Abstract
The aim of the study is to levels of nicotine and cotinine were elevated the oxidative stress malondialdehyde (MDA) and inflammation as nitric oxide (NO2 and NO3) may possibly be associated with decreased antioxidant enzyme activities and can sensitively indicate the production of reactive oxygen species (ROS). To evaluate the quantitative analysis of nicotine and cotinine levels and the alterations in the selected parameters of antioxidant metabolisms during nitroxidative stress in the saliva of smokeless tobacco consumers. Saliva nicotine and cotinine was measured by HPLC method and nitric oxide, lipid peroxidation and activities of antioxidant enzymes were estimated by spectrophotometric methods. Significant increase in concentrations of nicotine and cotinine levels of saliva in smokeless tobacco users in comparison to controls. Saliva lipid peroxidation was increased in experimental subjects (gutkha group 39.28% and khaini group 25.00%) as compared to controls and nitric oxide in the form of nitrites and nitrates was significantly increased in the saliva of smokeless tobacco users compared to controls. The activity levels of antioxidant enzymes were decreased in the saliva of the smokeless tobacco users in comparison with normal controls. A strong positive correlation of nicotine and cotinine with nitroxidative stress markers in gutkha and khaini users. Increased expression of inducible nitric oxide synthase (iNOS) enzyme leads to intoxication in saliva and indirectly induces inflammation process. Increased production of reactive oxygen species (ROS) and decrease in the activity levels of antioxidant enzymes in the saliva of smokeless tobacco users indicate conspicuous cell and tissue damage.
Abbreviations: SLT, Smokeless tobacco; ROS, Reactive oxygen species; RNS, Reactive nitrogen species; TBA, Thiobarbituric acid; TCA, Trichloro acetic acid; DCM, Dichloromethane; DEE, Diethyl ether; TBARS, Thiobarbituric acid reacting substance; MDA, malondialdehyde; GSH, Reduced glutathione; SOD, Superoxide dismutase; CAT, Catalase; GPx, Glutathione peroxidase; GST, Glutathione S-Transferase; TSNA, Tobacco- specific nitrosamines; NOS, Nitric oxide synthase; nNOS, Neuronal nitric oxide synthase; eNOS, Endothelial nitric oxide synthase; iNOS, Inducible nitric oxide synthase
Keywords: Smokeless tobacco, Nicotine, Cotinine, RP-HPLC, Free radicals, Antioxidant enzymes
Highlights
-
•
Tobacco is global burden of deaths and risks worldwide. The use of smokeless tobacco is through chewing or snuffing and the product is kept between the cheek or gums.
-
•
The main composition of smokeless tobacco is nicotine, tobacco specific nitrosamines, volatile nitrosamines, aldehydes and heavy metals.
-
•
The levels of cotinine and nicotine are increased and may cause chronic damage to oral tissues and inflammation.
-
•
Decreased enzymatic activities of antioxidants and uric acid levels act as markers of increased oxidative stress and nitrosative stress is also increased through increased levels of nitric oxide.
1. Introduction
Tobacco is consumed in the form of smoking and smokeless tobacco products and smokeless tobacco (SLT) is consumed without burning and can be used orally or nasally. Oral smokeless tobacco products are placed in the mouth, cheek or lip and sucked (dipped) or chewed [1]. India is one of the largest producers and consumers of tobacco, most of it in the smokeless tobacco form and available in different brands across the country [2]. It is estimated that 5500 adolescents consume tobacco every day in India and every year 4 million young people are starting the consumption of tobacco regularly [3]. India has the second highest number of tobacco users in the world with 229 million users and China has 311 million tobacco users [4], [5]. Low socioeconomic status, illiteracy, socially acceptable and easily addictive habit are the various multiple factors contributing to the consumption of more smokeless tobacco than smoking cigarettes. The smokeless tobacco constituents may also contribute to adverse health effects among consumers. Gutkha, panmasala with tobacco, khaini are the multiple forms of smokeless tobacco (SLT) that is locally made and consumed in India and contains several carcinogenic elements. The composition of gutkha product contains tobacco, areca nut, catechu, slaked lime and added flavors. It is available in the khaleja and rebel brands in India and khaleja brand is the most preferable and highly consumable form of gutkha. Khaini is the combination of tobacco and limestone paste, available in mahak chaini/khaini brand. Smokeless tobacco is placed between the cheek and gums during chewing and spit out leads to mucosal lesions of the oral cavity. Lime and catechu are actively involved the formation of reactive oxygen species (ROS) in the oral cavity [6]. High nicotine delivery and dependence potential of smokeless tobacco products may be a critical factor enabling it to compete with the most rapidly absorbed nicotine from smoking tobacco [7]. In humans, most of the intake of nicotine is metabolized into cotinine. It acts as major metabolite and specific marker of nicotine exposure and its concentration is determined by the rate of nicotine metabolism. Absorption of nicotine from smokeless tobacco occurs primarily through mucous membranes and accelerates quickly, becoming maximal at 5 min, but then declines over 30 min despite the continued presence of tobacco in the mouth [8].
Superoxide anion and hydro peroxides are the main sources of nicotine induced free radicals and act as markers of oxidative stress [9], [10]. Uric acid and reduced glutathione (GSH) are the non enzymatic antioxidants, scavenging the free radicals produced during metabolic reactions in the cells [11]. Cells continuously generate reactive oxygen species (ROS) as part of metabolic processes. The antioxidant defense system consisting of enzymes such as catalase, superoxide dismutase and glutathione peroxidase, which decreased the concentrations of oxidants in cells and tissues. Increased levels of nicotine and cotinine shows high consumption and addictive nature of the smokeless tobacco products and higher levels of nicotine indicate that chronic damage of oral tissues and cancers of the oral cavity. Therefore, the purpose of this study was to explore the concentrations of nicotine and cotinine, status of antioxidant enzymes, levels of lipid peroxidation and nitric oxide in saliva of smokeless tobacco consumers.
