Abstract
Correct sorting of proteins is essential to generate and maintain the identity and function of the different cellular compartments. In this study we demonstrate the role of lipid rafts in biosynthetic delivery of Pma1p, the major plasma membrane proton ATPase, to the cell surface. Disruption of rafts led to mistargeting of Pma1p to the vacuole. Conversely, Pma1-7, an ATPase mutant that is mistargeted to the vacuole, was shown to exhibit impaired raft association. One of the previously identified suppressors, multicopy AST1, not only restored surface delivery but also raft association of Pma1-7. Ast1p, which is a peripheral membrane protein, was found to directly interact with Pma1p inducing its clustering into a SDS/Triton X100-resistant oligomer. We suggest that clustering facilitates partition of Pma1p into rafts and transport to the cell surface.
INTRODUCTION
The organization of the secretory pathway of budding yeast Saccharomyces cerevisiae is characterized by the key feature that growth and secretion are coupled. Thus, addition of new membrane contributes to cell growth. At least two constitutive pathways originating in the Golgi complex transport proteins to the plasma membrane and to the extracellular medium (Harsay and Bretscher, 1995). The third major destination from the Golgi, involving at least three biosynthetic routes, is the vacuole. In yeast the vacuole serves complex functions such as osmoregulation, protein degradation, storage of amino acids, ions, and polyphosphates (Bryant and Stevens, 1998).
Correct localization of membrane proteins to their respective compartments is essential for the generation and maintenance of the different organelles. Specific and regulated mechanisms ensure proper sorting of membrane proteins. Although sorting determinants and delivery machinery for vacuolar membrane protein targeting have been identified (Cowles et al., 1997; Piper et al., 1997; Black and Pelham, 2000), the mechanisms for sorting proteins to the cell surface are still poorly defined (Stack et al., 1995). Recent experiments are starting to give insight into the processes involved. It has been shown that transmembrane domain-dependent sorting determines the final destination of some membrane proteins (Rayner and Pelham, 1997; Black and Pelham, 2000). Furthermore, association with lipid rafts has also been shown to play a role in biosynthetic delivery to the plasma membrane in yeast (Bagnat et al., 2000).
Lipid rafts, formed by lateral association of sphingolipids and cholesterol, were first conceived in mammalian MDCK cells as platforms for polarized lipid and protein sorting (Simons and Ikonen, 1997; Simons and van Meer, 1988). Their insolubility in cold nonionic detergents such as Triton X-100 (TX100) made isolation and biochemical characterization possible (Brown and Rose, 1992). Comprehensive work has established a role for lipid rafts in membrane traffic in different cell types (Brown and Rose, 1992; Keller and Simons, 1998; Lafont et al., 1998; Ledesma et al., 1998). Furthermore, a role for lipid rafts as signaling platforms has been proposed as a fundamental element of numerous signal transduction cascades and is thought to be crucial for immune system activation (reviewed by Simons and Toomre, 2000; and Dykstra et al., 2001).
In yeast, lipid rafts are composed of phosphoinositol-based sphingolipids and ergosterol and seem to be destined to the surface but not to the vacuole (Bagnat et al., 2000). In contrast to their mammalian counterparts, assembly of rafts starts early; glycophosphatidylinositol (GPI)-anchored proteins associate with rafts already in the endoplasmic reticulum (ER; Bagnat et al., 2000). The functional consequence of this may be the early segregation of GPI-anchored proteins from other secreted proteins (Muñiz et al., 2001).
In this work we have explored the role of lipid rafts in sorting of plasma membrane H+-ATPase (Pma1p) to the cell surface. We found that upon disruption of rafts, Pma1p was delivered to the vacuole where it was degraded in a PEP4-dependent manner. Importantly, a temperature-sensitive ATPase mutant (pma1-7) that is missorted to vacuole (Chang and Fink, 1995) was not able to associate with lipid rafts. This allowed us to identify one of the previously isolated suppressors, multicopy AST1, as a protein involved in Pma1p association with lipid rafts. Direct interaction with Ast1p and subsequent clustering restored mutant Pma1-7 interaction with detergent-resistant membranes (DRMs) and delivery to the cell surface. Thus, we have identified a mechanism involved in lipid raft association and surface delivery.
MATERIALS AND METHODS
Yeast Strains, Plasmids, and Media
Media were prepared as described previously (Sherman et al., 1986). Strains and plasmids used in this study are described in Table 1. MBY208 was generated in a one-step gene replacement by transformation of RH3804 with p1513, an URA3-marked PEP4 disruption construct provided by W. Zachariae (MPI-CBG, Dresden, Germany). ACX58 was made by transforming ACX28 with pCKR3A, a vps1Δ::LEU2 disruption construct (Nothwehr et al., 1995). ACX28 is a cross between ACY7 and L4364. WLY62 was made by directly transforming L3852 with pCKR3A.
Table 1.
Yeast strains used in this study
| Yeast strain | Genotype | Source |
|---|---|---|
| RH690-15D | Mata his4 leu2 ura3 lys2 bar1 | H. Riezman lab |
| RH3804 | Mata lcb1-100 trp1 leu2 ura3 lys2 bar1 | H. Riezman lab |
| MBY208 | Mata lcb1-100 trp1 leu2 ura3 lys2 bar1 pep4∷URA3 | This study |
| NY179 | Mata leu2-3,112 ura3-52 | P. Novick lab |
| NY778 | Mata sec6-4 leu2-3,112 ura3-52 | P. Novick lab |
| H891 | Mata sec18-1 trp1-289 leu2-3,112 ura3-52 his | S. Keranen lab |
| L3852 | Matα his3Δ200 lys2Δ201 leu2-3,112 ura3-52 ade2 | G. Fink lab |
| L4364 | Mata his3Δ200 lys2Δ201 leu2-3,112 ura3-52 ade2 | G. Fink lab |
| ACY7 | Matα his3Δ200 lys2Δ201 leu2-3,112 ura3-52 ade2 pma1-7 | Chang and Fink (1995) |
| ACY12 | Matα his3Δ200 lys2Δ201 leu2-3,112 ura3-52 ade2 pma1-7 pep4∷URA3 | Chang and Fink (1995) |
| ACX28 | Mata his3Δ200 lys2Δ201 leu2-3,112 ura3-52 ade2 pma1-7 | This study |
| ACX58 | Mata his3Δ200 lys2Δ201 leu2-3,112 ura3-52 ade2 pma1-7 vps1Δ∷LEU2 | This study |
| WLY62 | Mata his3Δ200 lys2Δ201 leu2-3,112 ura3-52 ade2 vps1Δ∷LEU2 | This study |
| RSY1396 | Mata lys2Δ201 leu2-3,112 ura3-52 ade2 gap1Δ∷LEU2 | Ljungdahl et al. (1992) |
| Plasmid | Source | |
| p1513 | pep4∷URA3 | W. Zachariae |
| pPL296 | GAP1:HA LYS2 CEN | Ljungdahl et al. (1992) |
| pCKR3A | vps1Δ∷LEU2 | Nothwehr et al. (1995) |
| pAC49 | AST1 URA3 2 μ | Chang and Fink (1995) |
| pAC64 | AST1∷myc3 LEU2 CEN | Chang and Fink (1995) |
SDS-PAGE
If samples were boiled, Pma1p aggregated. Therefore, for analysis of Pma1p samples were only preheated to 37°C for 10 min before SDS-PAGE as described (Bagnat et al., 2000).
