCB 25
2,3’,4-trichloro-4’-hydroxybiphenyl
- 4-OH PCB 52
2,2’,5, 5’-tetrachloro-4-hydroxybiphenyl
- PAPS
adenosine 3’-phosphate 5’-phosphosulfate
- PCB
polychlorinated biphenyl
- PCB 28
2,4,4’-trichlorobiphenyl
- 4’-PCB 3 sulfate
4-chloro-4’-biphenylsulfate
- 4-PCB 8 sulfate
2,4’-dichloro-4-biphenylsulfate
- 4-PCB 11 sulfate
3,3’-dichloro-4-biphenylsulfate
- 4’-PCB 25 sulfate
2,3’,4-trichloro-4’-biphenylsulfate
- 4-PCB 52 sulfate
2,2’,5, 5’-tetrachloro-4-biphenylsulfate
- SULT
human cytosolic sulfotransferase
- SULT1E1
human estrogen sulfotransferase 1E1
- SULT2A1
human hydroxysteroid sulfotransferase 2A1
Footnotes
Conflict of Interest The authors state that there are no conflicts of interest.
References
- Ampleman MD, Martinez A, DeWall J, Rawn DF, Hornbuckle KC, Thorne PS, 2015. Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements. Environ. Sci. Technol. 49, 1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assem M, Schuetz EG, Leggas M, Sun D, Yasuda K, Reid G, Zelcer N, Adachi M, Strom S, Evans RM, Moore DD, Borst P, Schuetz JD, 2004. Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J. Biol. Chem. 279, 22250–22257. [DOI] [PubMed] [Google Scholar]
- ATSDR, 2000. Toxicological profile for polychlorinated biphenyls (PCBs). US Department of Health and Human Services, Public Health Service Agency for Toxic Substances and Disease Registry, Atlanta, GA: 17, http://www.atsdr.cdc.gov/toxprofiles/tp17.html. [PubMed] [Google Scholar]
- Brouwer A, Morse DC, Lans MC, Schuur AG, Murk AJ, Klasson-Wehler E, Bergman A, Visser TJ, 1998. Interactions of persistent environmental organohalogens with the thyroid hormone system: mechanisms and possible consequences for animal and human health. Toxicol. Ind. Health 14, 59–84. [DOI] [PubMed] [Google Scholar]
- Coughtrie MW, 2016. Function and organization of the human cytosolic sulfotransferase (SULT) family. Chem. Biol. Interact. 259, 2–7. [DOI] [PubMed] [Google Scholar]
- Dhakal K, Gadupudi GS, Lehmler HJ, Ludewig G, Duffel MW, Robertson LW, 2017. Sources and toxicities of phenolic polychlorinated biphenyls (OH-PCBs). Environ. Sci. Pollut. Res. Int doi: 10.1007/s11356-017-9694-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffel MW, 2010. Sulfotransferases, in: McQueen CA (Ed.), Comprehensive Toxicology, Vol. 4 Biotransformation (F.P. Guengerich, Vol. Ed.), 2nd ed. Elsevier, Oxford, pp. 367–384. [Google Scholar]
- Ekuase EJ, Lehmler HJ, Robertson LW, Duffel MW, 2014. Binding interactions of hydroxylated polychlorinated biphenyls (OHPCBs) with human hydroxysteroid sulfotransferase hSULT2A1. Chem. Biol. Interact. 212, 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekuase EJ, Liu Y, Lehmler HJ, Robertson LW, Duffel MW, 2011. Structure-activity relationships for hydroxylated polychlorinated biphenyls as inhibitors of the sulfation of dehydroepiandrosterone catalyzed by human hydroxysteroid sulfotransferase SULT2A1. Chem. Res. Toxicol. 24, 1720–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmlinger MW, Kuhnel W, Ranke MB, 2002. Reference ranges for serum concentrations of lutropin (LH), follitropin (FSH), estradiol (E2), prolactin, progesterone, sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), cortisol and ferritin in neonates, children and young adults. Clin. Chem. Lab. Med. 40, 1151–1160. [DOI] [PubMed] [Google Scholar]
- Erickson MD, Kaley RG, 2nd, 2011. Applications of polychlorinated biphenyls. Environ. Sci. Pollut. Res. Int. 18, 135–151. [DOI] [PubMed] [Google Scholar]
- Falany CN, Comer KA, Dooley TP, Glatt H, 1995. a. Human dehydroepiandrosterone sulfotransferase. Purification, molecular cloning, and characterization. Ann. N. Y. Acad. Sci. 774, 59–72. [DOI] [PubMed] [Google Scholar]
- Falany CN, He D, Li L, Falany JL, Wilborn TW, Kocarek TA, Runge-Morris M, 2009. Regulation of hepatic sulfotransferase (SULT) 1E1 expression and effects on estrogenic activity in cystic fibrosis (CF). J. Steroid. Biochem. Mol. Biol. 114, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falany CN, Krasnykh V, Falany JL, 1995. b. Bacterial expression and characterization of a cDNA for human liver estrogen sulfotransferase. J. Steroid. Biochem. Mol. Biol. 52, 529–539. [DOI] [PubMed] [Google Scholar]