2. Materials
2.1. Chemicals
Nicotine was purchased with purity of ≥ 99% and cotinine purity of 98% from Sigma Aldrich, Bangalore was used as internal standards in HPLC method. Thiobarbituric acid (TBA), Trichloro acetic acid (TCA), Methanol, Dichloromethane (DCM), Diethyl ether (DEE), Acetonitrile (ACN), Sodium n-Heptane sulponic acid and Potassium di hydrogen phosphate (KH2PO4).
2.2. Subjects
The study population consisted of ninety human male volunteers and divided into three groups. Each group consists of thirty male volunteers: group I- control group II- gutkha chewers and group III- khaini chewers. The experimental subjects are mainly auto drivers, car mechanics and house builders by using a questionnaire. Gutkha chewers consume four to eight khaleja brand of gutkha sachets and khaini subjects consume five packets of mahak chaini brand daily for 2–6 years available in Ananthapuramu. The inclusion criteria are the habitual use of only gutkha and khaini packets by the gutkha and khaini users respectively and choose the unmarried and low economic status people. The exclusion criteria are the consumed either alcohol or smoking groups or religion of people are not preferred. Healthy subjects who did not use any form of tobacco or alcohol and had not been exposed to any kind of chemicals and were of the same age, sex, and socioeconomic strata as the experimental subjects were selected as controls. The study groups were ranging in age from 25 to 35 years and this study was approved by the Institutional Ethical Committee.
Group-I: Controls
Group-II: Gutkha chewers
Group-III: Khaini chewers
2.3. Saliva collection and analysis
Five milliliters of unstimulated whole salivary samples were obtained by expectoration, in the absence of chewing movements, in dry plastic vials with the test subject sitting in a relaxed position. The collected saliva samples were centrifuged at 3000 rpm for 10 min. The supernatants were stored at −70 °C until further analysis.
2.4. Biochemical estimations
Estimation of lipid peroxidation in saliva was done by measuring the thiobarbituric acid reacting substance (TBARS) and was expressed in terms of malonaldehyde (MDA) content [12]. The glutathione (GSH) levels in the samples were estimated using 5, 5′- dithiobis-2-nitrobenzoic acid (DTNB) method [13] and the amount of glutathione is expressed as mmoles/mg protein. Superoxide dismutase (SOD) activity was evaluated following the method [14] and is expressed as U/mg protein. Catalase (CAT) was determined by the method [15] and the activity of CAT is expressed as µmoles of H2O2 decomposed/min/mg protein or U/mg protein. Glutathione peroxidase (GPx) was assayed by the method [16] and the activity of GPx was expressed as mmoles of glutathione oxidized/min/mg protein. Glutathione S-Transferase (GST) conjugated with GSH and 1-chloro-2, 4-dinitrobenzene gives a complex, S-(2, 4-dinitrophenyl) glutathione, which is spectrophotometrically measured as increased in absorbance at 340 nm by the method [17]. Albumins, total proteins and uric acid were analyzed using auto analyzer kit methods. Nitric oxide (NO2 and NO3) by the method [18].
2.5. HPLC
HPLC system (Shimadzu, Japan) is equipped with binary gradient system with variable UV/VIS detector (SPD-20A) and Rheodyne injector with a 20 µl loop and LC-20 CE pumps and integrator. Reversed phase chromatographic analysis was performed in isocratic condition using C18 reverse phase column (5 µ) at 37 °C.
2.6. HPLC operating conditions19
Resolution of peaks was performed with the mobile phase consisting a mixture of 0.272 g of KH2PO4, 0.184 g of sodium n-heptane sulfonate, 820 ml of water (HPLC-grade), and 180 ml of methanol (HPLC grade). The pH of the mobile phase was adjusted by drop wise addition of ortho phosphoric acid (pH = 3.2). The flow rate used was 1.0 ml/min, and the wavelength was fixed at 256 nm for nicotine and 262 nm for cotinine as per the modified method of [19]. Nicotine and cotinine at the concentrations of 20 µM/ml were used as standards.
2.7. Sample analysis for HPLC
Sample analysis was processed by the modified method of [19]. A 0.1 ml aliquot of saliva was placed into a glass test tube was alkalinized with 20 µl of 5.0 M NaOH, then vortex mixed at 2800 rpm for 30 s. Equal amounts of dichloromethane-diethyl ether (1:1 v/v) was used for one-step single extraction, then vortex mixed at 2800 rpm for 2 min. The organic layer, after being centrifuged at 3500 rpm for 3 min, was transferred to a new glass tube containing 4 µl of 0.25 M HCl The organic layer, after being centrifuged at 3500 rpm for 3 min, was transferred to a new glass tube containing 4 µl of 0.25 M HCl. The organic phase was then evaporated under a stream of nitrogen at 35 °C until dryness and reconstituted in 50 µl of mobile phase. A 20 µl aliquot was injected into the HPLC for analysis.
2.8. Statistical data analysis
All the quantitative data were expressed as mean± SEM and Students t-test was used to determine the significance of the parameters between the groups and the Pearson correlation coefficient analyzed using Graph Pad Prism version 6.01 for Windows. P < 0.05 was considered statistically significant.