Immunoprecipitation, DRM Association, and Determination of Ergosterol in DRMs
Pma1p was immunoprecipitated with anti-Pma1p–specific antibodies (a gift of R. Serrano, UPV, Valencia, Spain) as described previously (Chang and Slayman, 1991). When Pma1p was immunoprecipitated from Optiprep (Nycomed, Oslo, Norway) density gradients, samples were diluted twofold and adjusted to 1% NP40, 1% Na-deoxycholate, 0.1% SDS, and 2 mM EDTA. For immunoprecipitation Ast1-myc samples were adjusted to 1% NP40, 0.1% SDS, 150 mM NaCl, and 2 mM EDTA (TNPS buffer) and incubated with anti–c-Myc rabbit polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) and protein A-Sepharose (Pharmacia, Piscataway, NJ) for 3 h. Immunoprecipitates were washed three times with TNPS and once with 20 mM Tris, pH 7.4. DRM association was done essentially as described previously (Bagnat et al., 2000). To simplify the analysis, gradient fractions containing detergent-resistant and soluble material were pooled (R and S, respectively) before processing by Western blotting or immunoprecipitation. Quantification of DRM association was done by metabolic labeling with [35S]methionine (NEN, Boston, MA) and SDS-PAGE/phosphorimaging analysis. To quantify the ergosterol in DRMs in subcellular fractions, cells were grown in rich medium and the lysates were subjected to the fractionation procedure used for the plasma membrane delivery assay (see below). PM and E/V fractions were recovered by diluting threefold in water and pelleting at 100,000 × g for 1 h at 4°C. PM, E/V, and P20 pellets were resuspended in TNE buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 5 mM EDTA) and incubated with buffer or 1% TX100 at 4°C for 30 min. Then, samples were subjected to Optiprep density gradient floatation as before, and sterols were extracted from the top fractions of the gradients as described previously (Bagnat et al., 2000). The dried lipids were resuspended in methanol, and free sterols were quantified with the use of the Amplex red cholesterol assay kit from Molecular Probes (Eugene, OR) in a microplate fluorometer (Labsystems, Franklin, MA). The percentage of free sterol in DRMs is the ratio of free sterol in the top fraction of the TX100-containing gradient to the one containing buffer only.
Plasma Membrane Delivery
The plasma membrane delivery assay was derived from a plasma membrane purification protocol developed by R. Serrano (1988). Cells (∼5 OD600) were grown in rich medium at 24°C to 1 OD600/ml, washed in synthetic medium (Bio101, Carlsbad, CA), resuspended in 3 ml of fresh methionine-free medium, and preincubated for 5 min at the indicated temperatures. Then, cells were pulse-labeled with 0.5 mCi [35S]methionine for 10 min and chased for 30 min at the indicated temperatures. Labeling was terminated by addition of NaN3 to 0.02%. Cells were lysed in 0.75 ml of buffer L (25 mM Tris, pH 8, 2.5 mM EDTA) with a protease inhibitor cocktail (1 mM PMSF, 2.5 μg/ml chymostatin, leupeptin, antipain, and pepstatin) by vortexing with glass beads. The lysates were then cleared of unbroken cells by centrifugation (700 × g for 10 min) and subjected to differential centrifugation at 20,000 × g for 20 min in a TLA45 rotor (Beckman Instruments, Fullerton, CA). The supernatants were then discarded, and the pellets (P20) were resuspended in 0.55 ml of 20% glycerol in buffer B (10 mM Tris, pH 7.4, 0.2 mM EDTA, 0.2 mM DTT), and 0.5 ml was loaded on top of a sucrose-step gradient (0.5 ml 53%, 1 ml, 43%, in buffer B). After centrifugation (2 h at 100,000 × g in TLS55 rotor; Sorvall, Newton, CT), six 320-μl fractions were collected from the top. Pma1p distribution was analyzed by immunoprecipitation, SDS-PAGE, and phosphorimaging.
Cross-linking
Cells expressing myc-tagged AST1 (pAC64) were grown at 24°C in rich medium and incubated for 10 min with NaN3 0.02% on ice. After washing with water, cells were lysed by vortexing with glass beads in 0.45 ml of PBS (150 mM NaCl, 20 mM Na-phosphate, pH 7.5). Then, clear lysates (300 μl) were mixed with the membrane-permeable cross-linker (dithiobis(succinimidylpropionate [DSP], 12 μl 10 mg/ml in DMSO; Pierce, Rockford, IL) and incubated for 2 h on ice. The reaction was terminated by a 15-min incubation with Tris, pH 8 (50 mM final), and 1 mM PMSF. Ast1p was immunoprecipitated with a rabbit polyclonal anti-myc antibody or coimmunoprecipitated with anti-Pma1p and subsequently detected with a mouse monoclonal anti-myc antibody.
Subcellular Fractionation
Fractionation was done essentially as described previously (Roberg et al., 1999). Briefly, cleared lysates were loaded on top of a 20–60% linear sucrose gradient and centrifuged at 100,000 × g for 18 h in a SW60 rotor (Beckman). Then 0.35-ml fractions were collected from the top, and the distribution of different organelle markers was determined by conventional Western blotting with specific antibodies: anti-Pma1p; anti-Tlg1p, and anti-Sed5p (a gift from H. Pelham, MRC, Cambridge, England), anti-Sec61p (a gift from S. Nock, Howard Hughes Medical Institute, San Francisco, CA); anti-Pep12p, and anti-Vph1p (Molecular Probes); and antic-Myc monoclonal 9E10 (Santa Cruz).
SDS/TX100 Velocity Gradients
Cell lysates were adjusted to 0.4% SDS and 0.2% TX100 and immediately loaded on top of a 5–20% linear sucrose velocity gradient containing detergent and centrifuged in a SW60 rotor (Beckman) for 16 h at 215.000 × g at 4°C. Fractions were collected from the top, and Pma1p distribution was analyzed by Western blotting. The approximate size was determined with the use of markers of known size (transferrin, catalase, ferritin, and thyroglobulin) as described previously (Scheiffele et al., 1996).
RESULTS
Pma1p Associates with Lipid Rafts in the Golgi Complex
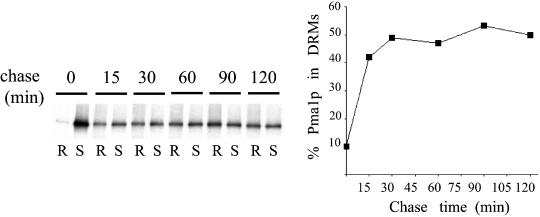
To gain insight into the process of lipid raft biogenesis, we decided to follow Pma1p during biosynthetic transport. Pma1p is one of the most abundant plasma membrane proteins (Serrano et al., 1986) and one of the major components of lipid rafts in yeast (Bagnat et al., 2000). We first determined the kinetics of Pma1p incorporation into (DRMs. Cells were pulse-labeled with [35S]methionine for 5 min at 30°C and chased for various times. Aliquots were taken, and cell lysates were treated with TX100 for 30 min at 4°C and subjected to Optiprep density gradient centrifugation. DRMs float to a light density fraction, whereas detergent-soluble proteins remain in the bottom fractions. Pma1p was then immunoprecipitated from detergent resistant (R) and soluble (S) fractions and analyzed by SDS-PAGE and phosphorimaging. Pma1p incorporation into DRMs was rapid and almost complete by 15 min, reaching a plateau after 30 min (Figure 1).
