Abstract
Aim
To evaluate whether hypofractionation with integrated boost to the tumour bed using intensity-modulated radiation therapy is an acceptable option and to determine whether this treatment compromises local control, toxicity and cosmesis.
Background
Retrospective studies have demonstrated that patients who are treated with HF and integrated boost experience adequate local control, a dosimetric benefit, decreased toxicity and acceptable cosmesis compared with conventional fractionation.
Materials and methods
A retrospective, observational and longitudinal study was conducted from January 2008 to June 2015 and included 34 patients with breast cancer (stage 0–II) who were undergoing conservative surgery.
The prescribed doses were 45 Gy in 20 fractions (2.25 Gy/fraction) to the breast and 56 Gy in 20 fractions (2.8 Gy/fraction) to the tumour bed.
Results
Thirty-four patients were included. The mean follow-up was 49.29 months, and the mean age was 52 years. The mean percentage of PTV from the mammary region that received 100% of the prescribed dose was 97.89% (range 95–100), and the mean PTV percentage of the tumour bed that received 100% of the dose was 98% (95–100).
The local control and the overall survival were 100%, and the cosmesis was good in 82% of the patients. Grade 1 acute toxicity was present in 16 patients (47%), and grade 1 chronic toxicity occurred in 6 cases (18%).
Conclusion
The results of the present study demonstrate that hypofractionation with integrated boost using intensity-modulated radiation therapy is an acceptable option that provides excellent local control and low toxicity.
Keywords: Hypofractionation, Concomitant boost, Early-stage breast cancer, Mexico, Intensity-modulated radiation therapy
1. Background
In patients with early-stage breast cancer, the finding that treatment with conservative surgery and adjuvant radiotherapy (RT) has greater efficacy in local control (LC, i.e., a decreased risk of recurrence of up to 70% within 5 years) and overall survival (OS, i.e., 5% absolute improvement over 15 years) has been established for several decades with the support of several randomized studies.1, 2, 3, 4
The boost to the surgical area was initially questioned, but 2 randomizphase III studies have confirmed that the increase to the tumour bed reduces local recurrence (LR) without deteriorating the cosmesis.4, 5, 6, 7, 8, 9 In the beginning of 2015, the results of the NCT0229033 study were reported10; this study was a 20-year follow-up in which the OS rates were similar in both arms, and the cumulative recurrences were 16.4% without boost vs. 12% with boost with an hazard ratio (HR) of 0.95 [99% CI (0.52–0.81), p < 0.0001].
Regarding conventional RT to the breast and concomitant boost to the tumour bed, there are few studies, and most of these are institutional11, 12, 13, 14, 15; these studies used fractions of 1.6–1.8 Gy with total doses of 45–51 Gy to the mammary gland and doses of 2.3–2.4 Gy per fraction (Fx) to the tumour bed for total doses of 60–73 Gy.
Hypofractionation (HF) plus sequential boost is a treatment that was proposed many years ago. The LRs over 5 years were similar in the Royal Marsden Hospital and START (A, B) studies and ranged from 9 to 14% and 2–5% to 10 years, respectively.16, 17
HF has expanded as an option and does not involve differences from the conventional schedule regarding LC, locoregional control (LRC) or OS.4, 18, 19, 20
There is not much phase III evidence regarding HF with concomitant boost to the tumour bed.21, 22, 23, 24 Few phase I—II studies have been published, and the available studies have heterogeneous numbers of patients and involve doses to the breast ranging from 2.5 to 2.7 Gy/Fx with totals of 15–20 Fx and doses to the tumour bed of 2.75–3.5 Gy/Fx.25, 26, 27, 28, 29, 30 Regarding patients over 70 years of age who have been treated with this schedule, the evidence is scarce, but the results have proven this approach to be an option for this population group.20, 31, 32, 33, 34, 35
The 2018 management guides of the National Comprehensive Cancer Network (NCCN) indicate that boost to the tumour bed is indicated for those who are <50 years of age and have high-grade or focally positive margins. The boost to positive margins requires an increase of the dose to the tumour bed and may increase fibrosis. Therefore, caution is required for an indication for an additional dose to this site.1
The present study utilized HF to the breast with concomitant boost to the tumour bed using intensity-modulated radiation therapy (IMRT) based on the schedule proposed by Freedman et al. in which the dose is maintained, while the boost energy is modified.
2. Aim
-
-
Evaluate whether HF with concomitant boost applied through IMRT is an acceptable technique.
-
-
Determine whether this radiation technique compromises local control, toxicity or cosmesis.