3. Results
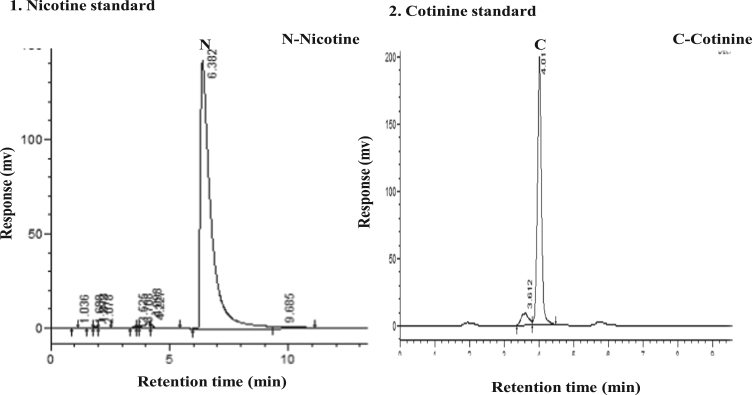
Data presented in (Table 1) indicated that the total proteins were decreased in the saliva of the gutkha group with no significant change and significant decrease in khaini group compared to controls. The salivary albumins (Gutkha group, −22.22% and khaini group, −37.03%) and globulins found that there was a decreased level in both groups of smokeless tobacco consumers compared to controls and showed no statistical difference. An increased malondialdehyde levels were observed in the saliva of experimental subjects when compared to controls (Gutkha chewers, 39.28% and khaini chewers, 25.00%). Smokeless tobacco consumers were exposed that the significantly increased levels of nitric oxide than controls. Data indicates significantly elevated the levels of nicotine and cotinine in saliva of smokeless tobacco users in comparison to controls were mentioned (Table 2). Although the normal control group had no considerable amounts of nicotine intake and tobacco exposure, but small concentrations of nicotine and cotinine levels were observed in control group due to environmental tobacco exposure and some food constituents. The range of retention time of standard nicotine is 5.3–6.3 min and showed a chromatogram peak at 6.00 min. The range of retention time of standard cotinine is 3.6–4.6 min and showed a chromatogram peak at 4.01 min (Fig. 1).
Table 1.
Effect of smokeless tobacco on salivary nitric oxide and lipid peroxidation.
| Parameter | Groups |
||
|---|---|---|---|
| Controls | Smokeless Tobacco users (SLT) |
||
| Gutkha users | Khaini users | ||
| Total proteins (g/dl) | 1.12 ± 0.03 | 1.06 ± 0.04# | 0.97 ± 0.05* |
| Albumins (g/dl) | 0.27 ± 0.05 | 0.21 ± 0.01 | 0.17 ± 0.01 |
| Globulins (g/dl) | 0.85 ± 0.08 | 0.83 ± 0.05 | 0.079 ± 0.03 |
| Malondialdehyde (µmoles/mg protein) | 0.28 ± 0.05 | 0.39 ± 0.09 | 0.35 ± 0.08 |
| Nitric oxide (NOx) (µmoles/L) | 47.91 ± 2.34 | 55.77 ± 1.13* | 54.46 ± 1.72* |
Data are represented as the mean± SEM and * denotes that data are significantly different with the other groups and # denotes that data are not significantly different with the other groups.
Table 2.
Concentrations of nicotine and cotinine levels in saliva.
| Parameter | Groups |
||
|---|---|---|---|
| Controls | Smokeless Tobacco users (SLT) |
||
| Gutkha users | Khaini users | ||
| Nicotine (ng/ml) | 34.23 ± 2.87 | 3712.12 ± 522.21* | 3556.96 ± 253.82* |
| Cotinine (ng/ml) | 40.91 ± 3.45 | 7248.04 ± 958.10* | 5326.90 ± 656.94* |
Data are represented as the mean ± SEM and * denotes that data are significantly different with the other groups.
Fig. 1.
HPLC Chromatograms of nicotine and cotinine standards.