Figure 1.
Raft association of Pma1p during biosynthetic transport. WT (RH690-15D) cells were radiolabeled with [35S]methionine for 5 min and chased for various times. Aliquots corresponding to the different time points were subjected to TX100 extraction, 1% at 4°C, and Optiprep density gradient centrifugation. Pma1p was immunoprecipitated from detergent-resistant (R) and soluble fractions (S) and analyzed by SDS-PAGE and phosphorimaging (right panel). Raft association is expressed as the percentage of total Pma1p present in the detergent insoluble fraction.
To test whether this fast kinetics was a result of raft association in the ER, as found before for GPI-anchored proteins (Bagnat et al., 2000) or in a later compartment, we performed a pulse-chase experiment in wild-type and sec18-1 mutant cells, in which ER-to-Golgi traffic is blocked at 37°C. After a short, 5-min pulse at 24°C a small fraction of Pma1p was found in DRMs in wild-type and sec18-1 mutant cells. The fraction of Pma1p in DRMs was increased to nearly steady state levels after 45 min of chase at 24°C in both types of cells. In contrast, at 37°C little Pma1p associated with DRMs in sec18-1 mutant cells (Figure 2A). Thus, unlike GPI-anchored proteins, raft association of Pma1p seems to take place mainly after ER exit. To test whether Pma1p associates with DRMs before arrival to the surface, we used sec6-4 mutant cells to block fusion of secretory vesicles with the plasma membrane. Either at permissive or restrictive temperature both in wild-type and sec6-4 mutant cells, Pma1p was found in DRMs (Figure 2B). Taken together, these data shows that Pma1p associates with rafts in the Golgi complex.
Figure 2.
Newly synthesized Pma1p associates with rafts, mainly, in the Golgi. (A) WT (NY179) and sec18 (H891) cells were radiolabeled for 5 min at 24 or 37°C and chased for 0 or 45 min at 24 or 37°C. After TX100 extraction (1% at 4°C) and density gradient centrifugation, Pma1p was immunoprecipitated from detergent-resistant (R) and soluble (S) fractions and analyzed by SDS-PAGE and phosphorimaging. ER-to-Golgi transport was monitored by following Gas1p processing from precursor (p) to mature (m) form (right panel). (B) WT (NY179) and sec6-4 (NY778) cells were radiolabeled for 30 min at 24 or 37°C and chased for 1 h at 24 or 37°C. Raft association of Pma1p was analyzed as in A.
Lipid Rafts Are Required for Plasma Membrane Sorting of Pma1p
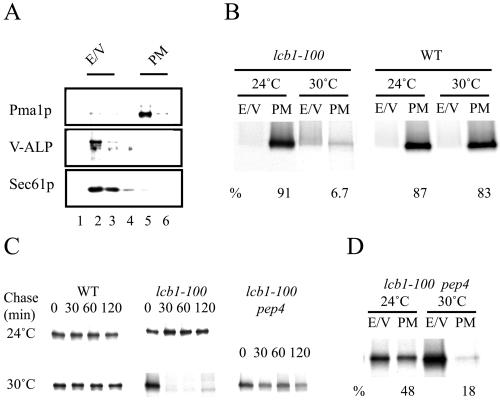
In sphingolipid-deficient strains, proton extrusion at low pH is impaired (Patton et al., 1992). This could be due to deficient transport of Pma1p to the surface. To explore the role of lipid rafts in Pma1p sorting, we used the lcb1-100, temperature-sensitive mutant, which cannot synthesize sphingolipids and disrupts Pma1p association with rafts upon shift to restrictive temperature (Bagnat et al., 2000) and adapted a plasma membrane purification protocol (Serrano, 1988) for our transport assay. After differential centrifugation and sucrose density gradient centrifugation, plasma membrane can be efficiently separated from ER and vacuole (Figure 3A). Golgi and endosomal elements are mostly excluded during the first centrifugation. Wild-type and lcb1-100 mutant cells were pulse-labeled for 10 min and chased for 30 min at 24 or 30°C. After fractionation, Pma1p was immunoprecipitated from plasma membrane (PM) and ER-vacuole (E/V) density gradients fractions and analyzed by SDS-PAGE and phosphorimaging. At 24°C in both types of cells the bulk of Pma1p was found in the plasma membrane fraction as expected. In contrast, at 30°C in lcb1-100, but not in wild-type cells, only a small fraction of the ATPase could reach the plasma membrane and was mostly degraded (Figure 3B). Next, we tested the possibility that degradation of newly synthesized Pma1p in lcb1-100 cells could be the result of missorting to the vacuole. To this end we deleted the PEP4 gene to inactivate vacuolar proteases and performed a pulse-chase experiment. Pma1p was degraded in lcb1-100 cells at 30°C as before but remained stable in the lcb1-100 pep4Δ double mutant (Figure 3C). To further confirm that Pma1p was delivered to the vacuole upon disruption of lipid rafts, we analyzed the transport of Pma1p in the lcb1-100 pep4Δ strain. On shift to nonpermissive conditions, the newly synthesized ATPase was found mostly in the E/V fraction (Figure 3D), thus reversing the distribution of wild-type cells. These data demonstrate that lipid rafts are required for plasma membrane delivery of Pma1p.
Figure 3.
PM delivery of Pma1p in lcb1-100 mutant. (A) Distribution of organelle markers in the last step of subcellular fractionation. Cells were lysed, and after clearing from cell debris, a 20,000 × g pellet (P20) was generated that was subsequently resuspended in 20% glycerol and loaded on top of a sucrose step gradient and centrifuged for 2 h at 100,000 × g. Then, six fractions were collected from the top, and the distribution of Pma1p, Sec61p, and vacuolar alkaline phophatase was analyzed by Western blotting. (B) PM delivery of Pma1p. WT (RH690-15D) and lcb1-100 (RH3804) mutant cells were radiolabeled for 10 min at 24 or 30°C and chased for 30 min at 24 or 30°C, and the lysates were fractionated as previously described. Pma1p was immunoprecipitated from fractions 2-3 (E/V) and 5-6 (PM) and analyzed by SDS-PAGE and phosphorimaging; the percentage of Pma1p in the PM fraction is indicated. ER and vacuole markers fractionated similarly in WT and lcb1-100 at 24 and 30°C. (C) Degradation of newly synthesized Pma1p in lcb1-100 cells is PEP4 dependent. WT (RH690-15D), lcb1-100 (RH3804), and lcb1-100 pep4 (MBY208) cells were radiolabeled for 10 min at 24 or 30°C and chased for 0, 30, 60, and 120 min at 24 or 30°C. Pma1p was immunoprecipitated from cell lysates and analyzed by SDS-PAGE and autoradiography. (D) Sorting of Pma1p in lcb1-100 pep4 cells. Cells (MBY208) were radiolabeled for 10 min at 24 or 30°C and chased for 30 min at 24 or 30°C. Cell lysates were fractionated as before and Pma1p was immunoprecipitated from E/V and PM fractions and analyzed by SDS-PAGE and phosphorimaging.