3. Materials and methods
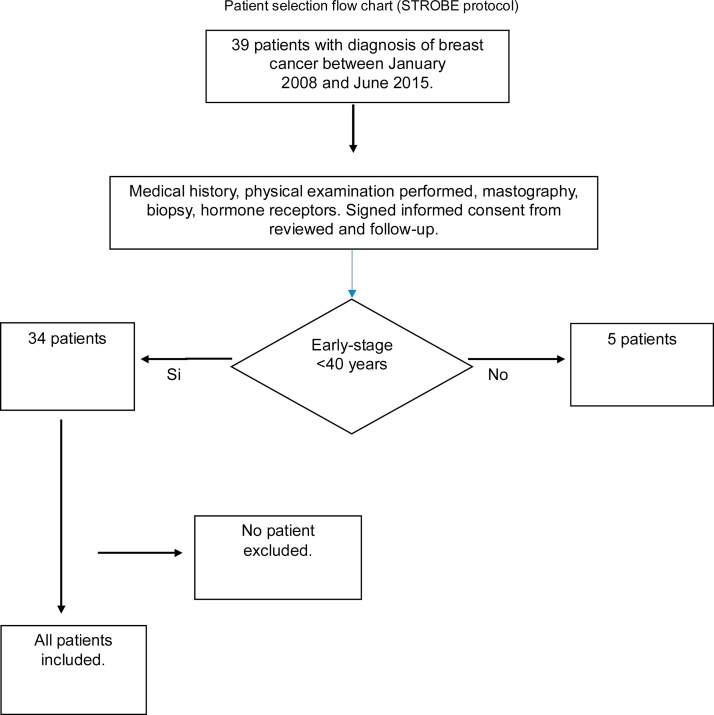
This is a retrospective, observational and longitudinal study that was conducted from January 2008 to June 2015 in the Radiotherapy Unit of the Hospital General de Mexico. Thirty-nine cases were reviewed, and 5 were excluded because they did not meet the inclusion criteria. Therefore, 34 patients were included in the study. The inclusion criteria were as follows: patients who had been subjected to conservative surgery with a histopathological diagnosis of breast cancer in the initial stage (pTis-T2) according to the American Joint Committee on Cancer (AJCC, 6th edition), tumours < 3 cm, negative lymph nodes (N0), and older than 40 years of age. The exclusion criteria were as follows: patients younger than 40 years, multifocal disease, positive lymph nodes, synchronous or metachronous disease, collagen disease, second primary disease, surgery outside the hospital, and previous radiation therapy (Fig. 1).
Fig. 1.
Flow chart of the selection of patients based on the guidelines from the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement.
The included patients received an HF schedule with concomitant boost to the tumour bed using IMRT. The dose to the mammary area was 45 Gy in 20 Fx, and the dose to the tumour bed was 56 Gy in the same number of fractions.
3.1. Simulation, volume definition and planning
The patients were placed in the supine position with both arms above the head level, and a ramp was used under the breast. In cases of patients with voluminous breasts, prone ramps were used. The patients were aligned, and we defined the origin using lasers. Radiopaque marks were placed on the surgical scar and on the drainage sites. Planning was performed using axial images from tomography with 5-mm cuts from the lower border of the jaw extending to 2–3 cm below the inframammary border.
The computed tomography (CT) scans were sent to the Eclipse planning system version 7.3.10 from 2004 to 2014 and to the Eclipse 13 version beginning in 2015. The volume definitions were based on the International Commission on Radiation Units and Measurements (ICRU) 50, and the volumes were defined for the mammary region and the tumour bed. The clinical target volume (CTV) of the breast was defined to include all mammary tissue, and the planning target volume (PTV) was created by allowing a 3-mm margin from the mammary CTV. The tumour bed was outlined from the surgical clips that were placed during surgery, a 3–5-mm margin was given to create the CTV from the site, and the PTV of the tumour bed was created by allowing a 5-mm margin from the CTV of the site. The mammary PTV was maintained 3 mm under the skin in all cases.
The risk organs were outlined and included the ipsilateral lung, heart (including the pericardium), pulmonary trunk and contralateral breast. The contralateral lung was outlined but was not included as a risk organ.
The treatment plan was executed in all patients with the forward IMRT technique with multiple beams (7–9 fields) using a step-and-shoot approach with a photon energy of 6 MV (MV). Fields with a perpendicular entrance to the breast were restricted. Both treatment volumes were included in the same radiation plan. Dose calculation was performed with pencil beam convolution and AAA. The dose homogeneity in the central axis was <7%. The PTV volume received at least 95% of the prescribed dose. The area that received 110% of the prescribed dose was kept <2 cc of the PTV volume.
Quality assurance for the IMRT treatments was performed until February of 2014 through verification with Kodak EDR2 film using a solid phantom of 30 cm × 30 cm in 6-cm depth and a VIDAR densitometer. The measurements of the absolute dose were performed with a Farmer Wellhofer ionization chamber and Wellhofer electrometer. Omni Pro Software was used for the data analysis. After this date, quality assurance was performed with Octavius PTW equipment and an Octavius 729 detector using the criterion of a gamma index ≤ 1 to ensure compliance with dose and distance restrictions. Data analysis was performed with MEPHYSTO Verisoft software.
3.2. Treatment dose and restriction
The applied radiation schedule was 45 Gy to the breast and 56 Gy to the tumour bed in 20 Fx given from Monday to Friday with rests on Saturday and Sunday. The daily radiation doses per fraction to the breast and tumour bed were 2.25 Gy and 2.8 Gy, respectively, and the doses were given concomitantly.