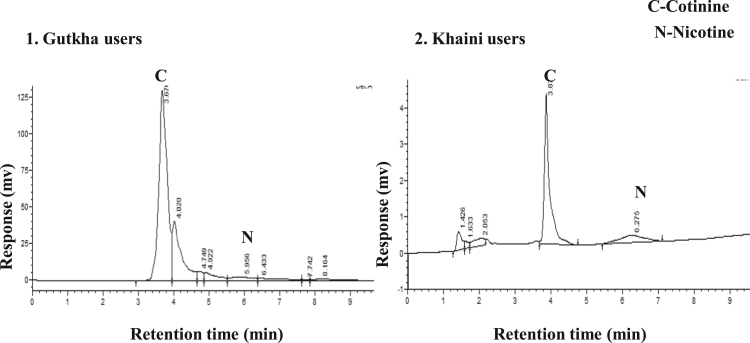
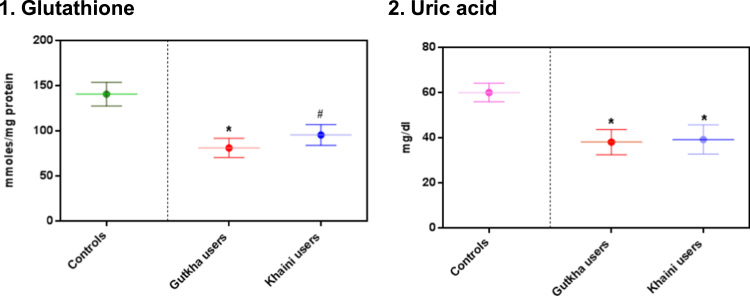
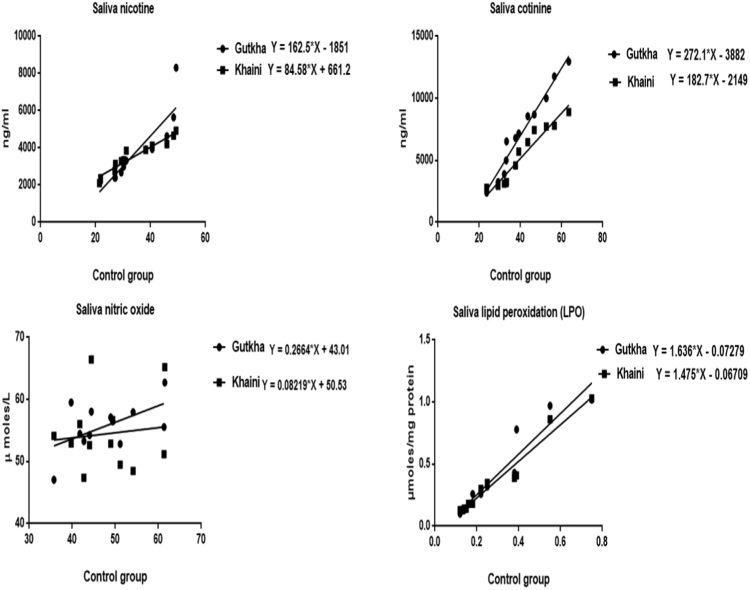
The data shown in (Fig. 2) indicates that there are no peaks observed in chromatograms of saliva in normal controls at the retention of 4.01 and 6.00 min of nicotine and cotinine. The group II consumers showed narrow nicotine chromatogram peak at retention time of 5.95 and cotinine peak at 3.67. In this gutkha group, large amount of nicotine is metabolized into cotinine. Nicotine showed chromatogram peak at 6.27 retention time and cotinine showed chromatogram peak at 3.87 min in group III consumers (Fig. 3). Uric acid levels demonstrated that the significantly decreased in the saliva of smokeless tobacco users than normal controls. Salivary glutathione levels were decreased significantly in gutkha chewers and khaini chewers showed decreased levels of glutathione with no significant difference compared to controls (Fig. 4). Data presented in (Fig. 5) represented that the linear regression plots of nicotine, cotinine, malondialdehyde (MDA) and nitric oxide in saliva between control and experimental subjects.
Fig. 2.
HPLC Chromatograms of the nicotine and cotinine levels in saliva samples of controls.
Fig. 3.
HPLC Chromatograms of the nicotine and cotinine levels in saliva samples of gutkha and khaini users.
Fig. 4.
Levels of glutathione and uric acid in saliva. Data are represented as the mean±SEM and * denotes that significant difference with the other groups and # denotes that data are not significantly different with the other groups.
Fig. 5.
Regression plots of comparison of saliva nicotine, cotinine levels, lipid peroxidation and nitric oxide between controls and experimental subjects.
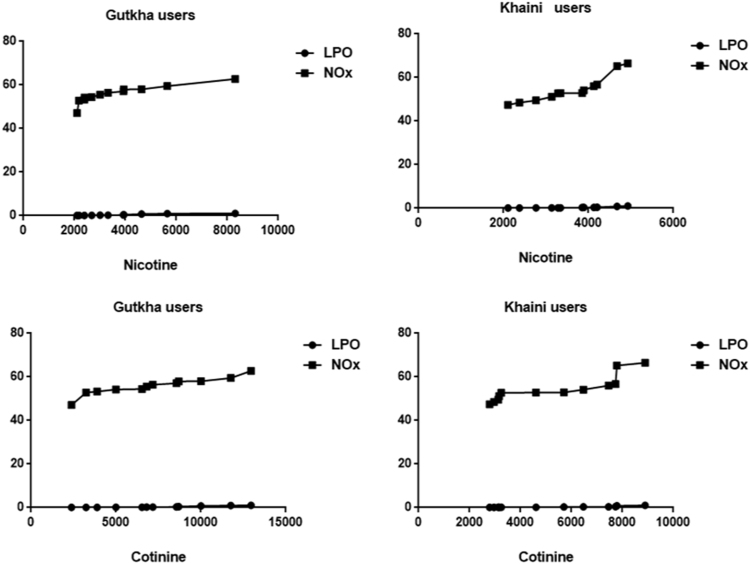
The decreased levels of superoxide dismutase in experimental subjects with no significant change. The levels of catalase and glutathione peroxidase were lower with significant difference. The group III chewers exposed that the significant decrease of glutathione S-transferase and decreased levels of GST in group II chewers with no significant change compared to controls (Table 3). Correlation analysis demonstrated that there was a significant uphill linear relationship occurs between nicotine and cotinine with malondialdehyde and nitric oxide in saliva of gutkha and khaini users and in many cases, the Pearson correlation coefficients were between 0.85 and 0.98. (Tables 4a and 4b).
Table 3.
Antioxidant enzymes status in saliva of smokeless tobacco users.
| Parameter | Groups |
||
|---|---|---|---|
| Controls | Smokeless Tobacco users (SLT) |
||
| Gutkha users | Khaini users | ||
| SOD (U/mg protein) | 1.38 ± 0.21 | 1.14 ± 0.28 | 1.33 ± 0.47 |
| CAT (µmoles/min/mg protein) | 22.51 ± 5.02 | 11.14 ± 2.72* | 20.70 ± 2.89* |
| GPx (mmoles/min/mg protein) | 17.61 ± 2.63 | 9.76 ± 1.32* | 10.44 ± 0.93* |
| GST (nmoles/min/mg protein) | 79.37 ± 12.20 | 58.39 ± 5.20 | 77.35 ± 5.66* |
Values are represented as the mean± SEM and * denotes that data are significantly different with the other groups.