Lipid Rafts Are the Major Phase in the Plasma Membrane
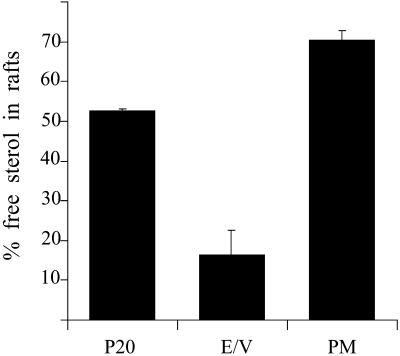
Two findings suggest that lipid rafts may be low in vacuoles. Firstly, vacuolar membrane proteins, in contrast to the plasma membrane proteins, are completely excluded from DRMs (Bagnat et al., 2000); second, the sphingolipid content of vacuoles is low (Hechtberger et al., 1994). To test this hypothesis more directly, we analyzed the detergent-insolubility of ergosterol in subcellular fractions. Cells were lysed and subjected to the fractionation protocol outlined above. Membranes containing both E/V and PM (P20) and the separated E/V and PM fractions were diluted and recovered by pelleting. Then, the membranes were resuspended and incubated with 1% TX100 or buffer at 4°C for 30 min and subjected to Optiprep density gradient centrifugation. Sterols were extracted from the top fractions, dried, and quantified. Although 75% of the free sterol present in the PM fraction was found in DRMs, only 15% of the E/V-free sterol was raft associated (Figure 4). Thus, in the plasma membrane, lipid rafts seem to be the major lipid phase in contrast to the E/V, where they constitute a minor phase.
Figure 4.
Raft association of free sterols in subcellular fractions. WT (RH690-15D) cells were grown in YPD medium at 24°C to mid log phase. Cell lysates were fractionated as in Figure 3, P20, E/V and PM membranes were diluted threefold in water and pelleted at 100,000 × g for 30 min. After treatment with TX100 or buffer, samples were subjected to Optiprep density gradient centrifugation. Sterols were extracted from the top fraction as described and determined with the use of the Amplex red cholesterol assay kit from Molecular Probes. The percentage of sterol in rafts was calculated as the ratio of sterol in the TX100 treated sample to the buffer treated one (n = 3).
A Vacuole-targeted Mutant ATPase Has Impaired Raft Association
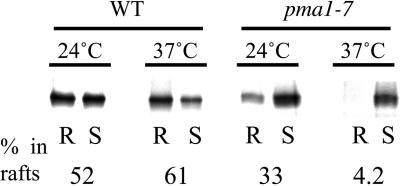
Because Pma1p is an essential protein, isolation of temperature-sensitive mutants that exhibit defective trafficking has been possible (Chang and Fink, 1995). Newly made Pma1 in one such conditional mutants, pma1-7, is delivered directly to the vacuole (Chang and Fink, 1995). Pma1-7 has two mutations: Pro434→Ala, near the catalytic domain, and Gly789→Ser, in a cytoplasmic loop between transmembrane segments 8 and 9 (Chang and Fink, 1995). With the use of this mutant we wanted to further test the role of lipid rafts in Pma1p sorting. Wild-type and pma1-7 mutant cells were pulse labeled for 10 min and chased for 30 min. Then, after TX100 treatment and Optiprep density gradient centrifugation, the ATPase was immunoprecipitated and analyzed as before. DRM association in pma1-7 mutant cells was significantly reduced at 24°C compared with wild-type cells and almost absent at 37°C (Figure 5). These data show that the mutant ATPase has defective raft association.
Figure 5.
Vacuole targeted H+-ATPase mutant has a low affinity for rafts. WT (L3852) and pma1-7 (ACY7) cells were radiolabeled for 10 min at 24°C and chased for 30 min at the same temperature. After TX100 extraction (1% at 4°C) and density gradient centrifugation the ATPase was immunoprecipitated from detergent resistant (R) and soluble (S) fractions and analyzed by SDS-PAGE and phosphorimaging.
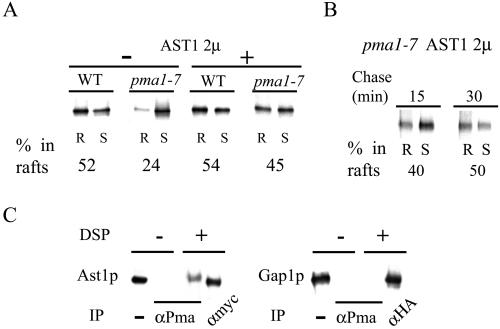
Ast1p Directly Interacts with Pma1p and Can Restore Raft Association of Mutant ATPase When Overexpressed
The fact that Pma1-7 is enzymatically active (Chang and Fink, 1995) allowed the isolation of suppressors that can redirect the mutant protein to the surface (Chang and Fink, 1995; Luo and Chang, 1997). Multicopy AST1 has been shown to suppress pma1-7 by routing mutant ATPase to the surface (Chang and Fink, 1995). This prompted us to investigate whether multicopy AST1 could also restore raft association of Pma1p. To do so, we pulse-labeled wild-type and pma1-7 cells with and without multicopy AST1 at 24°C for 10 min and chased them at the same temperature for 30 min. Then, DRM association of Pma1p was analyzed as before. Overexpression of Ast1p restored raft association of mutant ATPase to nearly wild-type levels but had no detectable effect in wild-type cells (Figure 6A). Raft association of mutant ATPase seemed to happen in the same compartment as that of the wild-type counterpart, because the effect was evident already after 15 min of chase (cf. Figures 1 and 6B).
Figure 6.
AST1 overexpression restores raft-association of mutant ATPase. (A) Raft association of ATPase in WT (L3852) and pma1-7 (ACY7) cells at 24°C. ATPase raft association, with and without high copy AST1 (pAC49), was determined after radiolabeling and immunoprecipitation from TX100 containing density gradients as before. (B) Mutant ATPase raft association in the presence of high copy AST1. pma1-7 (ACY7) cells harboring the pAC49 plasmid were radiolabeled for 10 min at 24°C and chased for 15 or 30 min at 24°C. Raft association of newly synthesized ATPase was determined as in A. (C) Protein–protein interaction between Ast1p and Pma1p. WT (L3852) cells expressing epitope-tagged AST1 (pAC64) grown at 24°C were lysed and incubated on ice with or without the cross-linker DSP for 2 h. Ast1p was immunoprecipitated with a rabbit polyclonal anti-myc antibody or coimmunoprecipitated with anti-Pma1p antibodies and subsequently detected with a mouse monoclonal anti-myc antibody. Ast1p is coimmunoprecipitated with Pma1p after treatment with DSP, the slightly lower mobility of Ast1p is probably due to cross-linking. Cells expressing Gap1-HA were grown in media containing urea as nitrogen source and subjected to the same procedure. Gap1-HA was immunoprecipitated with anti-HA–tag rabbit polyclonal antibodies or with anti-Pma1p antibodies and subsequently detected with a mouse monoclonal anti-HA–tag antibody.