- Flor S, He X, Lehmler HJ, Ludewig G, 2015. Estrogenicity and androgenicity screening of PCB sulfate monoesters in human breast cancer MCF-7 cells. Environ. Sci. Pollut. Res. Int. 23, 2186–2200, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamage N, Barnett A, Hempel N, Duggleby RG, Windmill KF, Martin JL, McManus ME, 2006. Human sulfotransferases and their role in chemical metabolism. Toxicol. Sci. 90, 5–22. [DOI] [PubMed] [Google Scholar]
- Gershon E, Hourvitz A, Reikhav S, Maman E, Dekel N, 2007. Low expression of COX-2, reduced cumulus expansion, and impaired ovulation in SULT1E1-deficient mice. FASEB J. 21, 1893–1901. [DOI] [PubMed] [Google Scholar]
- Glatt H, Boeing H, Engelke CE, Ma L, Kuhlow A, Pabel U, Pomplun D, Teubner W, Meinl W, 2001. Human cytosolic sulphotransferases: genetics, characteristics, toxicological aspects. Mutation Res. 482, 27–40. [DOI] [PubMed] [Google Scholar]
- Grimm FA, He X, Teesch LM, Lehmler HJ, Robertson LW, Duffel MW, 2015a. Tissue Distribution, Metabolism, and Excretion of 3,3’-Dichloro-4’-sulfooxy-biphenyl in the Rat. Environ. Sci. Technol. 49, 8087–8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm FA, Hu D, Kania-Korwel I, Lehmler HJ, Ludewig G, Hornbuckle KC, Duffel MW, Bergman A, Robertson LW, 2015b. Metabolism and metabolites of polychlorinated biphenyls. Critical Rev. Toxicol. 45, 245–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm FA, Lehmler HJ, He X, Robertson LW, Duffel MW, 2013. Sulfated metabolites of polychlorinated biphenyls are high-affinity ligands for the thyroid hormone transport protein transthyretin. Environ. Health Perspect. 121, 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulcan HO, Liu Y, Duffel MW 2008. Pentachlorophenol and other chlorinated phenols are substrates for human hydroxysteroid sulfotransferase hSULT2A1. Chem. Res. Toxicol. 21, 1503–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulcan HO, Duffel MW, 2011. Substrate inhibition in human hydroxysteroid sulfotransferase SULT2A1: studies on the formation of catalytically non-productive enzyme complexes. Arch. Biochem. Biophys. 507, 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guvenius DM, Aronsson A, Ekman-Ordeberg G, Bergman A, Noren K, 2003. Human prenatal and postnatal exposure to polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorobiphenylols, and pentachlorophenol. Environ. Health Perspect. 111, 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick RF, McClean MD, Meeker JD, Baxter LK, Weymouth GA, 2004. An unrecognized source of PCB contamination in schools and other buildings. Environ. Health Perspect. 112, 1051–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick RF, Meeker JD, Altshul L, 2011. Serum PCB levels and congener profiles among teachers in PCB-containing schools: a pilot study. Environ. Health 10, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Hornbuckle KC, 2010. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ. Sci. Technol. 44, 2822–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Lehmler HJ, Martinez A, Wang K, Hornbuckle KC, 2010. Atmospheric PCB congeners across Chicago. Atmos. Environ. 44, 1550–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihunnah CA, Wada T, Philips BJ, Ravuri SK, Gibbs RB, Kirisci L, Rubin JP, Marra KG, Xie W, 2014. Estrogen sulfotransferase/SULT1E1 promotes human adipogenesis. Mol. Cell. Biol. 34, 1682–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MO, 2014. Enzyme kinetics of conjugating enzymes: PAPS sulfotransferase. Methods Mol. Biol. 1113, 187–201. [DOI] [PubMed] [Google Scholar]
- James MO, Ambadapadi S, 2013. Interactions of cytosolic sulfotransferases with xenobiotics. Drug Metab. Rev. 45, 401–414. [DOI] [PubMed] [Google Scholar]