Table 4a.
Correlation of nicotine with lipid peroxidation and nitric oxide.
| Nicotine | Gutkha users |
Khaini users |
||
|---|---|---|---|---|
| r | P | r | P | |
| Malondialdehyde | 0.93 | < 0.0001 | 0.86 | 0.0004 |
| Nitric oxide | 0.86 | 0.0003 | 0.92 | < 0.0001 |
r = correlation coefficient.P < 0.0001 statistically significant difference.
Table 4b.
Correlation of cotinine with lipid peroxidation and nitric oxide.
| Cotinine | Gutkha users |
Khaini users |
||
|---|---|---|---|---|
| r | P | r | P | |
| Malondialdehyde | 0.92 | < 0.0001 | 0.85 | 0.0004 |
| Nitric oxide | 0.94 | < 0.0001 | 0.88 | 0.0002 |
r = correlation coefficient.P < 0.0001 statistically significant difference.
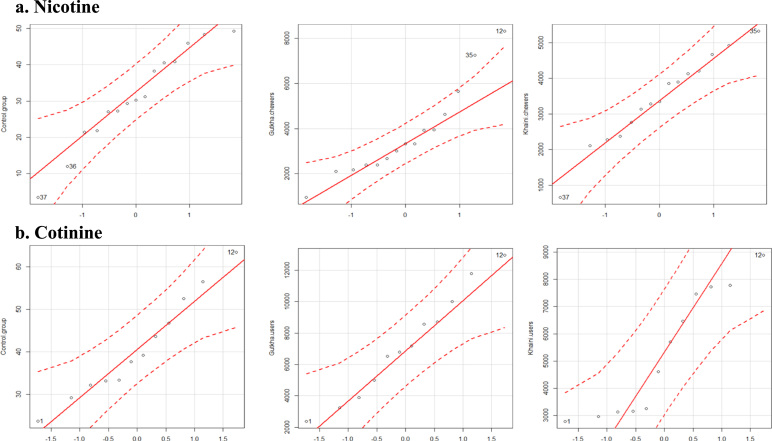
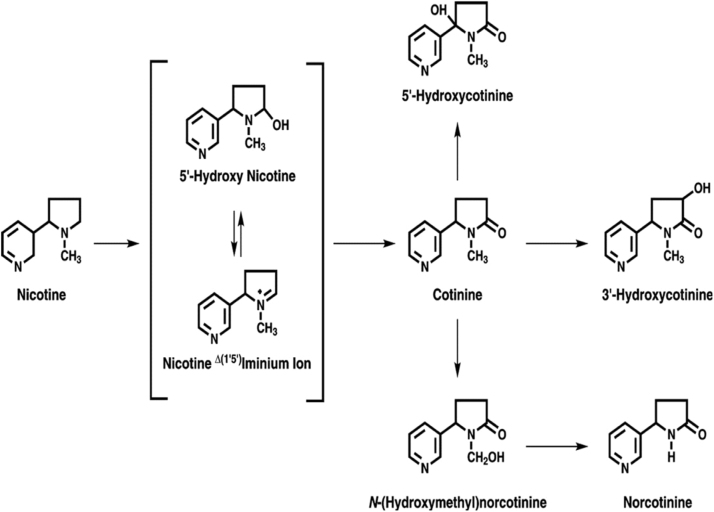
A data show in (Fig. 6) indicates that the correlation plots of nicotine and cotinine with malondialdehyde and nitric oxide in saliva of control and smokeless tobacco users. Quantile comparison plots show the distribution levels of nicotine and cotinine in controls and smokeless tobacco users (Fig. 7). The main metabolite of nicotine metabolism is cotinine and in addition to this, nicotine is extensively metabolized by the liver into other metabolites like 5′ hydroxy cotinine, 5′ hydroxy nicotine, nornicotine and nicotine iminium ion etc., (Fig. 8).
Fig. 6.
Correlation plots of nicotine and cotinine with nitric oxide and lipid peroxidation.
Fig. 7.
Quantile comparison plots of saliva nicotine and cotinine levels.
Fig. 8.
The mechanism of nicotine metabolism [20].