Ast1p behaves like a peripheral membrane protein (Chang and Fink, 1995). To test whether Ast1p is raft associated, we radiolabeled wild-type and pma1-7 mutant cells, expressing a myc-tagged version of Ast1p from a low copy vector, at 24 or 37°C and analyzed its partition into DRMs as before. A small fraction of Ast1 (∼10%) was found in DRMs in both cell types.
To find out whether Ast1p restored raft association of mutant ATPase by direct interaction, we grew wild-type cells expressing Ast1-myc and incubated cell lysates with the membrane-permeable cross-linker DSP. Ast1-myc was immunoprecipitated with rabbit anti-myc antibodies or coimmunoprecipitated with anti-Pma1p–specific antibodies and detected with an anti-myc mouse mAb. A fraction of Ast1-myc coimmunoprecipitated with Pma1p after cross-linking (Figure 6C, left), indicating that both proteins interact directly with each other. As a negative control we performed the same experiment in cells expressing HA-tagged Gap1p that were grown in media containing urea as the nitrogen source to allow efficient surface delivery (Roberg et al., 1999). In contrast to Ast1p, Gap1-HA did not coimmunoprecipitate with Pma1p after cross-linking (Figure 6C, right), indicating that the interaction between Ast1p and Pma1p is not result of the abundance of Pma1p.
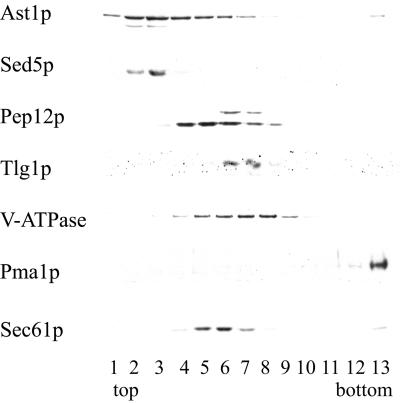
We, then examined the subcellular distribution of Ast1p with the use of sucrose density gradient fractionation. When expressed at low levels the peak of Ast1-myc cofractionated with Sed5p, an early Golgi marker, and a small fraction was also detected in the plasma membrane (Figure 7).
Figure 7.
Subcellular localization of Ast1p. WT (L3852) cells expressing epitope-tagged AST1 from a centromeric plasmid (pAC64) were lysed and fractionated in a 20–60%sucrose gradient (Roberg et al., 1999). Distribution of different organelle markers was visualized by Western blotting with the use of specific antibodies. The peak of Ast1p, detected with the use of an anti-myc antibody, coincides with that of Sed5p, an early Golgi marker, lower amounts are also found in fractions containing Pep12p, a late endosome marker, and to a small extent in fractions containing Pma1p, a plasma membrane marker.
Taken together these data indicate that Ast1p directly interacts with Pma1p, possibly in the Golgi, to restore raft association of mutant ATPase, thus correctly routing the protein to the plasma membrane in a lipid raft-dependent manner.
Restoration of Mutant ATPase Association with Lipid Rafts Is Not a Result of Surface Delivery
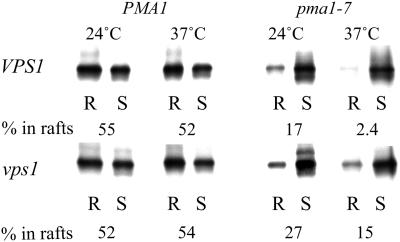
To find out whether the restoration of Pma1-7 association with rafts could simply be due to increased surface delivery, we made use of the vps1Δ mutant. VPS1 is required for Golgi to endosome traffic (Rothman et al., 1990), in its absence traffic toward the endosomes is deflected to surface (Nothwehr et al., 1995). Mutant ATPase is also delivered to the surface in the vps1 mutant where it remains stable (Luo and Chang, 2000). DRM association of wild-type and mutant ATPase was examined in VPS1 and vps1Δ mutant background at 24 and 37°C. As before, Pma1-7 showed impaired DRM association in VPS1 cells compared with Pma1p. In the vps1Δ mutant background only 27% of Pma1-7 partitioned into DRMs. Thus, unlike multicopy AST1, raft association was not restored to wild-type levels (cf. Figures 8 and 6A). This experiment shows that surface delivery of the mutant ATPase does not necessarily lead to increased raft association and further supports the idea that multicopy AST1 routes Pma1-7 to the surface by restoring lipid raft association.
Figure 8.
Plasma membrane rerouting of Pma1p in vps1 mutant does not restore its raft association. Pma1 and Pma1-7 association with DRMs was examined in VPS1 and vps1 background. Cells were radiolabeled for 10 min at 24 or 37°C and chased for 30 min at 24 or 37°C. ATPase distribution in R and S fractions was analyzed by immunoprecipitation and phosphorimaging.
Increased Raft Association of Mutant ATPase Involves Clustering by Ast1p
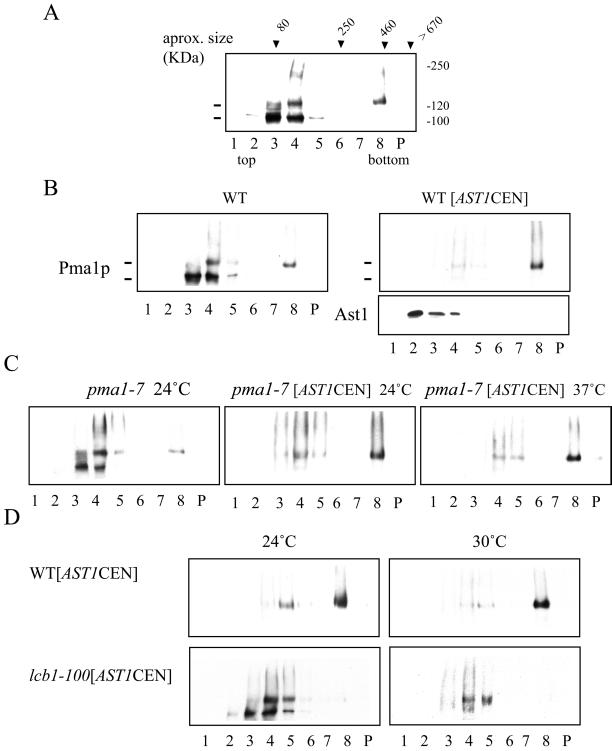
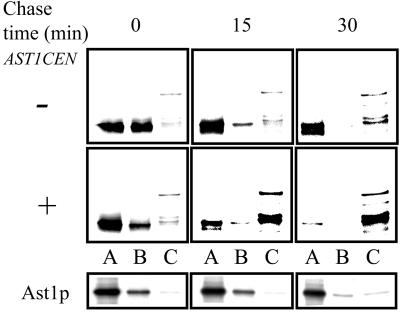
To explore how a peripheral membrane protein like Ast1p could mediate Pma1p association with lipid rafts, we used SDS/TX100 velocity gradients (Scheiffele et al., 1996) to monitor if changes in size due to complex formation occurred. Wild-type cells were grown at 24°C, and cleared lysates were adjusted to 0.4% SDS and 0.2% TX100 and loaded on top of a 5–30% sucrose velocity gradient. After centrifugation, Pma1p distribution in the different fractions was analyzed by Western blotting. Most of the protein was found in a fraction corresponding to the expected monomeric size (∼100 kDa). Interestingly, two bands were seen in SDS-PAGE for Pma1p when the samples were heated at 37°C before electrophoresis (see MATERIALS AND METHODS). Only the slower migrating band was present in a big, >400 kDa, SDS/TX100-resistant oligomer (Figure 9A). Next we analyzed the effect of AST1 overexpression on Pma1p. On moderate overexpression of AST1 from a centromeric vector, most of the ATPase migrated as the upper band and was present in the SDS/TX100-resistant complex (Figure 9B, left). Ast1p was not detected in this fraction, suggesting that the interaction with Pma1p is weak. Next, we checked whether this was also the case for Pma1-7. At 24°C in the absence of [AST1CEN], Pma1-7 behaved essentially as Pma1 but exhibited less of the SDS/TX100-resistant oligomer (Figure 9C, left). This tendency was more evident for the pma1-7 pep4 double mutant. On moderate overexpression of Ast1, Pma1-7 was shifted to the SDS/TX100-resistant oligomer both at 24 and 37°C (Figure 9C, right). To test whether SDS/TX100-resistant oligomer formation required the presence of lipid rafts, we performed the experiment in lcb1-100 mutant cells. Already at 24°C [AST1CEN]-induced oligomer formation was largely impaired and was abolished when cells were incubated at 30°C for 1 h.