- Joshi SN, Vyas SM, Duffel MW, Parkin S, Lehmler H-J, 2011. Synthesis of sterically hindered polychlorinated biphenyl derivatives. Synthesis, 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester MH, Bulduk S, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Bergman A, Safe SH, Kuiper GG, Schuur AG, Brouwer A, Visser TJ, 2000. Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs. Endocrinology 141, 1897–1900. [DOI] [PubMed] [Google Scholar]
- Kester MH, Bulduk S, van Toor H, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Schuur AG, Brouwer A, Visser TJ, 2002. Potent inhibition of estrogen sulfotransferase by hydroxylated metabolites of polyhalogenated aromatic hydrocarbons reveals alternative mechanism for estrogenic activity of endocrine disrupters. J. Clin. Endocrinol. Metab. 87, 1142–1150. [DOI] [PubMed] [Google Scholar]
- Khor VK, Dhir R, Yin X, Ahima RS, Song WC, 2010. Estrogen sulfotransferase regulates body fat and glucose homeostasis in female mice. Am. J. Physiol. Endocrinol. Metab. 299, E657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh WX, Hornbuckle KC, Marek RF, Wang K, Thorne PS, 2016. Hydroxylated polychlorinated biphenyls in human sera from adolescents and their mothers living in two U.S. Midwestern communities. Chemosphere 147, 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie F, Belanger A, Cusan L, Gomez J-L, Candas B, 1997. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J. Clin. Endocrinol. Metab. 82, 2396–2402. [DOI] [PubMed] [Google Scholar]
- Labrie F, Belanger A, Simard J, Van L-T, Labrie C, 1995. DHEA and peripheral androgen and estrogen formation: intracinology. Ann. N. Y. Acad. Sci. 774, 16–28. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Labrie C, Belanger A, Simard J, Lin SX, Pelletier G, 2003. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr. Rev. 24, 152–182. [DOI] [PubMed] [Google Scholar]
- Lehmler HJ, He XR, Li XS, Duffel MW, Parkin S, 2013. Effective synthesis of sulfate metabolites of chlorinated phenols. Chemosphere 93, 1965–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmler HJ, Robertson LW, 2001. Synthesis of hydroxylated PCB metabolites with the Suzuki coupling. Chemosphere 45, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Li X, Parkin S, Duffel MW, Robertson LW, Lehmler H-J 2010. An efficient approach to sulfate metabolites of polychlorinated biphenyls. Environ. Int. 36, 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Apak TI, Lehmler HJ, Robertson LW, Duffel MW, 2006. Hydroxylated polychlorinated biphenyls are substrates and inhibitors of human hydroxysteroid sulfotransferase SULT2A1. Chem. Res. Toxicol. 19, 1420–1425. [DOI] [PubMed] [Google Scholar]
- Meerts IA, Lilienthal H, Hoving S, van den Berg JH, Weijers BM, Bergman A, Koeman JH, Brouwer A, 2004. Developmental exposure to 4-hydroxy-2,3,3’,4’,5-pentachlorobiphenyl (4-OH-CB107): long-term effects on brain development, behavior, and brain stem auditory evoked potentials in rats. Toxicol. Sci 82, 207–218. [DOI] [PubMed] [Google Scholar]
- Quinete N, Esser A, Kraus T, Schettgen T, 2017. PCB 28 metabolites elimination kinetics in human plasma on a real case scenario: Study of hydroxylated polychlorinated biphenyl (OH-PCB) metabolites of PCB 28 in a highly exposed German Cohort. Toxicol. Lett. 276, 100–107. [DOI] [PubMed] [Google Scholar]
- Quinete N, Schettgen T, Bertram J, Kraus T, 2014. Occurrence and distribution of PCB metabolites in blood and their potential health effects in humans: a review. Environ. Sci. Pollut. Res. Int. 21, 11951–11972. [DOI] [PubMed] [Google Scholar]
- Robertson LW, Ludewig G, 2011. Polychlorinated Biphenyl (PCB) carcinogenicity with special emphasis on airborne PCBs. Gefahrst Reinhalt Luft 71, 25–32. [PMC free article] [PubMed] [Google Scholar]