4. Discussion
Chewing of smokeless tobacco is a very common and is easily addictable products to humans. The main composition of smokeless tobacco is nicotine, tobacco specific nitrosamines (TSNA), volatile nitrosamines, aldehydes and heavy metals, etc., Smokeless tobacco (SLT) mediated abnormalities in saliva have been investigated in detail and tobacco specific nitrosamines are the carcinogens in these products and synthesized from the nitration of nicotine during processing of tobacco. Tobacco consumption is the major factor for the generation of free radicals in cells and tissues [21]. Smokeless tobacco contains high amounts of nicotine than smoking tobacco is due to produce from Nicotiana rustica species. Saliva could constitute a first line of defense against free radical mediated oxidative stress and during metabolic reactions, increases lipid peroxidation reactions [22], [23]. Intriguingly, smokeless tobacco is associated with oral cancer and inflammation in saliva. Normal controls consisted minimal amounts of nicotine and cotinine and these low levels might be due to environmental tobacco exposure. Our results observed that significantly increased concentrations of nicotine observed in saliva of smokeless tobacco consumers. Feyerabend et al. (1982) [24] reported that cigarette smokers found that the significantly increased concentration of nicotine in saliva in comparison with non-smokers. Smokeless tobacco users demonstrated that significant increase in concentrations of cotinine levels of saliva compared to normal controls. An increased concentration of nicotine indicated the higher prevalence and consumption of smokeless tobacco. Pure nicotine and smokeless tobacco extract was induced the cell toxicity and oxidative stress by increasing the production of reactive oxygen species [25]. Nicotine has a half-life of 30–150 min and cotinine consisted of long half-life of approximately 20 h. Due to long half life of cotinine, concentrations of cotinine were relatively stable throughout the smoking day, reaching a maximum at the end of the day [26]. Previous results also stated that the significantly increased concentrations of cotinine in saliva of smokers than non-smokers [27]. During chewing movements large fraction of nicotine is metabolized into cotinine and it is preferred as major metabolite and biomarker. This indicates the tobacco exposure and nicotine dependence in smokeless tobacco consumers and the quantitative relation between cotinine clearance and intake of nicotine. Daily intake of smokeless tobacco had statistically significant association with cotinine concentrations in saliva of experimental subjects [28]. There was a significant strong positive correlation of nicotine and cotinine levels between control and gutkha group. Gutkha and khaini consumers indicated that nitrosative stress parameters showed that the positive correlation with nicotine and cotinine. The components of smokeless tobacco are nicotine and tobacco specific nitrosamines disrupt the antioxidant system leads to increased production of free radicals and oxidative degradation of oral tissues. Elevated levels of nitric oxide in cells could exert genotoxic effects include the formation of carcinogenic N-nitroso compounds, direct deamination of DNA bases and oxidation of DNA [29], [30]. Our observations suggest that total proteins, albumins and globulins were reduced in the saliva of smokeless tobacco users in comparison with controls. Albumin acts as powerful antioxidant [31] and decreased levels of albumins in saliva of smokeless tobacco consumers reflect the production of reactive oxygen species at higher levels than controls. Increased lipid peroxidation levels of experimental subjects in saliva than normal control group. The MDA levels were increased in the saliva of smokers versus non smokers [32]. Chewing of smokeless tobacco was increased, a simultaneous increase in the levels of MDA was observed. Significant increase in nitric oxide levels of saliva in smokeless tobacco users compared to non-users. Nitric oxide is degraded into nitrites and nitrates and is synthesized by nitric oxide synthase (NOS) and it exists in three isoforms: neuronal (nNOS), endothelial (eNOS) and inducible nitric oxide synthase (iNOS) forms. Inducible nitric oxide synthase is expressed in every cell and is up regulated in the saliva of experimental subjects. The NOx is a free radical released by NOS enzyme, which is produced from variety of cells and tissues [33].
Earlier reports reported that salivary nitric oxide levels were significantly increased in smokers compared to controls [34]. Nitric oxide has been suggested to play an important role in inflammation process when produced in excess amounts by inducible nitric oxide synthase [35], [36], [37]. Reactive nitrogen species (RNS) include nitric oxide and peroxynitrites and excessive production of these species creates imbalance between antioxidants and RNS leads to the formation of nitrosative stress. Aqueous extracts of areca nut and catechu were capable of generating superoxide anion and hydrogen peroxides and these free radicals play an important role in risk of oral cancer of experimental subjects [38]. The toxic components of smokeless tobacco are mainly nicotine and tobacco specific nitrosamines may also directly or indirectly cause the deterioration of periodontal tissues [39]. Our study showed that uric acid levels were significantly decreased in the saliva of smokeless tobacco consumers compared to controls. Uric acid is the product of purine catabolism and acts as a powerful antioxidant. It acts as a scavenger of singlet oxygen and free radicals by inhibiting oxidative stress [40] and this was consistent with previous findings showing that the significant decrease in uric acid levels in saliva of smokers than non smokers [41]. The GSH is a tripeptide consists of gamma gluatamyl cysteinyl glycine and acts as an antioxidant stabilizes the redox state of proteins. SOD enzyme converts the superoxide radical (O2- into the peroxide (H2O2)) and GPx also catalyzes the reduction of hydroperoxides [42]. The mean level of reduced glutathione was decreased significantly in the saliva of experimental groups as compared to non-chwers and this is also in line with the observation that a significant decrease in salivary GSH concentration was reported after smoking a single cigarette [43]. Salivary glutathione peroxidase, glutathione S-transferase, and catalase levels were decreased significantly in the saliva of smokeless tobacco users than controls. Previous reports had found that the antioxidant enzyme activities of GPx, GST and CAT levels were significantly decreased in liver and kidney of nicotine treated rats [11]. Superoxide dismutase enzyme activity show decreased levels in the experimental groups with no significant change compared to non-users. Consistent with the present study, superoxide dismutase levels showed an increased levels detected in smokers and non-smokers. In aggregate, our findings indicate that the imbalance of antioxidant enzymes, and increased enzyme activity of inducible nitric oxide synthase in saliva after smokeless tobacco chewing.
5. Conclusions
Decrease antioxidant status, uric acid levels, up regulation of lipid peroxidation and nitric oxide levels in the saliva of smokeless tobacco users when compared to controls to suggest that evaluation of these parameters may perhaps be used as markers of tobacco induced nitroxidative stress. One of the important implications for nicotine exposure is the production of reactive oxygen species in smokeless tobacco consumers. Chewing of smokeless tobacco is very dangerous and easily addictable product, awareness have been increased about the harmful effects of smokeless tobacco products and reduces the risk of exposure and consumption. Further studies are needed to correlate the toxic effects of prolonged use of smokeless tobacco on human health.