Figure 9.
Pma1p is present in SDS-TX100–resistant oligomers that are stabilized by overexpression of AST1. (A) PMA1 (RH690-15D) cells were grown at 24°C. Cell lysates were adjusted to 0.4% SDS and 0.2% TX100 and loaded on top of a 5–20% sucrose velocity gradient containing detergent and centrifuged for 16 h at 215,000 × g (at 4°C). Fractions were collected from the top and Pma1p distribution was analyzed by Western blotting. The approximate size was determined with the use of markers of known size (transferrin, catalase, ferritin, and thyroglobulin). The apparent molecular weight is shown on the right. (B) Overexpression of AST1 promotes the formation of a SDS/TX100-resistant ATPase oligomer. Wild-type cells (L3852) or cells expressing AST1 from a centromeric vector (pAC64) were grown at 24°C and treated as in A. The distribution of Pma1p and Ast1 was determined by Western blotting. (C) Overexpression of AST1 induces oligomer formation in pma1-7 mutant. pma1-7 (ACY7) and pma1-7 [AST1CEN] cells were grown at 24°C and incubated for 2 h at 24°C or 37°C and subjected to fractionation in velocity gradients as before. Distribution of mutant ATPase was determined by Western blotting. (D) Lipid rafts are required for Pma1p oligomer formation. WT [AST1CEN] and lcb1-100 [AST1CEN] cells were grown at 24°C and incubated for 1 h at 24 or 30°C and subjected to fractionation in velocity gradients as before. Oligomer formation is impaired in the lcb1-100 mutant at 24°C and completely abolished at 30°C.
Finally, we investigated whether the time course of the [AST1CEN]-induced oligomer formation correlated with the incorporation of Pma1p into DRMs. To do so we monitored the formation of the SDS/TX100-resistant complex during biosynthetic transport. Wild-type cells with and without [AST1CEN] were pulse-labeled for 10 min at 24°C and chased for 0, 15, or 30 min at the same temperature, and the lysates were subjected to SDS/TX100 velocity gradient centrifugation as before. Fractions 1-2-3 (A), 4-5-6 (B), and 7-8-9 (C) were pooled, and Pma1p and Ast1p were immunoprecipitated and analyzed as before. In control cells most of Pma1p was found in the top fractions (A and B) at all time points, and the SDS/TX100-resistant >400-kDa oligomer (fraction C) increased slowly during transport (Figure 10 top). Moderate overexpression of AST1 shifted the bulk of the ATPase to the SDS/TX100-resistant >400-kDa oligomeric form already after 15 min of chase (Figure 10), indicating that Ast1-induced oligomerization is an early event during surface delivery of Pma1p. A fraction of Ast1p (∼15%) was found in the middle of the gradient at early time points; after 30 min of chase most of Ast1p remained in top fractions, suggesting that the protein becomes more easily dissociated from the Pma1p oligomers. A small (2–4%) fraction of Ast1p was comigrating with the large Pma1p oligomers (fraction C).
Figure 10.
Ast1p mediates clustering of Pma1p during biosynthetic transport. PMA1 (L3852) and PMA1[AST1CEN] cells were pulse-labeled with [35S]methionine for 10 min at 24°C and chased for 0, 15, or 30 min at the same temperature. Cells lysates were subjected to SDS/TX100 velocity gradient fractionation as in Figure 9. Fractions 1-2-3 (A), 4-5-6 (B), and 7-8-9 (C) were pooled and Pma1p and Ast1-myc were immunoprecipitated and analyzed as before. Pma1p becomes oligomerized already after 15 min of chase. Note that the slower migrating form of Pma1p (see Figure 9) is not preserved during immunoprecipitation.
These data suggest that clustering of Pma1-7 into oligomers by Ast1p may mediate restoration of raft association.
DISCUSSION
In this study we have demonstrated the functional role of lipid rafts in Pma1p sorting to the plasma membrane and obtained evidence that supports a role for Pma1p clustering in lipid raft association.
Pma1p is one of the most abundant proteins of the yeast cell surface; it accounts for up to 20% of the protein content of the plasma membrane (Serrano et al., 1986). Not surprisingly, dedicated mechanisms operate in the transport of Pma1p through the secretory pathway. Lst1p is required for ER-to-Golgi transport (Roberg et al., 1999; Shimoni et al., 2000) and Ast1p in delivery from Golgi to the surface (Chang and Fink, 1995). After arrival to the surface Pma1 is very stable (half-life is >12 h), and there is no evidence for Pma1p recycling (Benito et al., 1991). Importantly, comprehensive mutational studies have uncovered structure/function relationships and allowed the isolation of sorting-defective alleles (Chang and Fink, 1995; Ambesi et al., 2000; Morsomme et al., 2000). One of these, pma1-7, is delivered directly to the vacuole at the nonpermissive temperature (Chang and Fink, 1995). These features, together with the fact that Pma1p is incorporated into lipid rafts during biosynthetic transport and is one of the major components of DRMs (Bagnat et al., 2000) make Pma1p a good model protein for studying biosynthetic protein delivery and lipid raft biogenesis.
Raft Association and Sorting
Sorting of plasma membrane proteins and raft association may be connected processes. Previously, we found that GPI-anchored proteins become detergent insoluble already in the ER (Bagnat et al., 2000). Despite the presence of rafts, Pma1p can only be incorporated into DRMs efficiently after leaving the ER (Figure 2). This suggests that incorporation of Pma1p into lipid rafts may require the presence of some factor(s) localized in the Golgi complex. Disruption of rafts leads to missorting of Pma1p to the vacuole (Figure 3) rather than to a block in the ER as shown for GPI-anchored proteins (Horvath et al., 1994; Skrzypek et al., 1997; Sutterlin et al., 1997). Mistargeting to the vacuole upon disruption of lipid rafts does not involve passage over the plasma membrane because endocytosis is also impaired in lcb1-100 mutant cells at the restrictive temperature (Sutterlin et al., 1997). Impaired raft association of vacuole-targeted Pma1-7 further supports a role for lipid rafts in surface delivery of plasma membrane H+-ATPase (Figure 5). Furthermore, overexpression of Ast1p restores raft association of Pma1-7, thus recovering normal sorting to the surface (Figure 6). Recovery of Pma1-7 raft association is not likely to be a consequence of increased surface delivery. Multicopy AST1 restores raft association of mutant ATPase at an early time point before plasma membrane delivery (Figure 6B), similar to what is found for wild-type Pma1p. Consistent with this study, Ast1p is present in the intracellular compartment where Pma1p is incorporated into DRMs (Figure 7). Moreover, in vps1Δ mutant cells Pma1-7 does not recover normal raft association (Figure 8), although the mutant ATPase is forced to be transported to the cell surface because vacuolar delivery is blocked.