The risk organ restrictions were V20 < 20% for the ipsilateral lung and V25 < 10% for the heart, and the maximum dose for the contralateral breast was <5 Gy. Treatment verification was performed twice per week by comparing portal imaging with a digitally reconstructed radiograph (DRR) using mammary tissue and bone anatomy for better localization.
3.3. Evaluation and statistical analysis
The patients were examined every 2 weeks during treatment. The Radiation Therapy Oncology Group (RTOG) scale was used to evaluate acute toxicity. Follow-ups after radiotherapy treatment were performed every 3 months during the first year, every 4 months during the second year and every 6 months after the third year. Chronic toxicity was evaluated with the Common Terminology Criteria Adverse Events scale 3.0 version (CTCAE v3.0), which includes telangiectasias, fibrosis, hyperpigmentation and atrophy. Cosmesis was not evaluated with a specific scale but was determined as good, regular or poor based on the chronic toxicity grade. The disease control evaluation was clinical and was conducted through imaging studies (i.e., mammogram, skeletal scintigraphy, or liver ultrasound).
The statistical analyses were performed with the SPSS programme version 22 (IBM, Chicago, IL, USA) using the frequency and central tendency measurements. The Spearman's rho test was applied for non-parametric variables, and survival was analysed with Kaplan–Meyer methods.
4. Results
Thirty-four patients were included in the present analysis. The mean follow-up was 49.2 months (range 20–72). The considered variables are presented in Table 1. The mean age was 52.6 years (50–80). The left breast was the most commonly affected (56%; 19 patients), and the right was affected in 15 patients (44%). The most commonly affected quadrant was the upper external quadrant (67.6%), and the mean size of the pathological tumour was 1.4 (0–3 cm). Twenty-one patients (62%) were in stage I (pT1N0M0), 9 patients (26%) were in stage IIA (pT2N0M0), and 4 patients (12%) were in stage 0 (pTisN0M0).
Table 1.
Characteristics of patients.
| No (%) | |
|---|---|
| Age (years) | |
| Mean | 52.62 (50–80) |
| Median | 54 (50–80) |
| >50–60 | 29 (85%) |
| >60–70 | 4 (12%) |
| >70–80 | 1 (3%) |
| Laterality | |
| Left-sided | 19 (56%) |
| Right-sided | 15 (44%) |
| Tumor size (cm) | |
| Mean | 1.46 (0–3) |
| T stage | |
| pTis | 4 (12%) |
| pT1 | 21 (62%) |
| pT2 | 9 (26%) |
| N stage | |
| pN0 | 34 (100%) |
| Histopathological subtype | |
| Ductal invasive | 13 (38%) |
| Ductal in situ | 9 (26%) |
| Ductal in situ + invasive | 5 (15%) |
| Lobular invasive | 3 (9%) |
| Ductal + lobular invasive | 3 (9%) |
| Mucinous | 1 (3%) |
| Histopathological factors | |
| aLVI−/PNI−/margins−/tumor bed− | 22 (65%) |
| aLVI+/PNI−/margins−/tumor bed− | 7 (21%) |
| aMargins+/LVI−/PNI−/tumor bed− | 5 (15%) |
| Hormone receptor status | |
| aER+/PR+ | 21 (62%) |
| aER+/PR− | 3 (9%) |
| aER−/PR+ | 2 (6%) |
| aER−/PR− | 8 (23%) |
| HER2/Neu status | |
| Negative | 28 (82%) |
| Positive | 6 (18%) |
| Adjuvant treatment | |
| Chemotherapy/radiotherapy | 18 (53%) |
| Only radiotherapy | 16 (47%) |
| Adjuvant hormonal therapy | |
| Yes | 26 (77%) |
| No | 8 (23%) |
| Hormonal therapy type | |
| Tamoxifen | 15 (44%) |
| Anastrozole | 11 (32%) |
Lymphovascular invasion (LVI); perineural invasion (PNI); estrogen receptors (ER); progesterone receptors.
In our findings, the most frequent histopathological subtype was invasive ductal carcinoma in 38% (13) of patients followed by in situ ductal carcinoma (DCIS) in 26% (9) patients and mixed (ductal in situ and invasive in 15%; the remaining cases were lobular and mucinous).
Regarding the histopathological factors, 22 patients (65%) had negative margins of the tumour bed, lymphovascular invasion (LVI) and perineural invasion (PNI), 7 patients (21%) were positive for LVI but negative for the other factors, and 5 patients (15%) had positive margins (not submitted to re-excision).
Regarding the hormonal receptors, 62% (21 patients) were oestrogen and progesterone +, 23% (8 patients) were receptor−, 9% (3 patients) were oestrogen+ and progesterone−, and 6% (2 patients) were oestrogen− and progesterone+. Regarding HER 2/Neu, 82% were reported to be negative, and 18% were positive.