Funding and Acknowledgements
Miss S. Fareeda Begum is a recipient of ICMR- Senior Research Fellow and financial assistance is greatly acknowledged.
Acknowledgments
Declaration of interest
The authors report no declarations of interest.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Smokeless Tobacco Fact Sheets, 3rd International conference on Smokeless Tobacco Advancing Science&Protecting Public Health. Sweden 22–25, 2002.
- 2.Nair S., Schensul J.J., Begum S., Pednekar M.S., Oncken C., Bilgi S.M., Pasi A.R., Donta B. Use of smokeless tobacco by Indian women aged 18-40 years during pregnancy and reproductive years. PLoS One. 2015;10:1–18. doi: 10.1371/journal.pone.0119814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha D.N., Gupta P.C., Pednekar M. Tobacco use among students in Bihar (India) Indian J. Public Health. 2004;48:111–117. [PubMed] [Google Scholar]
- 4.M. Eriksen, J. Mackay, H. Ross, The Tobacco Atlas 2012. 4th ed. Atlanta, GA. (2015) American Cancer Society; 2012. Www.tobaccoatlas.org. Accessed January 1, 2015.
- 5.Bhan N., Karan A., Srivastava S., Selvaraj S., Subramanian S.V., Millett C. Have Socioeconomic Inequalities in tobacco use in India Increased Over time? Trends from the National sample Surveys (2001–2012) Nicotine Tob. Res. 2016;18:1711. doi: 10.1093/ntr/ntw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nair U.J., Obe G., Friesen M., Goldberg M.T., Bartsch H. Role of lime in the generation of reactive oxygen species from betel-quid ingredients. Environ. Health Perspect. 1992;98:203–205. doi: 10.1289/ehp.9298203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foulds J., Ramstrom L., Burke M., Fagerstrom K. Effect of smokeless tobacco (snus) on smoking and public health in Sweden. Tob. Control. 2003;12:349–359. doi: 10.1136/tc.12.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benowitz N.L., Porchet H., Sheiner L., Jacob P., 3rd Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin. Pharmacol. Ther. 1988;44:23–28. doi: 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- 9.Benowitz N.L., Hukkanen J., Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helen A., Krishnakumar K., Vijayammal P.L., Augusti K.T. Antioxidant effect of onion oil (Allium cepa Linn.) on the damages induced by nicotine in rats as compared to alphatocopherol. Toxicol. Lett. 2000;116:61–68. doi: 10.1016/s0378-4274(00)00208-3. [DOI] [PubMed] [Google Scholar]
- 11.Dey S.K., Roy S. Role of reduced glutathione in the amelioration of nicotine-induced oxidative stress. Bull. Environ. Contam. Toxicol. 2010;84:385–389. doi: 10.1007/s00128-010-9948-5. [DOI] [PubMed] [Google Scholar]
- 12.Buege J.A., Aust S.D. Vol. 52. Academic press; New York: 1978. pp. 302–316. (Methods in Enzymology). [Google Scholar]
- 13.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 14.Kakkar P., Das B., Vishwanathan P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 15.Aebi H.E. Catalase. In: Bergmeyer H.U., editor. Methods of Enzymatic Analyses. Verlag Chemie; Weinheim: 1983. pp. 273–282. [Google Scholar]
- 16.Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B. Selenium: biochemical role as a component of glutathione peroxidise. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 17.Habig W.H., Pabst M.J., Jakoby W.B. Glutathone S-transferases, the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 18.Sastry K.V.H., Moudgal R.P., Mohan J., Tyag J.S., Rao G.S. Spectrophotometric determination of serum nitrite and nitrate by Copper-Cadmium alloy. Anal. Biochem. 2002;306:79–82. doi: 10.1006/abio.2002.5676. [DOI] [PubMed] [Google Scholar]
- 19.Massadeh A.M., Gharaibeh A.A., Omari K.W., Single-Step A. Extraction method for the determination of nicotine and cotinine in jordanian smokers blood and urine samples by RP-HPLC and GC-MS. J. Chromatogr. Sci. 2009;47:170–177. doi: 10.1093/chromsci/47.2.170. [DOI] [PubMed] [Google Scholar]
- 20.Patel B.P., Rawal U.M., Shah P.M., Prajapati J.A., Rawal R.M., Dave T.K., Patel P.S. Study of tobacco habits and alterations in enzymatic antioxidant system in oral cancer. Oncology. 2005;68:511–519. doi: 10.1159/000086995. [DOI] [PubMed] [Google Scholar]
- 21.Battino M., Ferreiro M.S., Gallardo I., Newman H.N., Bullion P. The antioxidant capacity of saliva. J. Clin. Periodontol. 2002;29:189–194. doi: 10.1034/j.1600-051x.2002.290301x.x. [DOI] [PubMed] [Google Scholar]
- 22.Miricescu D., Greabu M., Totan A., Didilescu A., Radulescu R. The antioxidant potential of saliva: clinical significance in oral diseases. Ther. Pharmacol. Clin. Toxicol. 2011;15:139–143. [Google Scholar]