This active role of Ast1p has to be reconciled with the fact that ast1Δ mutant does not show an obvious phenotype (Chang and Fink, 1995). In part this is explained by the existence of two related genes, AST2 and YIM1. Nevertheless, the ast1Δ pma1-7 double mutant has a synthetic growth defect that suggests a functional interaction between AST1 and PMA1. Indeed, we have now been able to show a direct protein–protein interaction (Figure 6C). These data support the idea that Ast1p, and perhaps other factors, by direct interaction with the cytoplasmic domain of Pma1p may facilitate its association with rafts.
Vacuole vs. Plasma Membrane
The outcome of this raft-mediated sorting process is illustrated by the dramatic difference in detergent insolubility of ergosterol in different subcellular compartments (Figure 4). This is consistent with the low sphingolipid content of vacuoles compared with that of the plasma membrane (Hechtberger et al., 1994) and the different tendency of plasma membrane and vacuolar membrane proteins to partition into DRMs (Bagnat et al., 2000). Results presented here demonstrate a clear role for lipid rafts in plasma membrane sorting and provide a framework for understanding how asymmetric distribution of lipids and proteins to post-Golgi destinations can be achieved.
Clustering and Raft Association
Central to the lipid raft hypothesis is that raft-associated proteins may not only spontaneously partition into rafts but that there are proteins that may be driven into sphingolipid/sterol-rich micro-domains by yet unidentified protein linkers (Simons and Ikonen, 1997). Glycosylated proteins are thought to rely on carbohydrate-interacting lectins, whereas in the case of nonglycosylated proteins other types of interactions may operate (Simons and Toomre, 2000). Pma1p undergoes extensive phosphorylation but not glycosylation during biosynthetic transport (Chang and Slayman, 1991) and requires some factor (protein or lipid) localized in the Golgi to be able to partition efficiently into DRMs. The results presented here suggest that Ast1p may be part of such a mechanism. In mammalian cells some raft-associated proteins like VIP17-MAL and caveolin have been shown to have a role in polarized sorting (Scheiffele et al., 1996; Cheong et al., 1999; Puertollano et al., 1999). In the first case there is evidence that raft association of influenza virus HA is affected by depletion of VIP17-MAL (Puertollano et al., 1999); however, mechanistic insights will demand further work. Here we have identified a protein that can regulate raft association and therefore promote surface rerouting of mutant ATPase, but the key question is how can a peripheral membrane protein recruit Pma1p into lipid rafts?
Indirect evidence suggest that oligomerization may be important for the recruitment of proteins to DRMs (Dykstra et al., 2001; Simons and Toomre, 2000, and references therein). The idea is that weak association of protein monomers with lipid rafts is increased by oligomerization. Studies have been difficult because interactions in membranes are often weak and transient. Indeed, only a small fraction of Pma1p can be detected in SDS/TX100-resistant oligomers in normal conditions (Figure 9A). However, oligomers could be stabilized by moderate overexpression of AST1 (Figure 9B). Importantly, antibody-mediated clustering increased raft association of GPI-anchored PLAP but did not make VSV-G, a nonraft marker protein, detergent-resistant (Harder et al., 1998), indicating that resistance to detergent extraction is not an intrinsic property of clustered membrane proteins. Interestingly, there is a shift in apparent molecular weight of Pma1p to a slower migrating band (also detected for HA-Pma1p with the use of anti-HA–tag monoclonal antibodies (our unpublished data) that is mostly in the oligomeric complex (see Figure 9). This might be due to a conformational change, which could lead to decreased mobility of the protein. Little of the faster migrating band is seen after immunoprecipitation of Pma1p (cf. Figures 9B and 10). This suggests that the postulated conformational change is not maintained under the conditions prevailing during immunoprecipitation.
Formation of Pma1p oligomers requires the presence of functional rafts (Figure 9D) and is an early event during partition of Pma1p into DRMs (Figure 10). Therefore, oligomerization is not an isolated event but rather part of the mechanism involved in partitioning of Pma1p into lipid rafts.
Clustering is starting to be a common theme in the biology of lipid rafts. Data presented here provides a direct evidence supporting oligomerization as a mechanism involved in protein recruitment into lipid rafts. Further work in this and other systems is needed to generalize this concept.
ACKNOWLEDGMENTS
We thank H. Pelham, S. Nock, and W. Zachariae for providing reagents and S. Keränen R. Schekman, and H. Riezman for providing yeast strains. We are most grateful to R. Serrano for providing the anti-Pma1p antibodies. We also thank T. Kurzchalia and J. Avila for critical reading of the manuscript. A.C. was supported by National Institutes of Health grant GM 58212.