After conservative surgery, 16 patients (47%) received only RT, and 18 (53%) received adjuvant chemotherapy/radiotherapy (QT/RT). In the latter group, chemotherapy (QT) was given before RT in 14 patients, 3 patients received 3 cycles before and 3 after radiation, and one patient received QT after radiation. Regarding the chemotherapy schedules, 17.6% of the patients were managed with doxorubicin/cyclophosphamide (DC), 14.7% received fluorouracil/doxorubicin/cyclophosphamide (FDC), and 11.8% received DC-paclitaxel; another 3 patients (8.67%) received one treatment on this schedule combined with trastuzumab, and the other 3 patients who were HER 2/Neu positive did not receive trastuzumab due to previous cardiopathy.
In reference to hormonal treatment, 26 patients (77%) received it, and 8 patients (23%) did not due to negativity for the receptors. Fifteen patients (44%) received tamoxifen, and 11 (32%) received anastrozole. In contingency tables, 4 patients were demonstrated to be triple negative, and 4 patients with negative receptors were Her 2 Neu-positive; the former group received only adjuvant RT. Among the 3 patients who received trastuzumab, one was positive for the receptors, the second was oestrogen+ and progesterone−, and the last patient was negative for the receptors.
Regarding the RT treatments, 33 patients were treated in the supine position, and only one patient with left breast cancer was treated in the prone position due to a large mammary volume. Thirty patients were treated with 7 beams, and 4 were treated with 9 beams. All patients received their treatments at the stipulated times.
The mean percentage of the PTV from the mammary region that received 100% of prescribed dose was 97.89% (range 95–100), and the mean percentage of the PTV from the tumour bed that received 100% of the dose was 98% (95–100). The mean percentage of the PTV from the breasts that received more than 110% of the prescribed dose was 0.29% (range 0–2). The mean breast volume was 957 cc (475–1578).
The mean percentage of the ipsilateral lung that received 20 Gy was 18.66% (4–25). One patient with a lesion in the right breast and a mammary volume of 1250 cc received 20 Gy on 25% of the volume of the ipsilateral lung, but the PTV coverage of the breast was not compromised. The mean dose of the hearts that received 25 Gy was 8.25% (0.02–22), and the mean maximum dose to the contralateral breast was 3.67 Gy (1.8–7.8). Another patient with a mammary volume of 1578 cc exceeded the restrictions for the heart and contralateral breast, but neither the prescription nor the PTV coverage of the breast were modified.
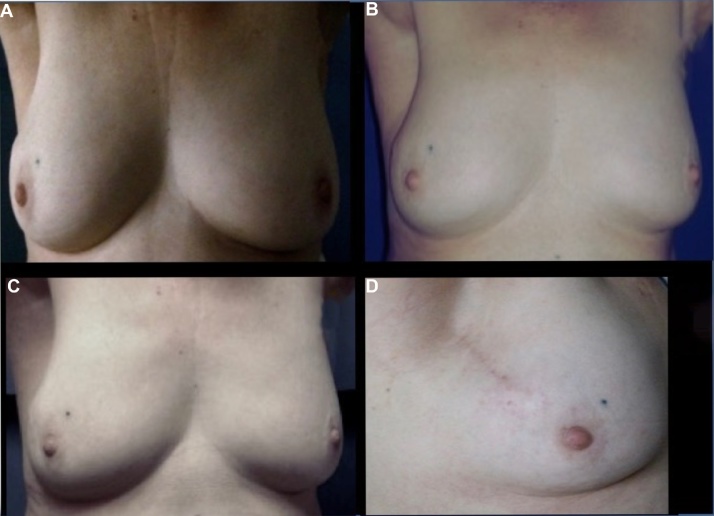
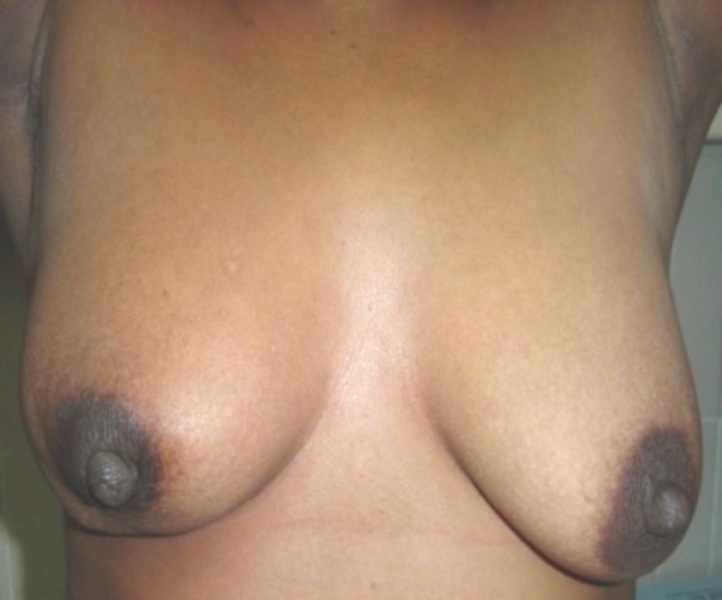
Acute toxicity was evaluated by the physician with the RTOG scale. During the treatment period, only 16 patients (47%) presented with acute toxicity as characterized by grade 1 dry radiodermatitis and were managed with topical treatment; the conditions reverted 2 weeks after radiation was finished. No patients presented with sub-acute toxicity (Fig. 2, Fig. 3 and Table 2).