- 23.Feyerabend C., Higenbottam T., Russell M.A. Nicotine concentrations in urine and saliva of smokers and non-smokers. Br. Med. J. (Clin ResEd) 1982;284:1002–1004. doi: 10.1136/bmj.284.6321.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yildiz D., Liu Y.S., Ercal N., Armstrong D.W. Comparison of pure nicotine and smokeless tobacco extractinduced toxicities and oxidative stress. Arch. Environ. Contam. Toxicol. 1999;37:434–439. doi: 10.1007/s002449900537. [DOI] [PubMed] [Google Scholar]
- 25.Benowitz N.L. Theuseof biologic fluid samples in assessing tobacco smoke consumption. NIDA Res. Monogr. 1983;48:6–26. [PubMed] [Google Scholar]
- 26.Schutte-Borkovec K., Heppel C.W., Heling A.K., Richter E. Analysis of myosmine, cotinine and nicotine in human toenail, plasma and saliva. Biomarkers. 2009;14:278–284. doi: 10.1080/13547500902898164. [DOI] [PubMed] [Google Scholar]
- 27.Huque R., Shah S., Mushtaq N., Siddiqi K. Determinants of salivary cotinine among smokeless tobacco users: a cross-sectional survey in Bangladesh. PLoS One. 2016;11:1–9. doi: 10.1371/journal.pone.0160211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu R.H., Hotchkiss J.H. Potential genotoxicity of chronically elevated nitric oxide: a review. Mutat. Res. 1995;339:73–78. doi: 10.1016/0165-1110(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 29.Hershkovich O., Shafat I., Nagler R.M.J. Age-related changes in salivary antioxidant profile: possible implications for oral cancer. Gerontol. A Biol. Sci. Med Sci. 2007;62:361–366. doi: 10.1093/gerona/62.4.361. [DOI] [PubMed] [Google Scholar]
- 30.Roche M., Rondeau P., Singh N.R., Tarnus E., Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008;582:1783–1787. doi: 10.1016/j.febslet.2008.04.057. [DOI] [PubMed] [Google Scholar]
- 31.Guentsch A., Preshaw P.M., Bremer-Streck S., Klinger G., Glockmann E., Sigusch B.W. Lipid peroxidation and antioxidant activity in saliva of periodontitis patients: effect of smoking and periodontal treatment. Clin. Oral. Investig. 2008;12:345–352. doi: 10.1007/s00784-008-0202-z. [DOI] [PubMed] [Google Scholar]
- 32.Wadhwa D., Bey A., Hasija M., Moin S., Kumar A., Aman S., Sharma V.K. Determination of levels of nitric oxide in smoker and nonsmoker patients with chronicperiodontitis. J. Periodontal Implant Sci. 2013;43:215–220. doi: 10.5051/jpis.2013.43.5.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ambe K., Watanabe H., Takahashi S., Nakagawa T., Sasaki J. Production and physiological role of NO in the oral cavity. Jpn. Dent. Sci. Rev. 2016;52:14–21. doi: 10.1016/j.jdsr.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moncada S., Higgs A. The L-argininenitric oxide pathway. New Eng. J. Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 35.Nussler A.K., Billiar T.R. Inflammation, immunoregulation, and inducible nitric oxide synthase. J. Leuk. Biol. 1993;54:171–178. [PubMed] [Google Scholar]
- 36.Lundberg J.O.N. Airborne nitric oxide: inflammatory marker and aerocrine messenger in man. Acta Phys. Sin. 1996;157:4–6. doi: 10.1111/apha.1996.157.s633.4. [DOI] [PubMed] [Google Scholar]
- 37.Nair U.J., Floyd R.A., Nair J., Bussachini V., Friesen M., Bartsch H. Formation of reactive oxygen species and of 8-hydroxydeoxyguanosine in DNA invitro with betel quid ingredients. Chem. Biol. Interact. 1987;63:157–169. doi: 10.1016/0009-2797(87)90095-0. [DOI] [PubMed] [Google Scholar]
- 38.Al-Tayar B., Tin-Oo M.M., Sinor M.Z., Alakhali M.S. Prevalence and association of smokeless tobacco use with the development of periodontal pocket among adults in Dawan Valley, Yemen: a cross swctional study. Tob. Induc. Dis. 2015;13:35–43. doi: 10.1186/s12971-015-0061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ames B.N., Cathcart R., Schweisrs E., Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant and radical caused ageing and cancer: a hypothesis. Proc. Natl. Acad. Sci. USA. 1981;73:6852–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arathi A., Benedicta Souza D., Sayanthan M., Raksha S., Buthesh G.A., Jisha K., Hegde M.C., Vivian Souza D. Salivary malondialdeyde and antioxidant status in oral squamous cell carcinoma patients and smokers. Biomed. Res. 2010;21:67–70. [Google Scholar]
- 41.Mates J.M., Sanchez-Jimenez F.M. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int. J. Biochem. Cell Biol. 2000;32:157–170. doi: 10.1016/s1357-2725(99)00088-6. [DOI] [PubMed] [Google Scholar]
- 42.Zappacosta B., Persichilli S., Mordente A., Minucci A., Lazzaro D., Meucci E., Giardina B. Inhibition of salivary enzymes by cigarettes smoke and the protective role of glutathione. Hum. Exp. Toxicol. 2002;21:7–11. doi: 10.1191/0960327102ht202oa. [DOI] [PubMed] [Google Scholar]
- 43.Agnihotri R., Pandurang P., Kamath S.U., Goyal R., Ballal S., Shanbhogue A.Y., Kamath U., Bhat G.S., Bhat K.M. Association of cigarette smoking with superoxide dismutase enzyme levels in subjects with chronic periodontitis. J. Periodontol. 2009;80:657–662. doi: 10.1902/jop.2009.080545. [DOI] [PubMed] [Google Scholar]