- Rodriguez EA, Li X, Lehmler HJ, Robertson LW, Duffel MW, 2016. Sulfation of Lower Chlorinated Polychlorinated Biphenyls Increases Their Affinity for the Major Drug-Binding Sites of Human Serum Albumin. Environ. Sci. Technol. 50, 5320–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner W, Hankinson SE, Sluss PM, Vesper HW, Wierman ME, 2013. Challenges to the measurement of estradiol: an endocrine society position statement. J. Clin. Endocrinol. Metab. 98, 1376–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandau CD, Ayotte P, Dewailly E, Duffe J, Norstrom RJ, 2000. Analysis of hydroxylated metabolites of PCBs (OH-PCBs) and other chlorinated phenolic compounds in whole blood from Canadian Inuit. Environ. Health Perspect. 108, 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuur AG, van Leeuwen-Bol I, Jong WM, Bergman A, Coughtrie MW, Brouwer A, Visser TJ, 1998. In vitro inhibition of thyroid hormone sulfation by polychlorobiphenylols: isozyme specificity and inhibition kinetics. Toxicol. Sci. 45, 188–194. [DOI] [PubMed] [Google Scholar]
- Sekura RD, 1981. Adenosine 3’-phosphate 5’-phosphosulfate. Methods Enzymol. 77, 413–415. [Google Scholar]
- Shanahan CE, Spak SN, Martinez A, Hornbuckle KC, 2015. Inventory of PCBs in Chicago and Opportunities for Reduction in Airborne Emissions and Human Exposure. Environ. Sci. Technol. 49, 13878–13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JJ, Sharma V, Duffel MW, 2001. Measurement of aryl and alcohol sulfotransferase activity. Current Protocols Toxicol. Chapter 4, Unit 4 5. [DOI] [PubMed] [Google Scholar]
- Squirewell EJ, Duffel MW, 2015. The effects of endoxifen and other major metabolites of tamoxifen on the sulfation of estradiol catalyzed by human cytosolic sulfotransferases hSULT1E1 and hSULT1A1*1. Drug Metab. Dispos. 43, 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squirewell EJ, Qin XY, Duffel MW, 2014. Endoxifen and Other Metabolites of Tamoxifen Inhibit Human Hydroxysteroid Sulfotransferase 2A1 (hSULT2A1). Drug Metab. Dispos. 42, 1843–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong MH, Jiang H, Liu P, Lawson JA, Brass LF, Song WC, 2005. Spontaneous fetal loss caused by placental thrombosis in estrogen sulfotransferase-deficient mice. Nat. Med. 11, 153–159. [DOI] [PubMed] [Google Scholar]
- Wada T, Ihunnah CA, Gao J, Chai X, Zeng S, Philips BJ, Rubin JP, Marra KG, Xie W, 2011. Estrogen sulfotransferase inhibits adipocyte differentiation. Mol. Endocrinol. 25, 1612–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LQ, James MO, 2007. Sulfonation of 17beta-estradiol and inhibition of sulfotransferase activity by polychlorobiphenylols and celecoxib in channel catfish, Ictalurus punctatus. Aquat. Toxicol. 81, 286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LQ, Lehmler HJ, Robertson LW, Falany CN, James MO, 2005. In vitro inhibition of human hepatic and cDNA-expressed sulfotransferase activity with 3-hydroxybenzo[a]pyrene by polychlorobiphenylols. Environ. Health Perspect. 113, 680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LQ, Lehmler HJ, Robertson LW, James MO, 2006. Polychlorobiphenylols are selective inhibitors of human phenol sulfotransferase 1A1 with 4-nitrophenol as a substrate. Chem. Biol. Interact. 159, 235–246. [DOI] [PubMed] [Google Scholar]
- White BA, Porterfield SP, 2013. Endocrine and Reproductive Physiology, 4th ed. Mosby, Philadelphia, PA, pp. 147–176. [Google Scholar]
- Xu Y, Yang X, Wang Z, Li M, Ning Y, Chen S, Yin L, Li X, 2013. Estrogen sulfotransferase (SULT1E1) regulates inflammatory response and lipid metabolism of human endothelial cells via PPARgamma. Mol. Cell. Endocrinol. 369, 140–149. [DOI] [PubMed] [Google Scholar]
- Zhang H, Varlamova O, Vargas FM, Falany CN, Leyh TS, 1998. Sulfuryl transfer: the catalytic mechanism of human estrogen sulfotransferase. J. Biol. Chem. 273, 10888–10892. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Mapuskar KA, Marek RF, Xu W, Lehmler HJ, Robertson LW, Hornbuckle KC, Spitz DR, Aykin-Burns N, 2013. A new player in environmentally induced oxidative stress: polychlorinated biphenyl congener, 3,3’-dichlorobiphenyl (PCB11). Toxicol. Sci. 136, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