Fig. 2.
Patient with early-stage breast cancer with conservative surgery plus partial breast irradiation with teletherapy treatment. (A) Evaluation at two years. (B) Evaluation at three years. (C) Evaluation at four years. (D) Evaluation at four years late view.
Fig. 3.
Patient with follow-up to four years after treatment with teletherapy.
Table 2.
Acute skin toxicity.
| Skin toxicity | Patients | % |
|---|---|---|
| No change skin | 16 | 53 |
| Grade 1 | 14 | 47 |
| Grade 2 | 0 | 0 |
| Grade 3 | 0 | 0 |
| Grade 4 | 0 | 0 |
Radiation Therapy Oncology Group (RTOG) scale.
Chronic toxicity, it was evaluated by the physician with the CTCAE v3.0, and the LENT-SOMA scale was not applied. Toxicity was evaluated 6 months after the radiation treatment was finished. We found that only 6 of 34 patients presented with toxicity that was characterized by grade 1 telangiectasias in 2 patients (6%) and grade 1 fibrosis on the site of the surgical scar in 4 patients (12%). In the evaluations of the cosmetic results, 82% (28 patients) had a good outcome (Table 3).
Table 3.
Late skin toxicity.
| Skin toxicity | Patients | % |
|---|---|---|
| No change skin | 28 | 82 |
| Fibrosis grade 1 | 4 | 12 |
| Telangiectasias grade 1 | 2 | 6 |
| Hyperpigmentation | 0 | 0 |
| Atrophy | 0 | 0 |
Common Terminology Criteria Adverse Events scale 3.0 version (CTCAE v3.0).
The mean follow-up was 4 years with a maximum of 6 years, and the mean disease-free interval was 38 months (range 12–60). Locoregional and distant control were achieved in 100% of the patients. Until the end of the study, no recurrence was found, 33 patients were alive and without tumour activity (97%), and 1 patient was lost at 36 months of follow-up; however, we reached this patient by phone and confirmed that she continued without tumour activity.
Kaplan–Meier curves for OS and disease-free survival (DFS) are not presented due to the lack of censual events, but 100% (95% IC) survivals were registered for both.
The correlations of the non-parametric variables were performed using Spearman's rho. In the analyses of the correlations of acute toxicity with breast PTV%, tumour bed PTV%, breast volume, adjuvant chemotherapy and hormonal treatment, no significant results were found (p = 0.74, p = 0.57, p = 0.78, p = 0.030 and p = 0.79, respectively). In terms of chronic toxicity, the presence of telangiectasias and fibrosis was significantly correlated with the mammary PTV% that received 100% of the dose (p = 0.036), but there were no significant correlations with the PTV% of the tumour bed, breast volume, adjuvant chemotherapy or hormonal treatment (p = 0.18, p = 0.69, p = 0.54, and p = 0.78, respectively). The pulmonary V20 was not significantly correlated with the breast PTV% or the tumour bed that received 100% of prescribed dose (p = 0.72 and p = 0.91, respectively), with breast volume (p = 0.66) or the affected breast (p = 0.30). Regarding the heart's V25, there was no significant correlation with the PTV% of the right breast that received 100% of prescribed dose (p = 0.60) and breast volume (p = 0.47), but there was a significant correlation between the heart V25 and the irradiation of the left breast (p = 0.001). Importantly, 56% of the patients were affected in the left breast.
These results indicate that IMRT to the breast and concomitant boost offers adequate tumour control without increasing toxicity while preserving cosmesis.
A quality of life evaluation of this treatment technique was not the objective of this publication, and this issue should be evaluated in the future, preferably with prospective studies.