Abbreviations used:
- TX100
Triton X-100
- GPI
glycophosphatidylinositol
- ER ,endoplasmic reticulum
DRMs, detergent resistant membranes
REFERENCES
- Ambesi A, Miranda M, Petrov VV, Slayman CW. Biogenesis and function of the yeast plasma-membrane H(+)-ATPase. J Exp Biol. 2000;203(Pt 1):155–160. doi: 10.1242/jeb.203.1.155. [DOI] [PubMed] [Google Scholar]
- Bagnat M, Keranen S, Shevchenko A, Simons K. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc Natl Acad Sci USA. 2000;97:3254–3259. doi: 10.1073/pnas.060034697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito B, Moreno E, Lagunas R. Half-life of the plasma membrane ATPase and its activating system in resting yeast cells. Biochim Biophys Acta. 1991;1063:265–268. doi: 10.1016/0005-2736(91)90381-h. [DOI] [PubMed] [Google Scholar]
- Black MW, Pelham HR. A selective transport route from Golgi to late endosomes that requires the yeast GGA proteins. J Cell Biol. 2000;151:587–600. doi: 10.1083/jcb.151.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Stevens TH. Vacuole biogenesis in Saccharomyces cerevisiae: protein transport pathways to the yeast vacuole. Microbiol Mol Biol Rev. 1998;62:230–247. doi: 10.1128/mmbr.62.1.230-247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Slayman CW. Maturation of the yeast plasma membrane [H+]ATPase involves phosphorylation during intracellular transport. J Cell Biol. 1991;115:289–295. doi: 10.1083/jcb.115.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Fink GR. Targeting of the yeast plasma membrane [H+]ATPase: a novel gene AST1 prevents mislocalization of mutant ATPase to the vacuole. J Cell Biol. 1995;128:39–49. doi: 10.1083/jcb.128.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong KH, Zacchetti D, Schneeberger EE, Simons K. VIP17/MAL, a lipid raft-associated protein, is involved in apical transport in MDCK cells. Proc Natl Acad Sci USA. 1999;96:6241–6248. doi: 10.1073/pnas.96.11.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles CR, Snyder WB, Burd CG, Emr SD. Novel Golgi to vacuole delivery pathway in yeast: identification of a sorting determinant and required transport component. EMBO J. 1997;16:2769–2782. doi: 10.1093/emboj/16.10.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra ML, Cherukuri A, Pierce SK. Floating the raft hypothesis for immune receptors: access to rafts controls receptor signaling and trafficking. Traffic. 2001;2:160–166. doi: 10.1034/j.1600-0854.2001.020302.x. [DOI] [PubMed] [Google Scholar]
- Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay E, Bretscher A. Parallel secretory pathways to the cell surface in yeast. J Cell Biol. 1995;131:297–310. doi: 10.1083/jcb.131.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechtberger P, Zinser E, Saf R, Hummel K, Paltauf F, Daum G. Characterization, quantification and subcellular localization of inositol-containing sphingolipids of the yeast Saccharomyces cerevisiae. Eur J Biochem. 1994;225:641–649. doi: 10.1111/j.1432-1033.1994.00641.x. [DOI] [PubMed] [Google Scholar]
- Horvath A, Sutterlin C, Manning-Krieg U, Movva NR, Riezman H. Ceramide synthesis enhances transport of GPI-anchored proteins to the Golgi apparatus in yeast. EMBO J. 1994;13:3687–3695. doi: 10.1002/j.1460-2075.1994.tb06678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P, Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafont F, Lecat S, Verkade P, Simons K. Annexin XIIIb associates with lipid microdomains to function in apical delivery. J Cell Biol. 1998;142:1413–1427. doi: 10.1083/jcb.142.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledesma MD, Simons K, Dotti CG. Neuronal polarity: essential role of protein-lipid complexes in axonal sorting. Proc Natl Acad Sci USA. 1998;95:3966–3971. doi: 10.1073/pnas.95.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungdahl PO, Gimeno CS, Styles CA, Fink GR. SHR3: a novel component of the secretory pathway specifically required for localization of amino acid permeases in yeast. Cell. 1992;71:463–478. doi: 10.1016/0092-8674(92)90515-e. [DOI] [PubMed] [Google Scholar]
- Luo W, Chang A. Novel genes involved in endosomal traffic in yeast revealed by suppression of a targeting-defective plasma membrane ATPase mutant. J Cell Biol. 1997;138:731–746. doi: 10.1083/jcb.138.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Chang A. An endosome-to-plasma membrane pathway involved in trafficking of a mutant plasma membrane ATPase in yeast. Mol Biol Cell. 2000;11:579–592. doi: 10.1091/mbc.11.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsomme P, Slayman CW, Goffeau A. Mutagenic study of the structure, function and biogenesis of the yeast plasma membrane H(+)-ATPase. Biochim Biophys Acta. 2000;1469:133–157. doi: 10.1016/s0304-4157(00)00015-0. [DOI] [PubMed] [Google Scholar]
- Muñiz M, Morsomme P, Riezman H. Protein sorting upon exit from the endoplasmic reticulum. Cell. 2001;104:313–320. doi: 10.1016/s0092-8674(01)00215-x. [DOI] [PubMed] [Google Scholar]
- Nothwehr SF, Conibear E, Stevens TH. Golgi and vacuolar membrane proteins reach the vacuole in vps1 mutant yeast cells via the plasma membrane. J Cell Biol. 1995;129:35–46. doi: 10.1083/jcb.129.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JL, Srinivasan B, Dickson RC, Lester RL. Phenotypes of sphingolipid-dependent strains of Saccharomyces cerevisiae. J Bacteriol. 1992;174:7180–7184. doi: 10.1128/jb.174.22.7180-7184.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Bryant NJ, Stevens TH. The membrane protein alkaline phosphatase is delivered to the vacuole by a route that is distinct from the VPS-dependent pathway. J Cell Biol. 1997;138:531–545. doi: 10.1083/jcb.138.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puertollano R, Martin-Belmonte F, Millan J, de Marco MC, Albar JP, Kremer L, Alonso MA. The MAL proteolipid is necessary for normal apical transport and accurate sorting of the influenza virus hemagglutinin in Madin-Darby canine kidney cells. J Cell Biol. 1999;145:141–151. doi: 10.1083/jcb.145.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner JC, Pelham HR. Transmembrane domain-dependent sorting of proteins to the ER and plasma membrane in yeast. EMBO J. 1997;16:1832–1841. doi: 10.1093/emboj/16.8.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberg KJ, Crotwell M, Espenshade P, Gimeno R, Kaiser CA. LST1 is a SEC24 homologue used for selective export of the plasma membrane ATPase from the endoplasmic reticulum. J Cell Biol. 1999;145:659–672. doi: 10.1083/jcb.145.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JH, Raymond CK, Gilbert T, O'Hara PJ, Stevens TH. A putative GTP binding protein homologous to interferon-inducible Mx proteins performs an essential function in yeast protein sorting. Cell. 1990;61:1063–1074. doi: 10.1016/0092-8674(90)90070-u. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Verkade P, Fra AM, Virta H, Simons K, Ikonen E. Caveolin-1 and -2 in the exocytic pathway of MDCK cells. J Cell Biol. 1996;140:795–806. doi: 10.1083/jcb.140.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F, Fink GR, Hicks JB. Methods in Yeast Genetics: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1986. [Google Scholar]
- Serrano R, Kielland-Brandt MC, Fink GR. Yeast plasma membrane ATPase is essential for growth and has homology with (Na++ K+), K+- and Ca2+-ATPases. Nature. 1986;319:689–693. doi: 10.1038/319689a0. [DOI] [PubMed] [Google Scholar]
- Serrano R. H+-ATPase from plasma membranes of Saccharomyces cerevisiae and Avena sativa roots: purification and reconstitution. Methods Enzymol. 1988;157:533–544. doi: 10.1016/0076-6879(88)57102-1. [DOI] [PubMed] [Google Scholar]
- Shimoni Y, Kurihara T, Ravazzola M, Amherdt M, Orci L, Schekman R. Lst1p and Sec24p cooperate in sorting of the plasma membrane ATPase into COPII vesicles in Saccharomyces cerevisiae. J Cell Biol. 2000;151:973–984. doi: 10.1083/jcb.151.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Skrzypek M, Lester RL, Dickson RC. Suppressor gene analysis reveals an essential role for sphingolipids in transport of glycosylphosphatidylinositol-anchored proteins in Saccharomyces cerevisiae. J Bacteriol. 1997;179:1513–1520. doi: 10.1128/jb.179.5.1513-1520.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack JH, Horazdovsky B, Emr SD. Receptor-mediated protein sorting to the vacuole in yeast: roles for a protein kinase, a lipid kinase and GTP-binding proteins. Annu Rev Cell Dev Biol. 1995;11:1–33. doi: 10.1146/annurev.cb.11.110195.000245. [DOI] [PubMed] [Google Scholar]
- Sutterlin C, Doering TL, Schimmoller F, Schroder S, Riezman H. Specific requirements for the ER to Golgi transport of GPI-anchored proteins in yeast. J Cell Sci. 1997;110:2703–2714. doi: 10.1242/jcs.110.21.2703. [DOI] [PubMed] [Google Scholar]