5. Discussion
HF has been demonstrated to be a safe, effective and a tolerable treatment strategy.14, 15, 16, 17, 18 The boost to the tumour bed has been completely demonstrated to reduce LR in patients with invasive breast cancer5, 6, 7, 8, 9, 10; intensifying the treatment by giving a boost to the tumour bed is not standard for patients with DCIS, and its use has been questioned. The results of retrospective studies demonstrate a reduction in the LR (3–6%) and a life expectancy greater than 10 years.4, 18, 27, 32, 33, 34 The boosts in the studies from the Royal Marsden Hospital and the START A and B were given sequentially.16, 17, 18 Regarding HF and concomitant boost, the results of phase III studies are few21, 22, 23, 24 as are the results of phase I and II studies, but the evidence in the literature demonstrates a reduction in LR; e.g., Clervide's and Freedman's studies.20, 25, 26, 27 The studies of HF and concomitant boost with IMRT have demonstrated a better dose homogeneity on the breast and tumour bed with adequate organ-at-risk protection.25, 26, 28, 29,32 Moreover, these studies have not demonstrated a dosimetric difference between the sequential and concomitant boost regimens.11, 12
Some reports of HF with concomitant boost have included patients with carcinoma in situ and concluded that, in this subtype, the treatment is safe and effective and provides adequate LC with moderate toxicity, but these studies require validation with prospective randomized studies.27, 32, 33, 34, 35 In the study from Clervide et al., 145 patients with carcinoma in situ received 42 Gy to the breast and 45.9 Gy to the tumour bed, and the rates of LR and CL were 4.1% and 96%, respectively; this study did not evaluate OS. Other studies have included patients with pT1-pT2 invasive carcinomas.13, 25
The IMPORT HIGH (CKUK/06/003) phase III study used dose scaling with accelerated HF in 2 schedules of concomitant boost vs. HF with a sequential boost. In 26 centres, this study evaluated the usage of IMRT with an inverse technique or direct technique for concomitant boost to the tumour bed that was marked with surgical clips and concluded that the IMPORT HIGH provides a guide and the necessary support for a safe integration of the appropriate RT techniques.22
In our study, 74% of the patients had invasive cancer, and 26% had in situ cancer. The cut-off age to receive HF with concomitant boost was >50 years old as utilized in phase I and II studies.25, 26
Adjuvant RT treatment was given to 16 patients, and 18 were treated with QT/RT. Although 65% of our patients did not have pathologic factors for bad prognoses for recurrence, they received the boost on the tumour bed due to the benefits observed in retrospective studies. Phase II studies do not recommend HF with concomitant boost in patients who have received adjuvant QT; however, in our study, these patients were included, and we did not observe any increase in toxicity.13 Hormonal treatment was given to 77% of the patients without increasing the toxicity as has been mentioned in some studies.
In phase II studies, HF with concomitant boost using IMRT has demonstrated a reduction in target inhomogeneity and, as demonstrated by the Emory University School of Medicine which has used this technique since 2003, also reduces the dose gradient and improves the treatment volume coverage.19, 26, 27, 28 Similarly, in the present study, the PTV mean percentage of the mammary area that received 100% of the prescribed dose was 97.89%, and the PTV of the tumour bed was 98%. Van der Lan reported that the dose was reduced by 20%, the mean volume of breast tissue outside the boost PTV that received 95% of the boost dose was reduced by 54%, and the mean heart and lung dose was reduced by 10%.13 Moreover, in our study, the risk organs were kept inside the marked restrictions, the mean percentage of the ipsilateral lung that received 20 Gy was 18.66%, the mean percentage of the heart that received 20 Gy was 8.25%, and the mean maximum dose to the contralateral breast was 3.6 Gy. These results are similar to what has been reported with the use of conventional fractionation with IMRT.11, 12, 13, 22 In terms of acute toxicity, 47% presented with grade 1 radiodermatitis, and 53% did not exhibit any toxicity.25, 26, 27, 28, 29, 30, 31, 32 Chronic toxicity was evaluated after 6 months, and 6 patients developed grade 1 toxicity (telangiectasias and fibrosis). Good cosmesis was present in 82%, which is very similar to that reported by Formenti et al.13, 19, 20 as well as the results from studies that have involved HF and concomitant boost.22, 23, 24, 25, 26, 27, 28, 29
Recently, Hammer et al. reported a prediction model based on a multivariate analysis for grade 2 or higher fibrosis in the tumour bed and discovered 3 influential independent variables, i.e., age, the CTV volume of the breast that receives more than 55 Gy (V55) and the maximum dose of radiation to the breast. According to this model, the chances of grade 2 or higher fibrosis increase as the Dmax, V55 and age increase. In our study, there were no significant correlations of fibrosis and telangiectasias with the breast volume, PTV percentage to the tumour bed, adjuvant QT or hormonal treatment, but there was a significant correlation with the PTV% of the breast that received 100% of the prescribed dose; although the fibrosis was grade 1. The model proposed by Hammer must be considered in prospective studies, but it is important to identify the other previously mentioned that promote fibrosis, such as the dose to the tumour bed, dose inhomogeneity, breast volume, energy (electrons), QT and hormonal therapy, age and post-surgical complications, such as haematoma, seroma or infection, to achieve better predictions.15
The present study had a mean follow-up of 49.29 months (range 20–60) and a mean disease-free interval of 38 months. Few studies of concomitant boost with IMRT have a 5-year follow-up. The OS was 100% without any local or distant recurrence at the time of evaluation. In the Clervide and Freedman studies, the local recurrences at 5 years were reported to be 4.1% and 2.7%, respectively; at Emory University, this value was 2.9%, and the reported OS of 97% is similar to what has been reported with conventional fractionation.19, 20, 25
The results from our study demonstrate that HF with concomitant boost using IMRT is a treatment option for patients with early-stage breast cancer that does not compromise CL or cosmesis, although for the latter, a longer period of evaluation is needed.
6. Conclusion
HF with concomitant boost using IMRT is a treatment option for patients with early-stage breast cancer that offers an adequate LC, less toxicity compared with the conventional schedule and acceptable cosmesis. Therefore, it is possible to use this RT modality if the technological conditions allow it in your hospital centre.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.National Comprehensive Cancer Network Guidelines (NCCN [Internet]). Invasive Breast Cancer. Version 1.2018 (last review April 05, 2018). Available in: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 2.Clarke M., Collins R., Darby S. Early Breast Cancer Trialist Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival. An overview of the randomized trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.Ceilley E., Jagsi R., Goldberg S. Radiotherapy for invasive breast cancer in North America and Europe: results of a survey. Int J Radiat Oncol Biol Phys. 2005;61(2):365–373. doi: 10.1016/j.ijrobp.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 4.Smith B.D., Bentzen S.M., Correa C.R. Fractionation for whole breast irradiation: an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;81(1):59–68. doi: 10.1016/j.ijrobp.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 5.Bartelink H., Horiot J.C., Poortmans P.M. Impact of a higher radiation dose on local control and a survival in breast-conserving therapy of early breast cancer : 10 years results of the randomized boost vesus no boost EORTC 22881-10-882 trial. J Clin Oncol. 2007;25(22):3259–3265. doi: 10.1200/JCO.2007.11.4991. [DOI] [PubMed] [Google Scholar]
- 6.Romestaing P., Lehingue Y., Carrie C. Role of a 10-Gy boost in the conservative treatment of early cancer: Results of a randomized clinical trial in Lyon, France. J Clin Oncol. 1997;15(3):963–968. doi: 10.1200/JCO.1997.15.3.963. [DOI] [PubMed] [Google Scholar]
- 7.Oh K.S., Kong F.M., Griffith K.A., Yanke B., Pierce L.J. Planning the breast tumor bed boost: changes in the excision cavity volume and surgical scar location after breast-conserving surgery and whole-breast irradiation. Int J Radiat Onco Biol Phys. 2006;66(3):680–686. doi: 10.1016/j.ijrobp.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 8.Bartelink H., Horiot J.C., Poortmans P. Recurrence rates after treatment of breast cnacer with or without additional radiation. N Engl J Med. 2001;345(19):1378–1387. doi: 10.1056/NEJMoa010874. [DOI] [PubMed] [Google Scholar]
- 9.Vrieling C., Collette L., Fourquet A. The influence of the boost in breast-conserving therapy on cosmetic outcome in the EORTC ``boost versus no boost" trial. EORTC Radiotherapy and Breast Cancer Cooperative Groups. European Organization for Research and Treatment of Cancer. Int J Radiat Oncol Biol Phys. 1999;45(3):677–685. doi: 10.1016/s0360-3016(99)00211-4. [DOI] [PubMed] [Google Scholar]
- 10.Bartelink H., Maingon P., Poortmans P. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomized phase 3 trial. Lancet Oncol. 2015;16(1):47–56. doi: 10.1016/S1470-2045(14)71156-8. [DOI] [PubMed] [Google Scholar]
- 11.Fiorentino A., Mazzola R., Ricchetti F. Intensity modulated radiation therapy with simultaneous integrated boost in early breast cancer irradiation. Report of feasibility and preliminary toxicity. Cancer Radiother. 2015;19(5):289–294. doi: 10.1016/j.canrad.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Lee H.H., Hou M.F., Chuang H.Y. Intensity modulated radiotherapy with simultaneous integrated boost vs conventional radiotherapy with sequential boost for breast cancer – a preliminary result. Breast. 2015;24(5):656–660. doi: 10.1016/j.breast.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Freedman G.M., White J.R., Arthur D.W., Allen Li X., Vicini F.A. Accelerated fractionation with a concurrent boost for early stage breast cancer. Radiother Oncol. 2013;106(1):15–20. doi: 10.1016/j.radonc.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Krug D., Souchon R. Radiotherapy of ductal carcinoma in situ. Breast Care. 2015;10(4):259–264. doi: 10.1159/000437452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammer C., Maduro J.H., Bantema-Joppe E.J. Radiation-induced fibrosis in the boost area after three-dimensional conformal radiotherapy with a simultaneous integrated boost technique for early-stage breast cancer: a multivariable prediction model. Radiother Oncol. 2017;122(1):45–49. doi: 10.1016/j.radonc.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 16.START Trialists' Group. Bentzen S.M., Agrawal R.K., Aird E.G. The UK Standardization of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomized trial. Lancet Oncol. 2008;9(4):331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.START Trialists' Group. Bentzen S.M., Agrawal R.K., Aird E.G. The UK Standardization of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomized trial. Lancet. 2008;371(9618):1098–1107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haviland J.S., Owen J.R., Dewar J.A. The UK Standardization of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomized controlled trials. Lancet Oncol. 2013;14(11):1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 19.Harnett A. Fewer fractions of adjuvant external beam radiotherapy for earlybreast cancer are safe and effective and can now be the standard of care. Why the UK's NICE accepts fewer fractions as the standard of care for adjuvant radiotherapy in early breast cancer. Beast. 2010;19(3):159–162. doi: 10.1016/j.breast.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 20.Murphy C., Anderson P.R., Li T. Impact of the radiation boost on outcomes after breast-conserving surgery and radiation. Int J Radiat Oncol Biol Phys. 2011;81(1):69–76. doi: 10.1016/j.ijrobp.2010.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.RTOG 1005: a phase III trial of accelerated whole breast irradiation with hypofractionation plus concurrent boost versus standard whole breast irradiation plus sequential boost for early-stage breast cancer. Last review November 17, 2017. Available in: https://www.rtog.org/clinicaltrials/protocoltable/studydetails.aspx?study=1005.
- 22.Tsang Y., Ciurlionis L., Kirby A.M. Clinical impact of IMPORT HIGH (CRUK/06/003) on breast radiotherapy practices in the United Kingdom. Br J Radiol. 2015;88(1056):20150453. doi: 10.1259/bjr.20150453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Askoxylakis V., Jensen A.D., Hafner M.F. Simultaneous integrated boost for adjuvant treatment of breast cancer-intensity modulated vs conventional radiotherapy: the IMRT-MC2 trial. BMC Cancer. 2011;11:249. doi: 10.1186/1471-2407-11-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Parijs H., Miedema G., Vinh-Hung V. Short course radiotherapy with simultaneous integrated boost for stage I–II breast cancer, early toxicity of a randomized trial. Radiat Oncol. 2012;7:80. doi: 10.1186/1748-717X-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerrero M., Li X.A., Earl M.A., Sarfaraz M., Kiggundu E. Simultaneous integrated boost for breast cancer using IMRT. A radiobiological and treatment planning study. Int J Radiat Oncol Biol Phys. 2004;59(5):1513–1522. doi: 10.1016/j.ijrobp.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Freedman G.M., Anderson P.R., Bleicher R.J. Five-year local control in a phase II study of hypofrationated intensity modulated radiation therapy with an incorporated boost for early stage breast cancer. Int J Radiat Oncol Biol Phys. 2012;84(4):888–893. doi: 10.1016/j.ijrobp.2012.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cante D., Franco P., Sciacero P. Five-year results of a prospective case series of accelerated hypofractionated whole breast radiation with concomitant boost to the surgical bed after conserving surgery for early breast cancer. Med Oncol. 2013;30(2):518–527. doi: 10.1007/s12032-013-0518-7. [DOI] [PubMed] [Google Scholar]
- 28.Franco P., Zeverino M., Migliaccio F. Intensity-modulated and hypofractionated simultaneous integrated boost adjuvant breast radiation employing static ports of tomotherapy (TomoDirect): a prospective phase II trial. J Cancer Res Clin Oncol. 2014;140(1):167–177. doi: 10.1007/s00432-013-1560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kyrgias G., Zygogianni A., Theodorou K. Accelerated hypofractionated whole-breast irradiation with concomitant daily boost in early breast cancer. Am J Clin Oncol. 2015;38(4):358–363. doi: 10.1097/COC.0b013e3182a46740. [DOI] [PubMed] [Google Scholar]
- 30.Ghannam A.A., Khedr R.A. An accelerated hypofractionated schedule with a daily concomitant boost after breast conservation surgery: the feasibility and toxicity. J Egypt Natl Canc Inst. 2016;28(1):39–44. doi: 10.1016/j.jnci.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Cante D., Franco P., Sciacero P. Hypofractionated whole-breast radiotherapy and concomitant boost after breast conservation in elderly patients. Tumori. 2016;102(2):196–202. doi: 10.5301/tj.5000402. [DOI] [PubMed] [Google Scholar]
- 32.McDonald M.W., Godette K.D., Whitaker D.J., Davis L.W., Johnstone P.A. Three years outcomes of breast intensity-modulated radiation therapy whit simultaneous integrated boost. Int J Radiat Oncol Biol Phys. 2010;77(2):523–530. doi: 10.1016/j.ijrobp.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 33.Cante D., Franco P., Sciacero P. Hypofractionation and concomitant boost to deliver adjuvant whole-breast radiation in ductal carcinoma in situ (DCIS): a subgroup analysis of a prospective case series. Med Oncol. 2014;31(2):838–844. doi: 10.1007/s12032-014-0838-2. [DOI] [PubMed] [Google Scholar]
- 34.Nilsson C., Valachis A. The role of boost and hypofractionation as adjuvant radiotherapy in patients with DCIS: a meta-analysis of observational studies. Radiother Oncol. 2015;114(1):50–55. doi: 10.1016/j.radonc.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Moran M.S., Zhao Y., Ma S. Association of radiotherapy boost for ductal carcinoma in situ with local control after whole-breast radiotherapy. JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2016.6948. Published online March 30. [DOI] [PMC free article] [PubMed] [Google Scholar]